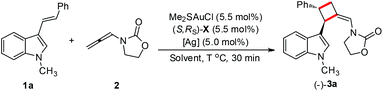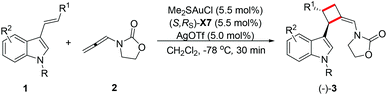Enantioselective gold-catalyzed intermolecular [2 + 2]-cycloadditions of 3-styrylindoles with N-allenyl oxazolidinone†
Haoxiang
Hu
a,
Yidong
Wang
a,
Deyun
Qian
a,
Zhan-Ming
Zhang
a,
Lu
Liu
*a and
Junliang
Zhang
*ab
aShanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N. Zhongshan Road, Shanghai 200062, China. E-mail: lliu@chem.ecnu.edu.cn; jlzhang@chem.ecnu.edu.cn
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, CAS, 345 Lingling Road, Shanghai 200032, China
First published on 20th April 2016
Abstract
The enantioselective intermolecular [2 + 2] cycloaddition of 3-styrylindoles with N-allenyl oxazolidinone has been achieved for the first time by the employment of a Xiang-Phos derived chiral gold-catalyst. The corresponding cycloadducts could be obtained in good yields (up to 95%) with up to 95% ee.
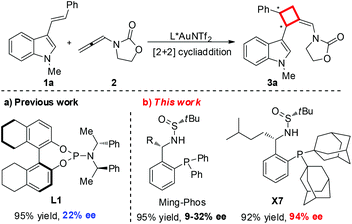
Gold catalysis, due to its remarkable catalytic capacity for the activation of C–C multiple bonds, has drawn tremendous attention and has been utilized broadly in the construction of complex molecules over the past decade.1 However, the development of asymmetric gold catalysis remains a long-standing challenge due to the inherent linear geometry of gold(I) complexes, which forces the catalytic active site far away from the chiral ligand, thus limiting the enantioselective control in the reaction process.2 To date, only a few of chiral ligands including atropisomeric biaryl phosphines, spirocyclic bisphosphines and phosphoramidites with bulky or extended substituents have been employed more successfully and maturely in gold catalysis.3,4 Therefore, the development of novel chiral ligands for gold catalysis would be highly desirable. Ideally, the salient features of the ligands include air and moisture stability, ease of handling, and being made from inexpensive and readily available chiral resources through a short synthetic route. Following these principles for the design of new chiral ligands, we recently developed a new type of air-stable chiral ligand (Ming-Phos) bearing the chiral sulfinamide moiety from readily available starting materials in two steps, which has been well applied in asymmetric gold-catalysis.5
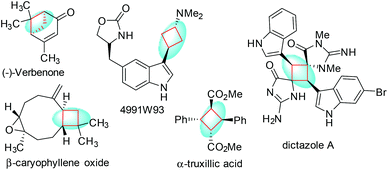
Chiral cyclobutanes are ubiquitous in natural products, bioactive compounds and versatile intermediates in synthetic chemistry (Fig. 1).6 Meanwhile, over the past decade, gold-catalyzed intermolecular cycloadditions of two unsaturated components have shown their power in the rapid access to various cyclic and polycyclic ring systems in an extremely efficient and stereoselective manner. In this context, asymmetric gold-catalyzed [2 + 2] cycloadditions7 of alkenes with N-allenamides8 are believed to be powerful and reliable methods to construct this skeleton. For example, González et al. disclosed the first enantioselective gold-catalyzed [2 + 2] cycloaddition reaction of N-tosyl allenamides and alkenes with the aid of chiral phosphoramidite ligands.7e Last year, M. Bandini et al. reported enantioselective gold catalyzed dearomative [2 + 2]-cycloaddition between indoles and allenamides.7f Very recently, we reported the first asymmetric [2 + 2] cycloaddition of N-tosyl allenamides and 3-styrylindoles using the phosphoramidite ligand L1, affording the corresponding cycloadducts in excellent enantioselectivity.9 However, this catalytic system cannot give acceptable enantioselectivity (22% ee) for the [2 + 2] cycloaddition of 3-styrylindole 1a with N-allenyl oxazolidinone (Scheme 1a). With diverse Ming-Phoses4 in hand, we wondered whether this new type of chiral ligand could address this low enantioselectivity issue or not. Unfortunately, no satisfactory results were obtained (9–32% ee, please see more details in the ESI†). We envisioned that the replacement of the phenyl group of the P substituents in the Ming-Phos by the more sterically bulky group might bring benefit to enhance the enantioselectivity control. Thus, a series of Xiang-Phoses bearing two bulky adamantyl groups on the P atom were designed and synthesized (Scheme 2a). Herein, we wish to share the performance of Xiang-Phos in excellent regio, diastereo and enantioselective gold-catalyzed asymmetric [2 + 2] cycloadditions between 3-styrylindoles and N-allenyl oxazolidinone (Scheme 1b).
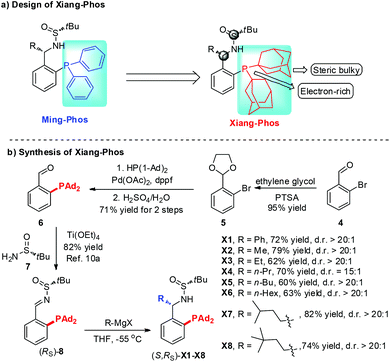 | ||
| Scheme 2 The design and concise synthetic approach to new chiral sufinamide monophosphines Xiang-Phos. | ||
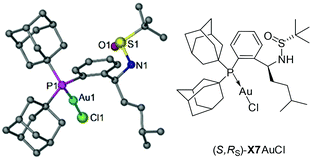
To test our hypothesis, a series of Xiang-Phoses (X1–X8) were prepared according to the synthetic route depicted in Scheme 2b. O-Phosphino benzaldehyde 6 was obtained in good yield from 2-bromobenzaldehyde 4via the process involving acetalation, C–P coupling with di-1-adamantylphosphine and hydrolysis. The condensation of 6 with chiral (RS)-sulfinamide 7, followed by diastereoselective addition with various Grignard reagents, would afford the corresponding Xiang-Phos (S,RS)-X1–X8 in good yields with high diastereoselectivities. The absolute configuration of the gold complex (S,RS)-X7AuCl was established by single-crystal X-ray analysis (Scheme 3).10
With a series of Xiang-Phoses in hand, we concentrated our investigation on their performance in the enantioselective [2 + 2] cycloaddition of 3-styrylindole 1a and allenamide 2 (Table 1). Compared to that of Ming-Phoses, the desired product (−)-3a was obtained in a better yield but with similar enantioselectivity when (S,RS)-X1 was employed (Table 1, entry 1). In contrast, the (S,RS)-X2 bearing a methyl group gave a promising enantioselectivity (Table 1, entry 2). Encouraged by this result, various Xiang-Phoses equipped with various alkyl groups (S,RS)-X3–X8 were examined (Table 1, entries 3–8). Gratifyingly, (−)-3a was obtained in 92% isolated yield with 88% ee by employing (S,RS)-X7 with the isopentyl substituent at −50 °C (Table 1, entry 7). The counteranion effect was also observed in this reaction. A mixture of a gold complex with AgOTf displayed a higher enantioselectivity (91% ee) than that with AgNTf2, AgBF4 and AgOMs (Table 1, entries 7, 9–11). Variation of the solvent did not lead to better result. However, a slightly better result was obtained by running the reaction at lower temperature (−78 °C) did not lead to better result, affording (−)-3a in 92% yield with 94% ee (Table 1, entry 15). Increasing or lowering the catalyst loading did not improve the yield and enantioselectivity (Table 1, entries 16 and 17). It should be noted that the reaction is not sensitive to moisture and it works well in wet solvent under air without any detriment to the enantioselectivity or yield.
| Entry | Ligand | [Ag] | Solvent | T (°C) | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|---|
| a Unless otherwise specified, all the reactions were carried out with 1a (0.11 mmol), 2 (0.1 mmol), Me2SAuCl (5.5 mol%), ligand (5.5 mol%), and [Ag] (5 mol%) in solvent (2 mL) for 30 min. b Yield of the isolated product. c Determined by HPLC analysis using a chiral stationary phase. d 10 mol% of the catalyst was used. e 2.5 mol% of the catalyst was used. | ||||||
| 1 | (S,RS)-X1 | AgNTf2 | CH2Cl2 | −50 | 84 | 19 |
| 2 | (S,RS)-X2 | AgNTf2 | CH2Cl2 | −50 | 87 | 74 |
| 3 | (S,RS)-X3 | AgNTf2 | CH2Cl2 | −50 | 86 | 82 |
| 4 | (S,RS)-X4 | AgNTf2 | CH2Cl2 | −50 | 85 | 83 |
| 5 | (S,RS)-X5 | AgNTf2 | CH2Cl2 | −50 | 88 | 85 |
| 6 | (S,RS)-X6 | AgNTf2 | CH2Cl2 | −50 | 91 | 83 |
| 7 | (S,RS)-X7 | AgNTf2 | CH2Cl2 | −50 | 92 | 88 |
| 8 | (S,RS)-X8 | AgNTf2 | CH2Cl2 | −50 | 89 | 70 |
| 9 | (S,RS)-X7 | AgOTf | CH2Cl2 | −50 | 91 | 91 |
| 10 | (S,RS)-X7 | AgBF4 | CH2Cl2 | −50 | 88 | 90 |
| 11 | (S,RS)-X7 | AgO3SCH3 | CH2Cl2 | −50 | 84 | 78 |
| 12 | (S,RS)-X7 | AgOTf | (CH2Cl)2 | −40 | 91 | 78 |
| 13 | (S,RS)-X7 | AgOTf | CHCl3 | −50 | 91 | 87 |
| 14 | (S,RS)-X7 | AgOTf | CH2Cl2 | −60 | 90 | 92 |
| 15 | (S,RS)-X7 | AgOTf | CH2Cl2 | −78 | 92 | 94 |
| 16d | (S,RS)-X7 | AgOTf | CH2Cl2 | −78 | 92 | 94 |
| 17e | (S,RS)-X7 | AgOTf | CH2Cl2 | −78 | 91 | 90 |
With the optimal conditions in hand, we then examined the generality of this asymmetric cycloaddition of N-allenyl oxazolidinone 2 with various 3-styrylindoles 1 (Table 2). The substrate scope is quite general and all the desired products 3 were obtained in good yields (up to 92%) with good to excellent enantioselectivities (up to 95% ee) as a single (Z)-stereoisomer (Table 2, entries 1–19). Notably, both electron-withdrawing groups (1b–1f) and electron-donating groups (1g–1i) attached to the phenyl rings and other electron-rich aromatic rings (1j–1k) on the alkenes slightly affect the reactivity and enantioselectivity of the reaction (Table 2, entries 1–10). The reaction of 1 with the substituents (methyl or bromo) on different positions of the indole moiety afforded the desired cycloadducts (3l–3p) in 59–84% yields with 81–91% ees (Table 2, entries 11–15). Furthermore, other N-substituents of 3-styrylindole 1 such as Bn and allyl are also suitable for this transformation (Table 2, entries 16–20). Unfortunately, the reaction of terminal olefin 1v gave a complicated mixture, which might be attributed to the self-polymerization (Table 1, entry 20). To our delight, the reaction of 1w with an aliphatic R1 (R1 = Me) group could afford the corresponding [2 + 2] cycloadduct 3w in 68% yield albeit with a moderate enantioselectivity (Table 2, entry 21, 69% ee), indicating that further ligand modification is necessary. The absolute configuration of the product (2S,3R)-3m was determined by single-crystal X-ray analysis,10 and all the other adducts were tentatively assigned.
| Entry | 1 | R/R1/R2 | 3 | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
| a Unless otherwise specified, all the reactions were carried out with 1a (0.11 mmol), 2a (0.1 mmol), Me2SAuCl (5.5 mol%), ligand (5.5 mol%), and [Ag] (5 mol%) in solvent (2 mL) for 30 min. b Isolated yield. c Determined by HPLC analysis using a chiral stationary phase. d The reaction is messy. | |||||
| 1 | 1b | Me/4-MeC6H4/H | 3b | 92 | 92 |
| 2 | 1c | Me/4-OMeC6H4/H | 3c | 86 | 93 |
| 3 | 1d | Me/4-tBuC6H4/H | 3d | 84 | 92 |
| 4 | 1e | Me/3-OMeC6H4/H | 3e | 83 | 91 |
| 5 | 1f | Me/3,5-MeC6H4/H | 3f | 77 | 95 |
| 6 | 1g | Me/4-BrC6H4/H | 3g | 89 | 89 |
| 7 | 1h | Me/4-ClC6H4/H | 3h | 84 | 91 |
| 8 | 1i | Me/4-FC6H4/H | 3i | 87 | 87 |
| 9 | 1j | Me/2-thienyl/H | 3j | 68 | 85 |
| 10 | 1k | Me/1-naphthyl/H | 3k | 72 | 89 |
| 11 | 1l | Me/Ph/4-Br | 3l | 84 | 84 |
| 12 | 1m | Me/4-BrC6H4/4-Br | 3m | 86 | 84 |
| 13 | 1n | Me/Ph/5-Me | 3n | 80 | 85 |
| 14 | 1o | Me/Ph/6-Me | 3o | 59 | 91 |
| 15 | 1p | Me/Ph/7-Me | 3p | 73 | 81 |
| 16 | 1q | Bn/Ph/H | 3q | 83 | 92 |
| 17 | 1r | Bn/4-OMeC6H4/H | 3r | 81 | 95 |
| 18 | 1s | Bn/4-tBuC6H4/H | 3s | 84 | 93 |
| 19 | 1t | Bn/4-ClC6H4/H | 3t | 86 | 86 |
| 20 | 1u | Allyl/Ph/H | 3u | 86 | 93 |
| 21d | 1v | Me/H/H | — | — | — |
| 22 | 1w | Me/Me/H | 3w | 68 | 69 |
It should be noted that this gold-catalyzed enantioselective [2 + 2] cycloaddition of 3-styrylindoles with N-allenyl oxazolidinone is easy to scale-up. A gram-scale reaction of 1 g of 1a and 500 mg of 2 was carried out under the standard conditions, furnishing 1.18 g of the desired product 3a in 82% isolated yield with a slightly better enantioselectivity (95% ee, Scheme 4).
In summary, a series of Xiang-Phoses were prepared in good yields with high diastereoselectivity from commercially available, inexpensive starting materials in short steps. Wide diverse structures with various side chains can be achieved easily by changing the Grignard reagents R-MgX. Moreover, the (S,RS)-X7 derived gold catalyst has shown good performance in the intermolecular [2 + 2] cycloaddition of various 3-styrylindoles with 3-(1,2-propadienyl)-2-oxazolidinone, leading to the chiral cyclobutane products in good yields with up to 95% ee. Xiang-Phos will become very attractive due to the salient features of these ligands including air and moisture stability, ease of handling, and diverse structural tuning. Further studies including the application of Xiang-Phos in other transition metal catalyzed asymmetric transformations will be reported in due course.
We are grateful to the Shanghai Pujiang Program (14PJ1403100), the 973 program (2015CB856600), the National Natural Science Foundation of China (21572065, 21425205, 21372084), and the Shanghai Eastern Scholar Program for financial support.
Notes and references
- For leading reviews on gold catalysis, see: (a) A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180 CrossRef CAS PubMed; (b) E. Jiménez-Núñez and A. M. Echavarren, Chem. Rev., 2008, 108, 3326 CrossRef PubMed; (c) D. J. Gorin, B. D. Sherry and F. D. Toste, Chem. Rev., 2008, 108, 3351 CrossRef CAS PubMed; (d) S. M. A. Sohel and R.-S. Liu, Chem. Soc. Rev., 2009, 38, 2269 RSC; (e) A. Fürstner, Chem. Soc. Rev., 2009, 38, 3208 RSC; (f) A. Corma, A. Leyva-Perez and M. J. Sabater, Chem. Rev., 2011, 111, 1657 CrossRef CAS PubMed; (g) C. Aubert, L. Fensterbank, P. Garcia, M. Malacria and A. Simonneau, Chem. Rev., 2011, 111, 1954 CrossRef CAS PubMed; (h) N. Krause and C. Winter, Chem. Rev., 2011, 111, 1994 CrossRef CAS PubMed; (i) M. Bandini, Chem. Soc. Rev., 2011, 40, 1358 RSC; (j) B.-L. Lu, L. Dai and M. Shi, Chem. Soc. Rev., 2012, 41, 3318 RSC; (k) D. Garayalde and C. Nevado, ACS Catal., 2012, 2, 1462 CrossRef CAS; (l) F. López and J. L. Mascareñas, Beilstein J. Org. Chem., 2013, 9, 2250 CrossRef PubMed; (m) W. Yang and A. S. K. Hashmi, Chem. Soc. Rev., 2014, 43, 2941 RSC; (n) F. López and J. L. Mascareñas, Chem. Soc. Rev., 2014, 43, 2904 RSC; (o) C. Obradors and A. M. Echavarren, Acc. Chem. Res., 2014, 47, 902 CrossRef CAS PubMed; (p) L. Zhang, Acc. Chem. Res., 2014, 47, 877 CrossRef CAS PubMed; (q) D. Qian and J. Zhang, Chem. Soc. Rev., 2015, 44, 677 RSC; (r) L. Liu and J. Zhang, Chem. Soc. Rev., 2016, 45, 506 RSC; (s) Z. Zheng, Z. Wang, Y. Wang and L. Zhang, Chem. Soc. Rev., 2016 10.1039/c5cs00887e.
- (a) A. S. K. Hashmi, Angew. Chem., Int. Ed., 2010, 49, 5232 CrossRef CAS PubMed; (b) D. J. Gorin and F. D. Toste, Nature, 2007, 446, 395 CrossRef CAS PubMed; (c) A. Fürstner and P. W. Davies, Angew. Chem., Int. Ed., 2007, 46, 3410 CrossRef PubMed; (d) M. C. Gimeno and A. Laguna, Chem. Rev., 1997, 97, 511 CrossRef CAS PubMed; (e) P. Pyykkö, Angew. Chem., Int. Ed., 2004, 43, 4412 CrossRef PubMed; (f) G. L. Hamilton, E. J. Kang, M. Mba and F. D. Toste, Science, 2007, 317, 496 CrossRef CAS PubMed.
- For asymmetric gold catalysis with chiral atropisomeric biaryl phosphines or spirocyclic bisphosphines, see: (a) F. Liu, D. Qian, L. Li, X. Zhao and J. Zhang, Angew. Chem., Int. Ed., 2010, 49, 6669 CrossRef CAS PubMed; (b) G. Zhou, F. Liu and J. Zhang, Chem. – Eur. J., 2011, 17, 3101 CrossRef CAS PubMed; (c) S. G. Sethofer, T. Mayer and F. Dean Toste, J. Am. Chem. Soc., 2010, 132, 8276 CrossRef CAS PubMed; (d) S. A. Gawade, S. Bhunia and R.-S. Liu, Angew. Chem., Int. Ed., 2012, 51, 7835 CrossRef CAS PubMed; (e) J. F. Briones and H. M. L. Davies, J. Am. Chem. Soc., 2012, 134, 11916 CrossRef CAS PubMed; (f) J. F. Briones and H. M. L. Davies, J. Am. Chem. Soc., 2013, 135, 13314 CrossRef CAS PubMed; (g) Z.-Y. Cao, X. Wang, C. Tan, X.-L. Zhao, J. Zhou and K. Ding, J. Am. Chem. Soc., 2013, 135, 8197 CrossRef CAS PubMed; (h) Y.-M. Wang, A. D. Lackner and F. D. Toste, Acc. Chem. Res., 2014, 47, 889 CrossRef CAS PubMed; (i) B. Ma, T. Miao, Y. Sun, Y. He, J. Liu, Y. Feng, H. Chen and Q.-H. Fan, Chem. – Eur. J., 2014, 20, 9969 CrossRef CAS PubMed; (j) D.-Y. Zhu, Z. Zhang, X.-Q. Mou, Y.-Q. Tu, F.-M. Zhang, J.-B. Peng, S.-H. Wang and S.-Y. Zhang, Adv. Synth. Catal., 2015, 357, 747 CrossRef CAS. For earlier examples, see: (k) M. P. Muz, J. Adrio, J. C. Carretero and A. M. Echavarren, Organometallics, 2005, 24, 1293 CrossRef; (l) M. J. Johansson, D. J. Gorin, S. T. Staben and F. D. Toste, J. Am. Chem. Soc., 2005, 127, 18002 CrossRef CAS PubMed; (m) Z. Zhang and R. A. Widenhoefer, Angew. Chem., Int. Ed., 2007, 46, 283 CrossRef CAS PubMed; (n) R. L. LaLonde, B. D. Sherry, E. J. Kang and F. D. Toste, J. Am. Chem. Soc., 2007, 129, 2452 CrossRef CAS PubMed.
- For asymmetric gold catalysis with chiral phosphoramidites, see: (a) I. Alonso, B. Trillo, F. López, S. Montserrat, G. Ujaque, L. Castedo, A. Lledós and J. L. Mascareñas, J. Am. Chem. Soc., 2009, 131, 13020 CrossRef CAS PubMed; (b) H. Teller, S. Flügge, R. Goddard and A. Fürstner, Angew. Chem., Int. Ed., 2010, 49, 1949 CrossRef CAS PubMed; (c) A. Z. González, D. Benitez, E. Tkatchouk, W. A. Goddard III and F. D. Toste, J. Am. Chem. Soc., 2011, 133, 5500 CrossRef PubMed; (d) H. Teller, M. Corbet, L. Mantilli, G. Gopakumar, R. Goddard, W. Thiel and A. Fürstner, J. Am. Chem. Soc., 2012, 134, 15331 CrossRef CAS PubMed; (e) S. Suárez-Pantiga, C. Hernández-Díaz, E. Rubio and J. M. González, Angew. Chem., Int. Ed., 2012, 124, 11720 ( Angew. Chem., Int. Ed. , 2012 , 51 , 11552 ) CrossRef; (f) B. Liu, K.-N. Li, S.-W. Luo, J.-Z. Huang, H. Pang and L.-Z. Gong, J. Am. Chem. Soc., 2013, 135, 3323 CrossRef CAS PubMed; (g) D. Qian, H. Hu, F. Liu, B. Tang, W. Ye, Y. Wang and J. Zhang, Angew. Chem., Int. Ed., 2014, 53, 13751 CrossRef CAS PubMed; (h) Y. Wang, P. Zhang, D. Qian and J. Zhang, Angew. Chem., Int. Ed., 2015, 54, 14849 CrossRef CAS PubMed; (i) Q. Cheng, Y. Wang and S.-L. You, Angew. Chem., Int. Ed., 2016, 55, 3496 CrossRef CAS PubMed.
- Z.-M. Zhang, P. Chen, W. Li, Y. Niu, X.-L. Zhao and J. Zhang, Angew. Chem., Int. Ed., 2014, 53, 4350 CrossRef CAS PubMed.
- (a) R. C. Nieuwendaal, M. Bertmer and S. E. Hayes, J. Phys. Chem. B, 2008, 112, 12920 CrossRef CAS PubMed; (b) G. Ohloff and W. Giersch, Helv. Chim. Acta, 1977, 60, 1496 CrossRef CAS; (c) W. R. Gutekunst and P. S. Baran, J. Org. Chem., 2014, 79, 2430 CrossRef CAS PubMed; (d) K. S. Jandu, V. Barrett, M. Brockwell, D. Cambridge, D. R. Farrant, C. Foster, H. Giles, R. C. Glen, A. P. Hill, H. Hobbs, A. Honey, G. R. Martin, J. Salmon, D. Smith, P. Woollardand and D. L. Selwood, J. Med. Chem., 2001, 44, 681 CrossRef CAS PubMed; (e) K. H. Schulte-Elte and G. Ohloff, Helv. Chim. Acta, 1968, 51, 494 CrossRef CAS.
- For racemic [2 + 2]-cycloadditions, see: (a) S. Suárez-Pantiga, C. Hernández-Díaz, M. Piedrafita, E. Rubio and J. M. González, Adv. Synth. Catal., 2012, 354, 1651 CrossRef; (b) X.-X. Li, L.-L. Zhu, W. Zhou and Z. Chen, Org. Lett., 2012, 14, 436 CrossRef CAS PubMed; (c) H. Faustino, P. Bernal, L. Castedo, F. López and J. L. Mascareñas, Adv. Synth. Catal., 2012, 354, 1658 CrossRef CAS; (d) P. Bernal-Albert, H. Faustino, A. Gimeno, G. Asensio, J. L. Mascareñas and F. López, Org. Lett., 2014, 16, 6196 CrossRef CAS PubMed. For asymmetric version, see: (e) S. Suárez-Pantiga, C. Hernández-Díaz, E. Rubio and J. M. González, Angew. Chem., Int. Ed., 2012, 51, 11552 CrossRef PubMed; (f) M. Jia, M. Monari, Q.-Q. Yang and M. Bandini, Chem. Commun., 2015, 51, 2320 RSC.
- For transformations of N-allenyl oxazolidinone, see: (a) L.-L. Wei, H. Xiong and R. P. Hsuang, Acc. Chem. Res., 2003, 36, 773 CrossRef CAS PubMed; (b) T. Lu, Z. Lu, Z.-X. Ma, Y. Zhang and R. P. Hsung, Chem. Rev., 2013, 113, 4862 CrossRef CAS PubMed; (c) M. M. Mastandrea, N. Mellonie, P. Giacinto, A. Collado, S. P. Nolan, G. P. Miscione, A. Bottoni and M. Bandini, Angew. Chem., Int. Ed., 2015, 54, 14885 CrossRef CAS PubMed; (d) V. Pirovano, L. Decataldo, E. Rossi and R. Viecnte, Chem. Commun., 2013, 49, 3594 RSC; (e) H. Faustino, I. Alonso, J. L. Mascareñas and F. López, Angew. Chem., Int. Ed., 2013, 52, 6526 CrossRef CAS PubMed; (f) H. Faustino, F. López, L. Castedo and J. L. Mascareñas, Chem. Sci., 2011, 2, 633 RSC; (g) S. Montserrat, H. Faustino, A. Lledós, J. L. Mascareñas, F. López and G. Ujaque, Chem. – Eur. J., 2013, 19, 15248 CrossRef CAS PubMed; (h) J. Francos, F. G. Carmona, H. Faustino, J. I. Sigüenza, E. Díez, I. Alonso, R. Fernández, J. M. Lassaletta, F. López and J. L. Mascareñas, J. Am. Chem. Soc., 2012, 134, 14332 CrossRef PubMed.
- Y. Wang, P. Zhang, Y. Liu, F. Xia and J. Zhang, Chem. Sci., 2015, 6, 5564 RSC.
- CCDC1456593 (3m), 1456594 (X7AuCl).
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1456593 and 1456594. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00087h |
| This journal is © the Partner Organisations 2016 |