Open Access Article
Open Access Article1-Phenyl-N-(benzothiazol-2-yl)methanimine derivatives as Middle East respiratory syndrome coronavirus inhibitors†
Min-Qi Hu‡
a,
Heng Li‡bc,
Ying Lina,
Ying Zhanga,
Jie Tangad,
Jian-Ping Zuob,
Li-Fang Yu a,
Xian-Kun Tong*bc,
Wei Tang*bc and
Fan Yang
a,
Xian-Kun Tong*bc,
Wei Tang*bc and
Fan Yang *a
*a
aShanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062, China. E-mail: fyang@chem.ecnu.edu.cn
bShanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203, China. E-mail: tangwei@simm.ac.cn; xktong@simm.ac.cn
cSchool of Pharmacy, University of Chinese Academy of Sciences, Beijing, 100049, China
dShanghai Greenchem & Biotech Co. Ltd., Shanghai, 200062, China
First published on 7th December 2020
Abstract
Middle East respiratory syndrome coronavirus (MERS-CoV) poses a serious threat to human health, and currently there are no effective or specific therapies available to treat it. Herein a series of 1-phenyl-N-(benzothiazol-2-yl)methanimine derivatives with inhibitory activity against MERS-CoV are described. The compound 4f with a 50% inhibition concentration value of 0.09 μM is a promising inhibitor that warrants further evaluation, towards the development of potential anti-MERS-CoV drugs.
Introduction
Middle East respiratory syndrome coronavirus (MERS-CoV) is a novel zoonotic virus in the Middle East.1,2 It first appeared in Saudi Arabia in June 2012,3 then spread to the rest of the Middle East and South Korea.4 MERS-CoV is primarily transmitted from animals to humans,5 but it can reportedly spread from human to human through close contact.6,7 It can cause severe respiratory disease, with symptoms of fever, coughing, and dyspnea, and may contribute to severe gastrointestinal diseases and renal failure.8 As at 03 June 2020, the World Health Organization had reported 2494 laboratory-confirmed cases of MERS-CoV infection, including 858 confirmed deaths (an approximately 35% fatality rate) in over 27 countries.MERS-CoV poses a severe threat to human health, as illustrated by the recent global coronavirus disease-2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2. Although some reported therapeutics for COVID-19 have recently been reported,9–17 their curative effect is still very limited and there can be side effects.18,19 Similarly, despite ongoing global efforts to develop small-molecule inhibitors,20–26 peptide inhibitors,27–30 neutralizing antibodies,31–33 and vaccines34–36 for the treatment and prevention of MERS-CoV, there is no effective approved specific antiviral therapy for MERS-CoV infection. Therefore, the development of specific antiviral drugs for the treatment of MERS-CoV is urgently needed.
Viral genomic studies indicate that MERS-CoV is an enveloped virus with positive-sense single-stranded RNA that belongs to lineage C in the genus Betacoronavirus of the family Coronaviridae under the Nidovirales order.3 It is the sixth coronavirus known to infect humans, and the first known lineage C Betacoronavirus associated with human infection.37,38 The MERS-CoV genome is approximately 30 kilobases in size and contains more than 10 open reading frames (ORFs).39 The large replicase ORF1a and ORF1b produce polyproteins PP1a and PP1ab, which are subsequently cleaved into 15 or 16 nonstructural proteins.40–42 The region downstream of ORF1b encodes at least four main structural proteins: spike (S), membrane (M), envelope (E), and nucleocapsid (N).
The S protein serves as the critical surface-located trimeric glycoprotein of MERS-CoV and induces entry into host cells,43 and it is composed of subunits S1 and S2. The receptor-binding domain on the S1 subunit attaches to dipeptidyl peptidase 4, which is a well-known target for diabetes.44–49 The conformation of the S2 subunit then changes and a six-helix bundle fusion core is formed, which enables the formation of the viral envelope, facilitating tight host cell membrane binding for fusion and subsequent host cell entry.50 Thus the S protein plays a pivotal role in mediating the entry of the virus into target host cells.
According to previous reports, MERS-CoV pseudovirus expressing S protein, which allows for single-cycle infection in cells expressing dipeptidyl peptidase 4, can be used to rapidly screen MERS-CoV entry inhibitors.51 In the current study, novel small-molecule inhibitors of entry events of pseudovirus expressing S protein were identified via high-throughput screening technology using a cell-based assay.
Materials and methods
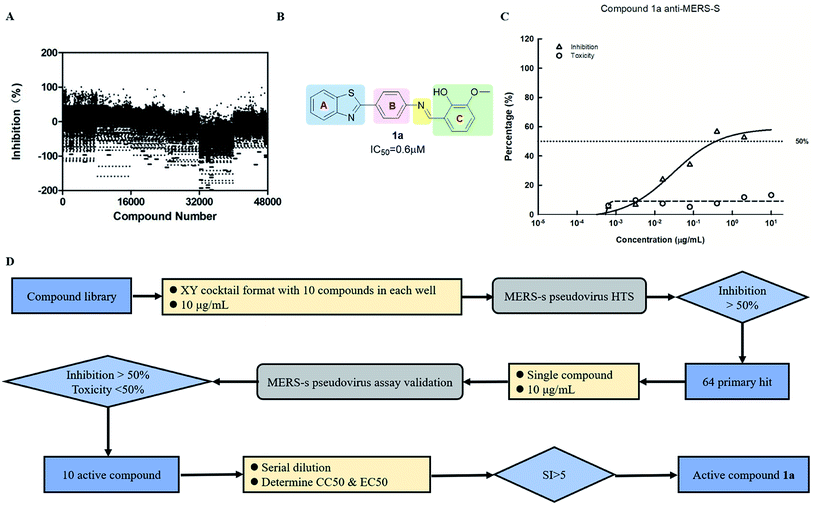
Screening was performed using an orthogonal cocktail library52 composed of 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 compounds that had never been screened for anti-MERS-CoV activity (Fig. 1A). Compound library samples were orthogonally pooled as mixtures of 10 per well at 2 μg mL−1 each, with duplicate representation for each compound (Fig. 1D). This bidirectional orthogonal pooling strategy enables greater screening efficiency and throughput when using large compound libraries. Using this approach, compound 1a was identified as a modest entry inhibitor (Fig. 1B) with a 50% inhibition concentration (IC50) of 0.73 μM (Fig. 1C). Using 1a as a hit compound, a series of 1-phenyl-N-(benzothiazol-2-yl)methanimine derivatives were designed and synthesized, and structure–activity relationships and their inhibitory potencies against MERS-S pseudovirus were investigated.
000 compounds that had never been screened for anti-MERS-CoV activity (Fig. 1A). Compound library samples were orthogonally pooled as mixtures of 10 per well at 2 μg mL−1 each, with duplicate representation for each compound (Fig. 1D). This bidirectional orthogonal pooling strategy enables greater screening efficiency and throughput when using large compound libraries. Using this approach, compound 1a was identified as a modest entry inhibitor (Fig. 1B) with a 50% inhibition concentration (IC50) of 0.73 μM (Fig. 1C). Using 1a as a hit compound, a series of 1-phenyl-N-(benzothiazol-2-yl)methanimine derivatives were designed and synthesized, and structure–activity relationships and their inhibitory potencies against MERS-S pseudovirus were investigated.
Results and discussion
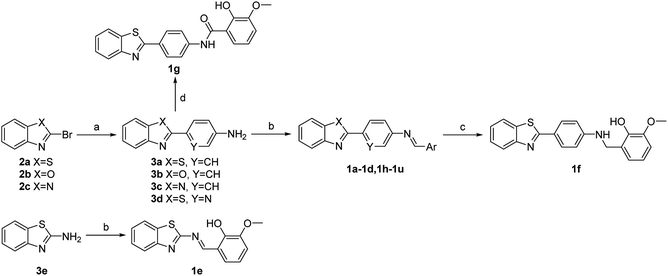
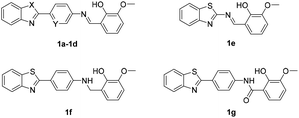
Four major regions were partitioned for diversification; the benzothiazole portion (blue), the linker region (pink), the imine portion (yellow), and the phenyl portion (green) (Fig. 1B). Compounds 1a–1g were synthesized first as outlined in Scheme 1. Commercially available aryl bromides were used as the starting material, and the corresponding intermediate amines 3a–3d were prepared using the Suzuki–Miyaura cross-coupling reaction.53 Compounds 1a–1e were generated using the reaction of an intermediate amine with the corresponding aromatic aldehyde in the presence of AcOH as catalyst.54 Compound 1g was generated by the reaction of 3a with 2-hydroxy-3-methoxybenzoyl chloride. Compound 1f was generated by a reduction reaction of 1a with NaBH4.55 Their IC50 values against MERS-S pseudovirus and 50% cytotoxic concentration (CC50) values are summarized in Table 1. The Schiff base structure of hit compound 1a was confirmed by comprehensive spectral data. The 1H NMR spectrum depicted a sharp singlet peak at δ 8.67 parts per million (ppm) indicating the presence of azomethine proton (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–). Phenolic-OH proton and methoxy protons exhibited singlet peaks at δ 13.44 and δ 3.94 ppm respectively. A multiplet signal at δ 6.87–8.14 ppm was assigned to the aromatic protons. The 13C nuclear magnetic resonance spectrum of 1a revealed corresponding peaks in the range of δ 114.2–166.1 ppm which were assigned to azomethine (–CH
N–). Phenolic-OH proton and methoxy protons exhibited singlet peaks at δ 13.44 and δ 3.94 ppm respectively. A multiplet signal at δ 6.87–8.14 ppm was assigned to the aromatic protons. The 13C nuclear magnetic resonance spectrum of 1a revealed corresponding peaks in the range of δ 114.2–166.1 ppm which were assigned to azomethine (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) carbon and aryl carbons. A peak at δ 55.2 ppm was identical to the methoxy carbon. The 1H–1H COSY spectrum demonstrated chemical shifts of three protons on ring C at δ 6.87–7.06 ppm, and two protons near the nitrogen atom side on ring B at δ 7.36–7.41 ppm. Correlation between the azomethine proton and the two protons near the nitrogen atom side on ring B was observed in the 1H–1H NOSEY correlation spectrum, indicating that the imine moiety of compound 1a is E-geometry. The HRMS (ESI) spectrum of 1a depicted a signal at m/z 383.0823, indicating a [M + Na]+ signal with the molecular formula C21H16N2NaO2S, which is concordant with the structure of 1a. Characteristic spectra and data are shown in the ESI.†
N–) carbon and aryl carbons. A peak at δ 55.2 ppm was identical to the methoxy carbon. The 1H–1H COSY spectrum demonstrated chemical shifts of three protons on ring C at δ 6.87–7.06 ppm, and two protons near the nitrogen atom side on ring B at δ 7.36–7.41 ppm. Correlation between the azomethine proton and the two protons near the nitrogen atom side on ring B was observed in the 1H–1H NOSEY correlation spectrum, indicating that the imine moiety of compound 1a is E-geometry. The HRMS (ESI) spectrum of 1a depicted a signal at m/z 383.0823, indicating a [M + Na]+ signal with the molecular formula C21H16N2NaO2S, which is concordant with the structure of 1a. Characteristic spectra and data are shown in the ESI.†
| Compound | X | Y | IC50a | CC50b |
|---|---|---|---|---|
| a IC50: 50% inhibitory concentration (μM).b CC50: 50% cytotoxic concentration (μM).c HRP: peptide mimics of heptad repeat domain in the MERS spike protein, which is responsible for conformational change during spike protein-mediated viral–host membrane fusion. | ||||
| 1a | S | CH | 0.73 ± 0.66 | >100 |
| 1b | O | CH | >10.0 | >100 |
| 1c | NH | CH | >10.0 | >100 |
| 1d | S | N | 2.7 ± 0.19 | >100 |
| 1e | — | — | >10.0 | >100 |
| 1f | — | — | >10.0 | >100 |
| 1g | — | — | >10.0 | >100 |
| HRPc | — | — | 2.0 | >100 |
Compounds with benzoxazole (1b) or benzimidazole (1c) motifs lost inhibitory activity, as did the compound without a phenyl group B (1e) in the linker region. Modest inhibitory activity was evident when the phenyl group B was replaced with a pyridyl group (1d). When the imine portion was reduced or replaced with amide (1f and 1g), inhibitory activity was markedly reduced. Modification was then focused on the phenyl ring C of the compound, and derivatives 1h–1m were designed and synthesized in a similar way as 1a. Their IC50 values on MERS-S pseudovirus and CC50 values are summarized in Table 2.
| Compound | Ar | IC50a | CC50b |
|---|---|---|---|
| a IC50: 50% inhibitory concentration (μM).b CC50: 50% cytotoxic concentration (μM).c HRP: peptide mimics of heptad repeat domain in the MERS spike protein, which is responsible for conformational change during spike protein-mediated viral–host membrane fusion. | |||
| 1h |  |
0.57 ± 0.11 | >100 |
| 1i |  |
0.52 ± 0.03 | >100 |
| 1j | 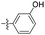 |
0.20 ± 0.05 | >100 |
| 1k | 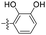 |
0.40 ± 0.01 | >100 |
| 1l | 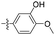 |
0.22 ± 0.03 | >100 |
| 1m | 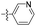 |
0.34 ± 0.24 | >100 |
| 1n | 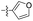 |
>10.00 | >100 |
| 1o | 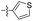 |
0.14 ± 0.06 | >100 |
| 1p | 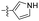 |
>10.00 | >100 |
| 1q | 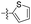 |
6.52 ± 1.14 | >100 |
| 1r | 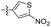 |
0.62 ± 0.31 | >100 |
| 1s | 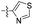 |
>10.00 | >100 |
| 1t | 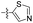 |
8.80 ± 0.01 | >100 |
| 1u | 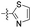 |
>10.00 | >100 |
| HRPc | — | 2.00 | >100 |
All the derivatives demonstrated inhibitory activities with IC50 values ranging from 0.20 μM to 0.57 μM, and no cell toxicity. Either changing the position of the hydroxyl group and methoxy group or the absence of these groups increased the activity slightly, the compounds containing 3-OH were more potent. Changing the phenyl ring C to a pyridine ring did not elicit significant changes in inhibitory activity.
Given the results of 1h–1m, further structural extensions were investigated in the phenyl ring C area. A series of five-member aromatic heterocycles were introduced to replace the phenyl ring C. Derivatives 1o and 1r, substituted by a 3-thienyl group, exhibited marked inhibitory activity with respective IC50 values of 0.14 μM and 0.62 μM. The introduction of a nitro group on the thiophene motif caused a slight reduction in inhibitory activity. Compound 1q exhibited modest inhibitory capacity, indicating that the substitution mode influenced activity. None of the compounds in this series exhibited any cytotoxicity.
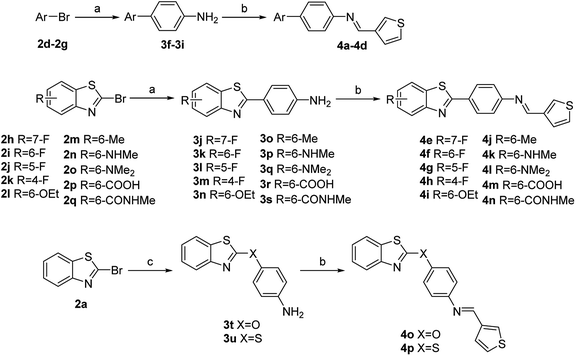
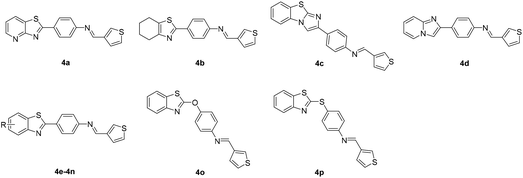
Based on the above results, while retaining the 3-thienyl group at ring C position a structure–activity relationship investigation was conducted in which structural extensions at the benzothiazole part of this compound were assessed. As shown in Scheme 2, commercially available aryl bromides were used as starting materials, and the corresponding intermediate amines 3f–3u were prepared using Suzuki–Miyaura cross-coupling or a nucleophilic substitution reaction. The final compounds 4a–4p were readily generated via a similar method that has been previously outlined in Scheme 1. Their IC50 values against MERS-S pseudovirus and their CC50 values are summarized in Table 3.
| Compound | R | IC50a | CC50b |
|---|---|---|---|
| a IC50: 50% inhibitory concentration (μM).b CC50: 50% cytotoxic concentration (μM).c HRP: peptide mimics of heptad repeat domain in the MERS spike protein, which is responsible for conformational change during spike protein-mediated viral–host membrane fusion. | |||
| 4a | — | >10.00 | >100 |
| 4b | — | >10.00 | >100 |
| 4c | — | >10.00 | >100 |
| 4d | — | >10.00 | >100 |
| 4e | 7-F | 1.67 ± 0.30 | >100 |
| 4f | 6-F | 0.09 ± 0.01 | >100 |
| 4g | 5-F | 1.00 ± 0.07 | >100 |
| 4h | 4-F | 0.53 ± 0.02 | >100 |
| 4i | 6-OEt | >10.00 | >100 |
| 4j | 6-Me | >10.00 | >100 |
| 4k | 6-NHMe | >10.00 | >100 |
| 4l | 6-NMe2 | >10.00 | >100 |
| 4m | 6-COOH | >10.00 | >100 |
| 4n | 6-CONHMe | >10.00 | >100 |
| 4o | — | >10.00 | >100 |
| 4p | — | >10.00 | >100 |
| HRPc | — | 2.00 | >100 |
Replacement of a benzothiazole unit (4a–4d) abolished the inhibitory activity (Table 3), further confirming that the benzothiazole motif played a pivotal role in inhibitory capacity. An atom of fluorine—the most electronegative of the halogens—was then introduced at different benzothiazole positions, and the derivatives obtained (4e–4h) exhibited diverse inhibitory capacity. Introduction of a fluorine atom at the 6-position of the phenyl ring A (4f) increased inhibition activity, with an IC50 value of 0.09 μM, and introduction of a fluorine atom at another position resulted in modest inhibitory potency. The introduction of other groups such as alkoxy, alkyl, amino, and carboxyl groups (4i–4n) reduced inhibitory activity. The reduction in inhibitory capacity is most likely due to the steric effect of the bulky group. To increase the flexibility of the compounds, heteroatom was introduced between the benzothiazole group and phenyl ring B to obtain 4o and 4p, but unfortunately inhibitory activity was abolished. The CC50 values of these compounds were greater than 100 μM, indicating no toxicity or at least lower toxicity to uninfected cells. The results of the study suggest that compound 4f has potential for the subsequent development of novel anti-MERS-CoV agents.
Conclusion
In summary, a series of new 1-phenyl-N-(benzothiazol-2-yl)methanimine derivatives was developed as MERS-CoV entry inhibitors via modification of a hit compound, which was obtained by screening an orthogonal cocktail library composed of 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 compounds. All of the compounds were assessed for their capacity to inhibit the entry of pseudovirus expressing MERS-CoV S protein. Structure–activity relationships indicated that benzothiazole was the best option for region A of the structure, and the presence of a phenyl ring B and an imine portion favored the retention of inhibitory activity against MERS-CoV. The introduction of a 6-member aromatic ring or a 3-thiophene ring at ring C position also favored inhibitory activity. Remarkably, compound 4f, with an IC50 value of 0.09 μM, was more potent than the initial hit compound. In addition, none of these types of structures exhibited cytotoxicity. The present study demonstrates an approach for the structural modification of hit compounds, and a novel skeleton for new anti-MERS-CoV drug research. Further research on this series of derivatives is ongoing.
000 compounds. All of the compounds were assessed for their capacity to inhibit the entry of pseudovirus expressing MERS-CoV S protein. Structure–activity relationships indicated that benzothiazole was the best option for region A of the structure, and the presence of a phenyl ring B and an imine portion favored the retention of inhibitory activity against MERS-CoV. The introduction of a 6-member aromatic ring or a 3-thiophene ring at ring C position also favored inhibitory activity. Remarkably, compound 4f, with an IC50 value of 0.09 μM, was more potent than the initial hit compound. In addition, none of these types of structures exhibited cytotoxicity. The present study demonstrates an approach for the structural modification of hit compounds, and a novel skeleton for new anti-MERS-CoV drug research. Further research on this series of derivatives is ongoing.
Experimental
Chemistry
Commercial reagents and solvents were purchased commercial suppliers and used without further purification. All non-aqueous reactions were under a nitrogen atmosphere and all non-aqueous reaction vessels were oven-dried. Flash column chromatography was performed using Qingdao Ocean silica gel (200–300) with the indicated eluents. All final compounds were characterized by their NMR and HRMS spectra, unless stated otherwise. 1H NMR spectra were recorded at a spectrometer frequency of 400 MHz, and 13C NMR spectra at 100 MHz on Bruker Avance 400. Chemical shifts are reported in δ (ppm) using signals of tetramethylsilane (TMS) as the internal standard. High-resolution mass spectra (HRMS) were measured on a Bruker ESI-TOF high-resolution mass spectrometer. Melting points (mp) were uncorrected and were recorded on a Buchi B-54 melting point apparatus.General procedure for the synthesis of compounds 3a–3d and 3f–3s
2a–2q (1 mmol), the corresponding pinacol borate (1.2 mmol) and Pd(dppf)Cl2·CH2Cl2 (0.05 mmol) were suspended in DMF (6 mL) in the presence of 2 M K2CO3 (2 mL) and stirred at 70 °C overnight under nitrogen. The reaction mixture was diluted with H2O (40 mL) and organics were extracted with EtOAc (3× 30 mL). The combined organic layers were washed with brine (30 mL), dried over MgSO4, filtered and concentrated in vacuo to afford crude mixture. The crude mixture was then purified by flash column chromatography in a mixture of petroleum ether and ethyl acetate to yield the pure products 3a–3d and 3f–3s.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)NH–), 8.48 (s, 1H, ArH), 7.95–7.88 (m, 2H, ArH), 7.79 (d, J = 8.4 Hz, 2H, ArH), 6.68 (d, J = 8.6 Hz, 2H, ArH), 5.99 (s, 2H, NH2), 2.82 (d, J = 4.4 Hz, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ 170.4, 166.1, 155.6, 152.5, 133.6, 130.4, 129.0 (2C), 125.3, 121.1, 121.1, 119.7, 113.5 (2C), 26.4.
O)NH–), 8.48 (s, 1H, ArH), 7.95–7.88 (m, 2H, ArH), 7.79 (d, J = 8.4 Hz, 2H, ArH), 6.68 (d, J = 8.6 Hz, 2H, ArH), 5.99 (s, 2H, NH2), 2.82 (d, J = 4.4 Hz, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ 170.4, 166.1, 155.6, 152.5, 133.6, 130.4, 129.0 (2C), 125.3, 121.1, 121.1, 119.7, 113.5 (2C), 26.4.Synthesis of 4-(benzo[d]thiazol-2-yloxy)aniline (3t)
4-Aminophenol (2 mmol) was dissolved in DMF (5 mL) followed by the addition of t-BuONa (2.4 mmol) and stirred at room temperature for 1 h. Then 2a (2 mmol) and K2CO3 (2.4 mmol) were added and stirred for another 4 h. The reaction mixture was diluted with H2O (40 mL) and organics were extracted with EtOAc (3× 30 mL). The combined organic layers were washed with brine (30 mL), dried over MgSO4, filtered and concentrated in vacuo to afford crude mixture. The crude mixture was then purified by flash column chromatography in a mixture of petroleum ether and ethyl acetate to yield the pure product 3t. Yellow oil, yield 80%. 1H NMR (400 MHz, chloroform-d) δ 7.73 (d, J = 8.1 Hz, 1H, ArH), 7.63 (d, J = 8.0 Hz, 1H, ArH), 7.40–7.34 (m, 1H, ArH), 7.25–7.21 (m, 1H, ArH), 7.12 (d, J = 8.7 Hz, 2H, ArH), 6.71 (d, J = 8.7 Hz, 2H, ArH), 3.74 (s, 2H, NH2). 13C NMR (100 MHz, chloroform-d) δ 173.6, 149.4, 147.2, 145.0, 132.2, 126.2, 123.8, 121.8 (2C), 121.6, 121.3, 115.9 (2C).Synthesis of 4-(benzo[d]thiazol-2-ylthio)aniline (3u)
4-Aminobenzenethiol (2 mmol) and 2a (2 mmol) was dissolved in MeCN (5 mL) followed by the addition of K2CO3 (2.4 mmol) and stirred at reflux for 3 h. Upon completion, the reaction was allowed to cold to room temperature. The reaction mixture was diluted with H2O (40 mL) and organics were extracted with EtOAc (3× 30 mL). The combined organic layers were washed with brine (30 mL), dried over MgSO4, filtered and concentrated in vacuo to afford crude mixture. The crude mixture was then purified by flash column chromatography in a mixture of petroleum ether and ethyl acetate to yield the pure product 3u. White solid; yield 50%. 1H NMR (400 MHz, chloroform-d) δ 7.84 (d, J = 8.2 Hz, 1H, ArH), 7.62 (d, J = 8.0 Hz, 1H, ArH), 7.50 (d, J = 8.4 Hz, 2H, ArH), 7.41–7.35 (m, 1H, ArH), 7.25–7.19 (m, 1H, ArH), 6.74 (d, J = 8.4 Hz, 2H, ArH), 4.02 (s, 2H, NH2). 13C NMR (100 MHz, chloroform-d) δ 172.4, 153.3, 148.0, 136.6 (2C), 134.4, 125.0, 122.8, 120.6, 119.7, 115.6, 114.9 (2C).General procedure for the synthesis of compounds 1a–1d, 1h–1u and 4a–4p
The catalytic amount of acetic acid was added to a solution of 3a–3d, 3f–3u (0.44 mmol) and the corresponding aromatic aldehyde (0.53 mmol) in MeOH (4 mL), the mixture was stirred at reflux for 2 h. The resulting precipitate was isolated by suction filtration and washed with MeOH (8 mL) to give the pure products 1a–1d, 1h–1u and 4a–4p.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.14 (d, J = 8.2 Hz, 2H, ArH), 8.07 (d, J = 8.2 Hz, 1H, ArH), 7.90 (d, J = 7.9 Hz, 1H, ArH), 7.52–7.48 (m, 1H, ArH), 7.41–7.36 (m, 3H, ArH), 7.06–6.99 (m, 2H, ArH), 6.93–6.87 (m, 1H, ArH), 3.94 (s, 3H, CH3). 13C NMR (100 MHz, chloroform-d) δ 166.1, 162.3, 153.1, 150.5, 149.3, 147.5, 134.0, 131.2, 127.7, 125.4, 124.2, 123.0, 122.2, 120.8, 120.6, 118.0, 117.7, 114.2, 55.2. HRMS (ESI) m/z: calculated for C21H16N2NaO2S [M + Na]+: 383.0825, found: 383.0823.
N–), 8.14 (d, J = 8.2 Hz, 2H, ArH), 8.07 (d, J = 8.2 Hz, 1H, ArH), 7.90 (d, J = 7.9 Hz, 1H, ArH), 7.52–7.48 (m, 1H, ArH), 7.41–7.36 (m, 3H, ArH), 7.06–6.99 (m, 2H, ArH), 6.93–6.87 (m, 1H, ArH), 3.94 (s, 3H, CH3). 13C NMR (100 MHz, chloroform-d) δ 166.1, 162.3, 153.1, 150.5, 149.3, 147.5, 134.0, 131.2, 127.7, 125.4, 124.2, 123.0, 122.2, 120.8, 120.6, 118.0, 117.7, 114.2, 55.2. HRMS (ESI) m/z: calculated for C21H16N2NaO2S [M + Na]+: 383.0825, found: 383.0823.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.31 (d, J = 8.3 Hz, 2H, ArH), 7.80–7.76 (m, 1H, ArH), 7.61–7.57 (m, 1H, ArH), 7.42 (d, J = 8.2 Hz, 2H, ArH), 7.38–7.34 (m, 2H, ArH), 7.06–7.01 (m, 2H, ArH), 6.94–6.88 (m, 1H, ArH), 3.95 (s, 3H, CH3). 13C NMR (100 MHz, chloroform-d) δ 162.7, 161.5, 150.6, 149.9, 149.8, 147.5, 141.2, 127.9 (2C), 124.6, 124.2, 123.7, 123.0, 120.8 (2C), 119.0, 118.0, 117.8, 114.3, 109.6, 55.2. HRMS (ESI) m/z: calculated for C21H16N2NaO3 [M + Na]+: 367.1053, found: 367.1041.
N–), 8.31 (d, J = 8.3 Hz, 2H, ArH), 7.80–7.76 (m, 1H, ArH), 7.61–7.57 (m, 1H, ArH), 7.42 (d, J = 8.2 Hz, 2H, ArH), 7.38–7.34 (m, 2H, ArH), 7.06–7.01 (m, 2H, ArH), 6.94–6.88 (m, 1H, ArH), 3.95 (s, 3H, CH3). 13C NMR (100 MHz, chloroform-d) δ 162.7, 161.5, 150.6, 149.9, 149.8, 147.5, 141.2, 127.9 (2C), 124.6, 124.2, 123.7, 123.0, 120.8 (2C), 119.0, 118.0, 117.8, 114.3, 109.6, 55.2. HRMS (ESI) m/z: calculated for C21H16N2NaO3 [M + Na]+: 367.1053, found: 367.1041.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.27 (d, J = 8.2 Hz, 2H, ArH), 7.68 (d, J = 7.5 Hz, 1H, ArH), 7.60 (d, J = 8.2 Hz, 2H, ArH), 7.54 (d, J = 7.5 Hz, 1H, ArH), 7.28 (d, J = 7.5 Hz, 1H, ArH), 7.23–7.20 (m, 2H, ArH), 7.15 (d, J = 7.7 Hz, 1H, ArH), 6.95–6.91 (m, 1H, ArH), 3.84 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ 163.9, 150.7, 150.6, 149.0, 147.9, 143.9, 135.0, 128.6, 127.6 (2C), 123.9, 122.6, 122.0 (2C), 121.7, 119.3, 118.8, 118.7, 115.8, 111.3, 55.9. HRMS (ESI) m/z: calculated for C21H17N3NaO2 [M + Na]+: 366.1213, found: 366.1198.
N–), 8.27 (d, J = 8.2 Hz, 2H, ArH), 7.68 (d, J = 7.5 Hz, 1H, ArH), 7.60 (d, J = 8.2 Hz, 2H, ArH), 7.54 (d, J = 7.5 Hz, 1H, ArH), 7.28 (d, J = 7.5 Hz, 1H, ArH), 7.23–7.20 (m, 2H, ArH), 7.15 (d, J = 7.7 Hz, 1H, ArH), 6.95–6.91 (m, 1H, ArH), 3.84 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ 163.9, 150.7, 150.6, 149.0, 147.9, 143.9, 135.0, 128.6, 127.6 (2C), 123.9, 122.6, 122.0 (2C), 121.7, 119.3, 118.8, 118.7, 115.8, 111.3, 55.9. HRMS (ESI) m/z: calculated for C21H17N3NaO2 [M + Na]+: 366.1213, found: 366.1198.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.61 (d, J = 2.4 Hz, 1H, ArH), 8.43 (d, J = 8.4 Hz, 1H, ArH), 8.09 (d, J = 8.2 Hz, 1H, ArH), 7.96 (d, J = 8.0 Hz, 1H, ArH), 7.74 (dd, J = 8.4, 2.5 Hz, 1H, ArH), 7.54–7.49 (m, 1H, ArH), 7.44–7.41 (m, 1H, ArH), 7.09–7.03 (m, 2H, ArH), 6.96–6.90 (m, 1H, ArH), 3.95 (s, 3H, CH3). 13C NMR (100 MHz, chloroform-d) δ 167.5, 163.8, 153.3, 150.4, 148.7, 147.5, 144.6, 142.3, 135.2, 127.6, 125.3, 124.7, 123.2, 122.5, 121.0, 120.3, 118.1, 117.9, 114.7, 55.2. HRMS (ESI) m/z: calculated for C20H15N3NaO2S [M + Na]+: 384.0777, found: 384.0786.
N–), 8.61 (d, J = 2.4 Hz, 1H, ArH), 8.43 (d, J = 8.4 Hz, 1H, ArH), 8.09 (d, J = 8.2 Hz, 1H, ArH), 7.96 (d, J = 8.0 Hz, 1H, ArH), 7.74 (dd, J = 8.4, 2.5 Hz, 1H, ArH), 7.54–7.49 (m, 1H, ArH), 7.44–7.41 (m, 1H, ArH), 7.09–7.03 (m, 2H, ArH), 6.96–6.90 (m, 1H, ArH), 3.95 (s, 3H, CH3). 13C NMR (100 MHz, chloroform-d) δ 167.5, 163.8, 153.3, 150.4, 148.7, 147.5, 144.6, 142.3, 135.2, 127.6, 125.3, 124.7, 123.2, 122.5, 121.0, 120.3, 118.1, 117.9, 114.7, 55.2. HRMS (ESI) m/z: calculated for C20H15N3NaO2S [M + Na]+: 384.0777, found: 384.0786.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.14 (d, J = 8.5 Hz, 2H, ArH), 8.08 (d, J = 8.2 Hz, 1H, ArH), 7.96–7.93 (m, 2H, ArH), 7.91 (d, J = 8.0 Hz, 1H, ArH), 7.52–7.47 (m, 4H, ArH), 7.41–7.36 (m, 1H, ArH), 7.32 (d, J = 8.4 Hz, 2H, ArH). 13C NMR (100 MHz, chloroform-d) δ 166.6, 160.1, 153.4, 153.2, 134.9, 134.0, 130.8, 130.2, 128.0 (2C), 127.8 (2C), 127.6 (2C), 125.3, 124.1, 122.1, 120.6, 120.5 (2C). HRMS (ESI) m/z: calculated for C20H14N2NaS [M + Na]+: 337.0770, found: 337.0752.
N–), 8.14 (d, J = 8.5 Hz, 2H, ArH), 8.08 (d, J = 8.2 Hz, 1H, ArH), 7.96–7.93 (m, 2H, ArH), 7.91 (d, J = 8.0 Hz, 1H, ArH), 7.52–7.47 (m, 4H, ArH), 7.41–7.36 (m, 1H, ArH), 7.32 (d, J = 8.4 Hz, 2H, ArH). 13C NMR (100 MHz, chloroform-d) δ 166.6, 160.1, 153.4, 153.2, 134.9, 134.0, 130.8, 130.2, 128.0 (2C), 127.8 (2C), 127.6 (2C), 125.3, 124.1, 122.1, 120.6, 120.5 (2C). HRMS (ESI) m/z: calculated for C20H14N2NaS [M + Na]+: 337.0770, found: 337.0752.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.19–8.17 (m, 3H, ArH), 8.07 (d, J = 8.1 Hz, 1H, ArH), 7.71 (d, J = 7.5 Hz, 1H, ArH), 7.60 (d, J = 8.4 Hz, 2H, ArH), 7.58–7.54 (m, 1H, ArH), 7.47–7.44 (m, 2H, ArH), 7.03–6.99 (m, 2H, ArH). 13C NMR (100 MHz, DMSO-d6) δ 166.6, 164.3, 160.3, 153.6, 150.7, 134.5, 133.7, 132.6, 131.1, 128.4 (2C), 126.7, 125.5, 122.8, 122.4 (2C), 122.3, 119.3, 119.3, 116.7. HRMS (ESI) m/z: calculated for C20H15N2OS [M + H]+: 331.0900, found: 331.0902.
N–), 8.19–8.17 (m, 3H, ArH), 8.07 (d, J = 8.1 Hz, 1H, ArH), 7.71 (d, J = 7.5 Hz, 1H, ArH), 7.60 (d, J = 8.4 Hz, 2H, ArH), 7.58–7.54 (m, 1H, ArH), 7.47–7.44 (m, 2H, ArH), 7.03–6.99 (m, 2H, ArH). 13C NMR (100 MHz, DMSO-d6) δ 166.6, 164.3, 160.3, 153.6, 150.7, 134.5, 133.7, 132.6, 131.1, 128.4 (2C), 126.7, 125.5, 122.8, 122.4 (2C), 122.3, 119.3, 119.3, 116.7. HRMS (ESI) m/z: calculated for C20H15N2OS [M + H]+: 331.0900, found: 331.0902.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.15–8.13 (m, 3H, ArH), 8.06 (d, J = 8.1 Hz, 1H, ArH), 7.57–7.54 (m, 3H, ArH), 7.48–7.42 (m, 4H, ArH), 7.14 (d, J = 8.2 Hz, 1H, ArH), 3.84 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ 166.8, 161.8, 159.5, 153.8, 153.6, 137.2, 134.4, 130.4, 130.0, 128.3 (2C), 126.6, 125.4, 122.7, 122.3, 122.0 (2C), 121.9, 118.1, 112.7, 55.2. HRMS (ESI) m/z: calculated for C21H17N2NaOS [M + H]+: 345.1056, found: 345.1071.
N–), 8.15–8.13 (m, 3H, ArH), 8.06 (d, J = 8.1 Hz, 1H, ArH), 7.57–7.54 (m, 3H, ArH), 7.48–7.42 (m, 4H, ArH), 7.14 (d, J = 8.2 Hz, 1H, ArH), 3.84 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ 166.8, 161.8, 159.5, 153.8, 153.6, 137.2, 134.4, 130.4, 130.0, 128.3 (2C), 126.6, 125.4, 122.7, 122.3, 122.0 (2C), 121.9, 118.1, 112.7, 55.2. HRMS (ESI) m/z: calculated for C21H17N2NaOS [M + H]+: 345.1056, found: 345.1071.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.19–8.13 (m, 3H, ArH), 8.07 (d, J = 8.1 Hz, 1H, ArH), 7.59 (d, J = 8.2 Hz, 2H, ArH), 7.57–7.54 (m, 1H, ArH), 7.49–7.45 (m, 1H, ArH), 7.15 (d, J = 6.9 Hz, 1H, ArH), 6.99 (d, J = 6.8 Hz, 1H, ArH), 6.84–6.80 (m,1H, ArH). 13C NMR (100 MHz, DMSO-d6) δ 166.6, 164.7, 153.6, 150.5, 149.4, 145.6, 134.5, 131.1, 128.4 (2C), 126.7, 125.5, 122.9, 122.8, 122.4 (2C), 122.3, 119.4, 119.3, 118.9. HRMS (ESI) m/z: calculated for C20H14N2NaO2S [M + Na]+: 369.0668, found: 369.0654.
N–), 8.19–8.13 (m, 3H, ArH), 8.07 (d, J = 8.1 Hz, 1H, ArH), 7.59 (d, J = 8.2 Hz, 2H, ArH), 7.57–7.54 (m, 1H, ArH), 7.49–7.45 (m, 1H, ArH), 7.15 (d, J = 6.9 Hz, 1H, ArH), 6.99 (d, J = 6.8 Hz, 1H, ArH), 6.84–6.80 (m,1H, ArH). 13C NMR (100 MHz, DMSO-d6) δ 166.6, 164.7, 153.6, 150.5, 149.4, 145.6, 134.5, 131.1, 128.4 (2C), 126.7, 125.5, 122.9, 122.8, 122.4 (2C), 122.3, 119.4, 119.3, 118.9. HRMS (ESI) m/z: calculated for C20H14N2NaO2S [M + Na]+: 369.0668, found: 369.0654.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.14–8.12 (m, 3H, ArH), 8.05 (d, J = 8.1 Hz, 1H, ArH), 7.57–7.52 (m, 1H, ArH), 7.47–7.44 (m, 2H, ArH), 7.39–7.35 (m, 3H, ArH), 7.06 (d, J = 8.3 Hz, 1H, ArH), 3.85 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ 166.9, 161.3, 154.3, 153.6, 151.3, 146.8, 134.4, 129.9, 128.9, 128.2 (2C), 126.6, 125.4, 122.9, 122.7, 122.3, 121.9 (2C), 113.7, 111.6, 55.6. HRMS (ESI) m/z: calculated for C21H17N2O2S [M + H]+: 361.1005, found: 361.0986.
N–), 8.14–8.12 (m, 3H, ArH), 8.05 (d, J = 8.1 Hz, 1H, ArH), 7.57–7.52 (m, 1H, ArH), 7.47–7.44 (m, 2H, ArH), 7.39–7.35 (m, 3H, ArH), 7.06 (d, J = 8.3 Hz, 1H, ArH), 3.85 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ 166.9, 161.3, 154.3, 153.6, 151.3, 146.8, 134.4, 129.9, 128.9, 128.2 (2C), 126.6, 125.4, 122.9, 122.7, 122.3, 121.9 (2C), 113.7, 111.6, 55.6. HRMS (ESI) m/z: calculated for C21H17N2O2S [M + H]+: 361.1005, found: 361.0986.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.73 (d, J = 4.7 Hz, 1H, ArH), 8.55 (s, 1H, ArH), 8.32 (d, J = 8.0 Hz, 1H, ArH), 8.15 (d, J = 8.1 Hz, 2H, ArH), 8.08 (d, J = 8.2 Hz, 1H, ArH), 7.91 (d, J = 8.0 Hz, 1H, ArH), 7.52–7.48 (m, 1H, ArH), 7.46–7.42 (m, 1H, ArH), 7.41–7.37 (m, 1H, ArH), 7.33 (d, J = 8.1 Hz, 2H, ArH). 13C NMR (100 MHz, chloroform-d) δ 167.3, 158.0, 154.2, 153.6, 152.3, 151.1, 135.1, 135.0, 131.8, 131.6, 128.6 (2C), 126.4, 125.2, 123.9, 123.2, 121.6, 121.5 (2C). HRMS (ESI) m/z: calculated for C19H14N3S [M + H]+: 316.0903, found: 316.0893.
N–), 8.73 (d, J = 4.7 Hz, 1H, ArH), 8.55 (s, 1H, ArH), 8.32 (d, J = 8.0 Hz, 1H, ArH), 8.15 (d, J = 8.1 Hz, 2H, ArH), 8.08 (d, J = 8.2 Hz, 1H, ArH), 7.91 (d, J = 8.0 Hz, 1H, ArH), 7.52–7.48 (m, 1H, ArH), 7.46–7.42 (m, 1H, ArH), 7.41–7.37 (m, 1H, ArH), 7.33 (d, J = 8.1 Hz, 2H, ArH). 13C NMR (100 MHz, chloroform-d) δ 167.3, 158.0, 154.2, 153.6, 152.3, 151.1, 135.1, 135.0, 131.8, 131.6, 128.6 (2C), 126.4, 125.2, 123.9, 123.2, 121.6, 121.5 (2C). HRMS (ESI) m/z: calculated for C19H14N3S [M + H]+: 316.0903, found: 316.0893.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.12 (d, J = 8.3 Hz, 2H, ArH), 8.07 (d, J = 8.1 Hz, 1H, ArH), 7.92–7.90 (m, 2H, ArH), 7.54–7.46 (m, 2H, ArH), 7.40–7.37 (m, 1H, ArH), 7.27 (d, J = 8.3 Hz, 2H, ArH), 6.97 (s, 1H, ArH). 13C NMR (100 MHz, chloroform-d) δ 166.6, 153.5, 153.2, 151.7, 146.2, 143.5, 134.0, 130.1, 127.5 (2C), 125.3, 124.9, 124.1, 122.0, 120.6, 120.4 (2C), 106.8. HRMS (ESI) m/z: calculated for C18H13N2OS [M + H]+: 305.0743, found: 305.0755.
N–), 8.12 (d, J = 8.3 Hz, 2H, ArH), 8.07 (d, J = 8.1 Hz, 1H, ArH), 7.92–7.90 (m, 2H, ArH), 7.54–7.46 (m, 2H, ArH), 7.40–7.37 (m, 1H, ArH), 7.27 (d, J = 8.3 Hz, 2H, ArH), 6.97 (s, 1H, ArH). 13C NMR (100 MHz, chloroform-d) δ 166.6, 153.5, 153.2, 151.7, 146.2, 143.5, 134.0, 130.1, 127.5 (2C), 125.3, 124.9, 124.1, 122.0, 120.6, 120.4 (2C), 106.8. HRMS (ESI) m/z: calculated for C18H13N2OS [M + H]+: 305.0743, found: 305.0755.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.12 (d, J = 8.2 Hz, 2H, ArH), 8.07 (d, J = 8.1 Hz, 1H, ArH), 7.90 (d, J = 7.9 Hz, 1H, ArH), 7.85 (s, 1H, ArH), 7.71 (d, J = 5.0 Hz, 1H, ArH), 7.51–7.47 (m, 1H, ArH), 7.41–7.36 (m, 2H, ArH), 7.29 (d, J = 8.2 Hz, 2H, ArH). 13C NMR (100 MHz, chloroform-d) δ 167.6, 155.0, 154.5, 154.3, 140.6, 135.1, 131.2, 130.9, 128.6 (2C), 126.9, 126.3, 126.0, 125.1, 123.1, 121.6, 121.5 (2C). HRMS (ESI) m/z: calculated for C18H13N2S2 [M + H]+: 321.0515, found: 321.0513.
N–), 8.12 (d, J = 8.2 Hz, 2H, ArH), 8.07 (d, J = 8.1 Hz, 1H, ArH), 7.90 (d, J = 7.9 Hz, 1H, ArH), 7.85 (s, 1H, ArH), 7.71 (d, J = 5.0 Hz, 1H, ArH), 7.51–7.47 (m, 1H, ArH), 7.41–7.36 (m, 2H, ArH), 7.29 (d, J = 8.2 Hz, 2H, ArH). 13C NMR (100 MHz, chloroform-d) δ 167.6, 155.0, 154.5, 154.3, 140.6, 135.1, 131.2, 130.9, 128.6 (2C), 126.9, 126.3, 126.0, 125.1, 123.1, 121.6, 121.5 (2C). HRMS (ESI) m/z: calculated for C18H13N2S2 [M + H]+: 321.0515, found: 321.0513.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.13 (d, J = 8.0 Hz, 1H, ArH), 8.08 (d, J = 8.1 Hz, 2H, ArH), 8.04 (d, J = 8.1 Hz, 1H, ArH), 7.56–7.52 (m, 1H, ArH), 7.47–7.43 (m, 2H, ArH), 7.30 (d, J = 8.1 Hz, 2H, ArH), 6.91 (s, 1H, ArH), 6.59 (s, 1H, ArH). 13C NMR (100 MHz, DMSO-d6) δ 167.1, 156.4, 155.6, 153.6, 134.3, 129.0, 128.2 (2C), 126.6, 125.3, 125.3, 123.0, 122.6, 122.2, 121.7 (2C), 120.5, 106.2. HRMS (ESI) m/z: calculated for C18H14N3S [M + H]+: 304.0903, found: 304.0894.
N–), 8.13 (d, J = 8.0 Hz, 1H, ArH), 8.08 (d, J = 8.1 Hz, 2H, ArH), 8.04 (d, J = 8.1 Hz, 1H, ArH), 7.56–7.52 (m, 1H, ArH), 7.47–7.43 (m, 2H, ArH), 7.30 (d, J = 8.1 Hz, 2H, ArH), 6.91 (s, 1H, ArH), 6.59 (s, 1H, ArH). 13C NMR (100 MHz, DMSO-d6) δ 167.1, 156.4, 155.6, 153.6, 134.3, 129.0, 128.2 (2C), 126.6, 125.3, 125.3, 123.0, 122.6, 122.2, 121.7 (2C), 120.5, 106.2. HRMS (ESI) m/z: calculated for C18H14N3S [M + H]+: 304.0903, found: 304.0894.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.12 (d, J = 8.1 Hz, 2H, ArH), 8.07 (d, J = 8.2 Hz, 1H, ArH), 7.90 (d, J = 8.0 Hz, 1H, ArH), 7.59–7.46 (m, 3H, ArH), 7.41–7.35 (m, 1H, ArH), 7.32 (d, J = 8.2 Hz, 2H, ArH), 7.18–7.14 (m, 1H, ArH). 13C NMR (100 MHz, chloroform-d) δ 167.6, 154.2, 153.7, 153.7, 142.6, 135.0, 133.0, 131.3, 131.1, 128.6 (2C), 127.9, 126.4, 125.1, 123.1, 121.7 (2C), 121.6. HRMS (ESI) m/z: calculated for C18H12N2NaS2 [M + Na]+: 343.0334, found: 343.0333.
N–), 8.12 (d, J = 8.1 Hz, 2H, ArH), 8.07 (d, J = 8.2 Hz, 1H, ArH), 7.90 (d, J = 8.0 Hz, 1H, ArH), 7.59–7.46 (m, 3H, ArH), 7.41–7.35 (m, 1H, ArH), 7.32 (d, J = 8.2 Hz, 2H, ArH), 7.18–7.14 (m, 1H, ArH). 13C NMR (100 MHz, chloroform-d) δ 167.6, 154.2, 153.7, 153.7, 142.6, 135.0, 133.0, 131.3, 131.1, 128.6 (2C), 127.9, 126.4, 125.1, 123.1, 121.7 (2C), 121.6. HRMS (ESI) m/z: calculated for C18H12N2NaS2 [M + Na]+: 343.0334, found: 343.0333.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.60 (s, 1H, ArH), 8.42 (s, 1H, ArH), 8.16–8.14 (m, 3H, ArH), 8.06 (d, J = 8.2 Hz, 1H, ArH), 7.57–7.53 (m, 1H, ArH), 7.48–7.43 (m, 3H, ArH). 13C NMR (100 MHz, DMSO-d6) δ 166.7, 155.4, 153.6, 153.1, 152.1, 138.7, 138.1, 134.4, 130.8, 128.3 (2C), 127.2, 126.7, 125.5, 122.8, 122.3, 122.1 (2C). HRMS (ESI) m/z: calculated for C18H12N3O2S2 [M + H]+: 366.0365, found: 366.0360.
N–), 8.60 (s, 1H, ArH), 8.42 (s, 1H, ArH), 8.16–8.14 (m, 3H, ArH), 8.06 (d, J = 8.2 Hz, 1H, ArH), 7.57–7.53 (m, 1H, ArH), 7.48–7.43 (m, 3H, ArH). 13C NMR (100 MHz, DMSO-d6) δ 166.7, 155.4, 153.6, 153.1, 152.1, 138.7, 138.1, 134.4, 130.8, 128.3 (2C), 127.2, 126.7, 125.5, 122.8, 122.3, 122.1 (2C). HRMS (ESI) m/z: calculated for C18H12N3O2S2 [M + H]+: 366.0365, found: 366.0360.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.17–8.10 (m, 3H, ArH), 8.07 (d, J = 8.2 Hz, 1H, ArH), 7.91 (d, J = 8.0 Hz, 1H, ArH), 7.53–7.47 (m, 1H, ArH), 7.42–7.36 (m, 3H, ArH). 13C NMR (100 MHz, chloroform-d) δ 167.5, 154.5, 154.2, 154.1, 153.7, 153.6, 135.1, 131.7, 128.6 (2C), 126.4, 125.2, 123.2, 122.3, 121.7 (2C), 121.6. HRMS (ESI) m/z: calculated for C17H11N3NaS2 [M + Na]+: 344.0287, found: 344.0275.
N–), 8.17–8.10 (m, 3H, ArH), 8.07 (d, J = 8.2 Hz, 1H, ArH), 7.91 (d, J = 8.0 Hz, 1H, ArH), 7.53–7.47 (m, 1H, ArH), 7.42–7.36 (m, 3H, ArH). 13C NMR (100 MHz, chloroform-d) δ 167.5, 154.5, 154.2, 154.1, 153.7, 153.6, 135.1, 131.7, 128.6 (2C), 126.4, 125.2, 123.2, 122.3, 121.7 (2C), 121.6. HRMS (ESI) m/z: calculated for C17H11N3NaS2 [M + Na]+: 344.0287, found: 344.0275.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.29 (s, 1H, ArH), 8.13 (d, J = 8.2 Hz, 2H, ArH), 8.07 (d, J = 8.2 Hz, 1H, ArH), 7.91 (d, J = 8.0 Hz, 1H, ArH), 7.53–7.47 (m, 1H, ArH), 7.41–7.37 (m, 1H, ArH), 7.32 (d, J = 8.2 Hz, 2H, ArH). 13C NMR (100 MHz, chloroform-d) δ 167.3, 157.0, 154.2, 153.0, 151.3, 147.8, 138.3, 135.0, 131.9, 128.6 (2C), 126.4, 125.2, 123.2, 121.7, 121.6 (3C). HRMS (ESI) m/z: calculated for C17H12N3S2 [M + H]+: 322.0467, found: 322.0471.
N–), 8.29 (s, 1H, ArH), 8.13 (d, J = 8.2 Hz, 2H, ArH), 8.07 (d, J = 8.2 Hz, 1H, ArH), 7.91 (d, J = 8.0 Hz, 1H, ArH), 7.53–7.47 (m, 1H, ArH), 7.41–7.37 (m, 1H, ArH), 7.32 (d, J = 8.2 Hz, 2H, ArH). 13C NMR (100 MHz, chloroform-d) δ 167.3, 157.0, 154.2, 153.0, 151.3, 147.8, 138.3, 135.0, 131.9, 128.6 (2C), 126.4, 125.2, 123.2, 121.7, 121.6 (3C). HRMS (ESI) m/z: calculated for C17H12N3S2 [M + H]+: 322.0467, found: 322.0471.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.15 (d, J = 8.1 Hz, 2H, ArH), 8.08 (d, J = 8.2 Hz, 1H, ArH), 8.03 (d, J = 3.1 Hz, 1H, ArH), 7.91 (d, J = 8.0 Hz, 1H, ArH), 7.55 (d, J = 3.0 Hz, 1H, ArH), 7.53–7.47 (m, 1H, ArH), 7.42–7.36 (m, 3H, ArH). 13C NMR (100 MHz, chloroform-d) δ 167.2, 166.9, 154.2, 153.8, 152.1, 144.8, 135.1, 132.5, 128.7 (2C), 126.4, 125.3, 123.2, 123.0, 121.9 (2C), 121.7. HRMS (ESI) m/z: calculated for C17H11N3NaS2 [M + Na]+: 344.0287, found: 344.0271.
N–), 8.15 (d, J = 8.1 Hz, 2H, ArH), 8.08 (d, J = 8.2 Hz, 1H, ArH), 8.03 (d, J = 3.1 Hz, 1H, ArH), 7.91 (d, J = 8.0 Hz, 1H, ArH), 7.55 (d, J = 3.0 Hz, 1H, ArH), 7.53–7.47 (m, 1H, ArH), 7.42–7.36 (m, 3H, ArH). 13C NMR (100 MHz, chloroform-d) δ 167.2, 166.9, 154.2, 153.8, 152.1, 144.8, 135.1, 132.5, 128.7 (2C), 126.4, 125.3, 123.2, 123.0, 121.9 (2C), 121.7. HRMS (ESI) m/z: calculated for C17H11N3NaS2 [M + Na]+: 344.0287, found: 344.0271.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.65 (d, J = 8.0 Hz, 1H, ArH), 8.27 (s, 1H, ArH), 8.20 (d, J = 8.1 Hz, 2H, ArH), 7.73–7.69 (m, 2H, ArH), 7.50–7.45 (m, 4.7 Hz, 1H, ArH), 7.42 (d, J = 8.1 Hz, 2H, ArH). 13C NMR (100 MHz, DMSO-d6) δ 170.1, 164.2, 156.5, 154.9, 148.4, 140.3, 133.1, 131.8, 129.7, 128.4 (2C), 128.3, 128.0, 125.4, 122.0 (2C), 120.3. HRMS (ESI) m/z: calculated for C17H11N3NaS2 [M + Na]+: 344.0287, found: 344.0289.
N–), 8.65 (d, J = 8.0 Hz, 1H, ArH), 8.27 (s, 1H, ArH), 8.20 (d, J = 8.1 Hz, 2H, ArH), 7.73–7.69 (m, 2H, ArH), 7.50–7.45 (m, 4.7 Hz, 1H, ArH), 7.42 (d, J = 8.1 Hz, 2H, ArH). 13C NMR (100 MHz, DMSO-d6) δ 170.1, 164.2, 156.5, 154.9, 148.4, 140.3, 133.1, 131.8, 129.7, 128.4 (2C), 128.3, 128.0, 125.4, 122.0 (2C), 120.3. HRMS (ESI) m/z: calculated for C17H11N3NaS2 [M + Na]+: 344.0287, found: 344.0289.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 7.91 (d, J = 8.2 Hz, 2H, ArH), 7.82 (s, 1H, ArH), 7.69 (d, J = 5.1 Hz, 1H, ArH), 7.41–7.37 (m, 1H, ArH), 7.22 (d, J = 8.2 Hz, 2H, ArH), 2.87–2.79 (m, 4H, CH2), 1.94–1.86 (m, 4H, CH2). 13C NMR (100 MHz, chloroform-d) δ 163.1, 153.5, 151.9, 150.4, 139.7, 130.8, 129.6, 128.0, 126.1 (2C), 125.8, 124.9, 120.3 (2C), 25.9, 22.7, 22.4, 22.0. HRMS (ESI) m/z: calculated for C18H17N2S2+[M + H]+: 325.0828, found: 325.0814.
N–), 7.91 (d, J = 8.2 Hz, 2H, ArH), 7.82 (s, 1H, ArH), 7.69 (d, J = 5.1 Hz, 1H, ArH), 7.41–7.37 (m, 1H, ArH), 7.22 (d, J = 8.2 Hz, 2H, ArH), 2.87–2.79 (m, 4H, CH2), 1.94–1.86 (m, 4H, CH2). 13C NMR (100 MHz, chloroform-d) δ 163.1, 153.5, 151.9, 150.4, 139.7, 130.8, 129.6, 128.0, 126.1 (2C), 125.8, 124.9, 120.3 (2C), 25.9, 22.7, 22.4, 22.0. HRMS (ESI) m/z: calculated for C18H17N2S2+[M + H]+: 325.0828, found: 325.0814.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 7.98 (s, 1H, ArH), 7.90 (d, J = 8.1 Hz, 2H, ArH), 7.81 (s, 1H, ArH), 7.73–7.68 (m, 2H, ArH), 7.62 (d, J = 8.1 Hz, 1H, ArH), 7.49–7.43 (m, 1H, ArH), 7.40–7.32 (m, 2H, ArH), 7.28 (d, J = 8.1 Hz, 2H, ArH). 13C NMR (100 MHz, chloroform-d) δ 153.0, 150.1, 147.1, 146.4, 139.9, 131.1, 130.6, 129.3, 129.2, 125.7, 125.2, 125.0 (2C), 124.9, 123.8, 123.4, 120.3 (2C), 111.6, 105.6. HRMS (ESI) m/z: calculated for C20H14N3S2 [M + H]+: 360.0624, found: 360.0609.
N–), 7.98 (s, 1H, ArH), 7.90 (d, J = 8.1 Hz, 2H, ArH), 7.81 (s, 1H, ArH), 7.73–7.68 (m, 2H, ArH), 7.62 (d, J = 8.1 Hz, 1H, ArH), 7.49–7.43 (m, 1H, ArH), 7.40–7.32 (m, 2H, ArH), 7.28 (d, J = 8.1 Hz, 2H, ArH). 13C NMR (100 MHz, chloroform-d) δ 153.0, 150.1, 147.1, 146.4, 139.9, 131.1, 130.6, 129.3, 129.2, 125.7, 125.2, 125.0 (2C), 124.9, 123.8, 123.4, 120.3 (2C), 111.6, 105.6. HRMS (ESI) m/z: calculated for C20H14N3S2 [M + H]+: 360.0624, found: 360.0609.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.10 (d, J = 6.8 Hz, 1H, ArH), 7.98 (d, J = 8.5 Hz, 2H, ArH), 7.85 (s, 1H, ArH), 7.80 (s, 1H, ArH), 7.70 (d, J = 5.1 Hz, 1H, ArH), 7.62 (d, J = 9.1 Hz, 1H, ArH), 7.40–7.36 (m, 1H, ArH), 7.28 (d, J = 8.3 Hz, 2H, ArH), 7.19–7.13 (m, 1H, ArH), 6.79–6.74 (m, 1H, ArH). 13C NMR (100 MHz, chloroform-d) δ 153.1, 150.6, 144.7, 144.5, 139.8, 130.5, 129.2, 125.8 (2C), 125.7, 124.9, 124.5, 123.6, 120.3 (2C), 116.5, 111.4, 106.9. HRMS (ESI) m/z: calculated for C18H14N3S [M + H]+: 304.0903, found: 304.0897.
N–), 8.10 (d, J = 6.8 Hz, 1H, ArH), 7.98 (d, J = 8.5 Hz, 2H, ArH), 7.85 (s, 1H, ArH), 7.80 (s, 1H, ArH), 7.70 (d, J = 5.1 Hz, 1H, ArH), 7.62 (d, J = 9.1 Hz, 1H, ArH), 7.40–7.36 (m, 1H, ArH), 7.28 (d, J = 8.3 Hz, 2H, ArH), 7.19–7.13 (m, 1H, ArH), 6.79–6.74 (m, 1H, ArH). 13C NMR (100 MHz, chloroform-d) δ 153.1, 150.6, 144.7, 144.5, 139.8, 130.5, 129.2, 125.8 (2C), 125.7, 124.9, 124.5, 123.6, 120.3 (2C), 116.5, 111.4, 106.9. HRMS (ESI) m/z: calculated for C18H14N3S [M + H]+: 304.0903, found: 304.0897.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.12 (d, J = 8.5 Hz, 2H, ArH), 7.88–7.85 (m, 2H, ArH), 7.71 (d, J = 5.1 Hz, 1H, ArH), 7.48–7.40 (m, 2H, ArH), 7.30 (d, J = 8.5 Hz, 2H, ArH), 7.13–7.09 (m, 1H, ArH). 13C NMR (100 MHz, chloroform-d) δ 167.6 (d, J = 1.7 Hz), 156.1 (d, J = 250.1 Hz), 156.0 (d, J = 2.5 Hz), 154.2, 153.8, 139.5, 130.2, 129.5, 127.7 (2C), 126.2 (d, J = 7.3 Hz), 125.9, 124.9, 121.1 (d, J = 16.5 Hz), 120.5 (2C), 117.9 (d, J = 3.5 Hz), 109.5 (d, J = 18.7 Hz). HRMS (ESI) m/z: calculated for C18H12FN2S2 [M + H]+: 339.0420, found: 339.0418.
N–), 8.12 (d, J = 8.5 Hz, 2H, ArH), 7.88–7.85 (m, 2H, ArH), 7.71 (d, J = 5.1 Hz, 1H, ArH), 7.48–7.40 (m, 2H, ArH), 7.30 (d, J = 8.5 Hz, 2H, ArH), 7.13–7.09 (m, 1H, ArH). 13C NMR (100 MHz, chloroform-d) δ 167.6 (d, J = 1.7 Hz), 156.1 (d, J = 250.1 Hz), 156.0 (d, J = 2.5 Hz), 154.2, 153.8, 139.5, 130.2, 129.5, 127.7 (2C), 126.2 (d, J = 7.3 Hz), 125.9, 124.9, 121.1 (d, J = 16.5 Hz), 120.5 (2C), 117.9 (d, J = 3.5 Hz), 109.5 (d, J = 18.7 Hz). HRMS (ESI) m/z: calculated for C18H12FN2S2 [M + H]+: 339.0420, found: 339.0418.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.08 (d, J = 8.3 Hz, 2H, ArH), 8.00 (dd, J = 8.8, 4.8 Hz, 1H, ArH), 7.86 (s, 1H, ArH), 7.70 (d, J = 4.9 Hz, 1H, ArH), 7.60–7.56 (m, 1H, ArH), 7.43–7.39 (m, 1H, ArH), 7.29 (d, J = 8.3 Hz, 2H, ArH), 7.25–7.19 (m, 1H, ArH). 13C NMR (100 MHz, chloroform-d) δ 166.3 (d, J = 3.3 Hz), 159.4 (d, J = 245.7 Hz), 154.1, 153.5, 149.8 (d, J = 1.8 Hz), 139.5, 135.0 (d, J = 11.1 Hz), 130.1, 129.8, 127.4 (2C), 125.9, 124.9, 122.9 (d, J = 9.2 Hz), 120.5 (2C), 113.9 (d, J = 24.6 Hz), 106.8 (d, J = 26.8 Hz). HRMS (ESI) m/z: calculated for C18H12FN2S2 [M + H]+: 339.0420, found: 339.0431.
N–), 8.08 (d, J = 8.3 Hz, 2H, ArH), 8.00 (dd, J = 8.8, 4.8 Hz, 1H, ArH), 7.86 (s, 1H, ArH), 7.70 (d, J = 4.9 Hz, 1H, ArH), 7.60–7.56 (m, 1H, ArH), 7.43–7.39 (m, 1H, ArH), 7.29 (d, J = 8.3 Hz, 2H, ArH), 7.25–7.19 (m, 1H, ArH). 13C NMR (100 MHz, chloroform-d) δ 166.3 (d, J = 3.3 Hz), 159.4 (d, J = 245.7 Hz), 154.1, 153.5, 149.8 (d, J = 1.8 Hz), 139.5, 135.0 (d, J = 11.1 Hz), 130.1, 129.8, 127.4 (2C), 125.9, 124.9, 122.9 (d, J = 9.2 Hz), 120.5 (2C), 113.9 (d, J = 24.6 Hz), 106.8 (d, J = 26.8 Hz). HRMS (ESI) m/z: calculated for C18H12FN2S2 [M + H]+: 339.0420, found: 339.0431.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.09 (d, J = 8.5 Hz, 2H, ArH), 7.85 (dd, J = 3.0, 1.2 Hz, 1H, ArH), 7.81 (dd, J = 8.8, 5.1 Hz, 1H, ArH), 7.73 (dd, J = 9.6, 2.5 Hz, 1H, ArH), 7.70 (d, J = 5.1 Hz, 1H, ArH), 7.40 (dd, J = 5.1, 2.9 Hz, 1H, ArH), 7.28 (d, J = 8.5 Hz, 2H, ArH), 7.17–7.13 (m, 1H, ArH). 13C NMR (100 MHz, chloroform-d) δ 169.0, 160.9 (d, J = 243.2 Hz), 154.1, 154.1 (d, J = 12.1 Hz), 153.6, 139.5, 130.1, 129.8, 129.3 (d, J = 1.9 Hz), 127.5 (2C), 125.9, 124.9, 121.2 (d, J = 9.9 Hz), 120.5 (2C), 112.7 (d, J = 25.0 Hz), 108.2 (d, J = 23.6 Hz). HRMS (ESI) m/z: calculated for C18H12FN2S2 [M + H]+: 339.0420, found: 339.0423.
N–), 8.09 (d, J = 8.5 Hz, 2H, ArH), 7.85 (dd, J = 3.0, 1.2 Hz, 1H, ArH), 7.81 (dd, J = 8.8, 5.1 Hz, 1H, ArH), 7.73 (dd, J = 9.6, 2.5 Hz, 1H, ArH), 7.70 (d, J = 5.1 Hz, 1H, ArH), 7.40 (dd, J = 5.1, 2.9 Hz, 1H, ArH), 7.28 (d, J = 8.5 Hz, 2H, ArH), 7.17–7.13 (m, 1H, ArH). 13C NMR (100 MHz, chloroform-d) δ 169.0, 160.9 (d, J = 243.2 Hz), 154.1, 154.1 (d, J = 12.1 Hz), 153.6, 139.5, 130.1, 129.8, 129.3 (d, J = 1.9 Hz), 127.5 (2C), 125.9, 124.9, 121.2 (d, J = 9.9 Hz), 120.5 (2C), 112.7 (d, J = 25.0 Hz), 108.2 (d, J = 23.6 Hz). HRMS (ESI) m/z: calculated for C18H12FN2S2 [M + H]+: 339.0420, found: 339.0423.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.15 (d, J = 8.6 Hz, 2H, ArH), 7.86 (dd, J = 2.9, 1.1 Hz, 1H, ArH), 7.70 (dd, J = 5.1, 0.9 Hz, 1H, ArH), 7.66 (dd, J = 8.0, 0.8 Hz, 1H, ArH), 7.41 (dd, J = 5.0, 2.6 Hz, 1H, ArH), 7.36–7.29 (m, 1H, ArH), 7.28 (d, J = 8.6 Hz, 2H, ArH), 7.19 (ddd, J = 10.4, 8.1, 0.8 Hz, 1H, ArH). 13C NMR (100 MHz, chloroform-d) δ 167.1, 154.8 (d, J = 257.2 Hz), 154.2, 153.7, 142.0 (d, J = 13.4 Hz), 139.5, 136.6 (d, J = 3.6 Hz), 130.1, 129.6, 127.8 (2C), 125.9, 124.9, 124.7 (d, J = 7.1 Hz), 120.5 (2C), 116.3 (d, J = 4.3 Hz), 111.0 (d, J = 18.0 Hz). HRMS (ESI) m/z: calculated for C18H12FN2S2 [M + H]+: 339.0420, found: 339.0417.
N–), 8.15 (d, J = 8.6 Hz, 2H, ArH), 7.86 (dd, J = 2.9, 1.1 Hz, 1H, ArH), 7.70 (dd, J = 5.1, 0.9 Hz, 1H, ArH), 7.66 (dd, J = 8.0, 0.8 Hz, 1H, ArH), 7.41 (dd, J = 5.0, 2.6 Hz, 1H, ArH), 7.36–7.29 (m, 1H, ArH), 7.28 (d, J = 8.6 Hz, 2H, ArH), 7.19 (ddd, J = 10.4, 8.1, 0.8 Hz, 1H, ArH). 13C NMR (100 MHz, chloroform-d) δ 167.1, 154.8 (d, J = 257.2 Hz), 154.2, 153.7, 142.0 (d, J = 13.4 Hz), 139.5, 136.6 (d, J = 3.6 Hz), 130.1, 129.6, 127.8 (2C), 125.9, 124.9, 124.7 (d, J = 7.1 Hz), 120.5 (2C), 116.3 (d, J = 4.3 Hz), 111.0 (d, J = 18.0 Hz). HRMS (ESI) m/z: calculated for C18H12FN2S2 [M + H]+: 339.0420, found: 339.0417.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.06 (d, J = 8.2 Hz, 2H, ArH), 7.93 (d, J = 8.9 Hz, 1H, ArH), 7.85 (s, 1H, ArH), 7.70 (d, J = 4.8 Hz, 1H, ArH), 7.41 (s, 1H, ArH), 7.34 (s, 1H, ArH), 7.29 (d, J = 8.2 Hz, 2H, ArH), 7.08 (d, J = 9.4 Hz, 1H, ArH), 4.11 (q, J = 6.9 Hz, 2H, CH2), 1.47 (t, J = 6.9 Hz, 3H, CH3). 13C NMR (100 MHz, chloroform-d) δ 164.0, 156.0, 153.9, 152.9, 147.7, 139.6, 135.3, 130.3, 129.9, 127.2 (2C), 125.9, 124.9, 122.5, 120.4 (2C), 115.0, 103.9, 63.1, 13.8. HRMS (ESI) m/z: calculated for C20H16N2NaOS2 [M + Na]+: 387.0596, found: 387.0613.
N–), 8.06 (d, J = 8.2 Hz, 2H, ArH), 7.93 (d, J = 8.9 Hz, 1H, ArH), 7.85 (s, 1H, ArH), 7.70 (d, J = 4.8 Hz, 1H, ArH), 7.41 (s, 1H, ArH), 7.34 (s, 1H, ArH), 7.29 (d, J = 8.2 Hz, 2H, ArH), 7.08 (d, J = 9.4 Hz, 1H, ArH), 4.11 (q, J = 6.9 Hz, 2H, CH2), 1.47 (t, J = 6.9 Hz, 3H, CH3). 13C NMR (100 MHz, chloroform-d) δ 164.0, 156.0, 153.9, 152.9, 147.7, 139.6, 135.3, 130.3, 129.9, 127.2 (2C), 125.9, 124.9, 122.5, 120.4 (2C), 115.0, 103.9, 63.1, 13.8. HRMS (ESI) m/z: calculated for C20H16N2NaOS2 [M + Na]+: 387.0596, found: 387.0613.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.10 (d, J = 8.3 Hz, 2H, ArH), 7.94 (d, J = 8.3 Hz, 1H, ArH), 7.85 (s, 1H, ArH), 7.72–7.68 (m, 2H, ArH), 7.43–7.38 (m, 1H, ArH), 7.32–7.27 (m, 3H, ArH), 2.50 (s, 3H, CH3). 13C NMR (100 MHz, chloroform-d) δ 166.6, 155.0, 154.2, 152.3, 140.6, 135.3, 135.2, 131.3, 131.0, 128.4 (2C), 127.9, 126.9, 125.9, 122.6, 121.5 (2C), 121.4, 21.6. HRMS (ESI) m/z: calculated for C19H15N2S2 [M + H]+: 335.0671, found: 335.0678.
N–), 8.10 (d, J = 8.3 Hz, 2H, ArH), 7.94 (d, J = 8.3 Hz, 1H, ArH), 7.85 (s, 1H, ArH), 7.72–7.68 (m, 2H, ArH), 7.43–7.38 (m, 1H, ArH), 7.32–7.27 (m, 3H, ArH), 2.50 (s, 3H, CH3). 13C NMR (100 MHz, chloroform-d) δ 166.6, 155.0, 154.2, 152.3, 140.6, 135.3, 135.2, 131.3, 131.0, 128.4 (2C), 127.9, 126.9, 125.9, 122.6, 121.5 (2C), 121.4, 21.6. HRMS (ESI) m/z: calculated for C19H15N2S2 [M + H]+: 335.0671, found: 335.0678.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.23 (s, 1H, ArH), 7.99 (d, J = 8.2 Hz, 2H, ArH), 7.72 (d, J = 8.8 Hz, 1H, ArH), 7.70–7.68 (m, 1H, ArH), 7.65–7.64 (m, 1H, ArH), 7.35 (d, J = 8.2 Hz, 2H, ArH), 7.04 (s, 1H, ArH), 6.81 (d, J = 8.8 Hz, 1H, ArH), 6.11 (q, J = 5.0 Hz, 1H, NH), 2.75 (d, J = 4.9 Hz, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.0, 155.7, 152.9, 148.4, 145.0, 140.4, 136.7, 132.6, 131.0, 127.8, 127.4 (2C), 125.4, 122.9, 121.8 (2C), 114.3, 100.3, 29.9. HRMS (ESI) m/z: calculated for C19H16N3S2 [M + H]+: 350.0780, found: 350.0757.
N–), 8.23 (s, 1H, ArH), 7.99 (d, J = 8.2 Hz, 2H, ArH), 7.72 (d, J = 8.8 Hz, 1H, ArH), 7.70–7.68 (m, 1H, ArH), 7.65–7.64 (m, 1H, ArH), 7.35 (d, J = 8.2 Hz, 2H, ArH), 7.04 (s, 1H, ArH), 6.81 (d, J = 8.8 Hz, 1H, ArH), 6.11 (q, J = 5.0 Hz, 1H, NH), 2.75 (d, J = 4.9 Hz, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.0, 155.7, 152.9, 148.4, 145.0, 140.4, 136.7, 132.6, 131.0, 127.8, 127.4 (2C), 125.4, 122.9, 121.8 (2C), 114.3, 100.3, 29.9. HRMS (ESI) m/z: calculated for C19H16N3S2 [M + H]+: 350.0780, found: 350.0757.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.24 (s, 1H, ArH), 8.02 (d, J = 8.6 Hz, 2H, ArH), 7.83 (d, J = 9.1 Hz, 1H, ArH), 7.71–7.69 (m, 1H, ArH), 7.65–7.64 (m, 1H, ArH), 7.36 (d, J = 8.5 Hz, 2H, ArH), 7.32 (s, 1H, ArH), 7.01 (d, J = 9.0 Hz, 1H, ArH), 3.00 (s, 6H, CH3). 13C NMR (100 MHz, DMSO-d6) δ 161.1, 155.8, 153.1, 148.7, 145.3, 140.4, 136.6, 132.7, 130.8, 127.9, 127.5 (2C), 125.4, 122.8, 1218 (2C), 113.3, 103.1, 40.5. HRMS (ESI) m/z: calculated for C20H18N3S2+[M + H]+: 364.0937, found: 364.0948.
N–), 8.24 (s, 1H, ArH), 8.02 (d, J = 8.6 Hz, 2H, ArH), 7.83 (d, J = 9.1 Hz, 1H, ArH), 7.71–7.69 (m, 1H, ArH), 7.65–7.64 (m, 1H, ArH), 7.36 (d, J = 8.5 Hz, 2H, ArH), 7.32 (s, 1H, ArH), 7.01 (d, J = 9.0 Hz, 1H, ArH), 3.00 (s, 6H, CH3). 13C NMR (100 MHz, DMSO-d6) δ 161.1, 155.8, 153.1, 148.7, 145.3, 140.4, 136.6, 132.7, 130.8, 127.9, 127.5 (2C), 125.4, 122.8, 1218 (2C), 113.3, 103.1, 40.5. HRMS (ESI) m/z: calculated for C20H18N3S2+[M + H]+: 364.0937, found: 364.0948.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.68 (s, 1H, ArH), 8.26 (s, 1H, ArH), 8.15 (d, J = 8.1 Hz, 2H, ArH), 8.12–8.06 (m, 2H, ArH), 7.71–7.69 (m, 1H, ArH), 7.66–7.64 (m, 1H, ArH), 7.40 (d, J = 8.1 Hz, 2H, ArH). 13C NMR (100 MHz, DMSO-d6) δ 170.4, 166.9, 156.4, 156.4, 154.6, 140.3, 134.5, 133.1, 129.8, 128.6 (2C), 127.9, 127.5, 127.5, 125.4, 124.3, 122.4, 121.9 (2C). HRMS (ESI) m/z: calculated for C19H13N2O2S2 [M + H]+: 365.0413, found: 365.0428.
N–), 8.68 (s, 1H, ArH), 8.26 (s, 1H, ArH), 8.15 (d, J = 8.1 Hz, 2H, ArH), 8.12–8.06 (m, 2H, ArH), 7.71–7.69 (m, 1H, ArH), 7.66–7.64 (m, 1H, ArH), 7.40 (d, J = 8.1 Hz, 2H, ArH). 13C NMR (100 MHz, DMSO-d6) δ 170.4, 166.9, 156.4, 156.4, 154.6, 140.3, 134.5, 133.1, 129.8, 128.6 (2C), 127.9, 127.5, 127.5, 125.4, 124.3, 122.4, 121.9 (2C). HRMS (ESI) m/z: calculated for C19H13N2O2S2 [M + H]+: 365.0413, found: 365.0428.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.61–8.55 (m, 2H, ArH and –(C
N–), 8.61–8.55 (m, 2H, ArH and –(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)NH–), 8.26 (s, 1H, ArH), 8.15 (d, J = 8.5 Hz, 2H, ArH), 8.09 (d, J = 8.5 Hz, 1H, ArH), 7.98 (d, J = 8.5 Hz, 1H, ArH), 7.72–7.68 (m, 1H, ArH), 7.66–7.65 (m, 1H, ArH), 7.40 (d, J = 8.5 Hz, 2H, ArH), 2.84 (d, J = 4.4 Hz, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ 169.2, 166.0, 156.4, 155.2, 154.5, 140.3, 134.4, 133.0, 131.5, 129.9, 128.5 (2C), 127.9, 125.6, 125.4, 122.2, 121.9 (2C), 121.6, 26.4. HRMS (ESI) m/z: calculated for C20H15N3NaOS2 [M + Na]+: 400.0549, found: 400.0565.
O)NH–), 8.26 (s, 1H, ArH), 8.15 (d, J = 8.5 Hz, 2H, ArH), 8.09 (d, J = 8.5 Hz, 1H, ArH), 7.98 (d, J = 8.5 Hz, 1H, ArH), 7.72–7.68 (m, 1H, ArH), 7.66–7.65 (m, 1H, ArH), 7.40 (d, J = 8.5 Hz, 2H, ArH), 2.84 (d, J = 4.4 Hz, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ 169.2, 166.0, 156.4, 155.2, 154.5, 140.3, 134.4, 133.0, 131.5, 129.9, 128.5 (2C), 127.9, 125.6, 125.4, 122.2, 121.9 (2C), 121.6, 26.4. HRMS (ESI) m/z: calculated for C20H15N3NaOS2 [M + Na]+: 400.0549, found: 400.0565.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.21 (s, 1H, ArH), 7.94 (d, J = 8.0 Hz, 1H, ArH), 7.73–7.67 (m, 2H, ArH), 7.66–7.61 (m, 1H, ArH), 7.49 (d, J = 8.4 Hz, 2H, ArH), 7.46–7.41 (m, 1H, ArH), 7.38–7.30 (m, 3H, ArH). 13C NMR (100 MHz, DMSO-d6) δ 171.9, 155.8, 152.1, 149.9, 148.6, 140.4, 132.4, 131.8, 127.8, 126.5, 125.4, 124.2, 122.4 (2C), 122.2, 121.7 (2C), 121.1. HRMS (ESI) m/z: calculated for C18H13N2OS2 [M + H]+: 337.0464, found: 337.0474.
N–), 8.21 (s, 1H, ArH), 7.94 (d, J = 8.0 Hz, 1H, ArH), 7.73–7.67 (m, 2H, ArH), 7.66–7.61 (m, 1H, ArH), 7.49 (d, J = 8.4 Hz, 2H, ArH), 7.46–7.41 (m, 1H, ArH), 7.38–7.30 (m, 3H, ArH). 13C NMR (100 MHz, DMSO-d6) δ 171.9, 155.8, 152.1, 149.9, 148.6, 140.4, 132.4, 131.8, 127.8, 126.5, 125.4, 124.2, 122.4 (2C), 122.2, 121.7 (2C), 121.1. HRMS (ESI) m/z: calculated for C18H13N2OS2 [M + H]+: 337.0464, found: 337.0474.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.27 (s, 1H, ArH), 7.94 (d, J = 8.0 Hz, 1H, ArH), 7.87–7.80 (m, 3H, ArH), 7.72–7.68 (m, 1H, ArH), 7.67–7.63 (m, 1H, ArH), 7.48–7.43 (m, 1H, ArH), 7.41–7.31 (m, 3H, ArH). 13C NMR (100 MHz, DMSO-d6) δ 169.7, 156.8, 154.0, 153.5, 140.2, 136.6 (2C), 134.8, 133.1, 127.9, 126.4, 125.4, 125.1, 124.4, 122.7 (2C), 121.7, 121.3. HRMS (ESI) m/z: calculated for C18H13N2S3 [M + H]+: 353.0235, found: 353.0232.
N–), 8.27 (s, 1H, ArH), 7.94 (d, J = 8.0 Hz, 1H, ArH), 7.87–7.80 (m, 3H, ArH), 7.72–7.68 (m, 1H, ArH), 7.67–7.63 (m, 1H, ArH), 7.48–7.43 (m, 1H, ArH), 7.41–7.31 (m, 3H, ArH). 13C NMR (100 MHz, DMSO-d6) δ 169.7, 156.8, 154.0, 153.5, 140.2, 136.6 (2C), 134.8, 133.1, 127.9, 126.4, 125.4, 125.1, 124.4, 122.7 (2C), 121.7, 121.3. HRMS (ESI) m/z: calculated for C18H13N2S3 [M + H]+: 353.0235, found: 353.0232.Synthesis of (E)-2-((benzo[d]thiazol-2-ylimino)methyl)-6-methoxyphenol (1e)
The catalytic amount of acetic acid was added to a solution of 3e (0.5 mmol) and 2-hydroxy-3-methoxybenzaldehyde (0.6 mmol) in MeOH (4 mL), the mixture was stirred at reflux for 2 h. The resulting precipitate was isolated by suction filtration and washed with MeOH to give the pure product 1e. Orange solid; yield 32%. Mp 170–172 °C. 1H NMR (400 MHz, chloroform-d) δ 12.44 (s, 1H, OH), 9.26 (s, 1H, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 7.96 (d, J = 8.2 Hz, 1H, ArH), 7.83 (d, J = 8.0 Hz, 1H, ArH), 7.51–7.45 (m, 1H, ArH), 7.39–7.33 (m, 1H, ArH), 7.13 (d, J = 7.9 Hz, 1H, ArH), 7.06 (d, J = 8.0 Hz, 1H, ArH), 6.95–6.89 (m, 1H, ArH), 3.93 (s, 3H, –CH3). 13C NMR (100 MHz, chloroform-d) δ 167.9, 166.4, 151.1, 150.5, 147.5, 133.7, 125.7, 124.3, 124.2, 122.0, 120.7, 118.3, 117.4, 115.8, 55.3. HRMS (ESI) m/z: calculated for C15H12N2NaO2S [M + Na]+: 307.0512, found: 307.0509.
N–), 7.96 (d, J = 8.2 Hz, 1H, ArH), 7.83 (d, J = 8.0 Hz, 1H, ArH), 7.51–7.45 (m, 1H, ArH), 7.39–7.33 (m, 1H, ArH), 7.13 (d, J = 7.9 Hz, 1H, ArH), 7.06 (d, J = 8.0 Hz, 1H, ArH), 6.95–6.89 (m, 1H, ArH), 3.93 (s, 3H, –CH3). 13C NMR (100 MHz, chloroform-d) δ 167.9, 166.4, 151.1, 150.5, 147.5, 133.7, 125.7, 124.3, 124.2, 122.0, 120.7, 118.3, 117.4, 115.8, 55.3. HRMS (ESI) m/z: calculated for C15H12N2NaO2S [M + Na]+: 307.0512, found: 307.0509.
Synthesis of 2-(((4-(benzo[d]thiazol-2-yl)phenyl)amino)methyl)-6-methoxy-phenol (1f)
NaBH4 (0.84 mmol) was added to a suspension of 1a (0.42 mmol) in MeOH (6 mL) and stirred at room temperature for 1 h. Upon completion, MeOH was removed under reduced pressure. The crude mixture was then purified by flash column chromatography in a mixture of petroleum ether and ethyl acetate to yield the pure product 1f. Light yellow solid; yield 66%. Mp 187–188 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.79 (s, 1H, OH), 8.00 (d, J = 7.4 Hz, 1H, ArH), 7.89 (d, J = 8.1 Hz, 1H, ArH), 7.78 (d, J = 8.4 Hz, 2H, ArH), 7.46–7.43 (m, 1H, ArH), 7.35–7.31 (m, 1H, ArH), 6.88–6.84 (m, 2H, ArH and NH), 6.82–6.80 (m, 1H, ArH), 6.73–6.68 (m, 3H, ArH), 4.30 (d, J = 5.9 Hz, 2H, CH2), 3.80 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ 168.0, 153.9, 151.6, 147.3, 143.8, 133.7, 128.6, 126.2, 125.5, 124.3, 121.8, 121.7, 120.0, 120.0, 118.6, 111.9, 110.4, 55.8, 40.8. HRMS (ESI) m/z: calculated for C21H19N2O2S [M + H]+: 363.1162, found: 363.1190.Synthesis of N-(4-(benzo[d]thiazol-2-yl)phenyl)-2-hydroxy-3-methoxybenzamide (1g)
2-Hydroxy-3-methoxybenzoyl chloride (0.53 mmol) was dissolved in Et2O (0.5 mL) and then added dropwise to a stirring solution of 3a (0.44 mmol) and NaHCO3 (0.88 mmol) in Et2O (1 mL) and H2O (0.5 mL) at 0 °C. Then the reaction was allowed to warm to room temperature and stirred for 12 h. The resulting precipitate was isolated by suction filtration and washed with H2O (2 mL), 2 N HCl (2 mL) and H2O (2 mL) sequentially to yield the pure product 1g. White solid; yield 14%. Mp 215–216 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.33 (s, 1H, OH), 10.60 (s, 1H, –(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)NH–), 8.13–8.09 (m, 3H, ArH), 8.04 (d, J = 8.2 Hz, 1H, ArH), 7.93 (d, J = 8.4 Hz, 2H, ArH), 7.53 (d, J = 7.8 Hz, 2H, ArH), 7.47–7.42 (m, 1H, ArH), 7.18 (d, J = 8.0 Hz, 1H, ArH), 6.96–6.90 (m, 1H, ArH), 3.84 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ 167.0, 166.8, 153.6, 148.4, 148.3, 141.1, 134.3, 128.4, 127.9 (2C), 126.6, 125.3, 122.6, 122.2, 120.9 (2C), 120.1, 118.6, 118.0, 115.5, 56.0. HRMS (ESI) m/z: calculated for C21H16N2NaO3S [M + Na]+: 399.0774, found: 399.0774.
O)NH–), 8.13–8.09 (m, 3H, ArH), 8.04 (d, J = 8.2 Hz, 1H, ArH), 7.93 (d, J = 8.4 Hz, 2H, ArH), 7.53 (d, J = 7.8 Hz, 2H, ArH), 7.47–7.42 (m, 1H, ArH), 7.18 (d, J = 8.0 Hz, 1H, ArH), 6.96–6.90 (m, 1H, ArH), 3.84 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ 167.0, 166.8, 153.6, 148.4, 148.3, 141.1, 134.3, 128.4, 127.9 (2C), 126.6, 125.3, 122.6, 122.2, 120.9 (2C), 120.1, 118.6, 118.0, 115.5, 56.0. HRMS (ESI) m/z: calculated for C21H16N2NaO3S [M + Na]+: 399.0774, found: 399.0774.
Plasmid and pseudovirus production
To produce MERS spike protein pseudotyped HIV virions, we constructed a MERS spike protein (MERS-S) expression plasmid. The codon-optimized gene of full-length S protein of MERS-CoV (Genebank: AFS88936), with replacement of the N-terminal signal peptide (aa 1–17) with CD5 signal sequence, were synthesized (Inovogen, China) and insertion into pcDNA3.1 vector, verified by sequencing. A HIV backbone vector pNL4-3. Luc.R-E- was used for pseudovirus package. 293T cells were co-transfected with MERS spike protein expression plasmid plus pNL4.3LucR-E- plasmid using X-tremeGENE DNA HP Transfection Reagent (Roche) according the manufacturer's instructions.29 At 72 h post-transfection, supernatants were harvested and filtered through 0.45 μm filter, the filtered supernatant was further concentrated 10 folds by ultracentrifugation with 100 kDa cut-off, aliquoted and stored at −80 °C as stock vial. The p24 level of pseudovirus stock solution was determined by ELISA kit according to the manual (ab218268, Abcam).Compound library and HTS screening
Compound library samples were orthogonally pooled as mixtures of 10 compounds per well at 2 μg mL−1 each, with duplicate representation for each compound. This bidirectional orthogonal pooling strategy allows for greater screening efficiency and throughput for large compound libraries. The pooling of compound library was prepared as described previously.52Test compounds were freshly prepared from initial dimethyl sulfoxide (DMSO) stocks, 80 cocktails per plate. Huh-7 cells were then trypsinized, counted and seeded at a final concentration of 104 cells per well. Plates were placed overnight in a CO2 incubator. The following day, samples were then added with an automatic liquid handler (FX from Beckman–Coulter). Finally, the pseudotyped virus, thawed and diluted immediately prior to use in cell culture medium, was added under a BSL-2 hood using Hydra (Thermo Fisher Scientific). The final concentration of DMSO in all wells was maintained at 2%. The plates were incubated at 37 °C in a humidified CO2 incubator for 48 h. Bright-Glo substrate (Promega) was added directly to each well and cell lysis was allowed to proceed in the dark for 5 min.
Cytotoxic effect of compounds was assayed by MTT method which evaluated the reduction product of MTT by cellular succinate dehydrogenase as an indicator of cell liability. Toxicity was calculated as 100% minus percentage of OD@570 readout each well against the control well with no drug, 100% − OD@570 (compound)/OD@570 (control) × 100%.
Luciferase activity was measured using the Envision microplate luminometer (PerkinElmer). Inhibition was calculated as 100% minus percentage of luciferase readout each well against the control well with no drug, 100% − Luc (compound)/Luc (control) × 100%.
Measurement of IC50 and CC50
For determining CC50 and IC50, compounds were serially diluted and assayed as described for the HTS screening, CC50 and IC50 were determined by regression analysis using 4 parameter regression.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the National Key Research and Development Program of China (2018YFC1200604). The pNL4.3.Luc.R-.E- and MERS-S expression plasmid was kindly gifted by Dr Shibo Jiang and Dr Lu Lu from Fudan University.Notes and references
- A. Zumla, D. S. Hui and S. Perlman, Lancet, 2015, 386, 995–1007 CrossRef
.
- Y. M. Arabi, H. H. Balkhy, F. G. Hayden, A. Bouchama, T. Luke, J. K. Baillie, A. Al-Omari, A. H. Hajeer, M. Senga, M. R. Denison, J. S. Nguyen-Van-Tam, N. Shindo, A. Bermingham, J. D. Chappell, M. D. Van Kerkhove and R. A. Fowler, N. Engl. J. Med., 2017, 376, 584–594 CrossRef
.
- A. M. Zaki, S. van Boheemen, T. M. Bestebroer, A. D. Osterhaus and R. A. Fouchier, N. Engl. J. Med., 2012, 367, 1814–1820 CrossRef CAS
.
- D. S. Hui, S. Perlman and A. Zumla, Lancet Respir. Med., 2015, 3, 509–510 CrossRef
.
- E. I. Azhar, S. A. El-Kafrawy, S. A. Farraj, A. M. Hassan, M. S. Al-Saeed, A. M. Hashem and T. A. Madani, N. Engl. J. Med., 2014, 370, 2499–2505 CrossRef CAS
.
- M. Cotten, S. J. Watson, P. Kellam, A. A. Al-Rabeeah, H. Q. Makhdoom, A. Assiri, J. A. Al-Tawfiq, R. F. Alhakeem, H. Madani, F. A. AlRabiah, S. A. Hajjar, W. N. Al-nassir, A. Albarrak, H. Flemban, H. H. Balkhy, S. Alsubaie, A. L. Palser, A. Gall, R. Bashford-Rogers, A. Rambaut, A. I. Zumla and Z. A. Memish, Lancet, 2013, 382, 1993–2002 CrossRef
.
- R. Breban, J. Riou and A. Fontanet, Lancet, 2013, 382, 694–699 CrossRef
.
- A. Assiri, J. A. Al-Tawfiq, A. A. Al-Rabeeah, F. A. Al-Rabiah, S. Al-Hajjar, A. Al-Barrak, H. Flemban, W. N. Al-Nassir, H. H. Balkhy, R. F. Al-Hakeem, H. Q. Makhdoom, A. I. Zumla and Z. A. Memish, Lancet Infect. Dis., 2013, 13, 752–761 CrossRef
.
- R. Alexpandi, J. F. De Mesquita, S. K. Pandian and A. V. Ravi, Front. Microbiol., 2020, 11, 1796 CrossRef
.
- W. R. Ferraz, R. A. Gomes, A. L. S. Novaes and G. H. G. Trossini, Future Med. Chem., 2020, 12, 1815–1828 CrossRef CAS
.
- D. Gentile, V. Patamia, A. Scala, M. T. Sciortino, A. Piperno and A. Rescifina, Mar. Drugs, 2020, 18, 225 CrossRef CAS
.
- A. K. Ghosh, M. Brindisi, D. Shahabi, M. E. Chapman and A. D. Mesecar, ChemMedChem, 2020, 15, 907–932 CrossRef CAS
.
- M. Hagar, H. A. Ahmed, G. Aljohani and O. A. Alhaddad, Int. J. Mol. Sci., 2020, 21, 3922 CrossRef CAS
.
- S. T. Ngo, N. Quynh Anh Pham, L. Thi Le, D. H. Pham and V. V. Vu, J. Chem. Inf. Model., 2020 DOI:10.1021/acs.jcim.0c00491
.
- O. O. Olubiyi, M. Olagunju, M. Keutmann, J. Loschwitz and B. Strodel, Molecules, 2020, 25, 3193 CrossRef CAS
.
- S. Shahinshavali, K. A. Hossain, A. Kumar, A. G. Reddy, D. Kolli, A. Nakhi, M. V. B. Rao and M. Pal, Tetrahedron Lett., 2020, 61, 152336 CrossRef
.
- L. Zhang, D. Lin, X. Sun, U. Curth, C. Drosten, L. Sauerhering, S. Becker, K. Rox and R. Hilgenfeld, Science, 2020, 368, 409–412 CrossRef CAS
.
- B. Cao, Y. Wang, D. Wen, W. Liu, J. L. Wang, G. Fan, L. Ruan, B. Song, Y. Cai, M. Wei, X. Li, J. Xia, N. Chen, J. Xiang, T. Yu, T. Bai, X. Xie, L. Zhang, C. Li, Y. Yuan, H. Chen, H. D. Li, H. Huang, S. Tu, F. Gong, Y. Liu, Y. Wei, C. Dong, F. Zhou, X. Gu, J. Xu, Z. Liu, Y. Zhang, H. Li, L. Shang, K. Wang, K. Li, X. Zhou, X. Dong, Z. Qu, S. Lu, X. Hu, S. Ruan, S. Luo, J. Wu, L. Peng, F. Cheng, L. Pan, J. Zou, C. Jia, J. Wang, X. Liu, S. Wang, X. Wu, Q. Ge, J. He, H. Zhan, F. Qiu, L. Guo, C. Huang, T. Jaki, F. G. Hayden, P. W. Horby, D. Zhang and C. Wang, N. Engl. J. Med., 2020, 382, 1787–1799 CrossRef
.
- M. Wang, R. Cao, L. Zhang, X. Yang, J. Liu, M. Xu, Z. Shi, Z. Hu, W. Zhong and G. Xiao, Cell Res., 2020, 30, 269–271 CrossRef CAS
.
- D. Falzarano, E. de Wit, A. L. Rasmussen, F. Feldmann, A. Okumura, D. P. Scott, D. Brining, T. Bushmaker, C. Martellaro, L. Baseler, A. G. Benecke, M. G. Katze, V. J. Munster and H. Feldmann, Nat. Med., 2013, 19, 1313–1317 CrossRef CAS
.
- V. Kumar, J. S. Shin, J. J. Shie, K. B. Ku, C. Kim, Y. Y. Go, K. F. Huang, M. Kim and P. H. Liang, Antiviral Res., 2017, 141, 101–106 CrossRef CAS
.
- A. H. de Wilde, D. Jochmans, C. C. Posthuma, J. C. Zevenhoven-Dobbe, S. van Nieuwkoop, T. M. Bestebroer, B. G. van den Hoogen, J. Neyts and E. J. Snijder, Antimicrob. Agents Chemother., 2014, 58, 4875–4884 CrossRef
.
- T. P. Sheahan, A. C. Sims, R. L. Graham, V. D. Menachery, L. E. Gralinski, J. B. Case, S. R. Leist, K. Pyrc, J. Y. Feng, I. Trantcheva, R. Bannister, Y. Park, D. Babusis, M. O. Clarke, R. L. Mackman, J. E. Spahn, C. A. Palmiotti, D. Siegel, A. S. Ray, T. Cihlar, R. Jordan, M. R. Denison and R. S. Baric, Sci. Transl. Med., 2017, 9(396), eaal3653 CrossRef
.
- T. P. Sheahan, A. C. Sims, S. R. Leist, A. Schafer, J. Won, A. J. Brown, S. A. Montgomery, A. Hogg, D. Babusis, M. O. Clarke, J. E. Spahn, L. Bauer, S. Sellers, D. Porter, J. Y. Feng, T. Cihlar, R. Jordan, M. R. Denison and R. S. Baric, Nat. Commun., 2020, 11, 222 CrossRef CAS
.
- M. H. Lin, D. C. Moses, C. H. Hsieh, S. C. Cheng, Y. H. Chen, C. Y. Sun and C. Y. Chou, Antiviral Res., 2018, 150, 155–163 CrossRef CAS
.
- H. Lee, J. Ren, R. P. Pesavento, I. Ojeda, A. J. Rice, H. Lv, Y. Kwon and M. E. Johnson, Bioorg. Med. Chem., 2019, 27, 1981–1989 CrossRef CAS
.
- L. Lu, S. Xia, T. Ying and S. Jiang, Emerging Microbes Infect., 2015, 4, e37 CAS
.
- J. Gao, G. Lu, J. Qi, Y. Li, Y. Wu, Y. Deng, H. Geng, H. Li, Q. Wang, H. Xiao, W. Tan, J. Yan and G. F. Gao, J. Virol., 2013, 87, 13134–13140 CrossRef CAS
.
- L. Lu, Q. Liu, Y. Zhu, K. H. Chan, L. Qin, Y. Li, Q. Wang, J. F. Chan, L. Du, F. Yu, C. Ma, S. Ye, K. Y. Yuen, R. Zhang and S. Jiang, Nat. Commun., 2014, 5, 3067 CrossRef
.
- C. Wang, S. Xia, P. Zhang, T. Zhang, W. Wang, Y. Tian, G. Meng, S. Jiang and K. Liu, J. Med. Chem., 2018, 61, 2018–2026 CrossRef CAS
.
- T. Ying, L. Du, T. W. Ju, P. Prabakaran, C. C. Lau, L. Lu, Q. Liu, L. Wang, Y. Feng, Y. Wang, B. J. Zheng, K. Y. Yuen, S. Jiang and D. S. Dimitrov, J. Virol., 2014, 88, 7796–7805 CrossRef
.
- Y. Li, Y. Wan, P. Liu, J. Zhao, G. Lu, J. Qi, Q. Wang, X. Lu, Y. Wu, W. Liu, B. Zhang, K. Y. Yuen, S. Perlman, G. F. Gao and J. Yan, Cell Res., 2015, 25, 1237–1249 CrossRef CAS
.
- J. H. Beigel, J. Voell, P. Kumar, K. Raviprakash, H. Wu, J.-A. Jiao, E. Sullivan, T. Luke and R. T. Davey, Lancet Infect. Dis., 2018, 18, 410–418 CrossRef CAS
.
- N. K. Alharbi, E. Padron-Regalado, C. P. Thompson, A. Kupke, D. Wells, M. A. Sloan, K. Grehan, N. Temperton, T. Lambe, G. Warimwe, S. Becker, A. V. S. Hill and S. C. Gilbert, Vaccine, 2017, 35, 3780–3788 CrossRef CAS
.
- B. L. Haagmans, J. M. A. van den Brand, V. S. Raj, A. Volz, P. Wohlsein, S. L. Smits, D. Schipper, T. M. Bestebroer, N. Okba, R. Fux, A. Bensaid, D. S. Foz, T. Kuiken, W. Baumgartner, J. Segales, G. Sutter and A. D. M. E. Osterhaus, Science, 2016, 351, 77–81 CrossRef CAS
.
- Y. Zhou, S. Jiang and L. Du, Expert Rev. Vaccines, 2018, 17, 677–686 CrossRef CAS
.
- S. Su, G. Wong, W. Shi, J. Liu, A. C. K. Lai, J. Zhou, W. Liu, Y. Bi and G. F. Gao, Trends Microbiol., 2016, 24, 490–502 CrossRef CAS
.
- J. F. Chan, S. K. Lau and P. C. Woo, J. Formosan Med. Assoc., 2013, 112, 372–381 CrossRef
.
- S. van Boheemen, M. de Graaf, C. Lauber, T. M. Bestebroer, V. S. Raj, A. M. Zaki, A. D. Osterhaus, B. L. Haagmans, A. E. Gorbalenya, E. J. Snijder and R. A. Fouchier, mBio, 2012, 3(6), e00473-12 CrossRef
.
- A. E. Gorbalenya, L. Enjuanes, J. Ziebuhr and E. J. Snijder, Virus Res., 2006, 117, 17–37 CrossRef CAS
.
- E. J. Snijder, P. J. Bredenbeek, J. C. Dobbe, V. Thiel, J. Ziebuhr, L. L. M. Poon, Y. Guan, M. Rozanov, W. J. M. Spaan and A. E. Gorbalenya, J. Mol. Biol., 2003, 331, 991–1004 CrossRef CAS
.
- J. Ziebuhr, E. J. Snijder and A. E. Gorbalenya, J. Gen. Virol., 2000, 81, 853–879 CrossRef CAS
.
- Z. Song, Y. Xu, L. Bao, L. Zhang, P. Yu, Y. Qu, H. Zhu, W. Zhao, Y. Han and C. Qin, Viruses, 2019, 11, 59 CrossRef CAS
.
- S. Jiang, L. Lu, L. Du and A. K. Debnath, J. Infect., 2013, 66, 464–466 CrossRef
.
- V. S. Raj, H. Mou, S. L. Smits, D. H. Dekkers, M. A. Muller, R. Dijkman, D. Muth, J. A. Demmers, A. Zaki, R. A. Fouchier, V. Thiel, C. Drosten, P. J. Rottier, A. D. Osterhaus, B. J. Bosch and B. L. Haagmans, Nature, 2013, 495, 251–254 CrossRef CAS
.
- N. Wang, X. Shi, L. Jiang, S. Zhang, D. Wang, P. Tong, D. Guo, L. Fu, Y. Cui, X. Liu, K. C. Arledge, Y. H. Chen, L. Zhang and X. Wang, Cell Res., 2013, 23, 986–993 CrossRef CAS
.
- S. H. Havale and M. Pal, Bioorg. Med. Chem., 2009, 17, 1783–1802 CrossRef CAS
.
- J. Ling, L. Ge, D. H. Zhang, Y. F. Wang, Z. L. Xie, J. H. Tian, X. H. Xiao and K. H. Yang, Acta Diabetol., 2019, 56, 7–27 CrossRef CAS
.
- C. F. Deacon, Nat. Rev. Endocrinol., 2020, 16, 642–653 CrossRef CAS
.
- D. Forni, G. Filippi, R. Cagliani, L. De Gioia, U. Pozzoli, N. Al-Daghri, M. Clerici and M. Sironi, Sci. Rep., 2015, 5, 14480 CrossRef CAS
.
- G. Zhao, L. Du, C. Ma, Y. Li, L. Li, V. K. Poon, L. Wang, F. Yu, B. J. Zheng, S. Jiang and Y. Zhou, J. Virol., 2013, 10, 266 CrossRef
.
- J. M. Garcia, A. Gao, P. L. He, J. Choi, W. Tang, R. Bruzzone, O. Schwartz, H. Naya, F. J. Nan, J. Li, R. Altmeyer and J. P. Zuo, Antiviral Res., 2009, 81, 239–247 CrossRef CAS
.
- G. Bort, M. Sylla-Iyarreta Veitía and C. Ferroud, Tetrahedron, 2013, 69, 7345–7353 CrossRef CAS
.
- M. Singh, S. K. Singh, B. Thakur, P. Ray and S. K. Singh, Anti-Cancer Agents Med. Chem., 2016, 16, 722–739 CrossRef CAS
.
- K. Potgieter, T. Gerber, E. Hosten and R. Betz, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2012, 68, o27 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra08442e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |