Nanocasting synthesis of Fe3O4@HTC nanocapsules and their superior electromagnetic properties†
Abstract
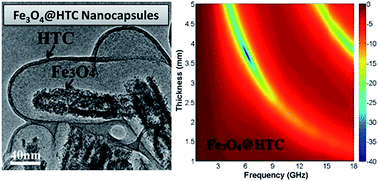
Magnetic nanocapsules with Fe3O4 nanorods as the core and hydrothermal carbon (HTC) as the shell have been synthesized using a nanocasting method. Due to the strong shape anisotropy of the nanorods, the nanocapsules exhibit a higher coercivity and increased resonance at the high frequency range. Benefiting from the improvement of dielectric loss and magnetic loss at high frequency, the Fe3O4@HTC nanocapsule composites show superior microwave attenuation properties.

 Please wait while we load your content...
Please wait while we load your content...