Visible light-mediated decarboxylative amination of indoline-2-carboxylic acids catalyzed by Rose Bengal†
Meng-Jie
Zhang
a,
Griffin M.
Schroeder
b,
Yan-Hong
He
*a and
Zhi
Guan
*a
aKey Laboratory of Applied Chemistry of Chongqing Municipality, School of Chemistry and Chemical Engineering, Southwest University, Chongqing 400715, PR China. E-mail: heyh@swu.edu.cn; guanzhi@swu.edu.cn
bChemistry Department, College of Saint Benedict and Saint John's University, MN 56374, USA
First published on 7th October 2016
Abstract
A visible light-induced decarboxylative amination of indoline-2-carboxylic acids and azodicarboxylate esters has been developed, in which the oxidation of α-amino acids provides a versatile CO2-extrusion platform to generate α-aminoalkyl radicals. The corresponding products were obtained with yields of up to 72% catalyzed by a metal-free photocatalyst under mild conditions.
Introduction
Visible light as a renewable energy source has the advantages of being inexpensive, nonpolluting and abundant.1 Photocatalysis driven by visible light under mild and green conditions has received widespread attention and has recently become an intriguing field of research in organic synthesis.2–4 Different types of photocatalysis have been developed. For example, chiral organocatalysts (such as thioureas) have been used for enantioselective photoreaction in which substrate is combined to the chiral catalyst through hydrogen bonds providing a chiral environment.5–12 Metal complexes have been reported as visible-light photocatalysts to perform a variety of photoredox reactions.13–15 Many common visible light photocatalysts are polypyridyl complexes ruthenium and iridium, such as Ru(bpy)32+ and [Ir(ppy)2(dtbbpy)]PF6.15 However, ruthenium and iridium salts have numerous disadvantages, including high cost, potential toxicity, and dwindling availability.16 Contrastingly, organic dyes—such as eosin Y,16–18 Mes-AcrClO4,19,20 fluorescein21 and Rose Bengal22–26—are more environmentally friendly, inexpensive and more readily available24–27 than their metallic counterparts. Recently, organic dyes have also been applied as effective visible light photoredox organocatalysts. Different types of mechanism for metal-free photoredox catalysis have been proposed, such as photoinduced electron or energy transfer process.28–35Over the past decade, decarboxylation reactions have widely received attention, largely due to the ability to change carboxylic acids—which are generally inexpensive and non-toxic—into more attractive reactants.36 The following methods are often used to realize the CO2-extrusion: transition metal catalyzed decarboxylation process37,38 and radical-mediated processes, such as persulfate oxidation decarboxylation method under high temperature39–41 and photoinduced single electron transformation.36,42–44 Generally, radical decarboxylation of carboxylic acids has several advantages for synthetic organic chemistry: the elimination of CO2 as the traceless byproduct does not influence the reaction system; furthermore, the resulting radical intermediates can be changed into more attractive, valuable chemical products with relative ease.45
The indoline substructure is considered an advantageous backbone because it is found in many types of organisms, including pharmaceutical molecules,46,47 naturally occurring alkaloids, and many biologically active molecules.48,49 Therefore, the synthesis of indoline derivatives has attracted much attention.50 In 2012, the Nishibayashi group reported amination of protected indoline via α-aminoalkyl radicals by using an iridium complex photocatalyst under visible light.51 Considering the importance of amination products of indoline, it is still desirable to develop more environmentally friendly methods for the synthesis of these compounds. Herein, we report the first metal-free decarboxylative amination reaction of N-protected indoline-2-carboxylic acids and azodicarboxylate esters catalyzed by an organic dye in the presence of visible light under mild conditions.
Results and discussion
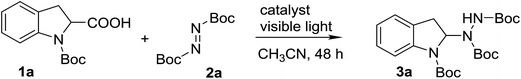
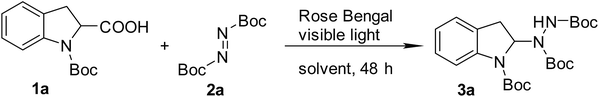
Initially, the model reaction of N-Boc-indoline-2-carboxylic acid (1a) with di-tert-butyl azodicarboxylate (2a) at room temperature in presence of a 32 W household fluorescent light bulb was employed to screen the reaction conditions.At the beginning, a preliminary organic dye screening was performed to determine the most efficient photocatalyst for the model reaction (Table 1) (Fig. 1). The model reaction without catalyst only gave the product 3a in a low yield of 20% under visible light (Table 1, entry 1). When eosin Y and fluorescein were introduced as photocatalysts, the product 3a was obtained in yields of 34% and 35%, respectively (Table 1, entries 2 and 3). The best result of 46% yield was obtained by using Rose Bengal (Table 1, entry 4). The data indicated that the tested organic dyes indeed promoted the reaction. Therefore, Rose Bengal was chosen as the suitable photocatalyst for the reaction. To verify the effect of the visible light, the model reaction with Rose Bengal (2 mol%) was performed in dark, and as expected no reaction was observed (Table 1, entry 5). This control experiment revealed that visible light is essential for the catalytic operation of this new decarboxylative amination reaction. Next, the influence of molar ratio on the model reaction was investigated. When the molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (1a
1 (1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a) was employed, the best yield of 52% was received (Table 1, entry 6). Thus, the molar ratio of 2
2a) was employed, the best yield of 52% was received (Table 1, entry 6). Thus, the molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (1a
1 (1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a) was selected as the optimal condition (for details, please see the ESI Table S1†).
2a) was selected as the optimal condition (for details, please see the ESI Table S1†).
| Entry | Catalyst | Yieldb (%) |
|---|---|---|
| a Reaction conditions: 1a (0.3 mmol), 2a (0.2 mmol), CH3CN (1.0 mL), and catalyst (2 mol%) under irradiation of 32 W fluorescent bulb at rt for 48 h. b Isolated yield. c 1a (0.4 mmol). | ||
| 1 | None | 20 |
| 2 | Eosin Y (5 mol%) | 34 |
| 3 | Fluorescein | 35 |
| 4 | Rose Bengal | 46 |
| 5 | Rose Bengal (in dark) | 0 |
| 6 | Rose Bengalc | 52 |
The choice of solvent is an important factor to promote this process. Various solvents were tested with 2 mol% of Rose Bengal as a photocatalyst under visible light (Table 2). Among the tested solvents, the best yield of 52% was obtained in CH3CN (Table 2, entry 1). The reactions in CH2Cl2 and ClCH2CH2Cl both resulted in a moderate yield of 50% (Table 2, entries 2 and 3). The reactions in the other tested solvents did not give better results (Table 2, entries 4–12). A trace amount of product 3a was observed in cyclohexane (Table 2, entry 13). Based on the results, CH3CN was selected as the most suitable solvent for the reaction.
| Entry | Solvent | Yieldb (%) |
|---|---|---|
| a Reaction conditions: 1a (0.4 mmol), 2a (0.2 mmol), solvent (1 mL), and Rose Bengal (2 mol%) under irradiation of 32 W fluorescent bulb at rt for 48 h. b Isolated yield. | ||
| 1 | CH3CN | 52 |
| 2 | CH2Cl2 | 50 |
| 3 | ClCH2CH2Cl | 50 |
| 4 | CHCl3 | 38 |
| 5 | EtOAc | 44 |
| 6 | n-BuOAc | 40 |
| 7 | EtOH | 38 |
| 8 | CH3OH | 33 |
| 9 | Toluene | 22 |
| 10 | DMF | 16 |
| 11 | DMSO | 9 |
| 12 | 1,4-Dioxane | 8 |
| 13 | Cyclohexane | Trace |
Next, the influence of solvent volume, catalyst dosage, and wattage of lamp were all investigated. The optimized reaction conditions were found to consist of the following: a volume of 0.5 mL (CH3CN), a Rose Bengal dosage of 1 mol%, and a fluorescent lamp wattage of 23 W (for details, please see the ESI Tables S2–S4†).
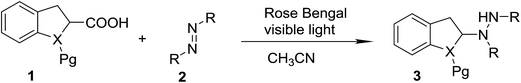
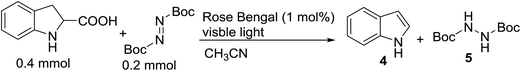
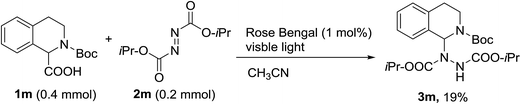
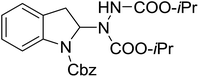
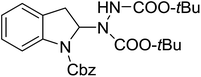
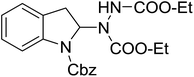
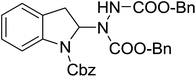
With the optimal conditions in hand, we investigated the substrate scope of the visible light-mediated Rose Bengal catalyzed decarboxylative amination reaction. In general, various azodicarboxylate esters readily participated in this reaction to generate amination products of indoline in moderate to good yields. Unfortunately, the 2,2-azobisisobutyric acid dimethyl ester (2n) failed to give the desired product 3n and the starting material 2n was intact (Table 3, entry 13), probably due to the electronic effect (N connects to alkyl instead of ester carbonyl group like the others). N-Boc, Cbz protected indoline carboxylic acids could be successfully applied and provided the desired products in moderate to good yields (Table 3, entries 1–8). When indoline carboxylate acid with strong electron withdrawing protecting group – sulfone was used, a low yield of 21% was observed (Table 3, entries 9 and 10). A low yield of 36% was obtained when N-benzoyl protected indoline carboxylate acid was employed as a substrate, likely due to its poor solubility in CH3CN (Table 3, entry 11). However, the presence of the free NH group of indoline carboxylic acid resulted in the formation of indole 4 and di-tert-butyl hydrazine-1,2-dicarboxylate 5 instead of amination product 3n (Scheme 1). The data indicated that the protecting group on nitrogen atom of indoline carboxylate acid has great influence on the decarboxylative amination reaction. This reaction can be expanded to other cyclic system that incorporates α-oxy group (2,3-dihydrobenzofuran-2-carboxylic acid) providing the corresponding amination adduct 3l in a low yield of 21% (Table 3, entry 12). In addition, N-Boc-1,2,3,4-tetrahydroisoquinoline-1-carboxylic acid (1m) can also participate in the reaction giving the corresponding product 3m with a yield of 19% (Scheme 2). For all the reactions, 2 equiv. of indoline carboxylate acids (1) was used to react with azodicarboxylate esters (2). In the most cases, nearly all azodicarboxylate esters (2) were consumed, with indoline carboxylate acids (1) left and unknown by-products were observed. For the model reaction, a conversion rate of 99% was determined after 48 h (5 generated from reduction of 2a was investigated as one of the by-prodcts) (Table 3, entry 1). This method represents a general procedure for the amination of indolines at the C-2 position. All the products obtained (3a–3m) are new compounds, and their structures have been determined by 1H NMR, 13C NMR and HRMS (for details, please see the ESI†).
| Entry | Product no. | Product | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1 (0.4 mmol), 2 (0.2 mmol), CH3CN (0.5 mL), and Rose Bengal (1 mol%) under irradiation of 23 W fluorescent bulb at rt. b Isolated yield (average of two trials, error: ±4%). c Conversion rate. | ||||
| 1 | 3a |
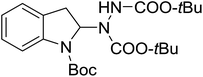
|
48 | 66 (99c) |
| 2 | 3b |
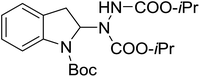
|
48 | 72 |
| 3 | 3c |
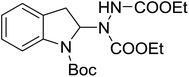
|
48 | 51 |
| 4 | 3d |
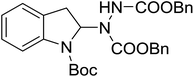
|
48 | 42 |
| 5 | 3e |
|
48 | 66 |
| 6 | 3f |
|
48 | 63 |
| 7 | 3g |
|
48 | 54 |
| 8 | 3h |
|
48 | 42 |
| 9 | 3i |
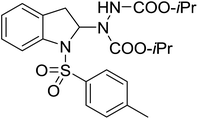
|
48 | 21 |
| 10 | 3j |
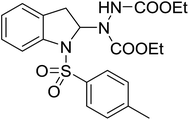
|
48 | 22 |
| 11 | 3k |
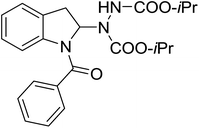
|
48 | 38 |
| 12 | 3l |
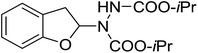
|
58 | 22 |
| 13 | 3n |
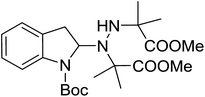
|
48 | — |
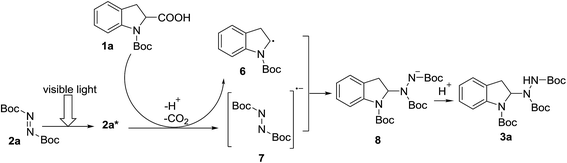
In order to gain more insight into the mechanism of the visible light-mediated Rose Bengal catalyzed decarboxylative amination reaction, fluorescence experiments with di-tert-butyl azodicarboxylate (2a) were performed. The absorption maximum of 2a is centered at λmax = 205 nm with a significant absorption in the UV region. However, 2a exhibits a weak absorption in the visible light region at λ = 402 nm (ξ = 36 L mol−1 cm−1) (the spectrum shown in the ESI†). Considering the product yield was 20% without the presence of a catalyst (Table 1, entry 1), we speculated that 2a could be the light absorber. In the absence of Rose Bengal, product is probably formed via the process shown in Scheme 3. Upon light absorption, 2a accepts a photon from the visible light to generate excited state 2a*. Reductive quenching of 2a* by N-Boc-indoline-2-carboxylic acid (1a) through single-electron transfer gives the corresponding radical anion (7). At the same time, α-aminoalkyl radical 6 is formed and CO2 is extruded. The radical–radical coupling of 6 and 7 gives intermediate 8. The protonation of 8 provides the product (3a). Because 2a has a weak absorption in the visible light region, the reaction without catalyst only gave a low yield.
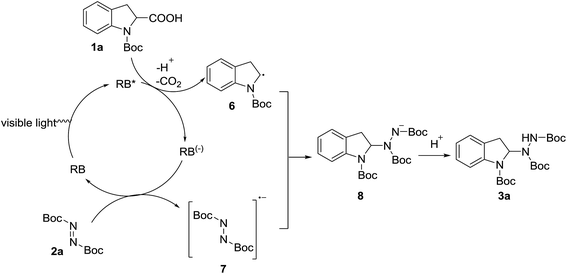
Finally, based on current literature,51–53 a plausible reaction pathway in the presence of Rose Bengal is proposed in Scheme 4. Upon light absorption, Rose Bengal (RB) accepts a photon from the visible light to generate excited state RB*. Subsequent single-electron oxidation of N-Boc-indoline-2-carboxylic acid (1a) by the visible-light-excited photocatalyst generates the reduced RB(−) and α-aminoalkyl radical 6, and CO2 is extruded. Then the reduction of the azodicarboxylate ester (2a) by RB(−) affords radical anion (7). Intermediate 8 is generated by the radical–radical coupling of 6 and 7, and the α-aminated product (3a) is obtained by the protonation of 8.
Conclusion
In summary, we have developed a visible light-mediated decarboxylative amination of N-protected indoline-2-carboxylic acid and azodicarboxylate esters. This reaction can be expanded to other cyclic system that incorporates α-oxy group and N-Boc-tetrahydroisoquinoline carboxylic acids. 13 new amination products were prepared with yields of up to 72%. The reaction was catalyzed by Rose Bengal as a metal-free photocatalyst under mild conditions. This process provides a general method for the amination of indolines at the C-2 position.General procedure for the decarboxylative amination
A round-bottom flask was charged with Rose Bengal (1 mol%), acids 1 (0.4 mmol) and azodicarboxylate esters 2 (0.2 mmol), to which CH3CN (0.5 mL) was introduced. The resultant mixture was stirred at rt under irradiation of 23 W fluorescent bulb for the specified reaction time and monitored by TLC. The crude mixture was concentrated in vacuo. The residue was purified by flash column chromatography (petroleum ether/ethyl acetate 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :1–3
:1–3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the products.
1) to give the products.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21276211, No. 21472152 and No. 21542014) and the Basic and Frontier Research Project of Chongqing (cstc2015jcyjBX0106).References
- G. Ciamician, Science, 1912, 36, 385–394 CrossRef CAS
.
- J. Xuan, X.-D. Xia, T.-T. Zeng, Z.-J. Feng, J.-R. Chen, L.-Q. Lu and W.-J. Xiao, Angew. Chem., Int. Ed., 2014, 53, 5653–5656 CrossRef CAS
.
- M. A. Ischay, M. E. Anzovino, J. Du and T. P. Yoon, J. Am. Chem. Soc., 2008, 130, 12886–12887 CrossRef CAS PubMed
.
- D. A. Nicewicz and D. W. C. MacMillan, Science, 2008, 322, 77–80 CrossRef CAS
.
- E. Kumarasamy, J. L. Jesuraj, J. N. Omlid, A. Ugrinov and J. Sivaguru, J. Am. Chem. Soc., 2011, 133, 17106–17109 CrossRef CAS
.
- V. Ramamurthy and J. Sivaguru, Chem. Rev., 2016, 116, 9914–9993 CrossRef CAS
.
- R. Raghunathana, S. Jockuschb, M. P. Sibia and J. Sivaguru, J. Photochem. Photobiol., A, 2016 DOI:10.1016/j.jphotochem.2015.12.023
.
- N. Vallavoju, S. Selvakumar, S. Jockusch, M. P. Sibi and J. Sivaguru, Angew. Chem., Int. Ed., 2014, 53, 5604–5608 CrossRef CAS
.
- N. Vallavoju, S. Selvakumar, B. C. Pemberton, S. Jockusch, M. P. Sibi and J. Sivaguru, Angew. Chem., Int. Ed., 2016, 55, 5446–5451 CrossRef CAS
.
- F. Mayr, R. Brimioulle and T. Bach, J. Org. Chem., 2016, 81, 6965–6971 CrossRef CAS
.
- A. Bauer, F. Westkämper, S. Grimme and T. Bach, Nature, 2005, 436, 1139–1140 CrossRef CAS
.
- M. M. Maturi and T. Bach, Angew. Chem., Int. Ed., 2014, 53, 7661–7664 CrossRef CAS
.
- X.-J. Dai, X.-L. Xu and X.-N. Li, Chin. J. Org. Chem., 2013, 33, 2046–2062 CrossRef CAS
.
- L. Shi and W.-J. Xia, Chem. Soc. Rev., 2012, 41, 7687–7697 RSC
.
- C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS
.
- M. Neumann, S. Fldner, B. Knig and K. Zeitler, Angew. Chem., Int. Ed., 2011, 50, 951–954 CrossRef CAS
.
- J. Davies, S. G. Booth, S. Essafi, R. A. W. Dryfe and D. Leonori, Angew. Chem., Int. Ed., 2015, 54, 14017–14021 CrossRef CAS
.
- J. Zhang, L. Wang, Q. Liu, Z. Yang and Y. Huang, Chem. Commun., 2013, 49, 11662–11664 RSC
.
- J. Xuan, X.-D. Xia, T.-T. Zeng, Z.-J. Feng, J.-R. Chen, L.-Q. Lu and W.-J. Xiao, Angew. Chem., Int. Ed., 2014, 53, 5653–5656 CrossRef CAS
.
- X.-X. Wu, C. Meng, X.-Q. Yuan, X.-T. Jia, X.-H. Qian and J.-X. Ye, Chem. Commun., 2015, 51, 11864–11867 RSC
.
- W. Guo, L.-Q. Lu, Y. Wang, Y.-N. Wang, J.-R. Chen and W.-J. Xiao, Angew. Chem., Int. Ed., 2015, 54, 2265–2269 CrossRef CAS
.
- C. Vila, J. Lau and M. Rueping, Beilstein J. Org. Chem., 2014, 10, 1233–1238 CrossRef
.
- X.-Y. Gu, X. Li, Y. Chai, Q. Yang, P. Li and Y.-M. Yao, Green Chem., 2013, 15, 357–361 RSC
.
- H.-J. Liu, W. Feng, C. W. Kee, Y.-J. Zhao, D. S. Leow, Y.-H. Pan and C.-H. Tan, Green Chem., 2010, 12, 953–956 RSC
.
- K. Tahara and Y. Hisaeda, Green Chem., 2011, 13, 558–561 RSC
.
- X. Li, X.-Y. Gu, Y.-J. Li and P.-X. Li, ACS Catal., 2014, 4, 1897–1900 CrossRef CAS
.
- D. A. Nicewicz and T. M. Nguyen, ACS Catal., 2014, 4, 355–360 CrossRef CAS
.
- J. Schwarz and B. König, Green Chem., 2016, 18, 4743–4749 RSC
.
- I. Ghosh, T. Ghosh, J. I. Bardagi and B. König, Science, 2014, 346, 725–728 CrossRef CAS
.
- D. P. Hari, P. Schroll and B. König, J. Am. Chem. Soc., 2012, 134, 2958–2961 CrossRef CAS
.
- E. Kumarasamy, R. Raghunathan, S. Jockusch, A. Ugrinov and J. Sivaguru, J. Am. Chem. Soc., 2014, 136, 8729–8737 CrossRef CAS
.
- A. Clay, N. Vallavoju, R. Krishnan, A. Ugrinov and J. Sivaguru, J. Org. Chem., 2016, 81, 7191–7200 CrossRef CAS
.
- A. Singh, C. J. Fennell and J. D. Weaver, Chem. Sci., 2016 10.1039/c6sc02422j
.
- R. Alonso and T. Bach, Angew. Chem., Int. Ed., 2014, 53, 4368–4371 CrossRef CAS
.
- N. Vallavoju, A. Sreenithya, A. J. L. Ayitou, S. Jockusch, R. B. Sunoj and J. Sivaguru, Chem.–Eur. J., 2016, 22, 11339–11348 CrossRef CAS
.
- Q.-Q. Zhou, W. Guo, W. Ding, X. Wu, X. Chen, L.-Q. Lu and W.-J. Xiao, Angew. Chem., Int. Ed., 2015, 54, 11196–11199 CrossRef CAS
.
- Z.-J. Fu, Z.-J. Li, Q.-H. Xiong and H. Cai, Chin. J. Org. Chem., 2015, 35, 984–996 CrossRef CAS
.
- J.-J. Dai, G.-Z. Wang, X.-L. Xu and H.-J. Xu, Chin. J. Org. Chem., 2013, 33, 2460–2468 CrossRef CAS
.
- S. Lu, Y. Gong and D. Zhou, J. Org. Chem., 2015, 80, 9336–9341 CrossRef CAS
.
- P.-F. Wang, Y.-S. Feng, Z.-F. Cheng, Q.-M. Wu, G.-Y. Wang, L.-L. Liu, J.-J. Dai, J. Xu and H.-J. Xu, J. Org. Chem., 2015, 80, 9314–9320 CrossRef CAS
.
- H. Wang, L.-N. Guo, S. Wang and X.-H. Duan, Org. Lett., 2015, 17, 3054–3057 CrossRef CAS PubMed
.
- L. Chu, C. Ohta, Z. Zuo and D. W. C. MacMillan, J. Am. Chem. Soc., 2014, 136, 10886–10889 CrossRef CAS PubMed
.
- Z. Zuo and D. W. C. MacMillan, J. Am. Chem. Soc., 2014, 136, 5257–5260 CrossRef CAS
.
- L. Gu, C. Jin, J. Liu, H. Zhang, M. Yuan and G. Li, Green Chem., 2016, 18, 1201–1205 RSC
.
- J. Xuan, Z. G. Zhang and W.-J. Xiao, Angew. Chem., Int. Ed., 2015, 54, 15632–15641 CrossRef CAS PubMed
.
- R. Viswanathan, C. R. Smith, E. N. Prabhakaran and J. N. Johnston, J. Org. Chem., 2008, 73, 3040–3046 CrossRef CAS
.
- N. Gruenfeld, J. L. Stanton, A. M. Yuan, F. H. Ebetino, L. J. Browne, C. Gude and C. F. Huebner, J. Med. Chem., 1983, 26, 1277–1282 CrossRef CAS
.
-
Modern alkaloids: structure, isolation, synthesis and biology, ed. E. Fattorusso and O. Taglialatela-Scafati, Wiley-VCH, Weinheim, 2008, p. 526 Search PubMed
.
- D.-S. Wang, Q.-A. Chen, S.-M. Lu and Y.-G. Zhou, Chem. Rev., 2012, 112, 2557–2590 CrossRef CAS
.
- Y. Miyake, K. Nakajima and Y. Nishibayashi, J. Am. Chem. Soc., 2012, 134, 3338–3341 CrossRef CAS
.
- Y. Miyake, K. Nakajima and Y. Nishibayashi, Chem.–Eur. J., 2012, 18, 16473–16477 CrossRef CAS
.
- L.-L. Chu, C. Ohta, Z.-W. Zuo and D. W. C. MacMillan, J. Am. Chem. Soc., 2014, 136, 10886–10889 CrossRef CAS
.
- Y.-H. Pan, C. W. Kee, L. Chen and C.-H. Tan, Green Chem., 2011, 13, 2682–2685 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra17524d |
| This journal is © The Royal Society of Chemistry 2016 |