Base-induced reversible H2 addition to a single Sn(ii) centre†
Abstract
A range of amines catalyse the oxidative addition (OA) of H2 to [(Me3Si)2CH]2Sn (1), forming [(Me3Si)2CH]2SnH2 (2). Experimental and computational studies point to ‘frustrated Lewis pair’ mechanisms in which 1 acts as a Lewis acid and involve unusual late transition states; this is supported by the observation of a kinetic isotope effect 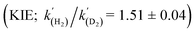 for Et3N. When DBU is used the energetics of H2 activation are altered, allowing an equilibrium between 1, 2 and adduct [1·DBU] to be established, thus demonstrating reversible oxidative addition/reductive elimination (RE) of H2 at a single main group centre.
for Et3N. When DBU is used the energetics of H2 activation are altered, allowing an equilibrium between 1, 2 and adduct [1·DBU] to be established, thus demonstrating reversible oxidative addition/reductive elimination (RE) of H2 at a single main group centre.

- This article is part of the themed collection: Tin Collection – a celebration of our 10th anniversary


 Please wait while we load your content...
Please wait while we load your content...