Photoinduced metal-free atom transfer radical polymerizations: state-of-the-art, mechanistic aspects and applications
Gorkem
Yilmaz
*a and
Yusuf
Yagci
 *ab
*ab
aDepartment of Chemistry, Istanbul Technical University, 34469 Maslak, Istanbul, Turkey. E-mail: a.gorkemyilmaz@gmail.com; yusuf@itu.edu.tr
bCenter of Excellence for Advanced Materials Research (CEAMR) and Department of Chemistry, Faculty of Science, King Abdulaziz University, P. O. Box 80203, Jeddah 21589, Saudi Arabia
First published on 16th March 2018
Abstract
Photoinduced atom transfer radical polymerization has recently been the center of intensive research in synthetic polymer chemistry because of the unique possibility of topological and temporal control in addition to precise control of macromolecular structure offered by conventional ATRP. Following the developments of new approaches to decrease the metal catalyst concentration, it was recently shown that ATRP can be carried out under metal-free conditions by using photochemical strategies. Various photocatalysts playing a role in oxidative and reductive pathways have been reported. This mini-review summarizes and highlights recent research efforts in the emerging area of photoinduced metal-free atom transfer radical polymerization. The proposed mechanisms for the activation and deactivation steps and potential applications have also been discussed.
1. Introduction
Photochemical protocols display an increasing pattern in not only replacing thermal synthetic pathways but also in finding applications in a variety of conventional strategies.1–3 This growing interest relies on the fact that it assembles a wide range of economic and ecological concerns.4 In contrast to thermal-based syntheses, which usually require elevated temperatures, photochemical syntheses can be performed at room temperatures and below. Furthermore, they offer temporal and 3D control over the processes and materials prepared, respectively.5The application of photochemistry to polymer science is mostly based on photopolymerization, which requires a photosensitive compound that converts light energy into chemical energy leading to the formation of reactive species capable of initiating polymerization. In this way, monomers are transformed to their corresponding polymers. For more than 30 years, photopolymerization has been the basis of numerous conventional applications in coatings, adhesives, inks, printing plates, optical waveguides, and microelectronics.6–8 Some other less traditional but interesting applications, including curing of acrylate dental fillings and production of 3D objects,9 are also available.
Free radical,10–18 cationic,6,19–26 anionic27 and even step-growth polymerizations28,29 can be performed by photochemical means. However, due to a higher number of photoinitiators and monomer formulations available, the free radical mode is in the advanced state. More recently, photochemistry has been applied to controlled/living polymerizations, which allows the syntheses of polymers with determined chain-end functionalities and controlled molecular weight characteristics.30–38 Specifically, photochemical energy has been successfully employed to reversible addition fragmentation39,40 and atom transfer radical polymerization (ATRP). However, the latter has gained more attention from the photochemical point of view due to the light sensitivity of the copper catalyst required.41
In this mini-review, we will focus on the light activated ATRP processes, specifically under metal-free conditions. In particular, the solutions to overcome problems associated with metal contamination in conventional photoATRP, challenges and potentials will be discussed. Historically, the fundamental concept of conventional photoATRP involving the photoinduced electron transfer (PET) reaction between radicals, sensitizers, nano-particles and copper catalysts laid the foundation for the next achievements in metal-free photoATRP as it is also based on light activated redox reactions. It seemed, therefore, appropriate first to discuss briefly conventional photoATRP with a special emphasis on work performed in the authors’ laboratory.
2. Photoinduced atom transfer radical polymerization (ATRP)
In traditional ATRP, generally, a low oxidation state transition metal complex (commonly CuX/L, X: Cl or Br and L: ligand) as a catalyst is necessary at high concentrations, which is prone to oxidation and difficult to remove after the reaction. At this point, photoinduced electron transfer (PET) reactions emerge as an alternative approach to carry out ATRP at lower catalyst concentrations and higher oxygen tolerance.42,43 Initially, the positive effect of light on ATRP systems was examined by Guan and Smart who performed ATRP by photochemical means in less Cu(X) concentrations.44 Subsequent studies demonstrated that the copper concentration could also be reduced by starting from copper(II) complexes (CuX2/L), which can be simultaneously reduced in the reaction medium to yield the actual catalyst. The photoreduction can be performed either by direct irradiation of the CuX2/L or by an indirect pathway where an additional photosensitive compound is necessary. The direct irradiation of the complex leads to an internal electron transfer from the host to the guest Cu(II) that yields Cu(I) responsible for the catalytic activation.41,45 The indirect irradiation, on the other hand, considers an electron transfer from an excited state sensitizer or a photogenerated radical to CuX2/L that produces CuX/L (Scheme 1).46–51Other photochemical strategies include the design and synthesis of specific copper complexes that enable photoinduced ATRP processes with very small concentrations.49,52–55
3. Photoinduced metal-free ATRP
Despite such noteworthy achievements, realizing ATRP under completely metal-free conditions was not possible until recently. The first example of photoinduced metal-free ATRP was shown by Hawker and co-workers who employed 10-phenylphenothiazine (PTZ) as a photocatalyst.56 The mechanism is believed to go through an oxidative quenching pathway, which considers a reduction of the alkyl halide initiator by the excited state PTZ. The thus formed alkyl radical initiates the polymerization of appropriate monomers as shown in Scheme 2.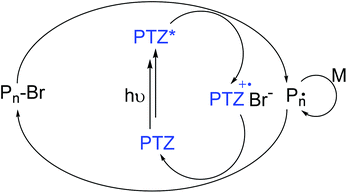 | ||
| Scheme 2 Oxidative quenching mechanism in photoinduced metal-free ATRP in the example of 10-phenylphenothiazine (PTZ). | ||
Detailed mechanistic studies reveal that both singlet and triplet states of PTZ are operative in reduction, whereas the latter is dominant as a result of its higher excited state lifetime and reactivity.57–59 Notably, the established mechanism is reported to be also operative when sulfonyl chlorides are used as initiating sites instead of alkyl halides.60 Later, other PTZ derivatives were also introduced and applied as photocatalysts for metal-free ATRP.61
However, the long procedures for the syntheses of PTZ compounds motivated researchers to investigate the probability of using naturally occurring and commercially available photosensitizers as catalysts for metal-free ATRP. Polynuclear aromatic compounds such as pyrene62 and perylene63 were shown to be competent catalysts favoured by their electron-rich structures that can reduce the alkyl halides easily upon light exposure. Both display very high efficiency in the production of monodisperse polymers with controlled chain end functionality as proved by chain extension and block copolymerization experiments. However, efforts on anthracene failed to produce polymers with narrow molecular weight distribution despite undergoing a fast PET reaction with the initiating alkyl halide. This has been explained by the transfer of the propagating sites to the labile positions on the inner ring of anthracene.62
More recently, dihydrophenazines64 and phenoxazines65 have also been applied as photocatalysts for metal-free ATRP. The similarity of their structures to that of PTZ makes them very good candidates for this purpose. In addition, they have light sensitivity at higher wavelengths, which allows the realization of ATRP at higher wavelengths in comparison with PTZ. Notably, these structurally similar compounds were found to display higher initiating efficiency in comparison with the above-mentioned polynuclear aromatics due to the stabilization of the halide ion by the heteroatoms on their structure.66 It was originally proposed that the triplet state and charge transfer make a major contribution to enhance control over the polymerization. However, latest studies on these dihydrophenazines claimed otherwise, indicating that the PET from short-lived excited singlet states can exert control of polymer molecular weight and dispersity by suppressing the steady-state concentration of the reactive debrominated radical. It was further suggested that PET rates for different photocatalysts are better interpreted in terms of the free energies of excited states than their electronic characters.67
In a very recent study, thienothiophene derivatives were also found to be an efficient catalyst for metal-free ATRP.68 Upon irradiation, these compounds also produced polymers with low dispersities through an oxidative quenching mechanism as evidenced by fluorescence quenching experiments.
Chart 1 shows the structures of photocatalysts that can activate photoinduced metal-free ATRP through an oxidative quenching mechanism.69
 | ||
| Chart 1 General structures of the photocatalysts utilized for metal-free ATRP that operate through oxidative quenching. | ||
Photoinduced metal-free ATRP processes are not limited only to electron-rich compounds that operate through an oxidative quenching mechanism. Recent studies showed that certain electron acceptor dyes in conjunction with amines could stimulate the process through a reductive quenching mechanism. Fluorescein,70 eosin Y71 and erythrosin B71 were shown to be efficient sensitizers under various colours of LED and industrially available visible light irradiation to produce monodisperse polymers of (meth)acrylates and vinyl monomers.
In another study, conventional Type II photoinitiators such as benzophenone, thioxanthone, isopropyl thioxanthone and camphorquinone were also shown to sensitize metal-free photoATRP when used together with a suitably selected amine and alkyl halide.72Chart 2 demonstrates the structures of the sensitizers that mediate photoinduced metal-free ATRP through a reductive quenching mechanism.
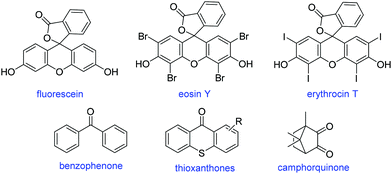 | ||
| Chart 2 General structures of the photocatalysts utilized for metal-free ATRP that operate through reductive quenching. | ||
Computational studies give evidence on the reductive quenching mechanism, which considers an electron transfer from the electron donating amine to the excited state dye to yield the radical anion of the dye and radical cation form of the amine. The radical anion form of the dye reduces the alkyl halide to give the radical responsible for the initiation. Scheme 3 shows the process using the example of conventional Type II initiators. The reversibility of the activation/deactivation processes ensures control over molecular weight distribution and chain-end fidelity as evidenced by chromatographic and spectroscopic observations as well as chain extension experiments.
 | ||
| Scheme 3 Proposed mechanism of the reductive quenching mechanism in photoinduced metal-free ATRP using the photosensitizer/amine initiating system. | ||
The overall comparison of the activators for photoinduced metal-free ATRP, following both oxidative and reductive pathways, is presented in Table 1.
| Sensitizer | Quenching mode | Irradiation source | Commentsa |
|---|---|---|---|
| a Comments are made in the example of methyl methacrylate polymerizations. Đ: Dispersity (Mw/Mn) and low Đ indicates values lower than 1.4. I*: Initiation efficiency: (Mn, theo)/Mn,GPC × 100. | |||
| Phenothiazines | Oxidative | UV-vis | – Low Đ |
| – High I* | |||
| Phenoxazines | Oxidative | UV or visible LED | – Low Đ |
| – High I* | |||
| Dihydrophenazines | Oxidative | Sunlight or white LED | – Low Đ |
| – High I* | |||
| Thienothiophenes | Oxidative | UV | – Low Đ |
| – Low I* | |||
| Perylene | Oxidative | Sunlight or white LED | – Low Đ |
| – Low I* | |||
| Pyrene | Oxidative | UV | – Low Đ |
| – Low I* | |||
| Fluorescein | Reductive | Various colours of LED | – Low Đ |
| – Low I* | |||
| Eosin Y | Reductive | Various colours of LED | – Low Đ |
| – Low I* | |||
| Erythrosin T | Reductive | Various colours of LED | – Low Đ |
| – Low I* | |||
| Benzophenone | Reductive | UV | – Low Đ |
| – Low I* | |||
| Thioxanthones | Reductive | UV | – Low Đ |
| – Low I* | |||
| Camphorquinone | Reductive | UV | – Low Đ |
| – Low I* | |||
4. Synthetic applications of photoinduced metal-free ATRP
Several efforts demonstrated the applications of photoinduced metal-free ATRP on the preparation of various polymeric materials. Hawker and co-workers used PTZ for photoinduced dehalogenation and coupling processes.73 This procedure was shown to efficiently work for both conjugated polyhalides as well as unconjugated substrates. For this purpose, PTZ derivatives with different reduction potentials were employed and, depending on this reactivity difference, dehalogenation processes can be chemoselectively realized. Scheme 4 shows the general representation of these photocatalyzed reactions. | ||
| Scheme 4 General representation of chemoselective dehalogenation and coupling reactions using different PTZ derivatives. | ||
A similar strategy was also employed for the chain-end and side chain transformation of various polymers.73,74 Thus, hydrogen atoms replace halogens and even in some cases, trithiocarbonate, so as to attain inert polymeric materials.
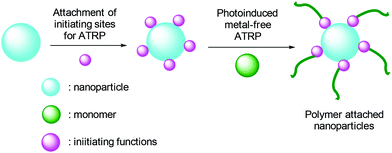
The application of the metal-free strategy is not limited to functionalization of polymers. Literature reports demonstrated the possibility of the synthesizing polymer brush nanostructures75 and modification of nanoparticles such as magnetic and silica-based materials.76,77 For this purpose, such nanoscale materials are modified with initiating sites for ATRP. The polymers are grown from these sites by photoATRP and composite materials are readily formed (Scheme 5).
We have recently shown that metal-free ATRP and Ring Opening Polymerization (ROP) processes can be performed in one reaction medium without affecting each other.78 By using a specifically designed bifunctional initiator possessing tertiary bromide and hydroxyl groups, vinyl and lactone monomers can be polymerized concurrently under sunlight, by metal-free approaches. In this way, block copolymers can be successfully synthesized in a one-shot manner (Scheme 6). The method not only applies metal-free and mild reaction conditions, but also offers a single step experimentation and purification process, which meet the ecological and economical demands.
 | ||
| Scheme 6 Synthesis of PMMA-b-PCL through concurrent metal-free controlled/living polymerizations under sunlight. | ||
In another study, we have shown the application of metal-free photoATRP for the preparation of hyperbranched polymers. Therein, monomer/inimer pairs were transformed into hyperbranched polymer structures under visible light irradiation using perylene as a photocatalyst (Scheme 7).79
It was also shown in the study that one can prepare hyperbranched-block copolymers by using the precursor hyperbranched polymers as macroinitiators, and polymerize the second segments with a typical photoinduced metal-free ATRP process.
There are also a few reports showing the use of photoinduced metal-free polymerizations to various applications including star polymer syntheses,80 flow systems81 and energy related areas.82
5. Summary and outlook
Despite the noteworthy efforts in reducing the amount of copper catalyst required for ATRP, complete removal of metal consumption was not possible until recently. Recent developments in the field showed that using certain sensitizers enable the syntheses of polymers with controlled functionalities and molecular weight characteristics. This helps temporal control over the process while suppressing the necessity of the work up necessary for the removal of the catalyst. Various sensitizers were developed that play a role in a broad range of light irradiation. Two distinctive mechanisms are postulated to be operative in the activation step, which are confirmed by detailed theoretical and spectroscopic analyses. As an emerging research area, photoinduced metal-free ATRP offers great opportunities for the preparation of a variety of complex macromolecular architectures and their application as biomaterials. To meet the demand for bioapplications, it is essential to develop novel photocatalysts that are non-toxic themselves and absorb light at higher wavelengths, preferably in the visible and near infrared region. Moreover, synergistic combinations with the other mode of the polymerizations are promising to significantly enhance bridging the gap between biological sciences and polymer science. Recent studies78 have already shown that biocompatible segments can successfully be attached to linear polymers by the combination of photoinduced ATRP and ROP processes under sunlight and metal-free conditions. This emerging area of research will certainly lead to new applications ranging from the synthesis of highly complex architectures to sophisticated surface patterning for biomedical applications. Moreover, this methodology is expected to allow the polymerization of a variety of monomers, which are prone to coordinate with metal catalysts as previously shown by Matyjaszewski in the case of acrylonitrile.59Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to Istanbul Technical University for financial support.Notes and references
- Y. Yagci, S. Jockusch and N. J. Turro, Macromolecules, 2010, 43, 6245–6260 CrossRef.
- S. Dadashi-Silab, S. Doran and Y. Yagci, Chem. Rev., 2016, 116, 10212–10275 CrossRef PubMed.
- M. Chen, M. Zhong and J. A. Johnson, Chem. Rev., 2016, 116, 10167–10211 CrossRef PubMed.
- J. H. Clark, Green Chem., 1999, 1, 1–8 Search PubMed.
- C. Brieke, F. Rohrbach, A. Gottschalk, G. Mayer and A. Heckel, Angew. Chem., Int. Ed., 2012, 51, 8446–8476 CrossRef PubMed.
- P. Xiao, J. Zhang, F. Dumur, M. A. Tehfe, F. Morlet-Savary, B. Graff, D. Gigmes, J. P. Fouassier and J. Lalevee, Prog. Polym. Sci., 2015, 41, 32–66 CrossRef.
- C. G. Roffey, Photopolymerization of surface coatings, Wiley, Chichester [u.a.], 1985 Search PubMed.
- J. V. Crivello, K. Dietliker, G. Bradley and S. T. Limited, Chemistry & technology of UV & EB formulation for coatings, inks & paints, 1998 Search PubMed.
- H.-B. Sun and S. Kawata, in NMR·3D Analysis·Photopolymerization, Springer, Berlin, Heidelberg, 2004, pp. 169–273, DOI:10.1007/b94405.
- G. Yilmaz, B. Aydogan, G. Temel, N. Arsu, N. Moszner and Y. Yagci, Macromolecules, 2010, 43, 4520–4526 CrossRef.
- G. Yilmaz, A. Tuzun and Y. Yagci, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 5120–5125 CrossRef.
- G. Yilmaz, G. Acik and Y. Yagci, Macromolecules, 2012, 45, 2219–2224 CrossRef.
- S. Kork, G. Yilmaz and Y. Yagci, Macromol. Rapid Commun., 2015, 36, 923–928 CrossRef PubMed.
- J. Lalevee, A. Dirani, M. El-Roz, X. Allonas and J. P. Fouassier, Macromolecules, 2008, 41, 2003–2010 CrossRef.
- J. Lalevee, F. Dumur, C. R. Mayer, D. Gigmes, G. Nasr, M. A. Tehfe, S. Telitel, F. Morlet-Savary, B. Graff and J. P. Fouassier, Macromolecules, 2012, 45, 4134–4141 CrossRef.
- M. A. Tehfe, J. Lalevee, F. Morlet-Savary, B. Graff, N. Blanchard and J. P. Fouassier, Macromolecules, 2012, 45, 1746–1752 CrossRef.
- J. P. Fouassier, X. Allonas and D. Burget, Prog. Org. Coat., 2003, 47, 16–36 CrossRef.
- J. Jakubiak, X. Allonas, J. P. Fouassier, A. Sionkowska, E. Andrzejewska, L. A. Linden and J. F. Rabek, Polymer, 2003, 44, 5219–5226 CrossRef.
- S. Erdur, G. Yilmaz, D. G. Colak, I. Cianga and Y. Yagci, Macromolecules, 2014, 47, 7296–7302 CrossRef.
- G. Yilmaz, S. Beyazit and Y. Yagci, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 1591–1596 CrossRef.
- M. U. Kahveci, M. A. Tasdelen and Y. Yagci, Macromol. Rapid Commun., 2008, 29, 202–206 CrossRef.
- M. A. Tasdelen, V. Kumbaraci, S. Jockusch, N. J. Turro, N. Talinli and Y. Yagci, Macromolecules, 2008, 41, 295–297 CrossRef.
- M. U. Kahveci, M. Uygun, M. A. Tasdelen, W. Schnabel, W. D. Cook and Y. Yagci, Macromolecules, 2009, 42, 4443–4448 CrossRef.
- J. Lalevee, M. El-Roz, X. Allonas and J. P. Fouassier, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 2008–2014 CrossRef.
- M. A. Tehfe, J. Lalevee, D. Gigmes and J. P. Fouassier, Macromolecules, 2010, 43, 1364–1370 CrossRef.
- Q. Michaudel, T. Chauvire, V. Kottisch, M. J. Supej, K. J. Stawiasz, L. X. Shen, W. R. Zipfel, H. D. Abruna, J. H. Freed and B. P. Fors, J. Am. Chem. Soc., 2017, 139, 15530–15538 CrossRef PubMed.
- R. B. Paul, J. M. Kelly, D. C. Pepper and C. Long, Polymer, 1997, 38, 2011–2014 CrossRef.
- B. Aydogan, A. S. Gundogan, T. Ozturk and Y. Yagci, Chem. Commun., 2009, 6300–6302, 10.1039/b914953h.
- S. Hurrle, A. S. Goldmann, H. Gliemann, H. Mutlu and C. Barner-Kowollik, ACS Macro Lett., 2018, 201–207, DOI:10.1021/acsmacrolett.7b01001.
- K. Matyjaszewski and J. H. Xia, Chem. Rev., 2001, 101, 2921–2990 CrossRef PubMed.
- V. Coessens, T. Pintauer and K. Matyjaszewski, Prog. Polym. Sci., 2001, 26, 337–377 CrossRef.
- M. Kamigaito, T. Ando and M. Sawamoto, Chem. Rev., 2001, 101, 3689–3745 CrossRef PubMed.
- K. Matyjaszewski, Macromolecules, 2012, 45, 4015–4039 CrossRef.
- J. S. Wang and K. Matyjaszewski, Macromolecules, 1995, 28, 7901–7910 CrossRef.
- G. Ng, J. Yeow, J. T. Xu and C. Boyer, Polym. Chem., 2017, 8, 2841–2851 RSC.
- S. Shanmugam, J. T. Xu and C. Boyer, Macromolecules, 2017, 50, 1832–1846 CrossRef.
- C. Y. Wu, S. Shanmugam, J. T. Xu, J. Zhu and C. Boyer, Chem. Commun., 2017, 53, 12560–12563 RSC.
- J. Yeow, R. Chapman, J. T. Xu and C. Boyer, Polym. Chem., 2017, 8, 5012–5022 RSC.
- B. Wenn and T. Junkers, Macromolecules, 2016, 49, 6888–6895 CrossRef.
- J. Tan, X. Rao, X. Wu, H. Deng, J. Yang and Z. Zeng, Macromolecules, 2012, 45, 8790–8795 CrossRef.
- M. A. Tasdelen, M. Uygun and Y. Yagci, Macromol. Chem. Phys., 2010, 211, 2271–2275 CrossRef.
- S. Dadashi-Silab, M. A. Tasdelen and Y. Yagci, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 2878–2888 CrossRef.
- X. Pan, M. A. Tasdelen, J. Laun, T. Junkers, Y. Yagci and K. Matyjaszewski, Prog. Polym. Sci., 2016, 62, 73–125 CrossRef.
- Z. B. Guan and B. Smart, Macromolecules, 2000, 33, 6904–6906 CrossRef.
- M. A. Tasdelen, M. Uygun and Y. Yagci, Macromol. Rapid Commun., 2011, 32, 58–62 CrossRef PubMed.
- M. Ciftci, M. A. Tasdelen and Y. Yagci, Polym. Chem., 2014, 5, 600–606 RSC.
- M. A. Tasdelen, M. Ciftci and Y. Yagci, Macromol. Chem. Phys., 2012, 213, 1391–1396 CrossRef.
- M. A. Tasdelen, M. Uygun and Y. Yagci, Macromol. Chem. Phys., 2011, 212, 2036–2042 CrossRef.
- D. Konkolewicz, K. Schroder, J. Buback, S. Bernhard and K. Matyjaszewski, ACS Macro Lett., 2012, 1, 1219–1223 CrossRef.
- O. S. Taskin, G. Yilmaz, M. A. Tasdelen and Y. Yagci, Polym. Int., 2014, 63, 902–907 CrossRef.
- T. Zhang, D. Gieseler and R. Jordan, Polym. Chem., 2016, 7, 775–779 RSC.
- A. Anastasaki, V. Nikolaou, F. Brandford-Adams, G. Nurumbetov, Q. Zhang, G. J. Clarkson, D. J. Fox, P. Wilson, K. Kempe and D. M. Haddleton, Chem. Commun., 2015, 51, 5626–5629 RSC.
- D. M. Haddleton, C. B. Jasieczek, M. J. Hannon and A. J. Shooter, Macromolecules, 1997, 30, 2190–2193 CrossRef.
- V. Nikolaou, A. Anastasaki, F. Alsubaie, A. Simula, D. J. Fox and D. M. Haddleton, Polym. Chem., 2015, 6, 3581–3585 RSC.
- X. C. Pan, N. Malhotra, A. Simakova, Z. Y. Wang, D. Konkolewicz and K. Matyjaszewski, J. Am. Chem. Soc., 2015, 137, 15430–15433 CrossRef PubMed.
- N. J. Treat, H. Sprafke, J. W. Kramer, P. G. Clark, B. E. Barton, J. Read de Alaniz, B. P. Fors and C. J. Hawker, J. Am. Chem. Soc., 2014, 136, 16096–16101 CrossRef PubMed.
- X. Pan, C. Fang, M. Fantin, N. Malhotra, W. Y. So, L. A. Peteanu, A. A. Isse, A. Gennaro, P. Liu and K. Matyjaszewski, J. Am. Chem. Soc., 2016, 138, 2411–2425 CrossRef PubMed.
- S. Jockusch and Y. Yagci, Polym. Chem., 2016, 7, 6039–6043 RSC.
- X. Pan, M. Lamson, J. Yan and K. Matyjaszewski, ACS Macro Lett., 2015, 4, 192–196 CrossRef.
- Y. Zhao, H. Gong, K. Jiang, S. Yan, J. Lin and M. Chen, Macromolecules, 2018, 51(3), 938–946 CrossRef.
- S. Dadashi-Silab, X. Pan and K. Matyjaszewski, Chem. – Eur. J., 2017, 23, 5972–5977 CrossRef PubMed.
- A. Allushi, S. Jockusch, G. Yilmaz and Y. Yagci, Macromolecules, 2016, 49, 7785–7792 CrossRef.
- G. M. Miyake and J. C. Theriot, Macromolecules, 2014, 47, 8255–8261 CrossRef.
- C.-H. Lim, M. D. Ryan, B. G. McCarthy, J. C. Theriot, S. M. Sartor, N. H. Damrauer, C. B. Musgrave and G. M. Miyake, J. Am. Chem. Soc., 2017, 139, 348–355 CrossRef PubMed.
- R. M. Pearson, C.-H. Lim, B. G. McCarthy, C. B. Musgrave and G. M. Miyake, J. Am. Chem. Soc., 2016, 138, 11399–11407 CrossRef PubMed.
- Y. Du, R. M. Pearson, C.-H. Lim, S. M. Sartor, M. D. Ryan, H. Yang, N. H. Damrauer and G. M. Miyake, Chem. – Eur. J., 2017, 23, 10962–10968 CrossRef PubMed.
- D. Koyama, H. J. A. Dale and A. J. Orr-Ewing, J. Am. Chem. Soc., 2018, 140, 1285–1293 CrossRef PubMed.
- C. Kutahya, A. Allushi, R. Isci, J. Kreutzer, T. Ozturk, G. Yilmaz and Y. Yagci, Macromolecules, 2017, 50, 6903–6910 CrossRef.
- J. C. Theriot, B. G. McCarthy, C.-H. Lim and G. M. Miyake, Macromol. Rapid Commun., 2017, 38 Search PubMed , 1700040.
- X. Liu, L. Zhang, Z. Cheng and X. Zhu, Polym. Chem., 2016, 7, 689–700 RSC.
- C. Kutahya, F. S. Aykac, G. Yilmaz and Y. Yagci, Polym. Chem., 2016, 7, 6094–6098 RSC.
- A. Allushi, C. Kutahya, C. Aydogan, J. Kreutzer, G. Yilmaz and Y. Yagci, Polym. Chem., 2017, 8, 1972–1977 RSC.
- K. M. Mattson, C. W. Pester, W. R. Gutekunst, A. T. Hsueh, E. H. Discekici, Y. Luo, B. V. K. J. Schmidt, A. J. McGrath, P. G. Clark and C. J. Hawker, Macromolecules, 2016, 49, 8162–8166 CrossRef.
- S. B. Tan, Y. F. Zhao, W. W. Zhang, P. Gao, W. W. Zhu and Z. C. Zhang, Polym. Chem., 2018, 9, 221–227 RSC.
- E. H. Discekici, C. W. Pester, N. J. Treat, J. Lawrence, K. M. Mattson, B. Narupai, E. P. Toumayan, Y. Luo, A. J. McGrath, P. G. Clark, J. Read de Alaniz and C. J. Hawker, ACS Macro Lett., 2016, 5, 258–262 CrossRef.
- Y. Yang, X. Liu, G. Ye, S. Zhu, Z. Wang, X. Huo, K. Matyjaszewski, Y. Lu and J. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 13637–13646 Search PubMed.
- J. Yan, X. Pan, M. Schmitt, Z. Wang, M. R. Bockstaller and K. Matyjaszewski, ACS Macro Lett., 2016, 5, 661–665 CrossRef.
- C. Aydogan, C. Kutahya, A. Allushi, G. Yilmaz and Y. Yagci, Polym. Chem., 2017, 8, 2899–2903 RSC.
- C. Aydogan, G. Yilmaz and Y. Yagci, Macromolecules, 2017, 50, 9115–9120 CrossRef.
- B. L. Buss, L. R. Beck and G. M. Miyake, Polym. Chem., 2018 10.1039/C7PY01833A.
- B. L. Ramsey, R. M. Pearson, L. R. Beck and G. M. Miyake, Macromolecules, 2017, 50, 2668–2674 CrossRef PubMed.
- F. Giustino and H. J. Snaith, ACS Energy Lett., 2016, 1, 1233–1240 CrossRef.
| This journal is © The Royal Society of Chemistry 2018 |