Stibine-protected Au13 nanoclusters: syntheses, properties and facile conversion to GSH-protected Au25 nanocluster†
Abstract
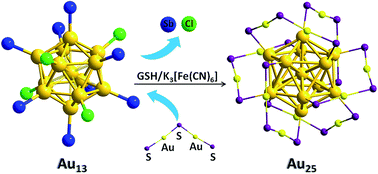
Monostibine-protected ionic Au13 nanoclusters, namely, [Au13(L)8(Cl)4][Cl] (L= SbPh3, 2a·Cl; Sb(p-tolyl)3, 2b·Cl) were prepared by the direct reduction of Au(L)Cl with NaBH4 in dichloromethane. Anion exchange with 2a·Cl afforded [Au13(SbPh3)8(Cl)4][X] (X = PF6, 2a·PF6; BPh4, 2a·BPh4). All these have been characterized by multinuclear NMR, ESI-MS and UV-Vis spectroscopy. Crystallographic analysis of 2a·BPh4 reveals that the cation possesses C2v symmetry and the tridecagold core adopts a closed icosahedron configuration. The weaker coordinating ability of the stibine ligands leads to the ready reaction of 2b·Cl with PPh3 or glutathione (GSH) to form the smaller phosphine-protected cluster [Au11(PPh3)8Cl2][Cl] or larger thiolate-protected cluster Au25(SG)18, respectively. In the latter reaction, the addition of a small amount (0.5 to 3.5 equivalents) of a suitable oxidant such as K3(Fe(CN)6 accelerates the conversion rate significantly.


 Please wait while we load your content...
Please wait while we load your content...