A new highly sensitive phosphor for carbon ion dosimetry†
Bhushan P. Korea,
N. S. Dhobleb,
S. P. Lochabc and
S. J. Dhoble*a
aDepartment of Physics, RTM Nagpur University, Nagpur-440033, India. E-mail: bhushan.kore084@gmail.com
bDepartment of Chemistry, Sevadal Mahila Mahavidyalaya, Nagpur-440009, India
cInter-University Accelerator Center, Aruna Asaf Ali Marg, New Delhi-110067, India
First published on 19th September 2014
Abstract
Dy3+-doped CaMg3(SO4)4 (CMS) phosphor was prepared by the acid distillation method and examined in detail with a thermoluminescence (TL) study whereby the phosphor was irradiated with γ-rays and a carbon ion beam. A good dosimetric glow curve was observed that is stable against both types of radiation. The CMS doped with 0.2 mol% of Dy3+ showed 3.5 times more sensitivity than commercially available CaSO4:Dy3+ TLD phosphor when irradiated with a carbon ion beam. The observed glow curve variation and resultant variation in the values of trapping parameters with a change in ion beam energy suggest more complex interactions of ion beam within the phosphor at higher energies.
1. Introduction
The demand for the dosimetry of charged particle beams has taken a decisive lead because of its utility in cancer diagnosis and therapy.1–3 Ion beam therapy has been found to be a crucial tool in clinical practice for curing tumors in comparison to conventional radiation beams.4,5 Using ion beams in cancer therapy is based on the phenomenon of dose deposition at Bragg's peak. In comparison to conventional radiation therapy such as γ-rays and high energy photon beams, heavy charge ion therapy is superior. The heavy charge particles (HCP) such as C5+ ions are heavier than the constituent particles in conventional radiation, which ensures penetration into the final treatment locations deep inside the body with minimum scattering and stiffer particle trajectories, low straggling effects, and sharper field edges.4 Ion beam therapy appears to be a more favorable option with several advantages when compared to conventional photon therapy. Ion beams have a well-defined range and small angular scattering compared with conventional photon or electron beam radiotherapy. Conventional photon beams have a limited depth of penetration proportional to the energy of the accelerator.Beams of protons and carbon ions have a much more favorable dose-depth distribution than photons and are the new frontiers of cancer radiation therapy.5 Heavy ion beams deliver a larger mean energy per unit length of their trajectory in the body than proton and photon beams.6 Bone and soft-tissue tumors are usually surgically removed because they are generally radio-resistant, and the effect of photon radiation is not sufficient for long-term control. However, when surgery is difficult to perform, radiotherapy is often employed as a sole treatment. In this sense, carbon ion radiotherapy exhibits more significant biological effects than other types of therapy.7 These factors are crucial for tumors located close to critical organs such as the eye or ear. Tumors that are to be treated with higher doses require special care so that healthy tissues are not influenced by the incident ion beam. This opens up a promising potential for their highly effective use in the treatment of intractable cancers. For this, the dose delivered to the tumor requires an accurate calibration of radiotherapy sources and their proper dosimetry. These ideas encourage us to find a suitable TLD phosphor for carbon ion dosimetry.
For the high energies of heavy charged particles in the entrance region, low ionization density commonly produce repairable damages. However, with increased energy, energy loss towards the Bragg's peak is more significant, which produce irreparable damages and results in a higher relative biological efficiency (RBE). The biological effectiveness refers to the difference in rate at which cells are killed with the same radiation dose. We have selected the carbon ion for study because heavier and lighter ions possess their own drawbacks. The limitation of using heavier ions such as neon or argon is that they cause irreparable damages in the entrance channel (surface), and thus significantly damage the healthy tissues in front of the tumor.8 For very light ions such as protons, the scattering effect is large, and therefore, no damage potentiation (the increase in strength of nerve impulses along pathways that have been used previously) can be observed in the target volume.8 Hence, carbon ion beams represent a most favorable option in heavy ion therapy and for enhancing the biological efficiency in tumor therapy.9
Sulphate-based thermoluminescence (TL) materials are known for their high sensitive TL response. It has been found that mixed sulfates form a class of TL phosphors with good TL characteristics when doped with the appropriate activators.10–13 This family includes several materials such as CaSO4:Dy3+, K2Ca2(SO4)3:Eu, LiNaSO4:Eu, Na21Mg(SO4)10Cl3:Dy3+, and K3Na(SO4)2:Eu, which are studied due to their excellent thermoluminescent properties such as high TL sensitivity, high TL efficiency, linear dose response over a wide range of doses, and reproducibility. But all these phosphors suffer from one or the other problem. More work is going on either to improve TL properties of these existing materials or to develop new high sensitive TL phosphors with ideal TL characteristic. Therefore, there is a great demand for highly efficient dosimetry materials for radiation dose assessment and to meet technological challenges. Such efforts are also crucial in a number of other areas such as human exploration in outer space.14 Several methods and treatments have been adopted (1) to improve TL sensitivity, (2) to know the responsible defects in these materials, and (3) to understand the phenomenon of TL in more detail.
To the best of our knowledge, the literature describing the effect of carbon ion beams on phosphor materials especially with reference to dosimetry is very limited. This study is an attempt to obtain useful data regarding the TL response of carbon beam-irradiated highly sensitive phosphor, which would be helpful in the dosimetry of carbon ion beams.
2. Experimental
2.1. Synthesis method
The phosphor studied in the present work was synthesized using the method described by Yamashita et al.15 For the preparation of the Dy3+-doped CaMg3(SO4)4 (CMS) phosphor, all of the starting materials used were of analytical grade. CMS doped with different concentrations of Dy3+ was prepared by dissolving CaSO4, MgSO4, and a stoichiometric amount Dy2O3 15 ml of hot sulfuric acid (the excess acid used). The mixture was allowed to heat at approximately 300 °C for 20 h, and during heating, the highly active acid vapors were condensed using a water-cooled condenser assembly so as to prevent any spontaneous reactivity. After cooling the mixture to room temperature, excess acid in the sample was repeatedly washed out with distilled water, and a water-insoluble compound was obtained in the form of small crystals. After washing the sample 4 to 5 times with distilled water, the remaining sample was dried in an oven at 80 °C. No further heat treatment was given to the samples.2.2. Experimental details
5 mg of CMS phosphor was exposed to γ-rays from 60Co and 137Cs sources for various doses to see the glow curve structure variation with dose and the linearity of the phosphor. The samples in the form of pellets were irradiated at room temperature by a C5+ ion beam at energies of 50 MeV and 75 MeV for different ion fluences in the range of 15 × 1010 to 30 × 1012 ions per cm2, using a 16 MV tandem Van de Graaff type electrostatic accelerator (15 UD pelletron) at the Inter-University Accelerator Center, New Delhi, India. The full details of this setup are given by Kanjilal et al.16 For irradiation, the sample pellets were mounted on a copper target ladder, as shown in Fig. 1(a) and (b). The copper ladder prevents heating of the sample during swift heavy ion (SHI) irradiation. For irradiation, the ladder was kept inside the evacuated irradiation chamber, as shown in Fig. 1(c). The ion beams were magnetically scanned on a 10 mm × 10 mm area of sample surfaces for uniform irradiation. The beam spot size used was 2.5 mm2. The pressure of the vacuum chamber during ion beam irradiation was 5 × 10−4 mbar.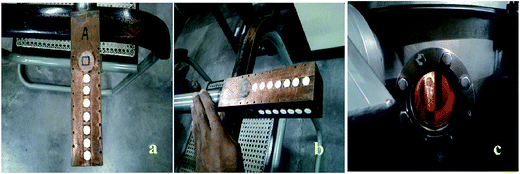 | ||
| Fig. 1 (a) and (b) Copper ladder with pellets of CMS sample mounted on it; (c) inner view of the C5+ ion irradiation chamber. | ||
The diffraction pattern of the CMS phosphor was examined using synchrotron XRD (SXRD) measurements at the ADXRD beamline (BL-12) of the Indus-2 synchrotron source at the Raja Ramanna Center for Advanced Technology (RRCAT), Indore, India.17,18 An image plate area detector (Mar 345 Dtb) was used to record the diffraction pattern. Lanthanum hexaborate (LaB6) was used as a standard material for calibration of the beam energy and the sample to detector distance. The wave-length used was 0.77774 Å. The TL glow curves were recorded using a Harshaw TLD reader (Model 3500) fitted with a 931B photomultiplier tube (PMT). The heating rate used was 5 °C s−1. The photoluminescence (PL) emission spectra of the samples were recorded using a RF-5301 PC Shimadzu spectrofluorophotometer. Emission and excitation spectra were recorded using a spectral slit width of 1.5 nm.
3. Results and discussion
3.1. X-ray diffraction
The X-ray diffraction pattern of the CMS phosphor was recorded using the beam energy of 15.94 keV with a sample-to-image plate distance of 151.2 mm. These refined values were obtained using the FIT2D program, and the image plate data files were also integrated using FIT2D, incorporating polarization correction.19 Fig. 2 shows more detail regarding the phase of this phosphor by presenting the high resolution synchrotron X-ray diffraction data collected at room temperature. The angle dispersive synchrotron XRD patterns are nearly pure phase and in good agreement with the standard ICDD file no. 19-0241. The different Dy3+ concentration-doped XRD patterns of the CMS phosphor are shown in Fig. S1.†,20 All four XRD patterns are almost identical, which suggests that the incorporation of Dy3+ into the CMS lattice does not influence the crystal structure. Fig. S2† shows the modification of the XRD pattern of the CMS phosphor after C5+ ion beam irradiation. A video illustrating the ion beam irradiation on sample pellets is also given in the ESI (file M1†). No new peaks were observed after ion beam irradiation, indicating no change in phase of the phosphor. Due to ion beam irradiation, the relative intensities of some dominant peaks change, and some minor peaks are diminished. This alteration is small, and indicates that there was a small reduction in the crystallinity of the phosphor after ion beam irradiation. Therefore, we can say that this phosphor is stable against C5+ ion beam irradiation. | ||
| Fig. 2 (a) High-resolution synchrotron X-ray diffraction patterns of the CMS phosphor; (b) the raw image plate X-ray diffraction data. | ||
3.2. SEM study
The SEM images of the as-synthesized samples are shown in Fig. 3. The large particles were formed when the acid distillation method was used, and some grains have a disk shape while others are irregularly shaped with fine surfaces. The figure shows the microstructures consisting of large particles in the 5–15 μm range, which would enable the phosphor to be useful in dosimetry application.213.3. TL study
To compare the TL sensitivity, we also measured the TL of the standard TLD (CaSO4:Dy3+) phosphor. It was observed that the sensitivity of the CMS phosphor is approximately 50% compared to the sensitivity of CaSO4:Dy3+. Fig. 4(b) shows the TL glow curve of the CMS phosphor for γ-ray irradiation from the 137Cs source at 2300 mRad. The glow curve shape of the 137Cs-irradiated samples was found to be similar to 60Co-irradiated samples, but there is a shift in the glow peak position towards lower temperatures when irradiated with the 137Cs source. The observed shift was approximately 3 °C for peak 1 and 28 °C for peak 2. This shift may be due to the alteration in the position of trapping levels inside the forbidden band gap. As can be seen from Fig. 4(c), the glow curve structures of CMS exposed to carbon ions (50 and 75 MeV) are similar to that of γ-irradiated samples. The glow curves of CMS exposed to two different energies of carbon ion beams are qualitatively similar, but quantitatively, there is a difference in the temperature and relative intensities of the glow peaks, which leads to the modifications in the trapping parameters. Various authors have reported significant variations in the glow curve structures and the positions when samples were irradiated with different sources.22–24 The CMS phosphor was found to possess a glow curve that is stable with variation in irradiation species, as the carbon ion- and γ-ray-irradiated samples show 11 and 8% fading, respectively, over 16 days of storage (see Section 3.6). In comparison to our phosphors, the standard TLD phosphor CaSO4:Dy3+ showed significant change in the shape of the glow curve when exposed to the carbon ion beam.
As can be seen in Fig. 4, the glow curve structure of CMS exposed to carbon ions at two different energies (i.e., 50 and 75 MeV) is similar to that of the 137Cs and 60Co γ-irradiated sample. There is a small difference between the TL glow peak temperature for the 60Co and 137Cs γ-irradiated sample. In the case of γ-irradiated samples using the 60Co source, the second peak at 260 °C is more prominent than the lower temperature peak at 146 °C when compared with TL glow curves of γ-irradiated samples using the 137Cs source. The lower temperature peak shows significant growth compared to the high temperature peak. This clearly indicates that the number of traps responsible for these peaks is not in the same proportion in the two cases. For the 75 MeV C5+ ion-irradiated samples, additional trapping levels form, which yield two more glow peaks compared to γ-ray-irradiated samples. The occurrence of additional glow peaks at 185 °C and 232 °C suggests the formation of intermediate trapping levels in-between the trapping levels responsible for the occurrence of glow peaks at 143 °C and 242 °C. Moreover, the C5+ ion-irradiated samples (50 MeV) show significant change in the position as well as intensity of the glow curve compared to glow curves of C5+ ion-irradiated samples at 75 MeV. The C5+ ion irradiation at 50 MeV not only alters the positions of trapping levels, causing a shift in the glow peak temperatures, but it also creates one additional trapping level that results in an additional glow peak. Upon irradiating the samples with C5+ ions, there may be the formation of new trapping and luminescent centers that are responsible for this anomalous behavior. Similar effects in the TL response of the CaSO4:Dy3+ phosphor upon C6+ ion beam irradiation have been reported by some authors.25
It is expected that upon irradiating samples with a highly energetic ion beam such as that of 50 and 75 MeV C5+ ions, there will be changes in the TL glow curve structure because the TL trapping and recombination mechanisms are very sensitive to any perturbation. The variation is more when atomic displacements occur due to non-elastic collisions, and ionization due to secondary particles takes place.26 The obtained results show that there is not much variation in the structure of the glow curve, but there are minor changes in glow peak temperature, number of glow peaks, and TL intensity, which are responsible for the variation in trapping parameters. These variations in trapping parameters are due to the disorganization of the initial localized energy levels in the mixed sulfate host as a result of the high energy ion irradiation.
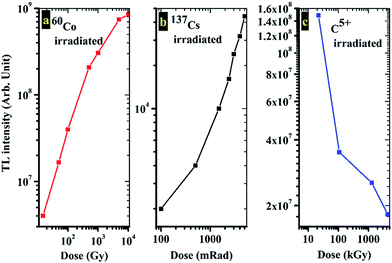 | ||
| Fig. 5 The TL response curves of the CMS phosphor irradiated by γ-rays from (a) 60Co, (b) 137Cs, and (c) C5+ ion beams. | ||
The influence of 50 and 75 MeV carbon ion beams is definitely more than that of γ-rays, and therefore, the corresponding cross section of C5+ ion tracks inside the CMS phosphor is higher. Low linear energy transfer (LET) radiation such as γ-rays and electrons confers higher luminescence efficiency as compared to high LET radiation consisting of heavily charged particles. However, high LET radiation may induce additional defects in the host material as compared to low LET radiation, and with increase in the radiation exposure, the density of defects inside the host increases, which leads to an increase in peak intensity.
The dose delivered by carbon beam was calculated using eqn (1):
 | (1) |
3.4. PL study
The photoluminescence (PL) excitation and emission spectra for different mol% concentrations of Dy3+-doped CMS pristine phosphor are shown in Fig. 6(a). The excitation spectrum of pristine CMS phosphor shows a number of excitation peaks with a prominent peak at approximately 349 nm. The emission spectrum that was recorded at this excitation wavelength shows characteristic emission peaks of Dy3+ at approximately 484 nm and 574 nm, which were assigned to the transitions 4F9/2 → 6H15/2 and 4F9/2 → 6H13/2, respectively.30 Fig. 6(b) shows the PL excitation and emission spectrum of 1 mol% Dy3+-doped CMS phosphor irradiated with 50 and 75 MeV carbon ion along with that of pristine phosphor. From the excitation and emission spectra, it was observed that phosphor exposed with a carbon ion beam exhibited a decrease in PL emission intensity, and with a further increase in the energy of the carbon ion beam, the emission intensity continued to decrease. There are several possible reasons for this decrease: (1) destruction of luminescence centers that are responsible for emission, (2) residual absorption of both the excitation light as well as emission light due to the irradiation, and (3) damage to the microstructure of the phosphor due to ion-induced defects.31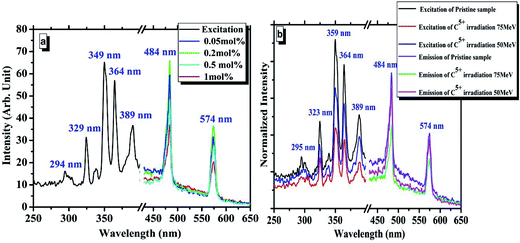 | ||
| Fig. 6 (a) PL of the CMS phosphor for different concentrations of Dy3+; (b) PL of carbon ion-irradiated CMS (Dy3+ = 0.2 mol%) phosphor at 50 and 75 MeV and pristine sample. | ||
At high ion fluences, structural changes occur inside the sample that may reduce its PL output. This effect is directly influenced by the energy loss of the ions and thus by the size and the damage concentration. The observed saturation in the TL glow curves and Dy3+ PL emission of the samples exposed to the 75 MeV C5+ ion beam might be due to the increased penetration depth of the ion beam. However, for the 50 MeV ion beam, the penetration is less and the backscattering could be more, resulting in a smaller number of implanted ions; therefore, it still exhibits a higher TL and PL efficiency.
3.5. Glow curve analysis
To further verify the energy levels and other kinetic parameters of the glow peaks in all three cases, the glow curve deconvolution was performed using glow curve deconvolution (GCD) functions as previously reported by Kitis et al.32For general order,
 | (2) |
These parameters were applied to the experimentally obtained glow curves to isolate each peak. Firstly, the order of kinetics and activation energy of one of the peaks was found using Chen's set of empirical formulae.33
The trap depth is calculated using the following equation, which is independent of order of kinetics:
| E = cγ(kTm2/γ) − bγ(2kTm) | (3) |
The frequency factor (s) is:
 | (4) |
To determine the order of kinetics (b), the form factor μg (μg = (T2 − Tm)/(T2 − T1)), which involves T1 and T2 (temperatures corresponding to half of the intensities on either side of the maximum), was calculated. This form factor μg is independent of the activation energy (E) and strongly depends on the order of kinetics (b). Finally, the peak was theoretically generated using these parameters and separated from the main experimental glow curve. The benefit of using the GCD method is that most of the parameters used for generating a theoretical curve are easily derived from the experimentally recorded glow curve. The thermal activation energy (E) was again calculated using the same set of equations. This procedure was repeated for each TL peak until a good fit between the experimental and theoretical glow curve was obtained.
The deconvolution of experimentally obtained glow peaks was carried out using the glow curve deconvolution program. The deconvolution of experimental glow curves was carried out in order to reveal the number of individual glow peaks present in a complex glow curve, as shown in Fig. 7. The isolated glow peaks were then examined to obtain data regarding trapping parameters. The generation of new absorption peaks with the increasing energy of the carbon ion beam also illustrates the changing position of the trapping levels (see Table 1).
| Type of irradiation | Peak number | Tm (°C) | μg | E (eV) | S (s−1) |
|---|---|---|---|---|---|
| 60Co | 1 | 146 | 0.44 | 0.60 | 4.11 × 106 |
| 2 | 258 | 0.48 | 0.61 | 9.07 × 104 | |
| 137Cs | 1 | 145 | 0.5 | 0.83 | 2.6 × 109 |
| 2 | 230 | 0.5 | 0.87 | 9.3 × 107 | |
| C5+ 75 MeV | 1 | 143 | 0.5 | 0.93 | 5.5 × 1010 |
| 2 | 185 | 0.5 | 0.67 | 4.44 × 106 | |
| 3 | 232 | 0.48 | 0.43 | 1.64 × 103 | |
| 4 | 242 | 0.5 | 1.21 | 2.3 × 1011 | |
| C5+ 50 MeV | 1 | 167 | 0.5 | 1.09 | 9.14 × 1011 |
| 2 | 234 | 0.48 | 0.36 | 2.9 × 102 | |
| 3 | 280 | 0.49 | 1.25 | 6.51 × 1010 |
3.6. Fading study
For the fading study, the γ-ray- and carbon ion-irradiated samples were stored for a few days without taking any precautions to shield them from light or moisture. The glow curves were then recorded for a period of approximately 16 days, as shown in Fig. 8, which illustrates the fading plot of the γ-ray- and carbon ion-irradiated phosphor. It was observed that the higher temperature glow peak is quite stable over the storage time. The carbon ion- and γ-ray-irradiated samples exhibited 11 and 8% fading, respectively, over 16 days of storage.3.7. Absorption spectra
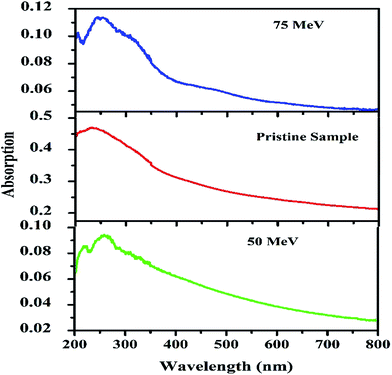
Fig. 9 shows the absorption spectra of the CMS phosphor for 0.2 mol% Dy3+ doping. The calculated band gap energy of the pristine sample is 5.34 eV. It was observed that the absorption of the phosphor increases with increasing energy of the ion beam, which is due to the generation of a large number of defects. The absorption was found to be shifted towards a higher wavelength with increases in beam energy. The difference between the band gap energy and the energy of the absorption peak represents the activation energy of trapped charge carriers. The sample irradiated by the 50 MeV ion beam demonstrated absorption at 219, 258, and 317 nm corresponding to 5.66, 4.80, and 3.911 eV energies, respectively; the 75 MeV ion beam resulted in absorption at 204, 243, 254, 313, and 459 nm corresponding to 6.07, 5.10, 4.88, 3.96, and 2.70 eV energies, respectively. The activation energies calculated from the absorption spectra are nearly same as those calculated by Chen's peak shape method.4. Conclusion
In conclusion, we provide insight for synthesizing a CMS phosphor by the acid distillation method with remarkable TL properties. The phosphor was found to be 3.5 times more sensitive than a standard CaSO4:Dy3+ phosphor with a stable dosimetric peak for C5+ ion irradiation. The TL response was nearly identical for different types of irradiation. The irradiation resulted in a slight variation in glow peak position, and ion beam irradiation caused additional glow peaks, which influences the trapping parameters. The obtained results can be easily correlated with the physical phenomenon, suggesting that this phosphor can be used for carbon ion beam dosimetry along with γ-ray dosimetry over a wide range of exposures.Acknowledgements
Authors are grateful to Inter University Accelerator Center (IUAC) New Delhi, India for providing financial assistance to carry out this work under research project UFR-56301. The authors would like to thank the Director of the Inter-University Accelerator Centre (IUAC), New Delhi, for providing beam time. The authors are also grateful to the Director of the Center for Advanced Technology, Indore, for providing access to the ADXRD measurement facility and Dr A. K. Sinha and Dr M. N. Singh for their support during the ADXRD measurement.References
- W. Barth, L. Dahl, J. Glatz, P. Forck and J. Klabunde, Proceedings of the Particle Accelerator Conference, Chicago, 2001, vol. 3281 Search PubMed.
- W. Barth, L. Dahl, J. Glatz, L. Groening, S. Richter and S. Yaramishev, Proceedings DIPAC, 2003, vol. 161 Search PubMed.
- T. Haberer, AIP Conf. Proc., 2002, vol. 610, p. 157 Search PubMed.
- H. Eickhoff, T. Haberer, G. Kraft, U. Krause, M. Richter, R. Steiner and J. Debus, Strahlenther. Onkol., 1999, 175, 21 CrossRef.
- U. Amaldi and G. Kraft, Rep. Prog. Phys., 2005, 68, 1861–1882 CrossRef CAS.
- H. Tsujii, J. Mizoe, T. Kamada, M. Baba, H. Tsuji, H. Kato, S. Kato, S. Yamada, S. Yasuda, T. Ohno, T. Yanagi, R. Imai, K. Kagei, H. Kato, R. Hara, A. Hasegawa, M. Nakajima, N. Sugane, N. Tamaki, R. Takagi, S. Kandatsu, K. Yoshikawa, R. Kishimoto and T. Miyamoto, J. Radiat. Res., 2007, 48, 1–13 CrossRef.
- H. Tsujii, T. Kamada, M. i. Baba, H. Tsuji, H. Kato, S. Kato, S. Yamada, S. Yasuda, T. Yanagi, H. Kato, R. Hara, N. Yamamoto and J. Mizoe, New J. Phys., 2008, 10, 075009 CrossRef.
- G. Kraft, Nucl. Instrum. Methods Phys. Res., Sect. A, 2000, 454, 1 CrossRef CAS.
- J. Heilmann, G. Taucher-Scholz, T. Haberer, M. Scholz and G. Kraft, Int. J. Radiat. Oncol., Biol., Phys., 1996, 34, 599 CrossRef CAS.
- P. D. Sahare and S. V. Moharil, J. Phys. D: Appl. Phys., 1990, 23, 567 CrossRef CAS.
- A. Pandey, P. D. Sahare, J. S. Bakare, S. P. Lochab, F. Singh and D. Kanjilal, J. Phys. D: Appl. Phys., 2003, 36, 2400 CrossRef CAS.
- B. P. Kore, N. S. Dhoble, K. Park and S. J. Dhoble, J. Lumin., 2013, 143, 337 CrossRef CAS PubMed.
- S. J. Dhoble, S. V. Moharil and T. K. Gundu Rao, J. Lumin., 2001, 93, 43 CrossRef CAS.
- T. Berger, G. Reitz, M. Hajek and N. Vana, Adv. Space Res., 2006, 37, 1716 CrossRef CAS PubMed.
- T. Yamashita, N. Nada, H. Onishi and S. Kitamura, Health Phys., 1971, 21, 295 CrossRef CAS.
- D. Kanjilal, S. Chopra, M. M. Narayanan, I. S. Iyer, V. Jha, R. Joshi and S. K. Datta, Nucl. Instrum. Methods Phys. Res., Sect. A, 1993, 328, 97 CrossRef.
- S. R. Kane, C. K. Garg and A. K. Sinha, AIP Conf. Proc., 2010, vol. 1234, p. 689 Search PubMed.
- A. K. Sinha, A. Sagdeo, P. Gupta, A. Kumar, M. N. Singh, R. K. Gupta, S. R. Kane and S. K. Deb, AIP Conf. Proc., 2011, vol. 1349, p. 503 Search PubMed.
- A. P. Hammersley, FIT2D V9.129 Reference Manual V3.1, ESRF Internal Report ESRF98HA01T, 1998 Search PubMed.
- See ESI.†.
- J. Manam and S. Das, J. Alloys Compd., 2010, 489, 84 CrossRef CAS PubMed.
- P. D. Sahare, N. Salah, S. P. Lochab, T. Mohanty and D. Kanjilal, J. Phys. D: Appl. Phys., 2005, 38, 3995 CrossRef CAS.
- B. Yang, L. Xie, Y. Xu and P. D. Townsend, Nucl. Instrum. Methods Phys. Res., Sect. B, 2002, 187, 408 CrossRef CAS.
- A. T. Davidson, A. G. Kozakiewicz, D. J. Wilkinson, J. D. Comins and T. E. Derry, Nucl. Instrum. Methods Phys. Res., Sect. B, 1998, 141, 523 CrossRef CAS.
- N. Salah, J. Phys. D: Appl. Phys., 2008, 41, 155302 CrossRef.
- O. B. Geiß, M. Krämer and G. Kraft, Nucl. Instrum. Methods Phys. Res., Sect. B, 1998, 142, 592 CrossRef.
- Y. S. Horowitz, D. Satinger, L. Oster, N. Issa, M. E. Brandan, O. Avila, M. Rodriguez-Villafuerte, I. Gamboa-deBuen, A. E. Buenfil and C. Ruiz-Trejo, Radiat. Meas., 2001, 33, 459 CrossRef CAS.
- Y. S. Horowitz, O. Avila and M. Rodriguez-Villafuerte, Nucl. Instrum. Methods Phys. Res., Sect. B, 2001, 184, 85 CrossRef CAS.
- J. F. Ziegler, J. P. Biersack and U. Littmark, The Stopping and Range of Ions in Solid, 1985 Search PubMed.
- B. P. Kore, N. S. Dhoble, S. P. Lochab and S. J. Dhoble, J. Lumin., 2014, 145, 299 CrossRef CAS.
- M. Batentschuka, A. Winnacker, K. Schwartz and C. Trautmann, J. Lumin., 2007, 125, 40 CrossRef PubMed.
- G. Kitis, J. M. Gomez-Ros and J. W. N. Tuyn, J. Phys. D: Appl. Phys., 1998, 31, 2636 CrossRef CAS.
- R. Chen, J. Electrochem. Soc., 1969, 116, 1254 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra08742a |
| This journal is © The Royal Society of Chemistry 2014 |