Recent research progress on polyoxometalate-based electrocatalysts in energy generation
Kai
Li
a,
Tao
Liu
b,
Jun
Ying
 *a,
Aixiang
Tian
*a,
Aixiang
Tian
 a and
Xiuli
Wang
a and
Xiuli
Wang
 *a
*a
aCollege of Chemistry and Materials Engineering, Bohai University, Jinzhou 121013, P. R. China. E-mail: ying@bhu.edu.cn; wangxiuli@bhu.edu.cn
bCollege of Sciences, North China University of Science and Technology, Tangshan, Hebei 063210, China
First published on 20th May 2024
Abstract
Sustainable energy generation is a pressing challenge in the society today, driven by the rapid exponential growth in industrialization. Thus, it has become an urgent priority to find sustainable solutions for energy generation. Polyoxometalates (POMs) are a class of materials characterized by diverse components, which provide numerous active sites, electronic regulatory effects, synergistic effects, structural diversity, and controllability. Researchers have made remarkable progress in regulating the catalytic performance of POM-based electrocatalysts by improving the configuration of POMs. Herein, we aim to review the recent research progress (from 2020 to present) on the relation between different types of POMs and their catalytic activities in electrocatalytic oxygen evolution (OER), electrocatalytic hydrogen evolution (HER) and CO2 reduction (CO2RR). By analyzing the application of POMs in different electrocatalytic fields, the suitability of POMs in these areas can be further determined and possible specific explanations can be summarized. The most important aspect is to provide relevant guidance for future researchers in the synthesis of POM-based electrocatalysts, where their electrocatalytic performance can be improved to the maximum extent through the reasonable selection of POMs. The utilization of POM-based electrocatalysts shows tremendous promise to address the pressing issues related to energy scarcity, providing a pathway to realize sustainable energy generation.
1. Introduction
Energy is of utmost importance in achieving sustainable development in society. Currently, approximately 80% of the global energy demand is met by fossil fuels, which face the risk of depletion.1 Thus, gradually transitioning from fossil fuels to sustainable and pollution-free non-fossil energy sources has become an inevitable development trend. According to market research institutions, the global hydrogen (H2) energy market reached around 90 billion US dollars in 2023, marking a 10% increase compared to 2022.2 Nevertheless, the majority of H2 production worldwide still relies on methods such as steam reforming and partial oxidation of hydrocarbons, which leads to significant carbon dioxide (CO2) emission.3,4 As reported by the International Energy Agency (IEA), the global energy-related CO2 emission is projected to reach 36.8 billion tons, reflecting a 1.1% increase compared to 2022.5 In view of this situation, the most effective and fundamental approach to address these challenges is to identify alternative renewable and clean energy sources that can meet global energy demands and facilitate the efficient transformation and utilization of CO2.6The energy crisis stems from the excessive dependence on fossil fuels and thus needs immediate attention, emphasizing the importance of utilizing renewable energy and promoting efficient energy conversion technology.7 Within this realm, electrochemical energy conversion has become a prominent field, especially in the domains of water splitting and CO2 reduction (CO2RR).8,9 These technologies show considerable potential in generating clean and sustainable energy carriers.10 Water splitting involves two types of redox reactions, hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), which hold great potential for generating environmentally friendly H2 and oxygen (O2).11,12 H2 plays a crucial role in energy generation, particularly as a fuel source for electricity generation in fuel cells.13 Fuel cells facilitate the reaction between H2 and O2, resulting in the production of water and the generation of electricity. This process is characterized by its clean and emission-free nature, making H2 an environmentally friendly energy choice. Alternatively, the electrocatalytic reduction of CO2 presents a promising avenue for the production of valuable carbon-based products. This process facilitates the effective recycling of natural carbon resources, fostering a circular and eco-friendly carbon economy. The resultant products include C1 (CH4, HCOOH, and CH3OH) and C2 (C2H4, C2H6, and CH3COOH) compounds.14–17 However, considering the high activation barrier of this type of reaction, efficient electrocatalysts with multiple catalytic sites are important to promote the necessary proton-assisted multi-electron transfer process to synthesize the required oxidation and reduction products.18
Polyoxometalates (POMs) are a class of multicomponent ion clusters composed of metal (VV, MoV, NbV, TaV, MoVI and WVI) and oxygen atoms, possessing diverse structural and chemical properties.19 POM molecules are formed by the connection of multiple metal centers and oxygen atoms through shared oxygen bridge bonds, resulting in stable spatial structures.20 To date, a wide range of POM structures has been reported, exhibiting exceptional characteristics that make them highly versatile in various fields.
POMs possess a tunable chemical composition and structure, enabling the syntheses of POM-based compounds with different chemical properties and reactivity by adjusting the synthetic method.21 POMs demonstrate excellent performance and stability, making them promising candidates for applications in covalent/coordination design and assembly of mixed organic–inorganic frameworks. By employing POMs as building blocks, interactions and assemblies with organic molecules can be achieved, facilitating the construction of composite materials with specific structures and properties.22 This organic–inorganic hybrid framework extends the functions and properties of traditional materials, especially in the field of electrocatalysis. In the field of electrocatalysis, POM-based catalysts have demonstrated advantages through both intrinsic and extrinsic regulation strategies (Scheme 1). Here, we provide a specific summary of the advantages of POM-based electrocatalysts: (I) POM-based compounds consist of various metal ions and polyoxoanions, providing rich compositional and structural control. This allows for tuning their catalytic performance by adjusting their composition and structural parameters, enabling optimization for specific reactions.23 (II) POMs possess abundant accessible active sites that can serve as catalytic centers for electrocatalytic reactions. Furthermore, their multi-metallic structure and highly dispersed ion coordination enable them to offer high activity and selectivity, resulting in an excellent performance in electrocatalytic reactions.24 (III) POMs exhibit good stability in electrocatalytic reactions. Their multi-metallic structure and discrete ion coordination enable them to resist corrosion and structural degradation in oxidative, reductive, acidic, and alkaline environments, thus prolonging the service life of materials.25 (IV) The structural parameters of POMs can be precisely controlled by adjusting their composition, topology, and pore structure. This tunability enables the customized design of POMs to meet the specific requirements of electrocatalytic reactions, leading to an optimal catalytic performance.26 Based on the aforementioned advantages, POM-based compounds have demonstrated potential as efficient electrocatalysts for energy generation. For instance, POMs exhibit remarkable catalytic activity and selectivity for crucial processes such as OER and HER involved in water splitting and CO2RR (Scheme 2).11,23 Moreover, POMs exhibit significant promise in reducing CO2 emissions, given that they can convert greenhouse gases into valuable fuels and chemicals.24
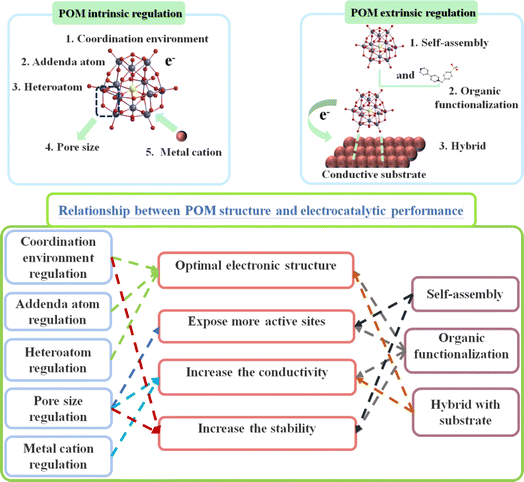 | ||
| Scheme 1 Relationship between structure control strategy and electrocatalytic performance of POM-based electrocatalysts. | ||
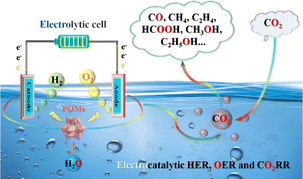 | ||
| Scheme 2 POM-based electrocatalysts for efficient electrocatalytic applications (HER, OER and CO2RR). | ||
It is well known that electrocatalytic reactions such as HER, OER, and CO2RR are reduction reactions, and the active sites for these reactions are typically metal sites. However, enhancing the reaction activity of electrocatalysts by increasing the number of active sites has been a major challenge for researchers. In recent years, the introduction of POMs in electrocatalysts has become a focal point. (I) The introduction of POMs allows control of the morphology, particle size, and pore structure of the material, thereby increasing the specific surface area of the electrocatalyst.25 A larger surface area means more exposed metal reaction sites, leading to improved activity in reduction reactions. (II) The structure of POMs is tunable, and by removing a structural unit, appropriate coordination atoms and transition metals can be selected to regulate their structure. Optimized structures can increase the number and activity of exposed metal reaction sites, thereby improving the efficiency of reduction reactions.26 (III) The presence of auxiliary heteroatoms such as nitrogen and sulfur in the material can alter the electronic structure and surface chemistry of POMs, enhancing the activity of the metal reaction sites. These auxiliary heteroatoms can create synergistic effects with metal atoms, promoting reduction reactions.27 (IV) Introducing modifying groups or functional groups on the surface of POMs can increase the surface active sites of the material.28 These groups can adsorb target reduction reactants, provide additional reduction reaction sites, and improve the interaction between the material and reactants.
Different from previous reviews, this review provides a detailed classification of POM-based electrocatalysis into POM-based single-crystal structures and POM-based nanostructures, providing a comprehensive overview of the electrocatalytic mechanisms and challenges in HER, OER, and CO2RR. More importantly, the suitability of POMs in these areas can be further determined by analysing their applications in different electrocatalytic fields. Based on the summarized possible explanations, the rational selection of POMs can maximize the electrocatalytic performance. Finally, this review systematically discusses the advantages, challenges, strategies, and prospects of POM-based electrocatalysts, which are crucial for the development of electrocatalysis in energy generation.
2. Development of POM-based electrocatalysts
2.1 Fundamental structures and redox activities of POMs
POMs are a type of anionic metal oxide species primarily composed of redox active metal centers.25 These electron-deficient molecular units are formed through the condensation of basic oxygen anions. The most common unit is the tetravalent oxygen anion [MO4]n, which is connected in the form of {MOn} in acidic media.26 POMs have emerged as highly attractive molecular catalysts and redox mediators, finding applications in catalytic systems involving multi-electron or multi-proton transfer.27–30 A deep understanding of the intrinsic properties of the original POMs is crucial for optimizing their electrocatalytic performance. It is essential to firmly grasp key concepts related to the original POMs, such as classical POM structure, structural evolution, composition adjustment and redox behavior, to rationally design suitable POM-based electrocatalysts for practical applications.Due to the pH-sensitive nature of POM structures, it is possible to construct isomers and vacancy-type POMs by modifying the {MO6} building blocks. The Keggin structure has five Baker–Figgis isomers (α, β, γ, δ, and ε), which are distinguished by the different rotation directions and angles of the trimeric metal clusters that constitute their primary structure.42 The derivative structures of Keggin-type POMs are constructed by removing one or more {MO6} building blocks, creating one or more vacancies that can accommodate external metal ions.43 Furthermore, the heteroatom at the center of POMs can be substituted with other elements, while maintaining their crystal structure (Fig. 1b). This characteristic makes POMs a versatile series of molecular clusters that can modify their structure and properties in response to various external stimuli.44 The unique structural distribution formed after substitution with new heteroatoms and introduction of metal ions can contribute to improved electrochemical performances.45
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds. These POMs do not undergo reversible redox processes because their reduction involves adding electrons to antibonding orbitals, leading to significant structural rearrangement of the polyanions. A representative example is the Anderson-type POMs or heptamolybdate [Mo7O24]6−.49 The fourth type is comprised of MO2L4 with Cs symmetry and MOL5 with C4v symmetry. These structural units exhibit reversible redox activity similar to MOL5 with C4v symmetry. The presence of abundant vacant sites allows them to coordinate various transition metals and oxygen-containing moieties, resulting in POMs with strong catalytic activity. Representative examples include vacant Keggin-type POMs ([XM11O39]n−, [XM9O34]n−) and vacant Dawson-type POMs [H7P8W48O184]33−.50–52 The fifth type represents POM configurations with pentagonal bipyramidal, MOL6 with C5v symmetry. This type has been widely used in wheel-shaped Mo clusters, where mixed-valence MoV/MoVI states are present.53 However, to date, despite the potential presence of mixed-valence metals in these POMs, their exploration remains challenging due to their intricate structure and complex mechanism.
O bonds. These POMs do not undergo reversible redox processes because their reduction involves adding electrons to antibonding orbitals, leading to significant structural rearrangement of the polyanions. A representative example is the Anderson-type POMs or heptamolybdate [Mo7O24]6−.49 The fourth type is comprised of MO2L4 with Cs symmetry and MOL5 with C4v symmetry. These structural units exhibit reversible redox activity similar to MOL5 with C4v symmetry. The presence of abundant vacant sites allows them to coordinate various transition metals and oxygen-containing moieties, resulting in POMs with strong catalytic activity. Representative examples include vacant Keggin-type POMs ([XM11O39]n−, [XM9O34]n−) and vacant Dawson-type POMs [H7P8W48O184]33−.50–52 The fifth type represents POM configurations with pentagonal bipyramidal, MOL6 with C5v symmetry. This type has been widely used in wheel-shaped Mo clusters, where mixed-valence MoV/MoVI states are present.53 However, to date, despite the potential presence of mixed-valence metals in these POMs, their exploration remains challenging due to their intricate structure and complex mechanism.
2.2 POM-based single-crystal structures
POMs exhibit the ability to self-assemble or combine with other metal elements and small organic molecules, resulting in the formation of unique single-crystal structures.54 Generally, conventional solution methods, hydrothermal methods, solvothermal methods, etc. are employed for the synthesis of POM-based single-crystal structures. It is worth noting that the utilization of POM-based single-crystal structures offers several notable advantages. Firstly, these structures enable a comprehensive analysis of the assembly and substitution sites within POMs through crystal structure determination, which provides valuable insights into the variations in their electronic properties and redox capability. Moreover, these structures play a vital role in comprehending the electron transfer pathways between the reactive sites of POMs and reactants involved in electrocatalytic processes. This understanding can be harnessed to design highly efficient electrocatalysts with a well-defined strategy. Simultaneously, there is a close correlation between POM-related structures and electrocatalytic performance. The interactions among multiple metal atoms in the cluster structure can regulate the selectivity of catalytic reactions.55 Thus, by adjusting the electronic structure of these clusters, such as controlling the electron transfer and redistribution between metal atoms, the electron transfer steps and intermediate species in catalytic reactions can be modulated. Additionally, the cluster structure has a large surface area and abundant pore structures, which help to enhance the specific surface area of the electrocatalyst and the diffusion rate of the reactants.56 Moreover, the stability of the cluster structure is crucial for maintaining the catalytic performance. An ideal coordination environment of POMs can suppress the disintegration of the cluster structure and the leaching of the metal atom, thereby extending the lifespan of the catalyst.57 Based on these advantages, a diverse range of POM structures has been developed in this field, including giant clusters, transition metal-substituted POMs and POM-based organic–inorganic hybrids. Consequently, this section focuses on the selection and discussion of three representative POM-based single-crystal structures.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O intermediates during organic catalytic processes. It may also be potentially formed by rapid intramolecular electron transfer within the capsule containing bridging O–W–O units, giving rise to the formation of binuclear Fe(IV)
O intermediates during organic catalytic processes. It may also be potentially formed by rapid intramolecular electron transfer within the capsule containing bridging O–W–O units, giving rise to the formation of binuclear Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species. These findings indicate that {FeIII30WVI72} serves as a promising electrocatalyst for iron-based inorganic analogs. In addition, other giant clusters with a host–guest structure have been explored, such as {Fe10P4W32}, {Mo124Ce4}, {Nb-288}, and {Mo154}.59–62 These colossal structures exhibit various geometries resulting from the different coordination of the {MOx} units, leading to the coexistence of multiple oxidation states of metals within the molecules. This phenomenon facilitates reversible redox reactions during the catalytic process, making them promising representatives of electrocatalysts with great potential.
O species. These findings indicate that {FeIII30WVI72} serves as a promising electrocatalyst for iron-based inorganic analogs. In addition, other giant clusters with a host–guest structure have been explored, such as {Fe10P4W32}, {Mo124Ce4}, {Nb-288}, and {Mo154}.59–62 These colossal structures exhibit various geometries resulting from the different coordination of the {MOx} units, leading to the coexistence of multiple oxidation states of metals within the molecules. This phenomenon facilitates reversible redox reactions during the catalytic process, making them promising representatives of electrocatalysts with great potential.
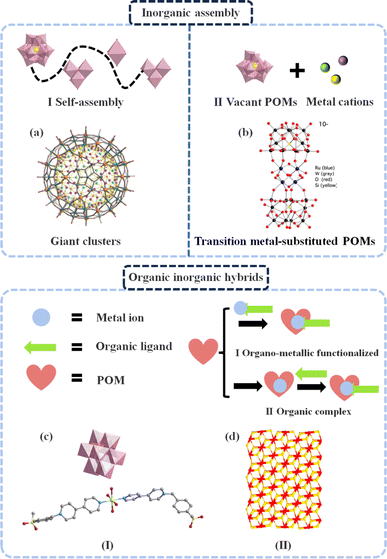 | ||
| Fig. 2 Detailed composition of POM-based single-crystal structures. (a) Structure of giant POMs. Reproduced from ref. 57. Copyright 2010, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (b) Structure of substituted POMs. Reproduced from ref. 67. Copyright 2016, the American Chemical Society. Structure of organic inorganic hybrids: (c) organo-metallic functionalized and (d) organic complex. Reproduced from ref. 72. Copyright 2022, the American Chemical Society. | ||
 | ||
| Fig. 3 Representative structure of POM-based electrocatalysts: (a) giant cluster. Reproduced from ref. 57. Copyright 2010, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (b) Transition metal-substituted POMs. Reproduced from ref. 67. Copyright 2016, the American Chemical Society. (c) POM-based organic–inorganic complexes. Reproduced from ref. 72. Copyright 2022, the American Chemical Society. (d) POM-substrate-based nanostructures. Reproduced from ref. 87. Copyright 2021, the American Chemical Society. (e) POM-assembled nanostructures. Reproduced from ref. 96. Copyright 2021, the American Chemical Society. (f) POM-encapsulated metal–organic framework nanostructures. Reproduced from ref. 98. Copyright 2019, the American Chemical Society. | ||
2.3 POM-based nanostructures
POM-based nanostructures are a class of nanomaterials assembled using POMs as structural units. Due to their high stability, redox activity and tunable charge, POMs play a significant role in controlling the shape and enhancing the performance of nanomaterials.73 POM-based nanostructures can be synthesized using various methods, such as solution-based synthesis, template-assisted fabrication and self-assembly.74–76 Depending on the synthesis conditions and assembly techniques, the resulting POM-based nanostructures can exhibit different morphologies, including nanoparticles, nanowires, nanotubes and thin films.77–80 Their size, shape and composition can be precisely controlled, enabling customized properties and functionalities. POM-based nanostructures serve as highly active electrocatalysts in various reactions due to their unique redox properties. Importantly, the synergistic effect between nanomaterials and POMs enhances their electrocatalytic performance, offering opportunities for innovation in the field of electrocatalysis. Their distinctive characteristics and controllable assembly provide opportunities for advancements in the field of electrocatalysis. Subsequently, several representative POM-based nanostructures will be introduced.3. POM-based single-crystal structures for electrocatalysis
Currently, the energy crisis continues to impact the development of countries worldwide. Therefore, it is urgent to replace disposable and environmentally harmful energy sources with eco-friendly, clean, and green energy.99 To achieve this primary goal, the fundamental concept is to efficiently produce sustainable and clean energy (H2) and convert greenhouse gases (CO2) into energy and chemicals. Consequently, the conversion of effective energy sources has attracted significant attention, such as water splitting (HER and OER), as well as CO2RR for converting greenhouse gases into chemicals. POM-based single-crystal structures have emerged as highly promising catalysts for a wide range of electrocatalytic reactions, which offer distinct advantages, including well-defined geometries, exceptional stability, adjustable redox properties and efficient electron and proton transfer capabilities. In this section, we summarize the applications of POM-based single-crystal structures in the field of electrocatalysis and provide detailed reports on the influence of different types of POMs on electrocatalytic performance, highlighting their potential in the fields of electrocatalysis and energy generation.3.1 Hydrogen evolution reaction
POM-based single-crystal structures can be utilized to create superior active electrocatalysts and modulate their electrocatalytic properties. For instance, (I) POM-based single-crystal structures exhibit enhanced catalytic activity for HER due to their unique atomic arrangement and surface properties. This enables efficient electron transfer and promotes the adsorption and activation of hydrogen molecules, enhancing the HER activity.72 (II) POM-based single-crystal structures enable a detailed understanding of the potential reaction mechanisms in HER. By utilizing advanced characterization techniques to explore active sites and reaction intermediates, complex electrocatalytic processes can be elucidated.101 It provides valuable insights for the design and development of more efficient HER electrocatalysts.Dawson-type POMs can be combined with transition metal complexes to enhance their HER performance under alkaline conditions. For example, Tian et al. successfully synthesized a POM-based hybrid derivative, denoted as (Hbipy)2[Mn(bipy)3]2[As2W18O62], through a one-step hydrothermal synthesis method.72 This approach involved the integration of transition metal complexes into the nanostructure of arsenic tungstate. The resulting compound exhibited a well-defined 3D topological network with organized 1D channels, which contributed to its exceptional performance in HER. In an alkaline solution of 1 M KOH, the compound demonstrated an overpotential of 105 mV at j = 10 mA cm−2. Furthermore, its Tafel slope was measured to be 98.9 mV dec−1, indicating its favorable catalytic kinetics.
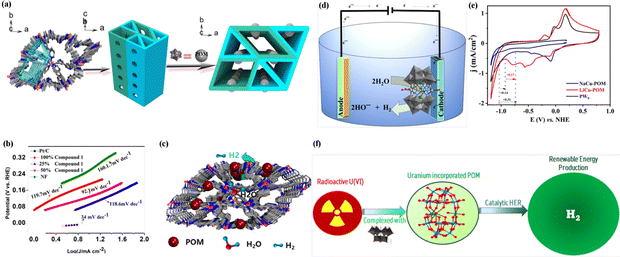 | ||
| Fig. 4 (a) 3D POM-encapsulated MOF architecture. (b) Tafel plots of compound-doped composites with different contents. (c) Electrocatalytic schematic mechanism of compound for HER. Reproduced from ref. 102. Copyright 2022, the American Chemical Society. (d) Schematic diagram of water reduction involving the utilization of sandwich-type POM synthesized. (e) Cyclic voltammogram profiles for NaCu-POM, LiCu-POM and PW9 were collected in 0.1 M potassium phosphate buffer used as an electrolyte. Reproduced from ref. 104. Copyright 2022, the American Chemical Society. (f) Schematic diagram of HER process involving the utilization of Finke-type POM synthesized with radioactive U(VI). Reproduced from ref. 106. Copyright 2023, the American Chemical Society. | ||
| Type of POM | Electrocatalyst | Electrolyte | Overpotential (mV)@10 mA cm−2 | Tafel slope (mV dec−1) | Durability | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| POM-based single-crystal structures | ||||||||
| Keggin | [Co-(phen)3]2[AsW12O40]·2H2O | 1 M KOH | 121 | 134.1 | 74 | |||
| {[(Ag2(H2biim)(H2biimCOO))2H2(SiW12O40)]·4H2O}n | 0.5 M H2SO4 | 112 | 77 | 1000 cycles, 10 h | 101 | |||
| [Na(H24C12O6)(CH3CN)(H2O)]2[Na(H24C12O6)(CH3CN)2][PW12O40] | 0.5 M H2SO4 | 114 | 229 | 10 h | 103 | |||
| Co2(3,3′-bpy)(3,5′-bpp)(4,3′-bpy)3[SiW12O40] | 1 M KOH | 92 | 92.1 | 48 h | 102 | |||
| (H2bib)5[Co4(H2O)2(AsW9O34)2]·2H2O | 1 M KOH | 78 | 78.9 | 1000 cycles, 24 h | 74 | |||
| Na6[{CoII(H2O)3}2{WVI(OH)2}2{BiIIIWVI9O33}2]·8H2O | 0.1 M K3PO4 | −726.04 | 173.8 | 1000 cycles, 10 h | 105 | |||
| Na4(Himi)2[{MnII(H2O)3}2{WVI(OH)2}2{BiIIIWVI9O33}2]·28H2O | 0.1 M K3PO4 | −643.52 | 152.1 | 1000 cycles, 10 h | 105 | |||
| Dawson | (Hbipy)2[Mn(bipy)3]2[As2W18O62] | 1 M KOH | 105 | 98.9 | 74 | |||
| {K2[(Ag2(H2biim)2)2(P2W18O62)]·2H2O}n | 0.5 M H2SO4 | 91 | 65 | 1000 cycles, 10 h | 101 | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| POM-based nanostructures | ||||||||
| Anderson | Cu/Mo2C (CuMo6) | 1 M KOH | 24 | 51.3 | 150 h | 123 | ||
| Ni-MoB2 (NiMo6) | 1 M KOH | 65.4 | 58.3 | 200 h | 124 | |||
| Co-MoB2 (CoMo6) | 1 M KOH | 91.3 | 76 | 124 | ||||
| Fe-MoB2 (FeMo6) | 1 M KOH | 131 | 82.7 | 124 | ||||
| Evans–Showell | 1T-Co-MoS2@HMCS (Co2MO10) | 1 M KOH | 74 | 81 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, 40 h 000 cycles, 40 h |
134 | ||
| 0.5 M H2SO4 | 132 | 78 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, 40 h 000 cycles, 40 h |
134 | ||||
| CoS2–MoS2 (Co2MO10) | 1 M KOH | 120 | 64 | 3000 cycles, 24 h | 135 | |||
| 0.5 M H2SO4 | 153 | 71 | 3000 cycles, 24 h | 135 | ||||
| Keggin | CoS2@MoS2@CC-30 h (GeMo12) | 1 M KOH | 87 | 87 | 1000 cycles, 20 h | 125 | ||
| 0.5 M H2SO4 | 65 | 122 | 1000 cycles, 20 h | 125 | ||||
| MoS2–Cu2S-CC-24 h (PMo12) | 1 M KOH | 159 | 109 | 1000 cycles, 24 h | 127 | |||
| Cu2S-MoS2@CC-1 (PMo12) | 0.5 M H2SO4 | 150 | 61 | 1000 cycles, 94 h | 128 | |||
| RuW/g-C3N4 (PW11Ru) | 0.5 M H2SO4 | 266 | 63 | 500 cycles | 130 | |||
| Isopolyoxomolybdates | Mo8O26-NbNxOy/NG (Mo8) | 1 M KOH | 32 | 47 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
131 | ||
| 0.5 M H2SO4 | 27 | 33 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
131 | ||||
| [(TBA)Mo12(AleThio)4] (Mo12) | 0.5 M H2SO4 | −77 | 80 | 2000 cycles | 133 | |||
3.2 Oxygen evolution reaction
The design and synthesis of efficient OER catalysts are crucial for improving the energy efficiency of water splitting.107 POM-based compounds have been explored as effective homogeneous/heterogeneous electrocatalysts for OER due to their rich redox chemistry. In particular, the combination of different transition metals in POM-based single-crystal structures can enhance the catalytic activity of POMs. This is because the different oxidation states and coordination geometries of transition metals influence the OER mechanism and their different degrees of activation in the electrolyte.110The assembly of large-ring POMs with [Pt(H2O)2(OH)4] moieties has been a topic of great interest in promoting electrocatalytic capabilities. Kuznetsova et al. successfully synthesized K22(NH4)9H3[{Pt(OH)3(H2O)}6P8W48O184]·79H2O by combining the large-ring inorganic Dawson-type POM cavity Li17(NH4)21H2[P8W48O184] with [Pt(H2O)2(OH)4].108 Remarkably, this compound possessed an enormous surface area with up to six {Pt(H2O)x(OH)4−x} building blocks coordinated within the Dawson-type POM, making it the largest Pt-enriched POM structure reported to date. Extensive electrochemical studies demonstrated its electrocatalytic activity for water oxidation, both in solution and as an electrode. Furthermore, comparing the cyclic voltammetry (CV) of the system with and without POM, it was concluded that the successful introduction of POM promoted the reduction of Pt(IV) to Pt(II) and its subsequent re-oxidation to Pt(IV). This finding opens up new avenues for exploring the electrocatalytic effects of introducing Pt-based moieties into large-ring POMs in aqueous solution.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and (ii) a chemically limiting step corresponding to O–O bond formation during a water nucleophilic attack (WNA), with an associated activation barrier of 0.86 eV (Fig. 5b). The latter mechanism is similar to other proposed mechanisms for water oxidation, where typically two protons and two electrons are released before WNA. The difference lies in the fact that the S0 (FeII–OH2) species undergoes natural PCET events in solution under aerobic conditions, while S1 (FeIII–OH) species are produced in crystalline solids. Notably, hydrogen bonding also played a role in the reaction, contributing to a reduction in the activation barrier for O–O bond formation. The lower Tafel slope observed for Co4-WS indicated faster kinetics compared to Fe4-WS, which is consistent with the lower activation barrier observed for Co-POM. Interestingly, despite the significant differences in the electronic structures of FeIII-POM and CoII-POM, a similar overpotential was observed. FeIII-POM proved to be stable under heterogeneous electrocatalytic conditions and exhibited activity as an OER catalyst. Consequently, several conclusions can be inferred: the activity of POM is influenced by its nuclearity, with the Finke-type POM displaying slightly superior electrocatalytic activity compared to the Keggin-type POM. Additionally, the significant role of hydrogen bonding should not be overlooked. Finke-type POMs show stronger hydrogen bonding ability compared with Keggin-type POMs, which can greatly reduce the activation energy of the reaction.
O) and (ii) a chemically limiting step corresponding to O–O bond formation during a water nucleophilic attack (WNA), with an associated activation barrier of 0.86 eV (Fig. 5b). The latter mechanism is similar to other proposed mechanisms for water oxidation, where typically two protons and two electrons are released before WNA. The difference lies in the fact that the S0 (FeII–OH2) species undergoes natural PCET events in solution under aerobic conditions, while S1 (FeIII–OH) species are produced in crystalline solids. Notably, hydrogen bonding also played a role in the reaction, contributing to a reduction in the activation barrier for O–O bond formation. The lower Tafel slope observed for Co4-WS indicated faster kinetics compared to Fe4-WS, which is consistent with the lower activation barrier observed for Co-POM. Interestingly, despite the significant differences in the electronic structures of FeIII-POM and CoII-POM, a similar overpotential was observed. FeIII-POM proved to be stable under heterogeneous electrocatalytic conditions and exhibited activity as an OER catalyst. Consequently, several conclusions can be inferred: the activity of POM is influenced by its nuclearity, with the Finke-type POM displaying slightly superior electrocatalytic activity compared to the Keggin-type POM. Additionally, the significant role of hydrogen bonding should not be overlooked. Finke-type POMs show stronger hydrogen bonding ability compared with Keggin-type POMs, which can greatly reduce the activation energy of the reaction.
 | ||
| Fig. 5 (a) Polyhedral representation of Co4-WS and Fe4-WS. (b) Water oxidation mechanism using Fe-POMs considered as single-site catalysts. Potentials and energies correspond to the catalytic cycle computed for the Fe4-WS electrocatalyst. The dark green square indicates the active species. Reproduced from ref. 110. Copyright 2022, The Royal Chemistry Society. (c) Scheme showing the assembly process of POV-based hybrid compounds 1–3. (d) Scheme showing the structural conversion of compound 1 during OER. Reproduced from ref. 111. Copyright 2020, Elsevier B.V. All rights reserved. | ||
Isopolyoxovanadates. Hu et al. synthesized three inorganic–organic compounds based on isopolyoxovanadates, [Ni2(BBTZ)(H2O)4]V4O12·2H2O (1), [Co2(BBTZ)(H2O)4]V4O12·2H2O (2) and [Ni(BBTZ)2]V2O6·2H2O (3) (BBTZ = 1,4-bis-(1,2,4-triazole-1-ylmethylene)benzene), which were investigated for their OER performance in 1 M KOH electrolyte (Fig. 5c).111 Compounds 1 and 2 possessed a 2D layered structure, while compound 3 featured a 3D framework with a five-fold interpenetrating metal–organic structure. During the OER process, structural transformations were observed in compounds 1–3. The organic BBTZ ligand and most of the isopolyoxovanadates species on the surface of compounds 1–3 were electrochemically separated at the anodic potential, dispersing them in the electrolyte and leaving V-doped transition metal hydroxide as the residual material. Importantly, the intermediate transition metal hydroxides formed during the OER process serve as the active centers for the electrocatalytic reaction, indicating that isopolyoxovanadate salts are not the active sites. Under alkaline electrocatalytic conditions for OER, this type of isopolyoxovanadate-based crystalline material undergoes a structural transformation process (Fig. 5d). Dissociation of the organic BBTZ ligand from the crystal lattice occurs, followed by the conversion of the original coordination network structure into a mixed intermediate comprised of NiOOH, NiV2O6, and V2O5. Kar et al. synthesized three compounds based on the coordination of the decavanadate anion ([V10O28]6−) with transition metal ions, with the general formula {[(H2O)2K-μ-(H2O)3M(H2O)3]2n[V10O28]n} (M = Mn2+, Co2+ and Ni2+).112 All these compounds, denoted as 1–3, were evaluated for their OER performance under alkaline conditions (0.1 M KOH). Similar to the previously mentioned compounds, these compounds exhibited structural instability during the OER process. Furthermore, it was observed that they produced V-doped metal hydroxides as the true active sites in alkaline media.
Isopolyoxomolybdates. The investigation of reaction mechanisms in giant POM clusters is often challenging due to their complex structures and oxidation states. However, in the groundbreaking study by Li et al. in 2023, they utilized an operando Raman system with flowing water to visualize the reaction process of isopolyoxomolybdates during the OER process.113 This innovative approach enabled the determination of the crystal structures of the clusters during the OER process, providing valuable insights into the intermediate active sites in cluster electrocatalysis. Starting with Na2MoO4 as the precursor and using H2SO4 as the electrolyte, a sequential acidification process was employed to create Mo7O246− ({Mo7}) and high-nuclearity Mo36O1128− ({Mo36}) clusters. The final polymerization product was identified as [H6K2Mo3O12(SO4)]n ({Mo3(SO4)}n). To precisely control the electrochemical synthesis of Mo-based compounds and generate molybdenum clusters with mixed oxidation states, a water-based flow-electrochemical synthesis cell was developed. The experimental evidence revealed that only {Mo36} and {Mo3(SO4)}n clusters were capable of transforming into the larger clusters {Mo154} and {Mo102} during the OER process. Furthermore, this study highlighted the crucial role of H+/e− under acidic conditions in the reduction of intermediate clusters. Acidic decomposition of {Mo36} and {Mo5(SO4)} clusters resulted in the formation of {Mo5} and {Mo3(SO4)}n monomers, which further self-assembled into the larger clusters {Mo154} and {Mo102} under the electrocatalytic conditions.
In parallel research, Zeng et al. compared the OER capabilities of conventional POMs with transition metal-incorporated derivatives.114 Three functional framework compounds, {CoO6Mo8O28}n (1), {γ-Mo8O26}n (2) and {α-Mo8O26} (3), were synthesized using the conventional solution method. Compound 1 possessed a pure 2D inorganic {CoO6Mo8O28}n framework, compound 2 had a 1D structure, and compound 3 exhibited a 0D structure. Compared to the traditional {Mo8O26} clusters, the 2D porous {CoO6Mo8O28}n catalyst exhibited a lower overpotential (293 mV at j = 10 mA cm−2) and smaller Tafel slope (53 mV dec−1) for water oxidation. The high-resolution XPS analysis indicated a synergistic effect between Co2p and Mo3d during the water oxidation process, highlighting the enhanced OER performance achieved through the synergistic doping of transition metal elements in conventional POMs.
| Type of POM | Electrocatalyst | Electrolyte | Overpotential (mV)@10 mA cm−2 | Tafel slope (mV dec−1) | Durability | Ref. |
|---|---|---|---|---|---|---|
| POM-based single-crystal structures | ||||||
| Keggin isopolyoxovanadates | (C5H7N2)6[NiW12O44] | 1 M KOH | 347 | 130 | 96 h | 109 |
| [Ni2(BBTZ)(H2O)4]V4O12·2H2O | 1 M KOH | 353 | 77.8 | 12 h | 111 | |
| [Co2(BBTZ)(H2O)4]V4O12·2H2O | 1 M KOH | 395 | 88.9 | 12 h | 111 | |
| [Ni(BBTZ)2]V2O6·2H2O | 1 M KOH | 433 | 81.8 | 12 h | 111 | |
| {[(H2O)2K-μ-(H2O)3Mn(H2O)3]2n[V10O28]n} | 0.1 M KOH | 290 | 122 | 112 | ||
| {[(H2O)2K-μ-(H2O)3Co(H2O)3]2n[V10O28]n} | 0.1 M KOH | 300 | 119 | 112 | ||
| {[(H2O)2K-μ-(H2O)3Ni(H2O)3]2n[V10O28]n} | 0.1 M KOH | 270 | 89 | 8 h | 112 | |
| Isopolyoxomolybdates | {CoO6Mo8O28}n | 1 M KOH | 293 | 53 | 24 h | 114 |
| {γ-Mo8O26}n | 1 M KOH | 303 | 59 | 114 | ||
| {α-Mo8O26} | 1 M KOH | 316 | 64 | 114 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| POM-based nanostructures | ||||||
| Anderson | AB&PS-13 (AlMo6) | 1 M KOH | 330 | 90 | 40 h | 136 |
| Fe-S-NiMoO4/MoO3@NF (FeMo6) | 0.1 M KOH | 490 | 60 | 10 h | 149 | |
| Finke | Co4(PW9)2@Co@C | 0.1 M KOH | 430 | 120 | 150 | |
| Co4(PW9)2@Co/Ni@C | 0.1 M KOH | 400 | 67 | 12 h | 150 | |
| Co4(PW9)2@N, S-Co@C | 0.1 M KOH | 410 | 62 | 12 h | 150 | |
| Co4(PW9)2@N,S-Co/Ni@C | 0.1 M KOH | 460 | 88 | 150 | ||
| Keggin | NiFe-LDH-PW12-X-12 h | 1 M KOH | 291 | 78.3 | 40 h | 138 |
| PW12/Ag/graphene-a | 0.1 M PBS | 540 | 190 | 139 | ||
| PW12/Ag/graphene-b | 0.1 M PBS | 610 | 192 | 139 | ||
| {Cu2SiW12O40}@HKUST-1 | 1 M KOH | 340 | 73 | 25 h | 140 | |
| PW12@amZIFs | 1 M KOH | 306 | 54 | 14 h | 141 | |
| SiW9Co3@ZIF-8 | 0.1 M KOH | 2730 | 69.4 | 143 | ||
| SiW9Co3@ZIF-67 | 0.1 M KOH | 470 | 113.6 | 12 h | 144 | |
| SiW9Co3[h]@ZIF-67 | 0.1 M KOH | 420 | 93.9 | 12 h | 144 | |
| PW6Mo6/ZIF-67@NF | 1 M KOH | 201 | 55 | 24 h | 146 | |
| GeW9@NiF | 1 M KOH | 370 | 84 | 8 h | 147 | |
| PBS (pH = 14) | 530 | 153 | 8 h | 147 | ||
3.3 CO2 reduction
The target of CO2RR is to obtain products through proton-coupled multi-electron transfer mechanisms, typically categorized as C1 and Cn ≥ 2 reduction products.115 However, the competitive HER often occurs during the electrocatalytic CO2 reduction process, greatly reducing the conversion efficiency of CO2. Therefore, for the development of CO2RR, it is very important to find efficient electrocatalysts that can inhibit HER. In this case, POMs can promote the formation of hydrogen bond networks near the CO2 coordination center, facilitating proton-coupled electron transfer when the proton source approaches the catalytic active center.116 Specifically, transition metal-substituted POMs may form reversible complexes with CO2 fixation or reaction, which appears favorable for electrocatalytic CO2RR, and can serve as attractive homogeneous or heterogeneous electrocatalysts.63 | ||
| Fig. 6 (a) Crystal structures of M-POMOFs. (b) Proposed reaction mechanism in PCR and ECR (photo- and electrocatalytic CO2 reduction) reactions for Fe-POMOF, respectively. Reproduced from ref. 115. Copyright 2022, The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. | ||
4. POM-based nanostructures for electrocatalysis
POM-based nanostructures demonstrate tremendous potential as highly efficient catalysts for a wide range of electrocatalytic reactions. By reducing the size of POM-based materials from bulk solids to the nanoscale, these nanostructures exhibit a significantly larger surface area and enhanced reaction activity.117 As a result, POM-based nanostructures offer improved efficiency and expanded application possibilities in electrocatalysis and energy conversion. Additionally, the combination of nanostructures and POM leads to a synergistic effect, in which the assembly structure facilitates shorter diffusion paths and provides more accessible active sites.118 The synthesis and modification of POM-based nanostructures can be precisely tailored to achieve the desired performance and enhanced catalytic activity.119 Strategies such as doping, surface functionalization and hybridization with other materials further enhance their electrocatalytic efficiency and stability. Overall, these exceptional characteristics position POM-based nanostructures as highly appealing candidates for electrocatalytic applications.4.1 Hydrogen evolution reaction
Functionalizing POM-based electrocatalysts on different types of conductive materials through covalent or non-covalent interactions has become an important direction for achieving a superior HER performance.120,121 With the synergistic effect of the combination of POMs with high redox ability and highly conductive materials, many advantages of POM-based nanostructures are revealed.122 (I) Their unique nanoscale morphology provides a large surface area-to-volume ratio, increasing the number of exposed active sites for electrocatalytic reactions. This increased surface area facilitates a higher density of catalytic active sites, thereby improving the HER performance. (II) POM-based nanostructures typically exhibit excellent electron conductivity. This characteristic allows for efficient charge transfer during the HER process, reducing the energy losses and improving the overall catalytic efficiency. (III) POM-based nanostructures can be easily functionalized or modified to adjust their catalytic properties. By introducing specific functional groups or different metal ions, the catalytic activity and selectivity of POM-based nanomaterials can be finely tuned. | ||
| Fig. 7 (a) Schematic illustration of the preparation of Cu/Mo2C. (b) SEM images of the as-synthesized Cu/Mo2C. Reproduced from ref. 123. Copyright 2023, Tsinghua University Press. (c) Schematic illustration of the guided synthesis of TM single atoms supported on MoB2 (TM = Ni, Co, Fe) applying Anderson-type POMs as molecular precursor. (d) Left: crystal structure of Ni-MoB2 with one Mo substituted by one Ni in a 2 × 2 × 2 supercell. Right: top view of the (100) facet cleaved from the crystal and the labeling of the possible reactive sites for intermediate adsorption. Reproduced from ref. 124. Copyright 2023, the American Chemical Society. (e) Strategy for the formation of CoS2@MoS2@CC. (f) Overpotentials of CoS2@MoS2@CC-30 h and the reported MoS2-based electrocatalysts for comparison at 10 mA cm−2 in 0.5 M H2SO4 and 1.0 M KOH electrolyte. Reproduced from ref. 125. Copyright 2020, Wiley-VCH GmbH. (g) Schematic illustration of Mo8O26-NbNxOy heterostructure. (h) Charge density difference diagram of Mo8O26–Nb4N5. (i) Side view of schematic models for H2O dissociation on Nb4N5 and Mo8O26–Nb4N5 surfaces. Reproduced from ref. 131. Copyright 2023, Wiley-VCH GmbH. | ||
The development of single-atom catalysts (SACs) and the exploration of the interactions between individual atoms and supports are crucial for identifying the active sites and elucidating catalytic mechanisms. Yang et al. utilized Anderson-type POMs (NiMo6, CoMo6 and FeMo6) as precursors and loaded a series of transition metals (TM = Ni, Co, Fe) onto MoB2 (TM-MoB2), achieving a remarkably high loading of TM (6.72–7.03 wt%) (Fig. 7c).124 The TM modification significantly enhanced the HER activity of MoB2, with Ni–MoB2 demonstrating the optimal performance in both alkaline and acidic solutions. DFT calculations demonstrated the significance of different surfaces, namely the NiMo-terminated and B-terminated (100) facets, when splitting the bulk Ni–MoB2 structure along the (100) plane (Fig. 7d). The (100) surface terminated with NiMo atoms is much more stable in terms of energy (6.29 eV) compared to the B-terminated (100) surface. It was revealed that the true active site is a MoB2-based SAC. The introduction of transition metals reduced the hydrolysis barrier of Mo in the system and enhanced its H adsorption capability, confirming that the Mo provided by the POM serves as the genuine chemical adsorption site. Interestingly, the introduction of TMs can enhance the HER activity, which can be attributed to the modulation of the different electronic states between the positively charged Ni and negatively charged material surface, thereby facilitating the adsorption of H by the electrocatalyst.
The incorporation of highly conductive compounds on the surface of nanomaterials has shown great potential in enhancing their overall electrocatalytic activity and durability. Transition metal phosphides (TMP) and graphitic carbon nitride (g-C3N4) have emerged as promising candidates in this research area. In the study by Shi et al., polypyrrole, PMo12, and Ni2+ were utilized as supramolecular precursors. Through electrostatic attraction and high-temperature calcination, carbon-coated porous nanospheres were successfully prepared as electrocatalysts (MoP/MoNiP@C).129 The introduction of conductive materials during the calcination process significantly improved the conductivity of the transition metals, thereby further enhancing the performance of the catalysts in HER. The use of polypyrrole and Keggin-type POM not only served as a source of phosphorus and molybdenum, respectively, but also facilitated charge transfer, leading to increased efficiency. Another study conducted by Pathan et al. involved the synthesis of highly dispersed RuW/g-C3N4 composite materials.130 This was achieved by complexing a ruthenium-substituted POM (PW11Ru) with melamine, followed by calcination under an inert atmosphere. The RuW/g-C3N4 composite displayed improved electrochemical activity for HER, with an overpotential of approximately 266 mV at j = 10 mA cm−2 and a Tafel slope of 63 mV dec−1.
To enhance electrocatalytic activity, Sakthinathan et al. employed a layer-by-layer (LbL) technique to attach nano-sized POM to a glassy carbon electrode (GCE), Na0.3[N(C4H9)4]7.7[(Mo3O8)4(O3PC(O)(C3H6NH2CH2C4H3S)PO3)4] [(TBA)Mo12(Alethio)4].133 The introduction of polyethyleneimine (PEI) not only improved the conductivity and electrocatalytic activity of [(TBA)Mo12(Alethio)4], but also served as a supporting material for the formation of closely interconnected nanolayers. The nanolayer electrode exhibited a significant synergistic effect between the POM and positively charged PEI, leading to high electrocatalytic activity in 0.5 M H2SO4, with a small initial potential of approximately −0.077 V and a low Tafel slope of 80 mV dec−1 at j = 10 mA cm−2.
4.2 Oxygen evolution reaction
Water oxidation is a crucial process in the fields of renewable energy and sustainable fuel production.136 It involves the conversion of water molecules into oxygen, protons, and electrons. Due to the sluggish kinetics of the electrochemical water splitting reaction, there has been a greater focus in the past few decades on developing efficient OER electrocatalysts to improve the electrode kinetics and stability under different electrolyte conditions.137 In recent years, significant efforts have been devoted to developing highly active and stable water oxidation catalysts, including incorporating POMs in surface-modified nanostructures. The high surface area and well-defined active sites of POMs contribute to improved catalytic performances. This high activity allows water molecules to efficiently release oxygen, making them promising catalysts for the water oxidation reaction.138 Meanwhile, their robust structures and strong metal–oxygen bonds ensure that the catalyst remains intact and active over long catalytic cycles. This stability is crucial for long-term catalytic performance and durability. More importantly, the characteristics of POM-based nanostructures provide high surface areas, facilitating the effective mass transfer of reactants and products.139 This promotes the accessibility of reactants to the active sites of the catalyst and enhances its overall catalytic efficiency.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, resulting in an approximately 10% improvement in OER performance upon integration with AB. The AB&PS-13 composite material exhibited a significant catalytic OER performance in 1 M KOH, with the AB&PS-13 composite demonstrating a stable overpotential of 330 mV at j = 10 mA cm−2 and Tafel slope of 90 mV dec−1 over a 40 h period. In another study by Zhang et al., an Anderson-type POM, (NH4)3[FeMo6O24H6]·7H2O, was used as a precursor to fabricate binder-free Fe-S-NiMoO4/MoO3 electrodes via a solvothermal method (Fig. 8a).137 These electrodes exhibited uniform growth on a nickel foam (NiF) in the form of a nanorod array (Fig. 8b). The DFT results demonstrated effective control of the OER process at each step through the presence of Fe heteroatoms (Fig. 8c). The ΔG1 value in Fe@MoO3(100) increases to 1.23 eV, indicating the weakened adsorption of *OH. Consequently, the potential-determining step on the surface of Fe@MoO3(100) shifts to step 2, which involves the conversion of *OH to *O. Importantly, after Fe doping, the overpotential for this step is reduced to 0.52 V, indicating a significant enhancement in OER activity. According to the above-mentioned conclusions, the introduction of Fe doping facilitated the adjustment of the MoO3 crystal facets, modulation of the electronic structure and enhancement of conductivity, thereby accelerating the reaction kinetics of OER. The Fe-S-NiMoO4/MoO3@NF electrode achieved an overpotential of 271 mV at j = 500 mA cm−2. Overall, these studies highlight the promising potential of Anderson-type POMs and their derivatives in advancing the field of electrocatalysis, particularly in the context of the oxygen evolution reaction.
2, resulting in an approximately 10% improvement in OER performance upon integration with AB. The AB&PS-13 composite material exhibited a significant catalytic OER performance in 1 M KOH, with the AB&PS-13 composite demonstrating a stable overpotential of 330 mV at j = 10 mA cm−2 and Tafel slope of 90 mV dec−1 over a 40 h period. In another study by Zhang et al., an Anderson-type POM, (NH4)3[FeMo6O24H6]·7H2O, was used as a precursor to fabricate binder-free Fe-S-NiMoO4/MoO3 electrodes via a solvothermal method (Fig. 8a).137 These electrodes exhibited uniform growth on a nickel foam (NiF) in the form of a nanorod array (Fig. 8b). The DFT results demonstrated effective control of the OER process at each step through the presence of Fe heteroatoms (Fig. 8c). The ΔG1 value in Fe@MoO3(100) increases to 1.23 eV, indicating the weakened adsorption of *OH. Consequently, the potential-determining step on the surface of Fe@MoO3(100) shifts to step 2, which involves the conversion of *OH to *O. Importantly, after Fe doping, the overpotential for this step is reduced to 0.52 V, indicating a significant enhancement in OER activity. According to the above-mentioned conclusions, the introduction of Fe doping facilitated the adjustment of the MoO3 crystal facets, modulation of the electronic structure and enhancement of conductivity, thereby accelerating the reaction kinetics of OER. The Fe-S-NiMoO4/MoO3@NF electrode achieved an overpotential of 271 mV at j = 500 mA cm−2. Overall, these studies highlight the promising potential of Anderson-type POMs and their derivatives in advancing the field of electrocatalysis, particularly in the context of the oxygen evolution reaction.
 | ||
| Fig. 8 (a) Schematic map of the synthesis of S-NiMoO4/MoO3@NF and Fe-S-NiMoO4/MoO3@NF electrodes. (b) HAADF-STEM of Fe-S-NiMoO4/MoO3@NF. (c) Free energy diagrams of Fe@MoO3(100) for the OER process. Reproduced from ref. 137. Copyright 2021, Elsevier B.V. All rights reserved. (d) Schematic illustration of synthesis of NiFe-LDH-PW12-12 h. (e) Mechanism of NiFe-LDH-PW12-12 h for OER in alkaline electrolyte. Reproduced from ref. 138. Copyright 2023, Elsevier Inc. All rights reserved. (f) Process for the synthesis of PW6Mo6/ZIF-67@NF. (g) Comparison of overpotential of PW6Mo6/ZIF-67@NF, PW12/ZIF-67@NF and PMo12/ZIF-67@NF. Reproduced from ref. 146. Copyright 2023, Hydrogen Energy Publications LLC. Published by Elsevier Ltd. All rights reserved. | ||
The utilization of MOF encapsulation to facilitate the interaction between POMs and MOFs is also a common strategy for the preparation of electrocatalysts. Liu et al. synthesized {Cu2SiW12O40}@HKUST-1 using a conventional solution method.140 The synergistic interaction between {Cu2SiW12O40} and HKUST-1 promoted the charge transfer and adsorption/desorption of the intermediates on {Cu2SiW12O40}@HKUST-1. As a result, this composite material exhibited excellent OER activity with a low overpotential (340 mV) and good cycling stability. Notably, {Cu2SiW12O40}@HKUST-1 demonstrated a superior performance compared to commercial RuO2. Zhao et al. synthesized carbon-supported metal oxides doped with PW12, namely PW12@amZIFs, via a series of chemical etching, cation exchange and thermal annealing steps using amorphous ZIF-67 as the precursor.141 The synergistic effect of POMs and ZIF-67 resulted in an enhanced electrocatalytic OER performance. The resulting composite material exhibited a superior structure and composition, leading to a low overpotential (306 mV, j = 10 mA cm−2) under OER conditions in 1.0 M KOH aqueous solution. Mukhopadhyay et al. were the first to report the used of an Fe-salen system for electrocatalytic OER. They prepared an efficient heterogeneous electrocatalyst, FSWZ-8 ((Fe-(salen)(OH) + H4[SiW12O40]·HCl)@ZIF-8), by co-encapsulating Fe-salen (Fe(salen)Cl) and SiW12 in the cavities of ZIF-8.142 This catalyst achieved OER under neutral conditions. POMs in the system played a role in reducing the charge transfer resistance and lowering the overpotential, facilitating a higher loading of Fe-salen and making the synthesis of nanomaterials easier. This study revealed the significant potential of POMs in material synthesis.
The introduction of transition metals in the Keggin-type POM framework enables the formation of hybrid systems with unique properties and enhanced catalytic performances. Transition metals possess a range of desirable characteristics, such as variable oxidation states and high electron mobility, which can significantly influence the electrocatalytic processes. In 2020, Abdelkader-Fernández et al. reported the synergistic effect of incorporating cobalt-based POMs in Co-containing ZIF-67, which enhanced the OER process.143 They synthesized two types of POM@ZIF nanocomposites, SiW9Co3@ZIF-8 and SiW9Co3@ZIF-67, using an in situ synthesis method at room temperature. These nanocomposites consisted of [SiW9Co3(H2O)3O37]10− (SiW9Co3) encapsulated within the ZIF-8 and ZIF-67 frameworks, respectively. Although these nanocomposites had similar structures, they exhibited different OER performances. The ZIF-67 framework demonstrated a synergistic effect, whereas no significant effect was observed in ZIF-8. SiW9Co3@ZIF-67 showed a superior OER performance, which could be attributed to the electron transfer from ZIF-67 to POM and the complementation of Co2+ nodes on the POM framework. In the same year, Abdelkader-Fernández et al. synthesized SiW9Co3@ZIF-67 and SiW9Co3[h]@ZIF-67 nanocomposites by encapsulating SiW11Co within the cavities of ZIF-67.144 Compared to the previous study, the POM composition changed with a lower Co2+ doping content. Despite their complex structure, according to the complete characterization of these high-POM-loaded materials, both nanocomposites exhibited enhanced OER activity compared to the original ZIF-67 framework, confirming previous findings. The improved OER performance of the nanocomposites with reduced Co2+ doping can be attributed to the presence of a higher quantity of POM clusters in the electrocatalyst and the significant structural modifications of the ZIF framework, which exposed more active sites.
Other transition metal-substituted POMs have also been studied. For example, Mulkapuri et al. synthesized a {Bi(OH2)2}3+-functionalized Keggin-type POM compound, K5[Bi(H2O)2SiW11O39]·13H2O (K51·13H2O), as a precursor and encapsulated it within the cavities of zeolitic imidazolate framework ZIF-8.145 The resulting host–guest-type composite, H5[Bi(H2O)2SiW11O39]@ZIF8 (H51@ZIF8), exhibited efficient electrocatalytic activity for water oxidation over a wide pH range (pH = 4.0–13.0). The {Bi(OH2)2}3+ moiety on the surface of the POM acted as the active site for electrocatalytic water oxidation. The experimental results also indicated that the bismuth–water complexes served as the active sites for OER. Furthermore, the OER performance was influenced by modulating the electronegativity of the coordinated metal ions. Song et al. prepared PW6Mo6/ZIF-67@NF as an OER electrocatalyst using a simple co-precipitation method.146 The introduction of PW6Mo6 induced the exposure of the Co nodes in ZIF-67, which acted as catalytic active sites for OER (Fig. 8f). The incorporation of PW6Mo6 improved the conductivity and electrochemically active surface area of ZIF-67, resulting in an ultra-low overpotential of 201 mV at j = 10 mA cm−2 (Fig. 8g).
4.3 CO2 reduction
Carbon cycling is achieved by reducing carbon dioxide into valuable products, which also helps reduce the dependence on fossil fuels.151 Electrocatalytic CO2 reduction utilizes electrical energy as a power source and employs catalysts to facilitate the reduction of CO2 molecules. This method allows the conversion of CO2 under mild conditions and enables the selective synthesis of various organic compounds. However, electrocatalytic CO2 reduction faces several challenges. (I) CO2 is a highly stable molecule, and thus its catalytic reduction reaction needs to overcome high energy barriers.152 (II) CO2 reduction reactions often compete with oxidative side reactions, resulting in low product selectivity.153 (III) Effective catalyst design and understanding the catalytic mechanisms are crucial areas that need to be studied.154 Thus, to address these challenges, researchers have extensively explored various catalyst systems, including noble metals, non-noble metals and organic catalysts. Noble metal catalysts such as copper, silver and gold exhibit good electrocatalytic activity for CO2 reduction, but their high cost and scarcity limit their application. Non-noble metal catalysts such as metal oxides, carbon-based materials, and polymers have broader resources and lower costs but require further improvement in terms of activity and selectivity. Organic catalysts, with their diverse structures and reaction properties, have emerged as promising catalyst systems, although their stability and efficiency still need improvement. POMs exhibit rich redox properties, tunable active sites, good selectivity and stability, making them attractive as both homogeneous and heterogeneous electrocatalysts for promoting the multi-electron reduction of CO2 into useful chemicals. Currently, nanostructured POM-based materials as CO2 reduction catalysts have received considerable attention. | ||
| Fig. 9 (a) Top: Schematic of the synthetic process and the detailed structure of POM@PCN-222(Co) composite. Bottom: Proposed electron-transfer scheme on the active single-metal site Co of H-POM@PCN-222(Co) for CO2RR. (b) Maximum FECO for six samples at −0.8 V (vs. RHE). (c) Mechanism of CO2RR in H-POM@PCN-222(Co). Reproduced from ref. 152. Copyright 2021, Wiley-VCH GmbH. (d) Crystal structures of PVMo11, PV2Mo10 and PV3Mo9. (e) Mechanism scheme of POM and indium synergistic catalysis of CO2RR. Reproduced from ref. 155. Copyright 2023, Elsevier Inc. All rights reserved. (f) Schematic diagram of electrochemical CO2 reduction reaction with Mo8@Cu/TNA. (g) Free energy diagrams and reaction pathway of CO2RR with Mo8@Cu/TNA under −0.58 V vs. RHE. Reproduced from ref. 157. Copyright 2020, Elsevier B.V. All rights reserved. | ||
Although indium (In) electrodes are widely used as electrochemical catalysts due to their excellent properties, the complex reaction pathways and high overpotentials associated with In electrocatalytic CO2RR pose challenges. In this case, due to the ability of POMs to transport and store electrons and protons, they show great potential for overcoming these limitations. The choice of coordinating metal atoms also influences the electrocatalytic processes, which is also an important factor worthy of attention. Sun et al. investigated the impact of V and Mo on CO2RR by combining In electrodes with different Keggin-type POMs (PVnMo(12−n)O40(n+3)−, n = 1, 2, 3) (Fig. 9d).155 The V atoms in the POM facilitated CO2 adsorption, while the Mo atoms played a role in electrode oxidation. However, the oxidation of Mo resulted in the loss of active sites on the In electrode, leading to the weak adsorption of *CO during electrolysis. In the PV3Mo9 system, the high proportion of active sites to electrode surface promoted C–C coupling and the production of the C2 product ethanol (Fig. 9e). Zha et al. demonstrated the crucial role of V centers in POM for the conversion of CO2 to C2 products.156 By combining Keggin-type POM [SiW9V3O40]7− (SiW9V3) with In, the efficient reduction of CO2 to acetic acid and formic acid was achieved with high FE (96.5%). These findings highlight the potential of POM electrolyte additives for regulating the microenvironment to enhance the CO2RR performance.
| Type of POM | Electrocatalyst | Electrolyte | Potential | Principal product | FE (%) | Ref. |
|---|---|---|---|---|---|---|
| Keggin | Fe-POMOFs (Zn-ε-Keggin) | 0.5 M KHCO3 | −0.71 V (vs. RHE) | CO | 92.1 | 115 |
| POM@PCN-222(Co) (CoW11Co) | 0.5 M KHCO3 | −0.8 V (vs. RHE) | CO | 96.2 | 152 | |
| Zn-CoTAPc/PMo12 | 0.5 M KHCO3 | −0.7 V (vs. RHE) | CO | 96.1 | 153 | |
| SiW12-MnL/KB | 0.5 M KHCO3 | −0.72 V (vs. RHE) | CO | 95 | 154 | |
| PW12-MnL/KB | 0.5 M KHCO3 | −0.72 V (vs. RHE) | CO | 80 | 154 | |
| PMo12-MnL/KB | 0.5 M KHCO3 | −0.52 V (vs. RHE) | CO | 65 | 154 | |
| PVMo11 + In | 0.1 M Na2SO4 | −0.5 V (vs. RHE) | C2H5OH | 53 | 155 | |
| PV2Mo10 + In | 0.1 M Na2SO4 | −0.3 V (vs. RHE) | C2H5OH | 80 | 155 | |
| PV3Mo9 + In | 0.1 M Na2SO4 | −0.3 V (vs. RHE) | C2H5OH | 93.4 | 155 | |
| PMo12 + In | 0.1 M Na2SO4 | −0.6 V (vs. RHE) | C2H5OH | 29.6 | 155 | |
| SiW9V3 + In | 0.1 M Na2SO4 | −0.71 V (vs. RHE) | CH3COOH | 96.5 | 156 | |
| Isopolyoxomolybdates | Mo8@Cu/TNA | The saturated NaHCO3 | −0.71 V (vs. RHE) | CH3COOH | 88.94 | 157 |
| The saturated NaHCO3 | −0.71 V (vs. RHE) | CH4 | 14.42 | 157 | ||
| The saturated NaHCO3 | −0.71 V (vs. RHE) | C2H4 | 28.34 | 157 |
5. Conclusions and outlooks
Herein, we presented a comprehensive review of the research advancements in the field of POMs and their derivatives as a class of electrocatalysts for HER, OER, and CO2RR, covering the period from 2020 to the present. This review was structured into two main sections, namely POM-based single-crystal structures and POM-based nanostructures. Within these sections, detailed discussions were presented on various structures, including giant clusters, assemblies, and organic–inorganic hybrid materials, with a specific focus on their correlation with electrocatalytic performance. Additionally, we extensively enumerated, summarized, and compared the effects and roles of different types of POMs in the field of electrocatalysis, with the ultimate aim of identifying the most suitable POM variants for electrocatalytic HER, OER, and CO2RR applications. However, despite the significant progress achieved in electrocatalysis for energy applications, there are still numerous problems that need to be addressed. In the subsequent sections, we provide a comprehensive discussion on the advantages, challenges, strategies, and outlooks in this domain (Fig. 10). | ||
| Fig. 10 Schematic illustration of the advantages, challenges, strategies and outlooks of POM-based electrocatalysts in practical applications. | ||
5.1 Advantages
POM-based electrocatalysts have tunable electronic structures and chemical compositions, facilitating electron transfer and promoting redox reactions to enhance the catalytic rates. Moreover, their multi-metallic nature allows for interconversion isomerism during catalytic processes, greatly facilitating the formation of the oxidation and reduction products required in most POM-based electrocatalysts for processes such as HER, OER, and CO2RR. Reductive POMs play a significant role in CO2RR, with the presence of vanadium enhancing their ability to reduce CO2 to C2 products. The desirable features of POMs, such as well-defined crystal structures, controllable morphologies, good solubility, and dispersibility, contribute to their popularity in the field of electrocatalysis. By summarizing the methods for the synthesis of the most efficient and stable POM-based electrocatalysts, we can roughly categorize them into the following five strategies:(I) Self-assembly of POM components into multinuclear clusters, leading to numerous redox reactions;
(II) Introduction of transition metal ions as heteroatoms in POM derivatives to achieve controlled electronic structures and enhanced electron transfer;
(III) Incorporation of varying numbers of transition metal active sites into vacancy-containing POMs to create single-cluster electrocatalysts with fixed electron transfer;
(IV) Doping POM into MOFs with porous materials to achieve heterogenization and increased active sites; and
(V) Loading POM onto different types of conductive substrates to improve the conductivity of composite materials and enhance the catalytic surface area.
5.2 Challenges
POM-based electrocatalysts have made significant progress in the fields of HER, OER, and CO2RR. However, there are still several challenges that need to be addressed and improved.(I) Although POM molecules generally have good solubility and adhesion in practical applications, their homogeneous development is limited due to the challenge of separating POMs from the bulk material. Therefore, it is necessary to explore better methods that can overcome this challenge and allow for the more effective utilization of the catalytic performance of POMs.
(II) POMs can be loaded onto various conductive substrates to achieve heterogenization, greatly improving the conductivity of electrocatalysts. However, the lack of precise structural information and the complexity of mechanisms due to structural diversity make it difficult to accurately identify the active sites and reaction intermediates in these composite materials. Consequently, further research is needed to establish a better understanding of the structure–performance relationship in POM composite materials.
(III) Both catalytic activity and stability are crucial factors for electrocatalysts. The large size of POMs poses a challenge in precisely controlling the size of POM molecules for better dispersion at interfaces or encapsulation within MOF frameworks, which can provide clear guidance for improving the stability.
(IV) POMs exhibit unique physicochemical properties, but current research on their CO2RR activity is relatively limited. Therefore, extensive studies are needed to explore the impact of multi-metal/heterometallic synergistic effects in POMs on CO2 reduction products.
(V) Many POM-based nanostructures and single-crystal structures have been reported as promising electrocatalysts for practical applications. However, the complexity of the methods for the synthesis POMs often limits their large-scale production in industrial settings. Therefore, the design of simpler synthetic routes plays a crucial role in achieving industrialization.
(VI) The attachment of POMs to a support matrix is crucial for maintaining their stability during the electrocatalytic process. However, achieving a strong and stable interface between the POMs and the support material can be challenging. Weak interactions or insufficient bonding can result in the detachment or leaching of POMs, leading to the loss of catalytic activity and reduced stability.
5.3 Strategies
To develop superior POM-based electrocatalysts, two key aspects that should be emphasized are improving their conductivity and stability. To achieve this, the following strategies can be employed:(I) Introducing hydrophobic or highly conductive functional organic ligands to modulate the physicochemical properties of POM-based electrocatalysts through coordination, thereby enhancing their catalytic capabilities.
(II) Enhancing the binding ability between the conductive substrate and POM to facilitate effective adhesion, charge transfer, and stability. Constructing a well-defined electron transfer pathway, such as Cu–O–Mo, can also enhance the synergistic interaction between the substrate and POM.
(III) Saturated POMs often exhibit weak catalytic activity due to their saturated coordination systems. To enhance both the conductivity and the number of active sites, transition metal ions can be introduced in POMs with vacant sites. Altering the coordinating metal ions can also modify the catalytic activity. For example, replacing PMo12 with PMo9V3 has been shown to enhance the reduction ability of CO2.
(IV) Investigating POM-based materials with smaller nano-sized dimensions is also important. The typical approach of grinding larger POMs into smaller sizes may damage their crystalline structure, making it challenging to accurately evaluate their catalytic activity. A more appropriate approach will be to incorporate effective modulators during the synthesis process to control their self-assembly. The common methods for the fabrication of POMs include hydrothermal synthesis and solvothermal methods. Additionally, reducing the heating time can decrease the degree of molecular aggregation and reduce the size of POM particles.
(V) To prevent the detachment or leaching of POMs during the electrocatalytic process and enhance the stability between POMs and the support materials, a protective coating can be formed between them to prevent the contact of POMs with the external environment. This can be achieved through methods such as chemical deposition, sol–gel method, and electrochemical deposition. The protective coating can provide an additional protective barrier, maintaining the stability and activity of POMs.
5.4 Outlooks
The tunable redox properties and rapid electron transfer of POM-based electrocatalysts are closely related to their structural changes. Therefore, designing more rational nanocrystal structures holds great application prospects for efficient POM-based electrocatalysts in HER, OER, and CO2RR, which is also a major development direction for future electrocatalysts.(I) In addition to the inherent properties of POMs, it is important to develop carriers with better conductivity or encapsulate POMs in MOFs with higher porosity to enhance their electrocatalytic performance by increasing their conductivity and specific surface area.
(II) More advanced strategies and fabrication processes should be developed to enhance the cohesion between POMs and organic ligands or materials, thereby improving the stability of nanostructures and single-crystal structures.
(III) POMs demonstrate adjustable redox capabilities, and it is well-established that reducible POMs can enhance the efficiency of CO2RR. More stable and accurate reactive sites should be constructed, which are conducive to the stable and efficient recovery of CO2 reduction into specific products.
(IV) The presence of POMs can lead to synergistic effects, enhancing the electrocatalytic capabilities, while making the reaction mechanism more complex. Therefore, developing in situ characterization devices to monitor the dynamic structural changes in POM-based electrocatalysts during electrocatalytic reactions and revealing the precise catalytic reaction principles based on identifying reactive intermediates are crucial.
(V) It is important to develop more novel POM-based electrocatalysts with inherent strong electrocatalytic activity, rather than relying on the addition of other transition metals during the synthesis process to enhance electrocatalytic activity.
(VI) In the evaluation of the HER and OER performance, it has been observed that isopolyoxovanadates and isopolyoxomolybdates demonstrate a superior performance. However, their application as POM-based electrocatalysts remains limited. Therefore, it is urgent to use polyoxometalates with Mo and V as coordination atoms to develop POM-based single-crystal structures and nanostructures. These research directions hold great potential for advancing POM-based electrocatalysts with enhanced catalytic activity and stability.
(VII) The utilization of protective coatings and encapsulation techniques can provide effective protection for POMs against the influence of harsh electrochemical environments. The research and development of new coating materials and deposition methods are indispensable for further improving the stability of POM-based electrocatalysts.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial supports of this research by Foundation for Innovative Research Groups of the National Natural Science Foundation of China (No. 21571023, 22271021, 21671025), Liaoning Revitalization Talents Program (XLYC1807049, XLYC1902011), General Program Fund for Education Department of Liaoning Province (LJKMZ20221479), Natural Science Foundation of Liaoning Province (2022-MS-369) and Construction of polyoxometalates Viologen Graphene Color Changing Assembly Key projects of Bohai University (2023, 0524xn057).Notes and references
- B. Li and N. Haneklaus, Energy Rep., 2021, 7, 783–791 CrossRef.
- J. Kurtz, M. Peters, M. Muratori and C. Gearhart, IEEE Electrific. Mag., 2018, 6, 8–18 Search PubMed.
- X. Chang, T. Wang and J. Gong, Energy Environ. Sci., 2016, 9, 2177–2196 RSC.
- S. Berardi, S. Drouet, L. Francas, C. Gimbert-Surinach, M. Guttentag, C. Richmond, T. Stoll and A. Llobet, Chem. Soc. Rev., 2014, 43, 7501–7519 RSC.
- A. M. Liu, W. X. Guan, K. F. Wu, X. F. Ren, L. G. Gao and T. L. Ma, Appl. Surf. Sci., 2021, 540, 7 Search PubMed.
- C. He, C. Xu and W. X. Zhang, ACS Appl. Mater. Interfaces, 2023, 15, 57015–57028 CAS.
- H. Semai and A. Bouhdjar, Environ. Sci. Pollut. Res., 2023, 30, 52692–52701 CrossRef PubMed.
- C. Y. Guo, Y. M. Shi, S. Y. Lu, Y. F. Yu and B. Zhang, Chin. J. Catal., 2021, 42, 1287–1296 CrossRef CAS.
- Z. F. Xin, J. J. Liu, X. J. Wang, K. J. Shen, Z. B. Yuan, Y. F. Chen and Y. Q. Lan, ACS Appl. Mater. Interfaces, 2021, 13, 54949–54956 Search PubMed.
- C. Zhang, Y. Liu, J. M. Wang, W. P. Li, Y. L. Wang, G. H. Qin and Z. G. Lv, Appl. Surf. Sci., 2022, 595, 10 Search PubMed.
- P. Zhou, G. Y. Zhai, X. S. Lv, Y. Y. Liu, Z. Y. Wang, P. Wang, Z. K. Zheng, H. F. Cheng, Y. Dai and B. B. Huang, Appl. Catal., B, 2021, 283, 9 CrossRef.
- A. Krishnan, R. Ajay, J. Anakha and U. S. K. Namboothiri, Surf. Interfaces, 2022, 30, 22 Search PubMed.
- G. Q. Zhang, Y. F. Zhou and F. L. Yang, Electrochim. Acta, 2019, 299, 672–681 CrossRef CAS.
- H. L. Dong, Y. Y. Li and D. E. Jiang, J. Phys. Chem. C, 2018, 122, 11392–11398 CrossRef CAS.
- Y. Qiao, W. C. Lai, K. Huang, T. T. Yu, Q. Y. Wang, L. Gao, Z. L. Yang, Z. S. Ma, T. L. Sun, M. Liu, C. Lian and H. W. Huang, ACS Catal., 2022, 12, 2357–2364 CrossRef CAS.
- Z. L. Zhao and G. Lu, J. Phys. Chem. C, 2019, 123, 4380–4387 CrossRef CAS.
- Z. Y. Guo, F. B. Yang, X. T. Li, H. W. Zhu, H. Do, K. L. Fow, J. D. Hirst, T. Wu, Q. L. Ye, Y. Q. Peng, H. Bin Wu, A. J. Wu and M. X. Xu, J. Energy Chem., 2024, 90, 540–564 CrossRef CAS.
- Q. K. Fan, X. Zhang, X. H. Ge, L. C. Bai, D. S. He, Y. T. Qu, C. C. Kong, J. L. Bi, D. W. Ding, Y. Q. Cao, X. Z. Duan, J. Wang, J. Yang and Y. Wu, Adv. Energy Mater., 2021, 11, 2101424 CrossRef CAS.
- S. J. Li, Y. B. Ma, Y. Zhao, R. J. Liu, Y. P. Zhao, X. S. Dai, N. A. Ma, C. Streb and X. N. Chen, Angew. Chem., Int. Ed., 2023, 62, e202314999 CrossRef CAS PubMed.
- S. S. Wang and G. Y. Yang, Chem. Rev., 2015, 115, 4893–4962 CrossRef CAS PubMed.
- N. I. Gumerova and A. Rompel, Nat. Rev. Chem, 2018, 2, 20 Search PubMed.
- L. L. Zhang, X. Ding, M. Y. Cong, Y. Wang and X. D. Zhang, Int. J. Hydrogen Energy, 2019, 44, 9203–9209 CrossRef CAS.
- L. Yang, Z. Zhang, C. N. Zhang and X. L. Wang, Rare Met., 2024, 43, 236–246 CrossRef CAS.
- S. Bhatt and S. Saha, Prog. Solid State Chem., 2023, 72, 27 CrossRef.
- L. Yang, Z. Zhang, C. N. Zhang and X. L. Wang, Rare Met., 2023, 43, 236–246 CrossRef.
- J. M. Cameron, G. Guillemot, T. Galambos, S. S. Amin, E. Hampson, K. M. Haidaraly, G. N. Newton and G. Izzet, Chem. Soc. Rev., 2022, 51, 293–328 RSC.
- M. R. Horn, A. Singh, S. Alomari, S. Goberna-Ferrón, R. Benages-Vilau, N. Chodankar, N. Motta, K. Ostrikov, J. MacLeod, P. Sonar, P. Gomez-Romero and D. Dubal, Energy Environ. Sci., 2021, 14, 1652–1700 RSC.
- T. Ueda, ChemElectroChem, 2018, 5, 823–838 CrossRef CAS.
- H. J. Lv, W. W. Guo, K. F. Wu, Z. Y. Chen, J. Bacsa, D. G. Musaev, Y. V. Geletii, S. M. Lauinger, T. Lian and C. L. Hill, J. Am. Chem. Soc., 2014, 136, 14015–14018 CrossRef CAS PubMed.
- S. Y. Lai, K. H. Ng, C. K. Cheng, H. Nur, M. Nurhadi and M. Arumugam, Chemosphere, 2021, 263, 23 CrossRef PubMed.
- J. F. Keggin, Nature, 1933, 131, 908 CrossRef CAS.
- G. P. Yang, Y. F. Liu, K. Li, W. Liu, B. Yu and C. W. Hu, Chin. Chem. Lett., 2020, 31, 3233–3236 CrossRef CAS.
- D. Ye, R. Y. Qu, Y. X. Zhang, W. H. Wu, S. J. Liu, C. H. Zheng and X. Gao, Mol. Catal., 2018, 448, 177–184 CrossRef CAS.
- J. L. Jios, N. Metzler-Nolte, P. G. Vázquez and G. P. Romanelli, Res. Chem. Intermed., 2016, 42, 977–986 CrossRef CAS.
- W. W. He, S. L. Li, H. Y. Zang, G. S. Yang, S. R. Zhang, Z. M. Su and Y. Q. Lan, Coord. Chem. Rev., 2014, 279, 141–160 CrossRef CAS.
- C. L. Yue, F. Y. Sun, N. Liu, Y. Liu, W. J. Bao, X. W. Zhang, C. Zhang, S. Y. Ma, Y. Zhou, C. Feng and Y. K. Lu, Fuel, 2024, 357, 129668 CrossRef CAS.
- A. Blazevic and A. Rompel, Coord. Chem. Rev., 2016, 307, 42–64 CrossRef CAS.
- N. V. Maksimchuk, V. Y. Evtushok, O. V. Zalomaeva, G. M. Maksimov, I. D. Ivanchikova, Y. A. Chesalov, I. V. Eltsov, P. A. Abramov, T. S. Glazneva, V. V. Yanshole, O. A. Kholdeeva, R. J. Errington, A. Solé-Daura, J. M. Poblet and J. J. Carbó, ACS Catal., 2021, 11, 10589–10603 CrossRef CAS.
- J. L. Liang, W. W. Wang, W. J. Wu, M. M. Wu, J. W. Hua, Y. Q. Liu and C. G. Liu, ChemistrySelect, 2023, 8, e202300004 CrossRef CAS.
- X. L. Wang, R. Zhang, X. Wang, H. Y. Lin, G. C. Liu and H. X. Zhang, Polyhedron, 2017, 135, 180–188 CrossRef CAS.
- W. G. Zhang, T. G. Zhang, G. Z. Lv, X. J. Cao and H. Y. Zhu, Sep. Purif. Technol., 2019, 218, 164–172 CrossRef CAS.
- B. Fabre, C. Falaise and E. Cadot, ACS Catal., 2022, 12, 12055–12091 CrossRef CAS.
- X. L. Yang, X. Y. Xie, S. Q. Li, W. X. Zhang, X. D. Zhang, H. X. Chai and Y. M. Huang, J. Hazard. Mater., 2021, 419, 12 Search PubMed.
- N. Li, J. Liu, B. X. Dong and Y. Q. Lan, Angew. Chem., Int. Ed., 2020, 59, 20779–20793 CrossRef CAS PubMed.
- J. J. Walsh, A. M. Bond, R. J. Forster and T. E. Keyes, Coord. Chem. Rev., 2016, 306, 217–234 CrossRef CAS.
- X. X. Li, D. Zhao and S. T. Zheng, Coord. Chem. Rev., 2019, 397, 220–240 CrossRef CAS.
- D. D. Li, P. T. Ma, J. Y. Niu and J. P. Wang, Coord. Chem. Rev., 2019, 392, 49–80 CrossRef CAS.
- L. W. Han, J. X. Lin, Q. Yin, B. Karadeniz, H. F. Li, J. Lü and R. Cao, Cryst. Growth Des., 2016, 16, 1213–1217 CrossRef CAS.
- Q. Gao, D. H. Hu, M. H. Duan and D. H. Li, J. Mol. Struct., 2019, 1184, 400–404 CrossRef CAS.
- T. V. Pinto, D. M. Fernandes, C. Pereira, A. Guedes, G. Blanco, J. M. Pintado, M. F. R. Pereira and C. Freire, Dalton Trans., 2015, 44, 4582–4593 RSC.
- M. Choudhari, J. J. Xu, A. I. McKay, C. Guerrin, C. Forsyth, H. Z. Ma, L. Goerigk, R. A. J. O'Hair, A. Bonnefont, L. Ruhlmann, S. Aloise and C. Ritchie, Chem. Sci., 2022, 13, 13732–13740 RSC.
- Y. Q. Jiao, C. Qin, X. L. Wang, C. G. Wang, C. Y. Sun, H. N. Wang, K. Z. Shao and Z. M. Su, Chem. - Asian J., 2014, 9, 470–478 CrossRef CAS PubMed.
- T. L. Lai, M. Awada, S. Floquet, C. Roch-Marchal, N. Watfa, J. Marrot, M. Haouas, F. Taulelle and E. Cadot, Chem.–Eur. J., 2015, 21, 13311–13320 CrossRef CAS PubMed.
- Y. L. Ren, L. Li, B. Mu, C. X. Li and R. D. Huang, J. Solid State Chem., 2017, 249, 1–8 CrossRef CAS.
- J. C. Liu, J. W. Zhao, C. Streb and Y. F. Song, Coord. Chem. Rev., 2022, 471, 23 Search PubMed.
- Z. B. Gan, J. S. Chen, J. Wang, C. M. Wang, M. B. Li, C. H. Yao, S. L. Zhuang, A. Xu, L. L. Li and Z. K. Wu, Nat. Commun., 2017, 8, 6 CrossRef PubMed.
- M. Bugnola, K. J. Shen, E. Haviv and R. Neumann, ACS Catal., 2020, 10, 4227–4237 CrossRef CAS.
- A. M. Todea, A. Merca, H. Bögge, T. Glaser, J. M. Pigga, M. L. K. Langston, T. Liu, R. Prozorov, M. Luban, C. Schröder, W. H. Casey and A. Müller, Angew. Chem., Int. Ed., 2010, 49, 514–519 CrossRef CAS PubMed.
- W. Jiang, X. M. Liu, J. Liu, J. Shi, J. P. Cao, X. M. Luo, W. S. You and Y. Xu, Chem. Commun., 2019, 55, 9299–9302 RSC.
- W. M. Xuan, R. Pow, N. Watfa, Q. Zheng, A. J. Surman, D. L. Long and L. Cronin, J. Am. Chem. Soc., 2019, 141, 1242–1250 CrossRef CAS PubMed.
- H. Y. Wang, S. R. Li, X. Wang, L. S. Long, X. J. Kong and L. S. Zheng, Sci. China: Chem., 2021, 64, 959–963 CrossRef CAS.
- Y. L. Wu, X. X. Li, Y. J. Qi, H. Yu, L. Jin and S. T. Zheng, Angew. Chem., Int. Ed., 2018, 57, 8572–8576 CrossRef CAS PubMed.
- Z. M. Wang, X. Xin, M. Zhang, Z. Li, H. J. Lv and G. Y. Yang, Sci. China: Chem., 2022, 65, 1515–1525 CrossRef CAS.
- A. A. Seddon, N. S. Hill, O. El-Zubir, A. Houlton, R. J. Errington, P. Docampo and E. A. Gibson, Chem. Commun., 2024, 60, 1876–1879 RSC.
- R. H. A. Jan, C. A. Biji, K. Shakeela, R. R. Shaikh and G. R. Rao, Catal. Lett., 2023, 154, 1631–1641 CrossRef.
- C. G. Liu, T. Zheng, S. Liu and H. Y. Zhang, J. Mol. Struct., 2016, 1110, 44–52 CrossRef CAS.
- Y. P. Liu, S. F. Zhao, S. X. Guo, A. M. Bond and J. Zhang, J. Am. Chem. Soc., 2016, 138, 2617–2628 CrossRef CAS PubMed.
- F. C. Shen, C. Guo, S. N. Sun, Z. Lei and Y. Q. Lan, Inorg. Chem., 2022, 61, 11182–11188 CrossRef CAS PubMed.
- J. M. Xu, Z. G. Zhang, K. Yang, W. W. He, X. D. Yang, X. M. Du, L. X. Meng, P. Y. Zhao and Z. Wang, J. Membr. Sci., 2020, 596, 15 CrossRef.
- Q. Chen, D. D. Zhang, M. M. Wang, X. W. Chen and J. H. Wang, J. Mater. Chem. B, 2015, 3, 6964–6970 RSC.
- Y. H. Zhu, J. B. Yang, X. M. Liu, J. L. Wang, Q. D. Ping, Z. Y. Du, J. N. A. Li, T. T. Zang, H. Mei and Y. Xu, Dalton Trans., 2022, 51, 3502–3511 RSC.
- R. Tian, L. P. Cui, K. Yu, J. H. Lv, Y. J. Ma, L. L. He and B. B. Zhou, ACS Appl. Nano Mater., 2022, 5, 14882–14892 CrossRef CAS.
- X. L. Yang, Y. S. Ye, Z. M. Wang, Z. H. Zhang, Y. L. Zhao, F. Yang, Z. Y. Zhu and T. Wei, ACS Omega, 2020, 5, 26230–26236 CrossRef CAS PubMed.
- Y. Du, W. X. Chen, Z. Y. Zhong, S. Z. Wang, L. A. Zhou, D. B. Xiong, Y. S. Liu, Z. H. Liu and K. Wang, J. Alloys Compd., 2023, 960, 10 Search PubMed.
- X. Q. Li, Z. Wang, C. Y. Hong, F. F. Feng, K. Yu and H. Liu, Macromolecules, 2022, 55, 9583–9593 CrossRef CAS.
- N. Sun, A. L. Wu, Y. Yu, X. P. Gao and L. Q. Zheng, Chem.–Eur. J., 2019, 25, 6203–6211 CrossRef CAS PubMed.
- M. Attoui, E. Pouget, R. Oda, D. Talaga, T. Buffeteau and S. Nlate, Inorg. Chim. Acta, 2019, 498, 9 CrossRef.
- Q. D. Liu and X. Wang, Angew. Chem., Int. Ed., 2023, 62, e202217764 CrossRef CAS PubMed.
- J. Q. Sha, X. Y. Yang, Y. Y. Chen, P. P. Zhu, Y. F. Song and J. Z. Jiang, ACS Appl. Mater. Interfaces, 2018, 10, 16660–16665 CrossRef CAS PubMed.
- Y. X. Guo, Y. J. Gong, Y. A. Gao, J. H. Xiao, T. Wang and L. Yu, Langmuir, 2016, 32, 9293–9300 CrossRef CAS PubMed.
- J. S. Li, Y. Wang, C. H. Liu, S. L. Li, Y. G. Wang, L. Z. Dong, Z. H. Dai, Y. F. Li and Y. Q. Lan, Nat. Commun., 2016, 7, 8 Search PubMed.
- K. Xia, K. Yamaguchi and K. Suzuki, Angew. Chem., Int. Ed., 2023, 62, e202214506 CrossRef CAS PubMed.
- W. J. Luo, J. Hu, H. L. Diao, B. Schwarz, C. Streb and Y. F. Song, Angew. Chem., Int. Ed., 2017, 56, 4941–4944 CrossRef CAS PubMed.
- T. Wei, M. Zhang, P. Wu, Y. J. Tang, S. L. Li, F. C. Shen, X. L. Wang, X. P. Zhou and Y. Q. Lan, Nano Energy, 2017, 34, 205–214 CrossRef CAS.
- X. Liu, J. Q. Huang, Q. Zhang and L. Q. Mai, Adv. Mater., 2017, 29, 1601759 CrossRef PubMed.
- X. Y. Luo, F. J. Li, F. Peng, L. Z. Huang, X. L. Lang and M. Q. Shi, ACS Appl. Mater. Interfaces, 2021, 13, 57803–57813 CrossRef CAS PubMed.
- Y. Hou, J. J. Xin, C. J. Gómez-García, B. X. Xiao, H. J. Pang, H. Y. Ma, X. M. Wang and L. C. Tan, ACS Appl. Energy Mater., 2021, 4, 13191–13198 CrossRef CAS.
- Z. H. Liu, L. Yuan, T. P. Wen, J. K. Yu and X. X. Xu, Int. J. Electrochem. Sci., 2023, 18, 9 CrossRef.
- Y. K. Lu, X. X. Guo, L. Y. Yang, W. F. Yang, W. T. Sun, Y. X. Tuo, Y. Zhou, S. T. Wang, Y. Pan, W. F. Yan, D. Sun and Y. Q. Liu, Chem. Eng. J., 2020, 394, 11 CrossRef.
- M. Guillen-Soler, N. V. Vassilyeva, E. P. Quirós-Díez, J. M. Vila-Fungueiriño, A. Forment-Aliaga and M. D. Gimenez-Lopez, Adv. Sustainable Syst., 2023, 8, 2300607 CrossRef.
- Shivaarun, P. Bhartiya, A. Naz, S. Rai, S. S. Narvi and P. K. Dutta, J. Polym. Mater., 2018, 35, 475–484 Search PubMed.
- A. Joshi, S. Acharya, N. Devi, R. Gupta, D. Sharma and M. Singh, Nanoscale Adv., 2023, 5, 6045–6052 RSC.
- S. Sun, L. P. Cui, K. Yu, M. L. Wang, J. H. Lv, S. H. Ge and B. B. Zhou, ACS Appl. Nano Mater., 2023, 7, 1310–1318 CrossRef.
- W. H. Xie, Y. Ren, F. L. Jiang, X. Y. Huang, B. J. Yu, J. H. Liu, J. C. Li, K. Y. Chen, Y. D. Zou, B. W. Hu and Y. H. Deng, Nat. Commun., 2023, 14, 9 CrossRef PubMed.
- R. Villanneau, A. Roucoux, P. Beaunier, D. Brouri and A. Proust, RSC Adv., 2014, 4, 26491–26498 RSC.
- H. K. Kolli, D. Jana and S. K. Das, Inorg. Chem., 2021, 60, 15569–15582 CrossRef CAS PubMed.
- H. Yang, J. Li, H. Y. Zhang, Y. Lv and S. Gao, Microporous Mesoporous Mater., 2014, 195, 87–91 CrossRef CAS.
- Q. Y. Li, L. Zhang, Y. X. Xu, Q. Li, H. G. Xue and H. Pang, ACS Sustain. Chem. Eng., 2019, 7, 5027–5033 CrossRef CAS.
- B. X. Xiao, S. U. Khan, G. N. Cui, L. M. Dong, C. J. Zhang, Q. Wu and H. J. Pang, Clean Technol. Environ., 2023, 25, 2629–2638 CrossRef CAS.
- C. Singh and S. K. Das, J. Chem. Sci., 2021, 133, 9 CrossRef.
- S. F. Li, J. S. Li, H. T. Zhu, L. Y. Zhang, X. J. Sang, Z. M. Zhu, W. S. You and F. X. Zhang, Dalton Trans., 2023, 52, 15725–15733 RSC.
- Z. F. Zhang, C. J. Gomez-Garcia, Q. Wu, J. J. Xin, H. J. Pang, H. Y. Ma, D. F. Chai, S. B. Li and C. Y. Zhao, Inorg. Chem., 2022, 61, 11830–11836 CrossRef CAS PubMed.
- J. H. Shi, Z. T. Wang, J. J. Zha, P. S. Wang, P. Wang, X. Y. Jiang, X. Liu, Q. Xu, X. Y. Liu, G. W. Diao and L. B. Ni, J. Mol. Struct., 2024, 1296, 7 CrossRef.
- S. Mulkapuri, A. Ravi, R. Nasani, S. K. Kurapati and S. K. Das, Inorg. Chem., 2022, 61, 13868–13882 CrossRef CAS PubMed.
- S. Mulkapuri, A. Ravi, S. Mukhopadhyay, S. K. Kurapati, V. Siby and S. K. Das, Inorg. Chem. Front., 2022, 9, 3566–3577 RSC.
- S. Mulkapuri, A. Siddikha, A. Ravi, P. Saha, A. V. Kumar, S. Boodida, M. Vithal and S. K. Das, Inorg. Chem., 2023, 62, 19664–19676 CrossRef CAS PubMed.
- M. X. Li, Y. Zhang, Z. M. Zhu, F. Su, L. C. Zhang and X. J. Sang, J. Coord. Chem., 2020, 73, 2437–2449 CrossRef CAS.
- A. A. Kuznetsova, V. V. Volchek, V. V. Yanshole, A. D. Fedorenko, N. B. Kompankov, V. V. Kokovkin, A. L. Gushchin, P. A. Abramov and M. N. Sokolov, Inorg. Chem., 2022, 61, 14560–14567 CrossRef CAS.
- P. Sood, A. Joshi and M. Singh, Nanoscale Adv., 2022, 4, 5015–5020 RSC.
- K. Azmani, M. Besora, J. Soriano-López, M. Landolsi, A. L. Teillout, P. de Oliveira, I. M. Mbomekallé, J. M. Poblet and J. R. Galán-Mascarós, Chem. Sci., 2021, 12, 8755–8766 RSC.
- N. Hu, J. Du, Y. Y. Ma, W. J. Cui, B. R. Yu, Z. G. Han and Y. G. Li, Appl. Surf. Sci., 2021, 540, 10 CrossRef.
- A. Kar, L. Sharma, A. Kumar, A. Halder and C. P. Pradeep, Eur. J. Inorg. Chem., 2022, 2022, 12 CrossRef.
- K. Li, S. Zhang, K. L. Zhu, L. P. Cui, L. Yang and J. J. Chen, J. Am. Chem. Soc., 2023, 145, 24889–24896 CAS.
- H. M. Zeng, Z. G. Jiang, H. W. Zhang, W. T. Mao, X. H. Gao and C. H. Zhan, Eur. J. Inorg. Chem., 2021, 2021, 2606–2610 CrossRef CAS.
- Q. Huang, Q. Niu, X. F. Li, J. Liu, S. N. Sun, L. Z. Dong, S. L. Li, Y. P. Cai and Y. Q. Lan, Sci. Adv., 2022, 8, 13 Search PubMed.
- J. J. Fang, Z. Liu, Y. L. Shen, Y. P. Xie and X. Lu, Chem. Sci., 2023, 14, 12637–12644 RSC.
- M. T. Peng, C. Chen, Y. Zhang, J. Y. Xu, Y. L. Teng and B. X. Dong, Dalton Trans., 2023, 52, 10737–10743 RSC.
- L. Yu and Q. Liang, New J. Chem., 2022, 46, 3073–3077 RSC.
- Y. Tang, Z. J. Zou, X. G. Wu, P. F. Zuo, L. Wang, G. W. Huang, J. Zhu and S. L. Zhong, New J. Chem., 2023, 47, 9887–9893 RSC.
- H. Zhou, Y. Y. Dong, X. Xin, M. Z. Chi, T. L. Song and H. J. Lv, J. Mater. Chem. A, 2022, 10, 19963–19971 RSC.
- M. Zhang, H. J. Li, J. H. Zhang, H. J. Lv and G. Y. Yang, Chin. J. Catal., 2021, 42, 855–871 CrossRef CAS.
- Y. Q. Feng, L. Qin, J. H. Zhang, F. Y. Fu, H. J. Li, H. Xiang and H. J. Lv, Chin. J. Catal., 2022, 43, 442–450 CrossRef CAS.
- D. Y. Jin, F. Qiao, Y. Zhou, J. F. Wang, K. C. Cao, J. Yang, J. K. Zhao, L. Zhou and H. T. Li, Nano Res., 2023, 17, 2546–2554 CrossRef.
- F. Yang, R. Q. Yao, Z. L. Lang, F. Y. Yu, H. L. Dong, Y. H. Wang, Y. G. Li and H. Q. Tan, ACS Energy Lett., 2023, 8, 5175–5183 CrossRef CAS.
- Y. Hou, H. J. Pang, J. J. Xin, H. Y. Ma, B. N. Li, X. M. Wang and L. C. Tan, Adv. Mater. Interfaces, 2020, 7, 9 Search PubMed.
- S. B. Li, J. W. Liang, X. G. Tan, F. B. Li, X. M. Wang, L. Ma, L. Zhang and K. Cheng, Int. J. Hydrogen Energy, 2024, 51, 1128–1137 CrossRef CAS.
- X. Y. Che, H. J. Pang, S. M. Hu, T. T. Yu, Z. X. Jin, Q. F. Zhen, H. Y. Ma, M. X. You and C. J. Zhang, Inorg. Chem. Commun., 2023, 155, 9 CrossRef.
- Z. X. Jin, H. J. Pang, K. Q. Li, M. L. Yang, J. J. Xin, H. Y. Ma, Q. Wu, X. M. Wang and G. X. Yang, ChemNanoMat, 2023, 10, e202300358 CrossRef.
- T. C. Shi, L. Men, X. H. Zhen, X. Li, J. Li, D. M. Wang and Z. M. Su, New J. Chem., 2023, 47, 21937–21943 RSC.
- S. Pathan, M. Ankitha, A. A. Mohan, N. Shabana, Y. Tong and P. A. Rasheed, Mater. Adv., 2024, 5, 274–281 RSC.
- Y. Yang, L. J. Liu, S. Chen, W. J. Yan, H. Q. Zhou, X. M. Zhang and X. J. Fan, Angew. Chem., Int. Ed., 2023, 62, e202306896 CrossRef CAS PubMed.
- M. L. Hu, Z. N. Wang, M. L. Li, K. M. Pan and L. P. Li, Int. J. Hydrogen Energy, 2021, 46, 28087–28097 CrossRef CAS.
- I. Sakthinathan, J. Koehling, V. Wagner and T. McCormac, ACS Appl. Mater. Interfaces, 2023, 15, 2861–2872 CrossRef CAS PubMed.
- C. L. Yue, Y. Zhou, Y. Liu, C. Feng, W. J. Bao, F. Y. Sun, Y. X. Tuo, Y. Pan, Y. Q. Liu and Y. K. Lu, Inorg. Chem. Front., 2022, 9, 2617–2627 RSC.
- C. H. Ma, S. S. Zhu, Y. C. Zhao, X. Y. Wang, T. Z. Zhan, L. H. Chen, J. N. Wang, Q. Ling, Z. C. Xiao, X. F. Wu, J. L. Cai and P. F. Wu, Langmuir, 2023, 40, 744–750 CrossRef.
- A. Joshi, P. Sood, A. Gaur, D. Rani, V. Madaan and M. Singh, J. Mater. Chem. A, 2022, 10, 12805–12810 RSC.
- Y. Zhang, H. R. Guo, J. K. Ren, X. P. Li, W. L. Ren and R. Song, Appl. Catal., B, 2021, 298, 9 Search PubMed.
- K. F. Shi, Z. M. Sun, M. W. Yuan, Y. L. Zhao and G. B. Sun, J. Colloid Interface Sci., 2024, 657, 37–45 CrossRef CAS PubMed.
- Y. Li, X. R. Chang, X. J. Sang, J. S. Li, Y. H. Luo, Z. M. Zhu and W. S. You, Eur. J. Inorg. Chem., 2019, 2019, 3597–3604 CrossRef CAS.
- H. Liu, L. G. Gong, C. X. Wang, C. M. Wang, K. Yu and B. B. Zhou, J. Mater. Chem. A, 2021, 9, 13161–13169 RSC.
- Y. P. Zhao, D. D. Gao, S. Liu, J. Biskupek, U. Kaiser, R. J. Liu and C. Streb, Chem.–Eur. J., 2023, 29, e202203220 CrossRef CAS PubMed.
- S. Mukhopadhyay, O. Basu, A. Kar and S. K. Das, Inorg. Chem., 2020, 59, 472–483 CrossRef CAS PubMed.
- V. K. Abdelkader-Fernández, D. M. Fernandes, S. S. Balula, L. Cunha-Silva and C. Freire, ACS Appl. Energy Mater., 2020, 3, 2925–2934 CrossRef.
- V. K. Abdelkader-Fernández, D. M. Fernandes, S. S. Balula, L. Cunha-Silva and C. Freire, J. Mater. Chem. A, 2020, 8, 13509–13521 RSC.
- S. Mulkapuri, A. Ravi and S. K. Das, Chem. Mater., 2022, 34, 3624–3636 CrossRef CAS.
- J. X. Song, P. F. Zhu, W. M. Ma and Y. X. Li, Int. J. Hydrogen Energy, 2024, 51, 327–337 CrossRef CAS.
- T. Ahmed, M. A. Asghar, A. Ali, Z. Akhter, S. Ali, I. Ullah, T. Nisar, V. Wagner, S. Touseef, A. Hussain and A. Haider, J. Alloys Compd., 2022, 909, 7 CrossRef.
- C. Lee, D. Jeon, J. Park, W. Lee, J. Park, S. J. Kang, Y. Kim and J. Ryu, ACS Appl. Mater. Interfaces, 2020, 12, 32689–32697 CrossRef CAS PubMed.
- R. H. Gong, D. D. Gao, R. J. Liu, D. Sorsche, J. Biskupek, U. Kaiser, S. Rau and C. Streb, ACS Appl. Energy Mater., 2021, 4, 12671–12676 CrossRef CAS.
- V. K. Abdelkader-Fernández, D. M. Fernandes, L. Cunha-Silva, A. J. S. Fernandes and C. Freire, Electrochim. Acta, 2021, 389, 12 CrossRef.
- Y. Q. Feng, F. Y. Fu, L. L. Zeng, M. Y. Zhao, X. Xin, J. K. Liang, M. Zhou, X. K. Fang, H. J. Lv and G. Y. Yang, Angew. Chem., Int. Ed., 2024, 63, e202317341 CrossRef CAS PubMed.
- M. L. Sun, Y. R. Wang, W. W. He, R. L. Zhong, Q. Z. Liu, S. Y. Xu, J. M. Xu, X. L. Han, X. Y. Ge, S. L. Li, Y. Q. Lan, A. M. Al-Enizi, A. Nafady and S. Q. Ma, Small, 2021, 17, 2100762 CrossRef CAS PubMed.
- H. Z. Yang, D. R. Yang, Y. Zhou and X. Wang, J. Am. Chem. Soc., 2021, 143, 13721–13730 CrossRef CAS PubMed.
- J. Du, Z. L. Lang, Y. Y. Ma, H. Q. Tan, B. L. Liu, Y. H. Wang, Z. H. Kang and Y. G. Li, Chem. Sci., 2020, 11, 3007–3015 RSC.
- W. C. Sun, D. Yao, Y. H. Tai, L. Zhou, W. X. Tian, M. Yang and C. X. Li, J. Colloid Interface Sci., 2023, 650, 121–131 CrossRef CAS PubMed.
- B. J. Zha, C. X. Li and J. J. Li, J. Catal., 2020, 382, 69–76 CrossRef CAS.
- D. J. Zang, Q. Li, G. Y. Dai, M. Y. Zeng, Y. C. Huang and Y. G. Wei, Appl. Catal., B, 2021, 281, 13 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |




