Open Access Article
Open Access ArticleResponse of chlorinated hydrocarbon transformation and microbial community structure in an aquifer to joint H2 and O2†
Cui Li a,
Rong Chenb,
Hui Liu*ac,
Yao Huang
a,
Rong Chenb,
Hui Liu*ac,
Yao Huang a,
Jintao Yua,
Weiwei Ouyanga and
Chen Xuea
a,
Jintao Yua,
Weiwei Ouyanga and
Chen Xuea
aSchool of Environmental Studies, China University of Geosciences, Wuhan, Hubei 430078, PR China
bSchool of Environmental and Biological Engineering, Wuhan Technology and Business University, Wuhan, Hubei 430065, PR China
cState Key Laboratory of Biogeology and Environmental Geology, China University of Geosciences, Wuhan, Hubei 430078, PR China
First published on 16th August 2022
Abstract
Hydrogen (H2) and oxygen (O2) are critical electron donors and acceptors to promote the anaerobic and aerobic microbial transformation of chlorinated hydrocarbons (CHCs), respectively. Electrochemical technology can effectively supply H2 and O2 directly to an aquifer. However, the response of CHC transformation and microbial community structure to joint H2 and O2 are still unclear. In this work, microcosms containing different combinations of H2 and O2 were constructed with natural sediments and nine mixed CHCs. The joint H2 and O2 microcosm (H2/O2 microcosm) significantly promoted the biotransformation of trichloroethylene (TCE), trans-dichloroethene (tDCE) and chloroform (CF). Illumina sequencing analyses suggested that a particular microbial community was formed in the H2/O2 microcosm. The specific microbial species included Methyloversatilis, Dechloromonas, Sediminibacterium, Pseudomonas, Acinetobacter, Curvibacter, Comamonas and Acidovorax, and the relative abundance of the tceA, phe and soxB genes synchronously increased. These results suggested that some specific microbes are potential CHC converters using H2 and O2 as energy sources, and aerobic and anaerobic transformations exist simultaneously in the H2/O2 microcosm. It provides a theoretical basis for establishing efficient green remediation technologies for CHC contaminated aquifers.
1 Introduction
Chlorinated hydrocarbons (CHCs) are widely used in various industrial applications.1 Due to improper disposal, CHCs have become common pollutants in soil and groundwater.2–4 Many of them accumulate in the fatty tissue of organisms and show various degrees of toxicity for humans and ecosystems,5 so it is necessary to study their migration and transformation. The previous research mainly focuses on individual CHC transformation, but CHCs are often present in aquifers as complex mixtures of contaminants.6 In addition, chloroform (CF), chlorinated ethenes, and chlorinated ethanes have been shown to inhibit the dechlorinating activity of organohalide respiring bacteria.6 Carbon tetrachloride (CT) and especially CF have been observed to inhibit the reductive dehalogenation of perchloroethylene (PCE) and trichloroethylene (TCE).7 Hence, a better understanding of mixed CHC transformation under laboratory conditions may provide a basis for groundwater remediation when multiple contaminants are present.Anaerobic and aerobic biotransformation has been proven to be suitable methods for the bioremediation of CHC contaminated sites.8,9 Organohalide respiration is an effective means of CHC transformation in anaerobic environments by microorganisms such as Dehalococcoides and Desulfuromonas.4,10,11 Reductive dehalogenase enzymes (RDases) are critical enzymes for organohalide respiration, cleaving the carbon–chlorine bond, such as the pceA gene encoded PCE-RDase and the tceA gene encoded TCE-RDase.10 Under aerobic conditions, CHCs can be co-metabolically degraded during microbial metabolism processes using other growth substrates or be directly used as growth substrates by some microbial species, such as Pseudomonas sp., Bacillus sp. and Stenotrophomonas sp.10,12,13 Monooxygenases are critical enzymes for aerobic biodegradation of chloroethene.14 Therefore, the above functional microorganisms play an essential role in CHC transformation.
However, the transformation of CHCs via microbial pathways is often limited by the restricted electron donors and electron acceptors. Several electron donors, including methanol, butyrate, lactate, benzoate and hydrogen (H2), have been reported to enhance the reductive dechlorination of CHCs in the field and laboratory studies.15–17 In most cases, H2 produced during the fermentation of organic compounds was the actual electron donor and showed the best ability to promote reductive dechlorination.15,18 However, when H2 stimulated the activity of dehalogenation microbes, it might also enhance the growth of competing microbial populations, such as methanogens, acetogens, sulphate and nitrate reducers, which was unfavourable for reductive dechlorination.10,19
Meanwhile, some microbes can utilize O2 as an electron acceptor to degrade CHCs.12,13 Therefore, introducing O2 into the subsurface through bioventing/biosparging or injecting O2 releasing materials (magnesium peroxide or calcium peroxide) becomes an effective strategy for the in situ bioremediation of organic-contaminated sites.20–22 It is well known that O2, as an excellent electron acceptor, can promote the growth of many aerobic and facultative microorganisms,23 but seriously inhibit anaerobic microbes, such as methanogens, acetogens, sulphate and nitrate reducers.4,24 Therefore, H2 and O2 play essential roles in regulating microbial communities and CHCs transformation.
In addition, due to the lower energetic yield of the metabolic reaction, bacteria are less inclined to undertake reductive dechlorination (anaerobic biotransformation) of low-chlorinated cis-dichloroethene (cDCE) and vinyl chloride (VC), thus they often accumulate at sites where PCE and TCE are transformed through organohalide respiration.10 Compared to anaerobic biotransformation, aerobic biotransformation is more efficient for CHCs with fewer chlorine substituents.8,10 Therefore, H2 and O2 were sequentially used to promote the transformation of CHCs.25–27 In recent years, the newly developed electrochemical technology provided both H2 and O2 simultaneously via water electrolysis to the aquifer and effectively converted CHCs.28 However, the transformation of CHCs triggered by the joint H2/O2 and their effects on the microbial communities remains unclear. The synergistic regulation mechanism needs to be explored.
In this work, the response of CHCs transformation and microbial communities to joint H2/O2 (produced from electrochemical technology) were studied in the lab, with nine mixed CHCs selected as representative contaminants, including chlorinated alkenes (PCE, TCE and trans-dichloroethene (tDCE)) and chlorinated alkanes (1,1,2,2-tetrachloroethane (1,1,2,2-TeCA), 1,1,2-trichloroethane (1,1,2-TCA), 1,2-dichloroethane (1,2-DCA), CT, CF and dichloromethane (DCM)). In addition, the quantification of microbial functional genes related to CHCs' aerobic and anaerobic transformation was detected to verify the relationship between the CHCs transformation and the microbial community composition. This work will provide a theoretical basis for establishing efficient green remediation technologies for CHCs contaminated aquifers.
2 Materials and methods
2.1 Chemicals
PCE (99%), TCE (99.5%), tDCE (98%), 1,1,2,2-TeCA (99.8%), 1,1,2-TCA (99%), 1,2-DCA (99%), CT (99%), CF (99%), and DCM (99.5%) were obtained from J&K Scientific Ltd., China. Sodium azide (NaN3) was obtained from Sinopharm Chemical Reagent Co., China. All chemicals used were of analytical grade or above. Ultrapure water (18.25 MU cm, ZOOMWO-M) was used for all the experiments. The H2 (99%), O2 (99%) and N2 (99%) were purchased from Wuhan Iron & Steel (Group) Oxygen Co., Ltd.2.2 Sediments characterization
The sediments for experiments were collected from an abandoned chemical factory site in Tianjin (China) at a depth of ∼5 m. The place was contaminated with high concentrations of chlorinated solvents. The contents of CHCs and other main chemical characteristics of the sediments are displayed in Table S1.†2.3 Chlorinated hydrocarbon transformation experiments
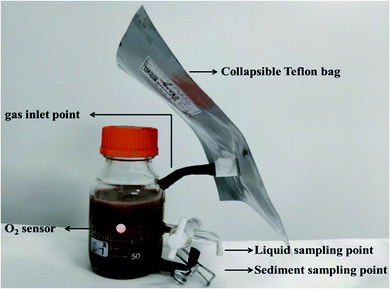
The experimental device used in this study for different microcosms was made of a 300 mL screw glass bottle, shown in Fig. 1. It consisted of a glass bottle and collapsible Teflon bag. A fluorine rubber hose connected the two units. The liquid and sediment sampling points were located at about 3.5 and 1 cm from the bottom of the glass bottle, respectively. The sole H2 and O2 were obtained by water electrolysis and collected in different collapsible Teflon bags. The gas content of joint H2/O2 microcosm is 100 mL H2 and 50 mL O2. The individual N2, H2 and O2 microcosms were conducted as control, with 150 mL of N2, H2 and O2, respectively. The microorganism was inhibited by 1 g L−1 sodium azide for abiotic control.The experiments were prepared in an anaerobic chamber (Coy Laboratory Products Inc., Michigan). Forty grams of wet sediment, 200 mL of deionized water (purged with N2 for 30 min to remove dissolved O2) and the mixed stocking solution of nine CHCs including PCE, TCE, tDCE, 1,1,2,2-TeCA, 1,1,2-TCA, 1,2-DCA, CT, CF, and DCM were added to the experimental devices. The initial concentration of each CHC was 30 μM. The Teflon bags containing gases were connected with the glass bottles. Microcosms were prepared in triplicates for each experimental treatment. The initial substrate concentrations were measured after one hour of shaking at 25 °C and 150 rpm. The initial concentration of each CHC in the aqueous phase is shown in Table 1.
| No. | Pollutants | Concentration (μM) |
|---|---|---|
| 1 | Perchloroethylene | 42.97 ± 8.10 |
| 2 | Trichloroethylene | 39.13 ± 5.76 |
| 3 | trans-Dichloroethene | 30.18 ± 2.35 |
| 4 | 1,1,2,2-Tetrachloroethane | 27.46 ± 3.45 |
| 5 | 1,1,2-Trichloroethane | 28.11 ± 1.90 |
| 6 | 1,2-Dichloroethane | 28.73 ± 1.80 |
| 7 | Carbon tetrachloride | 31.11 ± 3.17 |
| 8 | Chloroform | 33.42 ± 1.64 |
| 9 | Dichloromethane | 28.04 ± 2.03 |
The experimental devices were shaken at 25 °C on a rotary shaker at 150 rpm. One millilitre liquid sample was collected from the upper outlet of the bottle after several minutes of settlement and then added into a 42 mL brown bottle with 40 mL ultrapure water to determine the concentration of CHCs. Sediment samples were collected from the bottom outlet of the bottle and immediately frozen at −20 °C for further DNA extraction and 16S rRNA sequence analysis, which were prepared in triplicates. The sediments were digested in a Microwave Digestion System (MARS 5, CEM, USA) with concentrated nitric acid to determine cation components.
2.4 Analytical methods
The concentration of CHCs and possible transformation intermediates were determined by automatic purge and trap-gas chromatography-mass spectrometry (PT-GC-MS) (PT: Atomx, Teledyne Tekmar, USA; GC-MS: Thermo Fisher Scientific Inc., USA). Compounds were separated by an Aligent DB-624 capillary column (30 m × 0.25 mm × 1.4 μm), and the MS detector was operated in a full scan mode. The oven temperature was held at 35 °C for 2 min, heated at a rate of 5 °C min−1 to 100 °C, held for 2 min and then heated at a rate of 10 °C min−1 to 200 °C and held for 1 min. The inlet and MS transfer line temperatures were set at 220 and 280 °C, respectively.Total organic carbon (TOC) was measured by an Elemental Analyzer (multi EA 4000, JENA, Germany). Cation components in the sediments were measured by ICP-OES (Agilent 5100, USA), and anion components were measured by an ion chromatograph (Eco IC, Metrohm, Switzerland). Immediately after sampling, the oxidation–reduction potential (ORP) of the aqueous phase was measured by a pH meter (PHS-3C, Rex of Shanghai Co., Ltd. China) with an ORP composite electrode (Rex 501), and dissolved hydrogen (DH) was measured by a DH meter (DH200, CLEAN, USA). The dissolved oxygen (DO) was measured by a noninvasive oxygen meter (FIBOX 4, PreSens, Germany), with oxygen sensor spots previously glued onto the inner wall of the glass bottle.
2.5 DNA extraction and quantification of 16S rRNA gene
According to the manufacturer's instructions, DNA was extracted using PowerSoil® DNA Isolation Kits (MO BIO, USA). The primers of 341F (CCTACGGGAGGCAGCAG) and 515R (ATTACCGCGGCTGCTGGCA) were used to amplify the 16S rRNA gene.29 The qPCR was performed on an ABI QuantStudio 3 (Version 1.4.1 software, Applied Biosystems, USA), and each sample was duplicated.302.6 Taxonomic and functional microbial composition analyses
Sequencing was performed on an Illumina MiSeq instrument (MiSeq, Illumina, USA) at the Personal Biotechnology Company (Shanghai, China), using 338F (ACTCCTACGGGAGGCAGCA) and 806R (GGACTACHVGGGTWTCTAAT) primers to amplify the V3 and V4 regions of 16S rRNA genes. The microbial community was analysed using QIIME 2 (2019.4), and taxonomy was assigned using the Greengenes 13.8 database. Microbial diversity and abundance were estimated using the software Mothur (version 1.35.1, USA). The raw sequence data have been deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (BioProject accession number PRJNA797955).2.7 Quantification of functional genes by qPCR
The relative abundance of genes related to trichloroethylene transformation, phenol transformation and sulfur oxidation were quantified by qPCR. The primers of tceA-500F (TAATATATGCCGCCACGAATGG) and tceA-795R (AATCGTATACCAAGGCCCGAGG) were used to amplify the trichloroethylene dehalogenation gene tceA.31 The primers of TBMD-F (GCCTGACCATGGATGCSTACTGG) and TBMD-R (CGCCAGAACCACTTGTCRRTCCA) were used to amplify the phenol monooxygenase gene phe.32 The primers of 710F (ATCGGYCAGGCYTTYCCSTA) and 1184R (MAVGTGCCGTTGAARTTGC) were used to amplify the sulfur oxidation gene soxB.33 The 16S rRNA gene of each sample was used to normalize the data. The relative abundance was calculated by the 2−ΔΔCT method.233 Results
3.1 The transformation of chlorinated hydrocarbons in various gas conditions
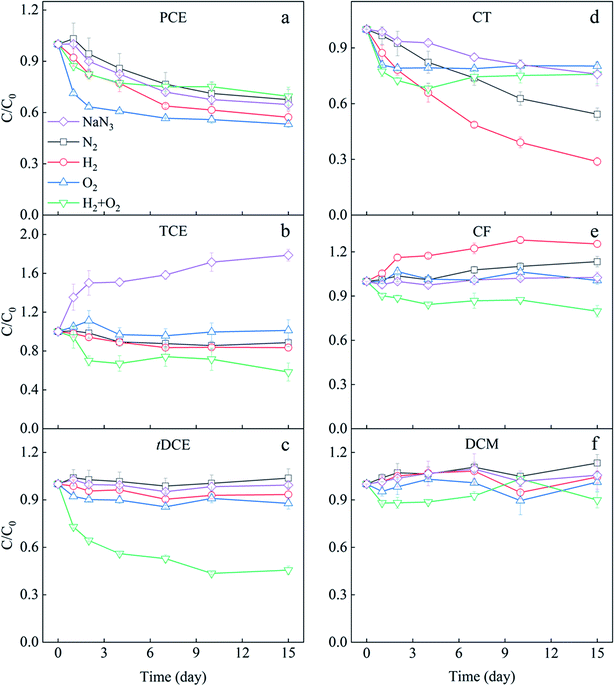
The transformation of each CHC under different H2 and O2 conditions in the mixed solution was observed (Fig. 2), and the mass balance and variance analysis of CHCs were listed in Table S2.† The microcosm with N2 served as an anaerobic control with no electron donor and acceptors addition, and that with NaN3 was set as an abiotic control because NaN3 could inhibit microbial activity. As shown in Fig. 2a, PCE concentration gradually decreased over time in the NaN3 microcosm, indicating the abiotic transformation of PCE. Some reduced components, mainly Fe(II)-bearing minerals, can directly reduce CHCs with higher chlorine substituents.34 There was a minimal difference between the PCE variation in NaN3 and N2 microcosms, indicating limited PCE biotransformation occurred in N2 microcosms. Generally, CHCs with more chlorine substituents, such as PCE, are easier to be transformed through anaerobic dechlorination.10 The minimal PCE transformation observed in this N2 microcosm might be due to the relatively higher ORP of the sediment (Fig. S6†). The addition of H2 slightly improved the PCE removal compared with NaN3 and N2 microcosms, suggesting H2 promoted the anaerobic biotransformation of PCE. As shown in Fig. S6,† the ORP in H2 microcosm decreased to −281 mV in two-day incubation, which should be the main reason for the stimulation of PCE anaerobic biotransformation. In the O2 and H2/O2 microcosms, the removal of PCE mainly occurred in the first two days. About 19.26 μM PCE was removed in the O2 microcosm, while the coexistence of H2 inhibited PCE reduction. Generally, compared to anaerobic biotransformation, aerobic biotransformation is more efficient for CHCs with fewer chlorine substituents.2,8,10 Therefore, the fast reduction of PCE in O2 and H2/O2 microcosms in the first two days was more likely due to chemical reaction. When subsurface sediment is exposed to oxygen, some reduced substances, such as Fe(II)-bearing minerals, can activate molecular O2 to produce hydroxyl radical (˙OH) and superoxide (O2˙−)35,36 to chemically oxidize chlorinated alkenes.37–39TCE remarkably increased 78.56% in the NaN3 microcosm in 15 days (Fig. 2b). TCE is a common intermediate in transforming other CHCs, such as 1,1,2,2-TeCA and PCE.10,17 About 16.74 μM PCE and 27.43 μM 1,1,2,2-TeCA in NaN3 microcosm were removed in our research (Table S2†). The total PCE and 1,1,2,2-TeCA reduction (44.17 μM) were much higher than the TCE increase (21.32 μM) in the NaN3 microcosm. Therefore, it is proposed that the accumulated TCE was intermediate during the abiotic transformation of PCE and 1,1,2,2-TeCA, and TCE might also be abiotically transformed further. Contrastingly, the TCE concentration did not significantly increase in all biotic microcosms, with the most apparent reduction occurring in joint H2/O2 microcosm (Fig. 2b). The difference in the TCE variations between the joint H2/O2 microcosm and the abiotic control was 38.39 μM, contributed by the biotransformation. Compared with sole O2 and sole H2 treatments, the joint H2/O2 promoted 17.51 and 9.98 μM TCE removal.
The concentration of tDCE in NaN3, N2, and H2 microcosms did not significantly change (Fig. 2c and Table S2†), suggesting neither chemically nor biologically reduction happened. The addition of O2 slightly promoted tDCE removal due to chemical oxidation or biological degradation.10 In contrast, tDCE decreased by 54% (14.59 μM) in H2/O2 microcosm. No other intermediates such as vinyl chloride (VC) or ethene were detected in our experiment systems.
The transformation of three chlorinated methanes in 15 days is displayed in Fig. 2d–f and Table S2.† CT showed different transformation trends in five microcosms (Fig. 2d). It continuously declined in the NaN3 microcosm, and 24% (7.70 μM) was removed in 15 days. In the N2 microcosm, CT decreased by 46% (13.10 μM), indicating anaerobic biotransformation of CT happened. The addition of H2 enhanced CT anaerobic transformation as 71% (23.92 μM) of CT was removed in 15 days. In O2 and H2/O2 microcosms, just like PCE, CT only decreased about 20% fastly on the first day, supposedly due to the oxidative transformation by the reactive oxygen species such as superoxide (O2˙−).40,41
CF contents in NaN3 and O2 microcosms did not significantly change after 15 day incubation (Fig. 2e and Table S2†), meaning no chemical and biological transformation occurred. In N2 and H2 microcosms, CF increased 13% (4.42 μM) and 25% (8.27 μM) in 15 days, respectively. CF is a potential intermediate of CT dechlorination.42 The increased CF should come from CT dechlorination. However, the increased CF amounts were less than the decreased CT, indicating that CF was further transformed in these two microcosms. By calculating the difference between decreased CT and increased CF, the CF lessened were 8.68 and 15.64 μM in N2 and H2 microcosms, respectively. Therefore, H2 also promoted CF transformation. In H2/O2 microcosm, 20% CF (7.28 μM) decreased in 15 days, demonstrating CF transformation was significantly enhanced compared with the sole H2 and O2 microcosms. Previous studies have shown that CF can be co-metabolized under anaerobic and aerobic conditions.43 Bouwer & McCarty observed that a significant fraction of radiolabeled CF was converted to CO2 in anaerobic bioreactors, indicating alternative processes other than reductive hydrogenolysis.43
DCM showed no significant variation in the microcosms except that 2.53 μM DCM decreased in H2/O2 microcosm, indicating a slight promotion of DCM transformation by H2/O2. However, no further intermediates were detected.
The three chlorinated ethanes, 1,1,2,2-TeCA, 1,1,2-TCA, and 1,2-DCA, showed no remarkable differences among the five microcosms (Fig. S1†), even though 1,1,2,2-TeCA decreased almost entirely in 15 days. It has been previously reported that 1,1,2,2-TeCA was transformed abiotically by dehydrochlorination, a non-redox reaction without electrons.17,44
In conclusion, TCE, CT and CF could be biologically transformed under anaerobic N2 and H2 conditions, and TCE and tDCE could be degraded under O2 conditions. The joint H2/O2 promoted the biotransformation of TCE, CF and tDCE.
3.2 Microbial community shift during chlorinated hydrocarbons transformation
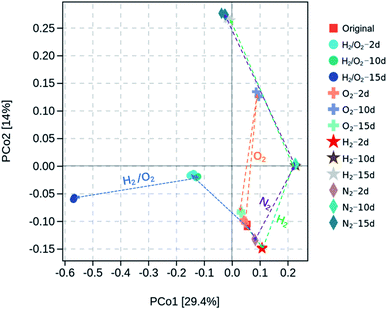
Microorganisms play essential roles in the transformation of CHCs. After adding CHCs and incubating in various microcosms, the total biomass was evaluated through quantity of 16S rRNA by qPCR, and microbial community information/structure were analyzed by high-throughput sequence analysis of 16S rRNA gene amplicons. Fig. S2† showed that the microbial numbers per gram sediment in the four gas-treated microcosms were in the magnitude of 6–7 and exhibited a slight increase after 2 d incubation but with minimal differences among the treatments. Clustering and principal coordinate analysis (PCoA) was conducted to show the dissimilarity of the microbial communities in different microcosms (Fig. 3). The first two axes in the PCoA graph accounted for 29.4% and 14% of the community structure variation, respectively. It was demonstrated that the microbial communities with N2 and H2 treatments followed a similar evolution route along the PCo2 direction. After 15 d incubation in N2 and H2 microcosms, the final microbial communities were very close. The microbial community with O2 treatment shifted in the same direction as that in N2 and H2 microcosms in 10 days and returned to the original state after 15 d incubation. However, the microbial community in joint H2 and O2 shifted along the PCo1 direction, and the final microbial community after the 15 d experiment was far from the other treatments, suggesting a distinct microbial community. After 15 d incubation, the microbial species abundance (observed species and Chao 1 indices) and diversity (Shanon and Simpson indices) were remarkably different (Fig. S3†). The joint H2/O2 treatment induced higher microbial species abundance than the original and O2-treated sediment but lower than that treated with N2 and H2. In contrast, the diversity in H2/O2 microcosm was the highest among all treatments.The most abundant ten microbial phyla in different microcosms with incubation time are displayed in Fig. S4.† Firmicutes was the major phylum constituting 95.9% of total bacterial reads in the initial sediment sample. The relative abundance of Proteobacteria and Actinobacteria remarkably increased after 15 d cultivation in the four microcosms. In the H2/O2 microcosm, the abundance of Proteobacteria (65.2%) was much higher than that in N2, H2 and O2 microcosms (15.6, 20.5, and 3.2%, respectively). Besides, Bacteroidetes also obviously increased in the H2/O2 microcosm (6.9%) compared to the N2, H2 and O2 microcosms (1.0%, 1.3% and 0.2%, respectively) after 15 d cultivation.
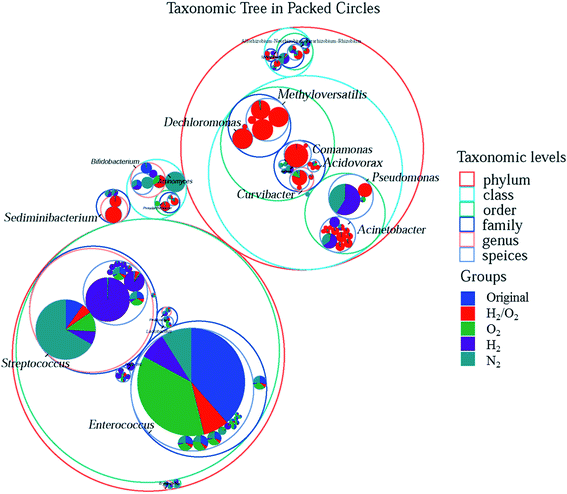
To more clearly distinguish the taxonomic differences among treatments and find out the specific microbial species and their taxonomic relationship, the taxonomic tree from phylum to species in packed circles were drawn, and the first ten genera were marked in Fig. 4. The red circles (the size represented its abundance) were the specific species in the H2/O2 microcosm. Almost all the genera of Methyloversatilis and Dechloromonas, which belong to the same family, and Sediminibacterium were only observed in the H2/O2 microcosm. Some species in the genera Pseudomonas, Acinetobacter, Curvibacter, Comamonas, and Acidovorax were specific in the H2/O2 microcosm.
To clarify the microbes potentially involved in CHCs transformation, the variation of genera with time in different microcosms was analyzed (Fig. 5). The results showed that Pseudomonas significantly increased in the later stage (10–15 days) of cultivation in N2 and H2 microcosms (Fig. 5a). The addition of H2 promoted the growth of Pseudomonas (0.1–10.3%) compared to that in the N2 microcosm (0.1–7.5%). It increased in the first ten days and then obviously decreased in the O2 microcosm, with the highest abundance of 2.0%. Pseudomonas rose dramatically in the first two days and fluctuated in the H2/O2 microcosm, with the highest abundance of 3.9%. Curvibacter increased considerably in the first ten days and then obviously decreased in the O2 microcosm, and the highest abundance was 3.7% (Fig. 5b), while Curvibacter (0–4.2%) increased throughout the experiment in H2/O2 microcosm. Acinetobacter (highest abundance 12.6%) significantly increased in H2/O2 microcosm while only increased in the later stage (10–15 days) in N2 and H2 microcosms. The addition of H2 promoted the growth of Acinetobacter (0.1–5.0%) compared to the N2 microcosm (0.1–1.8%) (Fig. 5c). Meanwhile, Acinetobacter showed an ignorable change in the O2 microcosm. Throughout the experiment, the relative abundance of Sediminibacterium (0–6.8%), Methyloversatilis (0–22.1%), and Dechloromonas (0–7.2%) strongly increased in H2/O2 microcosm (Fig. 5d–f), which were only found in minor proportions (<0.3% abundance) in other microcosms.
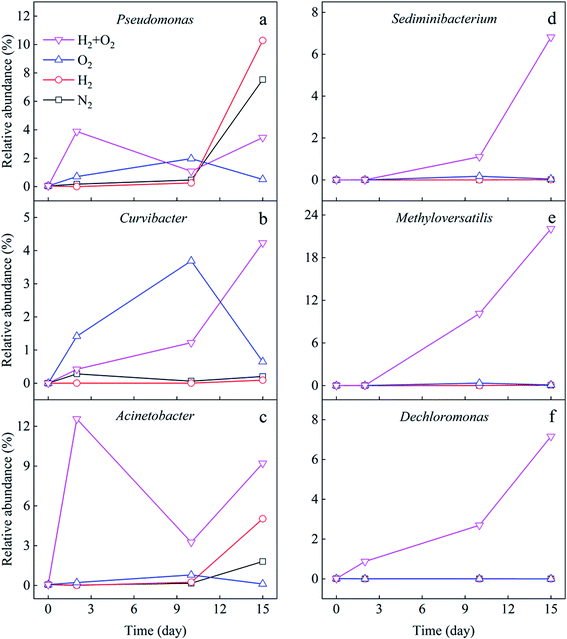 | ||
| Fig. 5 Relative abundance variation of different genera (a to f) potentially involved in CHCs transformation. | ||
The promotion of joint H2 and O2 on the CHCs transformation may be due to the aerobic and anaerobic microbes. Therefore, the quantification of microbial functional genes related to CHCs' aerobic and anaerobic transformation was detected. The reductive dehalogenases encoded by the tceA gene is responsible for TCE reduction.45 The phenol monooxygenase encoded by the phe gene can degrade TCE co-metabolically under aerobic conditions.46 The soxB gene encodes subunit of the sox enzyme system that is essential for sulfur-oxidizing bacteria, which has been found to degrade chloroethylenes.23,47 Fig. 6 shows the changes in the relative abundance of the above functional genes with experimental time. In H2/O2 microcosm, the relative abundance of the tceA gene increased significantly along with the time (Fig. 6a), and after 15 days of cultivation, the tceA relative abundance was up to 16 ± 5 folds. Comparatively, the relative abundance of the tceA gene did not increase in the O2 microcosm, and it was even below detection in N2 and H2 microcosms throughout the experiment. The phe gene only increased in H2/O2 microcosm, and the relative abundance was up to 936 ± 123 folds after 15 days of cultivation (Fig. 6b). The relative abundance of soxB gene in H2/O2 microcosm increased in the first ten days and decreased after that, which was finally raised to 1973 ± 250 folds after 15 d cultivation (Fig. 6c).
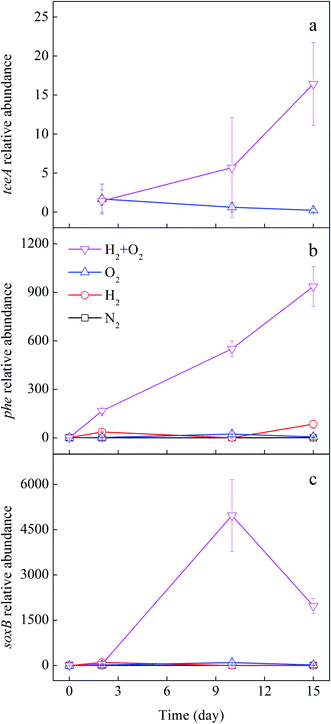 | ||
| Fig. 6 Fold change of relative abundance of tceA (a), phe (b) and soxB (c) genes in different microcosms. | ||
4 Discussion
4.1 Joint H2/O2 enhanced chlorinated hydrocarbons transformation
Our results demonstrated that, compared to the N2, H2, and O2 controls, the joint H2/O2 promoted TCE, tDCE, and CF transformation (Fig. 2). Under anaerobic conditions, reductive microbial dechlorination is the main route for CHCs biotransformation.4 In this process, the CHCs serve as the terminal electron acceptor, and molecular H2 typically serves as the electron donor.4 The CHCs with a high degree of chlorine substitution are generally more readily bio-transformed under anaerobic conditions but are often recalcitrant to aerobic degradation.48Under aerobic conditions, microbial degradation of CHCs mainly occurs through aerobic metabolic degradation (CHCs used as electron donors) and aerobic co-metabolic degradation (with degradation of CHCs occurring fortuitously during microbial metabolism processes using other growth substrates).10 O2 is an effective electron acceptor, and oxidative CHCs degradation is more efficient with decreasing number of chlorine substituents.8,10 TCE, tDCE, and CF can be biotransformed under either anaerobic or aerobic conditions, while biotransformation of PCE and CT occurs almost exclusively under anaerobic conditions.43,49 This research proves that joint H2/O2 can enhance the transformation of CHCs with two and three chlorine substituents, which could be biotransformed under both anaerobic and aerobic conditions.
4.2 Specific microbes in joint H2/O2
Compared with N2, H2 and O2 microcosms, the joint H2/O2 microcosm possesses a particular microbial community with specific species in the genera Methyloversatilis, Dechloromonas, Sediminibacterium, Pseudomonas, Acinetobacter, Curvibacter, Comamonas, and Acidovorax. In contrast, the relative abundance of the tceA, phe and soxB genes increased significantly.Previous studies have indicated that Pseudomonas was capable of aerobic metabolic and anaerobic reductive dechlorination of TCE,10,49 possibly determined by different species, which can explain the diverse behaviours of this genus in aerobic and anaerobic microcosms. The Pseudomonas in N2 and H2 microcosms increased after ten days, indicating that the anaerobic Pseudomonas grew slowly. Those in H2/O2 microcosm rose in the first two days, suggesting aerobic or facultative aerobic Pseudomonas in it.
Acinetobacter can utilize 3-chloroaniline and 4-chlorobenzoic acid under anaerobic conditions.50,51 It may also play an essential role in the degradation of tDCE under aerobic conditions.52 Sediminibacterium is a facultative anaerobe, which exists in groundwater polluted by CHCs, and it is associated with the aerobic degradation of VC.53 Methyloversatilis universalis is the only species identified in the Methyloversatilis genus. This aerobic versatile methylotrophic bacterium can grow with chlorinated herbicide benazolin-ethyl (4-chloro-2-oxobenzothiazolin-3-yl-acetic acid) as the sole carbon source.54 Dechloromonas was possibly responsible for CF reductive dechlorination.55 Our results show that Acinetobacter, Sediminibacterium, Methyloversatilis, and Dechloromonas are the specific genera and bloom in H2/O2 microcosm, indicating that they potentially transform CHCs through an aerobic or anaerobic pathway.
The genus Curvibacter is an aerobic chemoorganotroph,54 which might be associated with the degradation of organic contaminants such as phthalate ester.56 Our results show that this genus gradually increases in H2/O2 microcosm, possibly relating to aerobic metabolic or co-metabolic degradation of CHCs. Further studies are needed to prove this hypothesis.
In addition, in H2/O2 microcosm, the relative abundance of phe and soxB functional genes increased. Some genera, such as Acinetobacter, Pseudomonas and Dechloromonas, have been associated with the bioremediation of aromatic hydrocarbons.55,57 Many aromatic hydrocarbons degrading bacteria can co-metabolically degrade chloroethene, such as TCE, cDCE and VC.10 Acinetobacter can utilize dimethyl sulfide (DMS) as the sole sulfur source and degrade TCE and three DCE isomers.47 Hence, the H2/O2 microcosm might be conducive to the aerobically co-metabolic degradation of CHCs. In the microcosms, sediment from the chlorinated hydrocarbon-contaminated aquifer was used. The organic compounds in the sediment could be used as co-metabolic substrates. Meanwhile, the reductive dechlorination gene tceA also increased significantly in H2/O2 microcosm, indicating the anaerobic transform of TCE also existed, even though the anaerobic dechlorinating bacteria were not dominated in the systems.
4.3 Mechanisms of chlorinated hydrocarbons transformation in joint H2/O2
According to the microbial community analysis, the enhanced TCE, tDCE, and CF transformation by joint H2/O2 might follow the mechanism as below.(1) The specific microbes adapted to the joint H2/O2 environment have a transformation function. The microbial species in the genera Methyloversatilis, Dechloromonas, Sediminibacterium, Pseudomonas and Acinetobacter, can potentially transform CHCs. Some might use H2 and O2 as energy sources for transformation. It was also observed that the H2 concentration sharply increased to 0.5 mg L−1 in two days in H2 and H2/O2 microcosms (Fig. S5a†). However, it decreased to 0.4 mg L−1 after two days in the H2/O2 microcosm. On the other hand, the O2 concentration in H2/O2 microcosm was lower than that in the sole O2 microcosm throughout the experiment (Fig. S5b†), indicating that more H2 and O2 were consumed in the H2/O2 microcosm than in the sole H2 and O2 systems. These results proposed that the microbes utilizing H2 or O2 co-existed or some microbes consuming both H2 and O2 existed in the H2/O2 microcosm. Hydrogen-oxidizing bacterias (HOB) are facultative autotrophic bacteria that can use H2 as electron donor and O2 as an electron acceptor to fix carbon dioxide.58 In addition, in H2/O2 microcosm, the specific genera Pseudomonas has been reported as HOB, and genera Methyloversatilis and Dechloromonas belong to the same family Rhodocyclaceae, some genera of which have been reported as HOB, such as Paracoccus.59,60 Microbes capable of simultaneously utilizing H2 and O2 may have the ability to transform CHCs. Further study is needed to isolate the specific microbes and identify their transformation ability together with consumption of H2 and O2 in the microcosms with different proportions of H2 and O2.
(2) The aerobic and anaerobic transformations of CHCs may co-exist in the joint H2/O2 environment and simultaneously transform CHCs, which are confirmed by the synchronous increase of aerobic phe and soxB genes and anaerobic tceA gene in this system (Fig. 6). Previous studies have demonstrated that under aerobic conditions, anaerobic dechlorination bacteria and aerobic VC degraders co-exist in the sediment of a hyporheic riverbed zone with high organic carbon, and both reductive dechlorination and aerobic co-metabolic degradation of VC occur at the same time.61,62 Recent findings have revealed that the surface of sediment particles can form biofilms, and the presence of facultative aerobic bacteria colonizing the outer layers of sediment biofilms, which rapidly consume O2 and protect the strict anaerobes such as organohalide-respiring bacteria in core microniches.61 Fig. S6† shows the ORP variation in different microcosms. The ORP value was between −104 to −195 mV in H2/O2 microcosm. Thus, reductive dechlorination might be possible, especially in the inner section of sediment particles in such a low ORP environment. However, further exploration is needed to clarify the aerobic and anaerobic zonation for chlorinated hydrocarbon transformation.
(3) Hydrogen can promote the aerobic biodegradation of CHCs with fewer chlorine substituents.2 O2 is toxic to anaerobic microorganisms.63 Hydroxyl radical (˙OH) and superoxide (O2˙−) produced by the oxidation of reduced substances in anaerobic sediments can kill some microorganisms.64,65 In the H2/O2 microcosm, the O2 concentration was lower than that in the sole O2 microcosm throughout the experiment, especially in the first seven days (Fig. S5b†). Therefore the presence of H2 may relieve the oxidative stress on the anaerobe or facultative anaerobes, which may be another reason for the promotion of chlorinated alkene removal.
5 Conclusions
The joint H2/O2 enhanced the transformation of TCE, tDCE, and CF. A particular microbial community with higher diversity formed. The specific microbes in joint H2/O2 were Methyloversatilis, Dechloromonas, Sediminibacterium, Pseudomonas, Acinetobacter, Curvibacter, Comamonas, and Acidovorax, one or more of them potentially transforming CHCs using H2 and O2 as energy sources. The relative abundance of the tceA, phe and soxB genes synchronously increased, indicating the coexistence of aerobic and anaerobic transformation of CHCs. Further studies are needed to clarify the mechanism of the CHCs transformation by these specific microbes.Author contributions
Cui Li: conceptualization, methodology, validation, formal analysis, software, writing – original draft; Rong Chen, Weiwei Ouyang, Chen Xue, Minghui Liu: writing – review & editing; Hui Liu: supervision, project administration, funding acquisition, conceptualization, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key Research & Development Program of China (Grant No. 2018YFC1802504) and the National Natural Science Foundation of China (32061133002 and 41521001).References
- J. Jesus, D. Frascari, T. Pozdniakova and A. S. Danko, J. Hazard. Mater., 2016, 309, 37–52 CrossRef CAS PubMed.
- D. Frascari, G. Zanaroli and A. S. Danko, J. Hazard. Mater., 2015, 283, 382–399 CrossRef CAS PubMed.
- M. Zeppilli, E. Dell'Armi, L. Cristiani, M. P. Papini and M. Majone, Water, 2019, 11, 2579 CrossRef CAS.
- Z. Xiao, W. Jiang, D. Chen and Y. Xu, Ecotoxicol. Environ. Saf., 2020, 202, 110925 CrossRef CAS PubMed.
- J. Zimmermann, L. J. S. Halloran and D. Hunkeler, Chemosphere, 2020, 244, 125476 CrossRef CAS PubMed.
- A. Trueba-Santiso, D. Fernández-Verdejo, I. Marco-Rius, J. M. Soder-Walz, O. Casabella, T. Vicent and E. Marco-Urrea, Chemosphere, 2020, 240, 124877 CrossRef CAS PubMed.
- M. F. Azizian and L. Semprini, J. Contam. Hydrol., 2016, 190, 58–68 CrossRef CAS PubMed.
- S. Gaza, K. R. Schmidt, P. Weigold, M. Heidinger and A. Tiehm, Water Res., 2019, 151, 343–348 CrossRef CAS PubMed.
- C.-H. Chang, H.-Y. Yang, J.-M. Hung, C.-J. Lu and M.-H. Liu, Int. Biodeterior. Biodegrad., 2017, 117, 150–157 CrossRef CAS.
- I. Dolinova, M. Strojsova, M. Cernik, J. Nemecek, J. Machackova and A. Sevcu, Environ. Sci. Pollut. Res., 2017, 24, 13262–13283 CrossRef CAS PubMed.
- L. L. Wen, J. X. Chen, J. Y. Fang, A. Li and H. P. Zhao, Front. Microbiol., 2017, 8, 1439 CrossRef PubMed.
- T. E. Mattes, A. K. Alexander and N. V. Coleman, FEMS Microbiol. Rev., 2010, 34, 445–475 CrossRef CAS PubMed.
- N. B. Varzaghani, S. Shokrollahzadeh and A. Farazmand, Korean J. Chem. Eng., 2019, 36, 1305–1312 CrossRef CAS.
- N. B. Le and N. V. Coleman, Biodegradation, 2011, 22, 1095–1108 CrossRef CAS PubMed.
- F. Aulenta, J. M. Gossett, M. P. Papini, S. Rossetti and M. Majone, Biotechnol. Bioeng., 2005, 91, 743–753 CrossRef CAS PubMed.
- M. F. Azizian, I. P. Marshall, S. Behrens, A. M. Spormann and L. Semprini, J. Contam. Hydrol., 2010, 113, 77–92 CrossRef CAS PubMed.
- F. Aulenta, M. Potalivo, M. Majone, M. P. Papini and V. Tandoi, Biodegradation, 2006, 17, 193–206 CrossRef CAS PubMed.
- I. S. Lee, J. H. Bae and P. L. McCarty, J. Contam. Hydrol., 2007, 94, 76–85 CrossRef CAS PubMed.
- X. Wang, J. Xin, M. Yuan and F. Zhao, Water Res., 2020, 183, 116060 CrossRef CAS PubMed.
- M. N. Goltz, R. K. Gandhi, S. M. Gorelick, G. D. Hopkins, L. H. Smith, B. H. Timmins and P. L. McCarty, Environ. Sci. Technol., 2005, 39, 8963–8970 CrossRef CAS PubMed.
- H. Li, S. Y. Zhang, X. L. Wang, J. Yang, J. D. Gu, R. L. Zhu, P. Wang, K. F. Lin and Y. D. Liu, Environ. Technol., 2015, 36, 667–674 CrossRef CAS PubMed.
- S. Ko, M. Crimi, B. K. Marvin, V. Holmes and S. G. Huling, J. Environ. Manage., 2012, 108, 42–48 CrossRef CAS PubMed.
- J. Ma, H. Liu, C. Zhang, K. Ding, R. Chen and S. Liu, Sci. Total Environ., 2020, 720, 137587 CrossRef CAS PubMed.
- S. Atashgahi, Y. Lu and H. Smidt, Overview of known organohalide-respiring bacteria—phylogenetic diversity and environmental distribution, Springer-Verlag Berlin Heidelberg, 2016 Search PubMed.
- C. H. Chang, H. Y. Yang, S. K. Chen, J. M. Hung, C. J. Lu and M. H. Liu, Int. Biodeterior. Biodegrad., 2018, 132, 251–258 CrossRef CAS.
- S. T. Lohner, D. Becker, K. M. Mangold and A. Tiehm, Environ. Sci. Technol., 2011, 45, 6491–6497 CrossRef CAS PubMed.
- S. T. Lohner and A. Tiehm, Environ. Sci. Technol., 2009, 43, 7098–7104 CrossRef CAS PubMed.
- S. H. Yuan, Y. Liu, P. Zhang, M. Tong and H. Liu, Sci. China: Technol. Sci., 2021, 64, 251–260 CrossRef CAS.
- L. Hermon, J. Hellal, J. Denonfoux, S. Vuilleumier, G. Imfeld, C. Urien, S. Ferreira and C. Joulian, Front. Microbiol., 2019, 10, 89 CrossRef PubMed.
- X. Luo, S. Han, X. Fu, X. Li, L. Wang, S. Peng, W. Chen and Q. Huang, Appl. Soil Ecol., 2019, 135, 174–181 CrossRef.
- J. M. Fung, R. M. Morris, L. Adrian and S. H. Zinder, Appl. Environ. Microbiol., 2007, 73, 4439–4445 CrossRef CAS PubMed.
- H. Li, Q. Zhang, X. L. Wang, X. Y. Ma, K. F. Lin, Y. D. Liu, J. D. Gu, S. G. Lu, L. Shi, Q. Lu and T. T. Shen, Bioresour. Technol., 2012, 124, 129–136 CrossRef CAS PubMed.
- M. Tourna, P. Maclean, L. Condron, M. O'Callaghan and S. A. Wakelin, FEMS Microbiol. Ecol., 2014, 88, 538–549 CrossRef CAS PubMed.
- A. B. Cundy, L. Hopkinson and R. L. Whitby, Sci. Total Environ., 2008, 400, 42–51 CrossRef CAS PubMed.
- M. Tong, S. Yuan, S. Ma, M. Jin, D. Liu, D. Cheng, X. Liu, Y. Gan and Y. Wang, Environ. Sci. Technol., 2015, 50, 214–221 CrossRef PubMed.
- G. W. Luther, Aquat. Geochem., 2009, 16, 395–420 CrossRef.
- A. Teel, Water Res., 2001, 35, 977–984 CrossRef CAS PubMed.
- X. Wang and M. L. Brusseau, Environ. Toxicol. Chem., 1998, 17, 1689–1694 CrossRef CAS.
- R. J. Watts and A. L. Teel, J. Environ. Eng., 2005, 131, 612–622 CrossRef CAS.
- H. Che and W. Lee, Chemosphere, 2011, 82, 1103–1108 CrossRef CAS PubMed.
- B. A. Smith, A. L. Teel and R. J. Watts, Environ. Sci. Technol., 2004, 38, 5465–5469 CrossRef CAS PubMed.
- K. E. Vickstrom, M. F. Azizian and L. Semprini, Chemosphere, 2017, 182, 65–75 CrossRef CAS PubMed.
- J. A. Field and R. Sierra-Alvarez, Rev. Environ. Sci. Bio/Technol., 2004, 3, 185–254 CrossRef CAS.
- B. Jung and B. Batchelor, J. Hazard. Mater., 2008, 152, 62–70 CrossRef CAS PubMed.
- N. Liu, L. Ding, H. Li, P. Zhang, J. Zheng and C.-H. Weng, Bioresour. Technol., 2018, 261, 133–141 CrossRef CAS PubMed.
- S. H. Liang, J. K. Liu, K. H. Lee, Y. C. Kuo and C. M. Kao, J. Hazard. Mater., 2011, 198, 323–330 CrossRef CAS PubMed.
- L. Yee, A. Hosoyama, S. Ohji, K. Tsuchikane, J. Shimodaira, A. Yamazoe, N. Fujita, C. Suzuki-Minakuchi and H. Nojiri, Genome Announc., 2014, 2, e01048-14 CrossRef PubMed.
- F. Maphosa, S. H. Lieten, I. Dinkla, A. J. Stams, H. Smidt and D. E. Fennell, Front. Microbiol., 2012, 3, 351 Search PubMed.
- P. Pant and S. Pant, J. Environ. Sci., 2010, 22, 116–126 CrossRef CAS.
- H. D. Duc, Appl. Biol. Chem., 2016, 59, 703–709 CrossRef CAS.
- K. Kobayashi, K. Katayama-Hirayama and S. Tobita, J. Gen. Appl. Microbiol., 1997, 43, 105–108 CrossRef CAS PubMed.
- A. Olaniran, W. Stafford, D. Cowan, D. Pillay and B. Pillay, J. Microbiol. Biotechnol., 2007, 17, 560–570 CAS.
- F. P. Wilson, X. K. Liu, T. E. Mattes and A. M. Cupples, Environ. Sci. Pollut. Res., 2016, 23, 19062–19070 CrossRef CAS PubMed.
- R. Eugene, F. D. Edward, L. Stephen, S. Erko and T. Fabiano, The Prokaryotes Alphaproteobacteria and Betaproteobacteria, Springer-Verlag Berlin Heidelberg, 4th edn, 2014 Search PubMed.
- Y. S. Lai, A. Ontiveros-Valencia, T. Coskun, C. Zhou and B. E. Rittmann, Biotechnol. Bioeng., 2019, 116, 1439–1448 CrossRef CAS PubMed.
- D. Ma, Z. Hao, R. Sun, M. Bartlam and Y. Wang, Genome Announc., 2016, 4, e01510–01515 Search PubMed.
- Y. M. Chen, T. F. Lin, C. Huang, J. C. Lin and F. M. Hsieh, J. Hazard. Mater., 2007, 148, 660–670 CrossRef CAS PubMed.
- E. Ehsani, C. Dumolin, J. B. A. Arends, F. M. Kerckhof, X. Hu, P. Vandamme and N. Boon, Appl. Microbiol. Biotechnol., 2019, 103, 8241–8253 CrossRef CAS PubMed.
- J. Dou, Y. Huang, H. Ren, Z. Li, Q. Cao, X. Liu and D. Li, Appl. Biochem. Biotechnol., 2019, 187, 338–351 CrossRef CAS PubMed.
- T. G. Volova, E. G. Kiselev, E. I. Shishatskaya, N. O. Zhila, A. N. Boyandin, D. A. Syrvacheva, O. N. Vinogradova, G. S. Kalacheva, A. D. Vasiliev and I. V. Peterson, Bioresour. Technol., 2013, 146, 215–222 CrossRef CAS PubMed.
- S. Atashgahi, F. Maphosa, E. Dogan, H. Smidt, D. Springael and W. Dejonghe, FEMS Microbiol. Ecol., 2013, 84, 133–142 CrossRef CAS PubMed.
- S. Atashgahi, Y. Lu, J. Ramiro-Garcia, P. Peng, F. Maphosa, D. Sipkema, W. Dejonghe, H. Smidt and D. Springael, Environ. Sci. Technol., 2017, 51, 1626–1634 CrossRef CAS PubMed.
- B. K. Amos, K. M. Ritalahti, C. Cruz-Garcia, E. Padilla-Crespo and F. E. Loffler, Environ. Sci. Technol., 2008, 42, 5718–5726 CrossRef CAS PubMed.
- S. Ma, M. Tong, S. Yuan and H. Liu, ACS Earth Space Chem., 2019, 3, 738–747 CrossRef CAS.
- R. Chen, H. Liu, P. Zhang, L. Zhao, K. Ding and S. Yuan, Sci. Total Environ., 2019, 694, 133660 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra04185e |
| This journal is © The Royal Society of Chemistry 2022 |