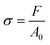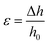Open Access Article
Open Access ArticleAn integrated design strategy coupling additive manufacturing and matrix-assisted pulsed laser evaporation (MAPLE) towards the development of a new concept 3D scaffold with improved properties for tissue regeneration
Teresa
Russo†
a,
Valentina
Peluso†
a,
Antonio
Gloria
*b,
Valentina
Gargiulo
c,
Michela
Alfe
 *c and
Giovanni
Ausanio
*d
*c and
Giovanni
Ausanio
*d
aInstitute of Polymers, Composites and Biomaterials, National Research Council of Italy, 80125 Naples, Italy
bDepartment of Industrial Engineering, University of Naples Federico II, 80125 Naples, Italy. E-mail: antonio.gloria@unina.it
cInstitute of Sciences and Technologies for Sustainable Energy and Mobility (STEMS), National Research Council of Italy, 80125 Naples, Italy. E-mail: michela.alfe@stems.cnr.it
dDipartimento di Fisica “Ettore Pancini”, Università degli Studi di Napoli Federico II, 80125 Napoli, Italy. E-mail: giovanni.ausanio@unina.it
First published on 12th April 2024
Abstract
Bioinspired strategies for scaffold design and optimization were improved by the introduction of Additive Manufacturing (AM), thus allowing for replicating and reproducing complex shapes and structures in a reliable manner, adopting different kinds of polymeric and nanocomposite materials properly combined according to the features of the natural host tissues. Benefiting from recent findings in AM, a Matrix-Assisted Pulsed Laser Evaporation (MAPLE) technique was employed for obtaining graphene-like material (GL) uniform coatings on 3D scaffolds for tissue repair strategies, towards the development of a new concept 3D scaffold with controlled morphological/architectural and surface features and mechanical and biological properties. The effect of the material-design combination through an integrated technological approach (i.e., MAPLE deposition of GL on 3D AM PCL scaffolds) was assessed through scanning electron microscopy, atomic force microscopy, contact angle measurements, mechanical measurements and biological analyses (cell viability assay and alkaline phosphatase activity) in conjunction with confocal laser scanning microscopy. The differentiation of hMSCs towards the osteoblast phenotype was also investigated analysing the gene expression profile. The obtained findings provided a further insight into the development of improved strategies for the functionalization or combination of GL with other materials and 3D structures in a hybrid fashion for ensuring a tighter adhesion onto the substrates, improving cell fate over time, without negatively altering the mechanical properties and behaviour of the neat constructs. In particular, the results provided interesting information, making 3D AM GL-coated scaffolds potential candidates for bone tissue engineering.
1. Introduction
The introduction of additive manufacturing (AM) technologies has led to a strong improvement in developing 3D constructs replicating the functional architecture and complex structure of native tissues and organs.1,2AM technologies operate in a layer-by-layer fashion and are crucial for the design of 3D scaffolds with controlled morphological and architectural features as well as fully interconnected pore networks.3–5
AM techniques based on injection/extrusion methods (e.g., 3D fiber deposition) may involve the possibility of processing both acellular and cellular materials into biological constructs for disease modelling, tissue engineering or regenerative medicine applications.3–7
Even though the great potential of 3D bioprinting techniques can be considered as not fully explored, many systems are currently favouring a gradual shift with regard to the clinical landscape. In this context, the combination of image capture and analysis techniques with AM should enhance the fabrication of patient-specific devices showing a better performance in terms of tissue regeneration.5,7
Among synthetic polymers employed in biomedicine, aliphatic polyesters and in particular poly(ε-caprolactone) (PCL) are widely investigated. PCL is a semi-crystalline and Food and Drug Administration (FDA)-approved polymer generally employed to manufacture scaffolds for tissue regeneration, as a consequence of its biocompatibility, long lasting mechanical characteristics and degradation features.8
Over the past few years, the use of hydroxyapatite (HA), magnetite (Fe3O4) or iron-doped hydroxyapatite (FeHA) nanoparticles8–13 has also been considered for the development of 3D AM nanocomposite (PCL/HA, PCL/Fe3O4, and PCL/FeHA) scaffolds with integrated functionalities for enhanced bone tissue engineering, providing interesting results.14–17
Great efforts have been made to push the research towards the identification of novel materials, innovative methodological approaches and technologies aiming to design 3D devices with peculiar properties.
From a materials science point of view, carbon-based and in particular graphene-related materials (GRMs) are emerging biocompatible coatings.18 GRMs comprise a wide pool of materials differing in terms of the structure (few-layer and multi-layered structures), composition and properties (even within the same GRM category). In addition to graphite, C-based sources for GRM production include carbon black, coke, carbonized/pyrolyzed wastes, biomasses and organic bio-precursors (e.g. plant-based, amino acids, and carbohydrates) through many approaches (e.g. thermal decomposition, ball milling, microwave sintering, flash Joule heating (FJH), hydrothermal methods, template-based confinement method and combined approaches).19–27
Graphene-like materials (GL) obtained from a top-down demolition of a nanostructured carbon black exhibit antimicrobial activity toward living cells through a bacteriostatic action28 and a proven biocompatibility on a vertebrate model.29 GL was further inspected30 by a biological survey toward target cellular lines (murine fibroblast NIH3T3, human keratinocytes HaCAT and the human cervical adenocarcinoma epithelial-like HeLa) demonstrating no perturbations in the different biological parameters evaluated, including the cytotoxic potential. GL composites are also capable of allowing Murine Embryonic Stem Cell (ESC) and Rat Microglial Cell (MC) growth and prolification.31 Furthermore, a very recent study successfully tested the compatibility of GL hybrids with neuron and beating cardiomyocyte differentiation.32
For the above reported evidence, GRMs (and specifically GL) can be considered as interesting coatings for several devices in biomedical applications,33,34 even if the studies on such coatings are still in their early stages.
The control of coating uniformity and thickness results in a crucial feature concerning the surface coating. Currently, there are many approaches for finely tailoring the surface chemistry and characteristics of coatings (e.g., physical vapor deposition, chemical and photochemical deposition, dip-coating, spin coating, and sputtering deposition).35
However, such techniques generally lead to inappropriate adherence related to the use of solvents.
A strict control of the coating and a good adherent film may be achieved benefiting from technological advances. In this scenario, the matrix-assisted pulsed laser evaporation (MAPLE) technique would be one of the intriguing and efficient options, as it represents a solvent-free technique especially employed to obtain ultra-thin, homogeneous and well adherent coatings on different kinds of substrates, without altering the physical and chemical properties of the deposited material.30,36,37
MAPLE can also allow coating nonplanar substrates with specific biomolecules and drugs, while maintaining their activity and peculiar features.30,36,37 Consequently, MAPLE ensures an optimal design of surface properties in terms of coating adhesion on the biomedical surface, and a better control over antibacterial properties can be achieved in many cases.30
For the first time MAPLE was recently employed to deposit GL on silicone slices, and the potential use as a functional coating was investigated for invasive devices for specific medical applications (i.e., indwelling urinary catheters). Specifically, interesting results were already obtained through coating of flexible polydimethylsiloxane (PDMS) substrates using MAPLE with GL,30 thus providing a further insight into the development of improved strategies for the functionalization or combination of GL with other materials in a hybrid fashion for ensuring a tighter adhesion onto the substrates.
Accordingly, benefiting from the previous results, the aim of the current research was to develop a new concept 3D scaffold (i.e., GL-coated PCL) with controlled morphological/architectural and surface features and mechanical and biological properties, through the material-design combination using an integrated technological approach (AM and MAPLE).
Contact angle measurements, and morphological/topographical, mechanical and preliminary biological analyses were performed to assess the effect of GL coating on the performance of 3D AM scaffolds as well as the feasibility of the integrated technological approach.
2. Materials and methods
2.1. 3D scaffold design and preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Sigma-Aldrich, St. Louis, MO) pellets were used and 3D scaffolds (length l0 of 10.0 mm, width w0 of 10.0 mm, and height h0 of 7.0 mm) were fabricated by the Fused Deposition Modeling/3D fiber deposition technique, using an extrusion-based system (EnvisionTEC GmbH, Germany). The pellets were heated to 100 °C and the 3D scaffolds were built layer by layer. A nitrogen pressure of 8.0 bar was employed for the processing of the material. The molten polymer was extruded/injected through a needle with an inner diameter of 400 μm. The filaments were deposited according to a lay-down pattern of 0/90°. A fiber spacing (i.e., centre-to-centre distance between two fibers) and a layer thickness of 750 and 320 μm were employed, respectively. A deposition speed of 40 mm min−1 was used.
000, Sigma-Aldrich, St. Louis, MO) pellets were used and 3D scaffolds (length l0 of 10.0 mm, width w0 of 10.0 mm, and height h0 of 7.0 mm) were fabricated by the Fused Deposition Modeling/3D fiber deposition technique, using an extrusion-based system (EnvisionTEC GmbH, Germany). The pellets were heated to 100 °C and the 3D scaffolds were built layer by layer. A nitrogen pressure of 8.0 bar was employed for the processing of the material. The molten polymer was extruded/injected through a needle with an inner diameter of 400 μm. The filaments were deposited according to a lay-down pattern of 0/90°. A fiber spacing (i.e., centre-to-centre distance between two fibers) and a layer thickness of 750 and 320 μm were employed, respectively. A deposition speed of 40 mm min−1 was used.
GL nanoparticles were prepared by means of a top-down demolition of a nanostructured carbon black (furnace type, CB N110, provided by Sid Richardson Carbon Co., Fort Worth, TX, USA) according to a previously reported approach.30,38 Briefly, carbon black powder (500 mg) was ground in a mortar and treated under stirring and reflux for 90 h with 10 mL of concentrated nitric acid (HNO3, 67%) at 100 °C. After the oxidation step the recovered sample was reduced with hydrazine hydrate (35 μL of hydrazine hydrate for each mg of sample) at 100 °C under stirring and reflux for 24 h. Following the reduction step, the hydrazine excess was neutralized with diluted nitric acid and the reduced sample (hereinafter GL) was recovered by centrifugation and further purified from reactant traces by under-vacuum filtration (Durapore® Membrane Filter, 0.22 μm). The GL, recovered on the filter, was diluted with distilled water establishing a concentration of 1 wt% (0.01 g mL−1) and stored. Before use, the GL suspension was treated by ultrasonic agitation (15 min at room temperature) to ensure concentration homogeneity before each deposition.
2.2. Surface morphology and topography analysis
Scanning Electron Microscopy (SEM) was employed to analyse GL as well as PCL scaffolds after MAPLE deposition treatment, using a FEI Quanta 200 FEG apparatus (The Netherlands). Before analysis, all samples were capped with a thin conductive gold layer.Atomic Force Microscopy (AFM) was performed on flat PCL substrates using an AFM Digital Instruments Nanoscope IIIa equipped with a sharpened silicon tip with a radius of less than 10 nm. The images of the surface profiles were obtained by operating the microscope in the tapping mode, with a scan size and rate of 2 μm and 1 Hz, respectively.
2.3. Contact angle measurements
Contact angle measurements were conducted on PCL and GL-coated PCL membranes by means of DATAPHYSICS OCA 20 apparatus, using deionized water and methylene iodide. The liquids were added dropwise on the membranes in different sites and the contact angle was assessed. CCD cameras recorded the process of the droplet addition on the membrane. The drop profile was extracted as already reported in the literature.41–43 Contact angles and surface free energy were evaluated and reported as mean value ± standard deviation.2.4. Mechanical measurements
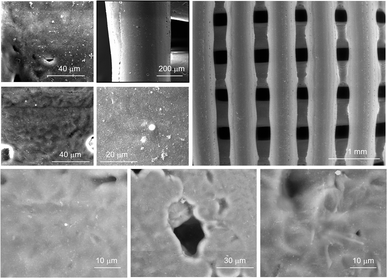
Mechanical compression tests were performed on two different kinds of 3D AM scaffolds (PCL and GL-coated PCL), using an INSTRON 5566 testing machine. Specifically, the block-shaped specimens (l0 = 10.0 mm, w0 = 10.0 mm and h0 = 7.0 mm) were compressed to a strain of 20% at a rate of 1 mm min−1.The engineering stress (σ) and strain (ε) were calculated as reported below:
where F is the measured force, A0 (l0 × w0) represents the initial cross-section area of the specimen, and Δh and h0 are the height variation and the initial height of the specimen, respectively.
2.5. Cell culture
Bone marrow human mesenchymal stem cells (hMSCs, Millipore, Germany) were cultured in Dulbecco's modified eagle medium (DMEM, Microtech, Italy) supplemented with 10% (v/v) fetal bovine serum (FBS, Gibco, Thermo Fisher Scientific, Waltham, MA, USA), 2 mM glutamine, and antibiotics (penicillin G sodium 100 U mL−1 and streptomycin 100 g mL−1) at 37 °C and 5% CO2.PCL and GL-coated PCL scaffolds were prepared by soaking the structures in a solution of ethanol and antibiotics (penicillin/streptomycin), washed in phosphate-buffered saline (PBS, Sigma-Aldrich, Milan, Italy), and pre-wetted in FBS. hMSCs were seeded onto the scaffolds using a density of 1.0 × 105 cells per sample. After seeding, the cell-laden structures were incubated for 2 h (37 °C and 5% CO2) and, successively, 1.5 mL of culture medium was added to each well in a 48-well plate.
2.6. Cell viability assay
Alamar Blue assay (AbD Serotec Ltd., UK) was used to assess cell viability and proliferation. At 3, 7, 14, and 21 days after cell seeding, the cell-laden scaffolds were rinsed with PBS (Sigma-Aldrich, Italy) and 200 μL of DMEM without phenol red (HyClone, UK) containing 10% (v/v) Alamar Blue was added for each sample. The samples were incubated in a 5% CO2 diluted atmosphere for 4 h at 37 °C.After removing one hundred microliters of the solution, it was transferred to a well plate. A spectrophotometer (Sunrise, Tecan, Männedorf, Zurich, Switzerland) was utilized, and the optical density was measured at wavelengths of 570 and 595 nm. The experiments were done at least three times in triplicate.
2.7. Alkaline phosphatase activity
Samples were removed from the medium and washed twice with PBS on days 7, 14 and 21. The PCL and GL-coated PCL cell-laden scaffolds were then incubated in 1 mL of a lysis buffer and centrifugated. A cell density of 1.0 × 105 cells per sample was employed.The alkaline phosphatase (ALP) activity was measured with an enzymatic assay (SensoLyte pNPP alkaline phosphatase assay kit – AnaSpec Inc., Fremont, CA, USA), which was based on p-nitrophenyl phosphate (pNPP). According to the manufacturer's protocol, the working solutions were prepared, and the procedure was followed.
2.8. Confocal laser scanning microscopy
Confocal laser scanning microscopy (CLSM) was also employed as an optical imaging technique for the analysis of cell adhesion and spreading at 14 and 21 days after seeding, using a Zeiss LSM 510/ConfoCor 2 system (Oberkochen, Germany) equipped with argon and helium–neon lasers and with a 10× and 20× objective. Cells were fixed with 4% paraformaldehyde for 20 min at room temperature, and permeabilized in 0.5% Triton-X100 and 1% BSA in PBS for 10 minutes. Actin filaments were stained with Phalloidin-AttoRho6G (100 μm), all from Sigma-Aldrich. Phalloidin fluorescence was collected in a spectral window of 500 to 530 nm.2.9. Gene expression profile
The extraction of total RNA was carried out using a Total RNA Purification Plus Kit. The isolation of the total RNA samples and qRT-PCR assays were performed using a Bio-Rad CFX96 Real-Time PCR System (Hercules, CA, USA), according to the procedures already reported in the literature.44The expression of osteogenesis-related genes including runt-related transcription factor 2 (RUNX2), osteopontin (OPN) and osteocalcin (OC) was analysed on days 7, 14 and 21. Data analysis was conducted using a method described in the literature.44
The obtained results were reported as fold change of target genes on GL-coated PCL compared with neat PCL (as control).
2.10. Statistical analysis
Each experiment was repeated at least three times in triplicate unless otherwise stated. The results were reported as mean value ± standard deviation. Statistical analysis was performed by one-way or two-way ANOVA, followed by the Bonferroni test with multiple comparisons among the distinct groups. The results were evaluated as statistically significant considering different confidence levels.3. Results
3.1. Scanning electron microscopy
SEM images of the GL-coated PCL scaffolds are reported in Fig. 1. The surface morphological and topographical features of the nanocomposites were clearly influenced by the presence of GL deposits that appeared well distributed on the scaffold fibre as well as inside the pores of the morphologically controlled scaffold.This evidence confirms the suitability of the MAPLE technique for the production of well-adherent coatings on complex 3D scaffolds. Its higher versatility allows for the deposition of delicate compounds (e.g., proteins or polymers) in the form of thin films without altering the stability of their functional characteristics.45 This important aspect is particularly evident when MAPLE is used on cryogenic targets made from the compounds of interest in an appropriate solvent.
The laser beam hits the target placed in the vacuumed deposition chamber and transfers the compounds without any damage.
The surface morphology and topography of the GL-coated PCL scaffolds were also inspected by AFM and compared with neat PCL. The AFM images (Fig. 2) demonstrated that the surface of the PCL substrate was completely covered by GL without discontinuity at the nanometric level.
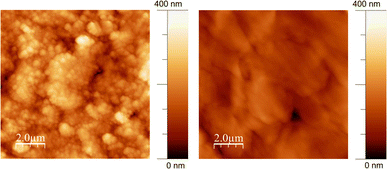 | ||
| Fig. 2 Non-contact representative AFM images (10 μm × 10 μm) of GL-coated PCL scaffolds (left) and the PCL substrate (right). | ||
The surface roughness parameters of GL-coated PCL scaffolds have been estimated (mean roughness, Ra, and Root Mean Square, RMS) and compared with those of bare PCL. The results were Ra = 41 ± 3 nm and RMS = 50 ± 4 nm for the GL-coated PCL scaffolds and Ra = 21 ± 3 nm and RMS = 30 ± 2 nm for the PCL. The higher RMS detected for the GL-coated PCL scaffolds is consistent with the MAPLE deposition characteristics where clusters of dry GL impacted the substrate without any settlement due to typical GL self-assembling phenomena arising upon slow drying from water suspension.46
3.2. Contact angle measurements
Contact angle testing provided information on the wettability and surface free energy. In particular, Table 1 reports the water contact angle and surface free energy in the case of PCL and GL-coated PCL membranes.| Membrane | Water contact angle (°) | Surface free energy (mJ m−2) |
|---|---|---|
| PCL | 89.1 ± 1.0 | 42.3 ± 1.1 |
| GL-coated PCL | 75.5 ± 0.7 | 48.1 ± 1.0 |
The water contact angles obtained for GL-coated PCL (75.5 ± 0.7°) were significantly lower than those obtained for neat PCL (89.1 ± 1.4°). As a consequence, GL-coated PCL structures appear to be significantly more hydrophilic than the PCL counterparts.
The presence of the GL coating led to a significant increase in the surface free energy.
3.3. Mechanical measurements
The mechanical compression tests performed on the two kinds of scaffolds showed a behaviour similar to that already reported in the literature for 3D AM scaffolds.5,14Fig. 3 reports the typical stress–strain curves obtained for PCL and GL-coated PCL scaffolds.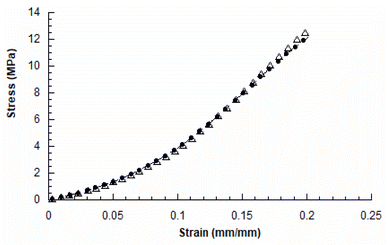 | ||
| Fig. 3 Typical stress–strain curves obtained for 3D AM PCL (●) and GL-coated PCL (△) scaffolds (rate of 1 mm min−1 and final strain of 20%). | ||
The compressive modulus was evaluated as the slope of the initial linear region of the stress–strain curve. The compressive modulus (E) and maximum stress (σmax) were reported as mean value ± standard deviation (Table 2).
| 3D AM scaffold | E (MPa) | σ max (MPa) |
|---|---|---|
| PCL | 27.5 ± 2.1 | 12.3 ± 0.6 |
| GL-coated PCL | 27.8 ± 2.0 | 12.9 ± 0.7 |
In particular, the neat PCL scaffolds showed a compressive modulus of 27.5 ± 2.1 MPa and a maximum stress of 12.3 ± 0.6 MPa, whilst values of 27.8 ± 2.0 MPa and 12.9 ± 0.7 MPa were obtained for the GL-coated PCL structures. However, no statistically significant differences (p > 0.05) were found between the two groups, in terms of compressive modulus and maximum stress. Thus, the presence of the functional GL coating did not significantly affect the mechanical properties and behaviour of the 3D PCL constructs.
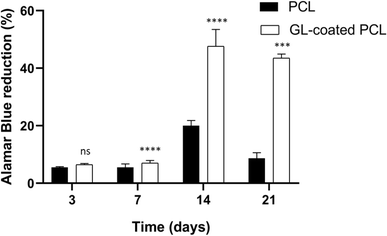
3.4. Cell viability assay
All samples supported the adhesion and proliferation of hMSCs. As reported, the number of viable cells was associated with the magnitude of dye reduction. A significant increase of Alamar Blue reduction was evident for PCL and GL-coated PCL scaffolds up to 14 days after seeding (Fig. 4). The results indicated that hMSCs were viable on both PCL and GL-coated PCL scaffolds. However, even though on day 3 no statistically significant differences were observed between the two groups, higher values of Alamar Blue reduction were found for GL-coated PCL scaffolds on 7, 14 and 21 days, compared to the neat PCL structures.3.5. Alkaline phosphatase activity (ALP)
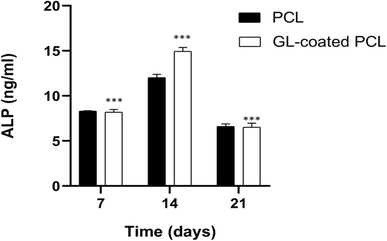
The alkaline phosphatase activity was measured on 7, 14 and 21 days for each group (PCL and GL-coated PCL). Fig. 5 reports some differences in terms of results. ALP peaked at 14 days after seeding. However, at each time point, the GL-coated PCL scaffolds showed significantly higher values of ALP activity, in comparison to the PCL ones. Accordingly, the presence of the GL coating strongly improved ALP activity.3.6. Confocal laser scanning microscopy
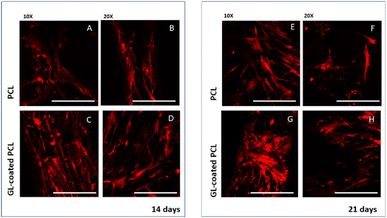
CLSM performed on cell-laden structures at 14 and 21 days after seeding provided qualitatively results in terms of cell adhesion and spreading over the observation time. The number of viable cells seems to increase over time (Fig. 4) with some modifications in the morphology, changing from a geometry characterized by a few ramifications to a thread-like geometry, with an increased number of ramifications. Cells appeared uniformly distributed on both PCL and GL-coated PCL scaffold surfaces. However, a better spreading was evident in the case of GL-coated PCL scaffolds (Fig. 6).3.7. Gene expression profile
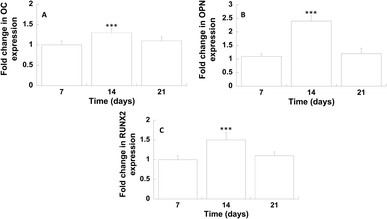
The differentiation of hMSCs towards the osteoblast phenotype on GL-coated PCL surfaces was investigated analysing the gene expression profile (Fig. 7).On day 14, the results showed a significantly improved mRNA expression level of OC and OPN, compared to the neat PCL. Analogously, on day 14, with regard to the transcription factor RUNX2, in the case of GL-coated PCL structures, the level was 1.5 (±0.2) fold higher than that of the neat PCL.
4. Conclusions
Graphene-like materials (GL) were obtained from a top-down demolition of a nanostructured carbon black and adopted as antimicrobial and biocompatible coatings of 3D morphologically controlled scaffolds fabricated in a layer-by-layer fashion by means of an Additive Manufacturing (AM) technique, with the aim to propose an integrated strategy for finely tuning scaffold features for tissue repair and regeneration.As frequently reported, the control of coating uniformity and thickness clearly remains a fundamental aspect for surface coating. The matrix-assisted pulsed laser evaporation (MAPLE) technique was adopted as an intriguing and efficient solvent-free option to obtain ultra-thin, homogeneous and well adherent coatings, without altering the physical and chemical properties of the deposited material, allowing coating complex and nonplanar objects with specific biomolecules and drugs also maintaining their activity and peculiar features.30,36,37
Accordingly, benefiting from the previous results, the current research was properly pursued to develop a new concept 3D scaffold with controlled properties through the material-design combination using an integrated technological approach (i.e., MAPLE deposition of GL on 3D AM PCL scaffolds). The results obtained from SEM analysis revealed a uniform GL coating onto the fiber of the 3D scaffolds, also highlighting that the GL coating was visible on the fibers of the deeper layers of 3D AM scaffolds. On the other hand, compression tests performed on PCL and GL-coated PCL scaffolds confirmed that the presence of the GL coating did not negatively alter the mechanical properties and behaviour of the 3D PCL scaffolds. Furthermore, the results from biological measurements highlighted that hMSCs were viable on both PCL and GL-coated PCL scaffolds. However, higher values of Alamar Blue reduction (on 7, 14 and 21 days) and alkaline phosphatase activity were found for GL-coated PCL scaffolds. In particular, at each time point, the higher ALP values found for the GL-coated scaffolds suggested that the material-design combination through the integrated technological approach provided the possibility of developing a new concept 3D scaffold able to better support the osteogenic differentiation of hMSCs.
Such results were also in good agreement with those from CLSM analysis which showed cell morphological changes.
On the other hand, even though water contact angle data provide important information on the surface wettability, the surface free energy clearly affects further surface features and properties. The surface free energy represents an important parameter which characterises the solid surface as well as the interaction with other materials.47 This parameter is related to the wettability and to further fundamental properties at the surface interface (e.g., adhesion and friction). Thus, its values are important for the application of the analysed materials according to different fields.47
In the biomedical field, scientific studies on the effects of cell attachment and differentiation were already carried out by measuring the wettability and surface free energy, also reporting that hydrophilic substrates with high surface free energy resulted in high biocompatibility and osteogenic responses.48
Accordingly, in the current work, the contact angle measurements were in agreement with those obtained from cell viability assay, ALP activity and CLSM analysis. Specifically, it was demonstrated that the structure with higher hydrophilicity and free energy (i.e., GL-coated PCL) improved cell adhesion, spreading and differentiation, differently from a surface with low free energy, which could also inhibit cell behavior in many cases. Thus, the improved wettability led to a better cell spreading, even if the role of the surface free energy should also be considered as the adhesion depends on the polar and nonpolar components of the surface free energy; in particular, the polar component mainly affects the polar molecules (e.g., water and proteins).49
For this reason, the wettability and surface free energy could represent an important indicator of cell behavior to guide the design and development of AM scaffolds. However, it is worth noting that the combination of such properties with the surface topography clearly represents a further important feature. Indeed, the role of surface topography combined with those of the wettability and surface free energy has been frequently stressed in the literature.49 For this reason, AFM was also performed, highlighting differences in terms of roughness (Ra = 41 ± 3 nm and RMS = 50 ± 4 nm for the GL-coated PCL scaffolds and Ra = 21 ± 3 nm and RMS = 30 ± 2 nm for the PCL).
Even though the stability/resistance of the GL deposits was also preliminarily assessed as the GL-coated samples (in the form of 3D porous scaffolds and 2D membranes) were immersed in the cell medium (see 2.5. Cell culture) and then digitally imaged at each time point (7, 14 and 21 days) and compared with those before the immersion (data not shown), nano-scratch tests should be performed to analyse the adhesion of the coating to the substrate.
The gene expression profile of osteogenic markers was employed to analyse the differentiation of hMSCs towards the osteoblast phenotype on GL-coated PCL surfaces. On day 14, the gene expression of OC and OPN, which are the major non-collagenous proteins secreted by pre-osteoblasts and osteoblasts,50 was significantly enhanced. It is well known how these proteins play an important role in the deposition and organization of the bone matrix. Furthermore, on day 14, a significant increase in the expression of RUNX2 was also found, which represents the major transcription factor functional for the osteoblast differentiation,51 regulating several bone-related genes.
As frequently reported in the literature,52–55 the improvement in the cell behaviour observed for GL-coated scaffolds should be ascribed to the synergistic contribution of the surface chemistry, surface topography and roughness.
Finally, the peculiar characteristics of 3D AM scaffolds were preliminarily combined with the MAPLE deposition technique, thus allowing direct further research towards the development of smart and improved strategies for surface functionalization or combination of GL with other materials and 3D structures in a hybrid fashion for ensuring a tighter adhesion onto the substrates and improving cell behaviour, without altering the mechanical performance of the neat constructs.
However, in the field of regenerative medicine, the analysis of the inflammation process clearly represents a key aspect. In general, the inflammatory response potentially induced by a scaffold, including the release of inflammatory factors and the polarization of macrophages, could be studied in different ways. Just as an example, basal levels of inflammatory markers should be quantified adopting in vitro models of inflammation and measuring basal levels of different pro-inflammatory interleukins (e.g., interleukin 1β (IL-1β)), interleukin-6 (IL-6) or anti – inflammatory interleukins (e.g., interleukin-10 (IL-10)). A further methodology could be related to the investigation of specific signalling pathways (TLRs, NF-kB, MAPK, NLRP3, etc.) or M1-phenotype macrophage surface marker (Ccr7, CD86, and CD68) or M2-phenotype macrophage surface marker (CD163, Il-1rn, and Arg1) gene expression over the experimental period. Further improvements and strategies will be properly carried out in future studies.
Future efforts will be devoted to: (i) further studies for analysing and/or eventually optimizing the penetration depth of the MAPLE treatment, even if preliminary experimental tests already evidenced interesting results (data not shown), in terms of distribution of GL deposits inside the pores, i.e. on the scaffold fibre of the inner layers, due to the fully interconnected pore network; (ii) analysis of the disperse and polar components of the energy; (iii) investigation of the effect of different concentrations of GL suspension, analysis of the dose–response curves and the half maximal inhibitory concentration (IC50), as a measure of the potency of a substance in inhibiting a specific biochemical or biological process by half; (iv) the development of functionally graded structures for tissue repair and regeneration, providing tailored properties at different scale levels, which could result from the combination of gradually distributed porosity, lattice architectures and material compositions.
Author contributions
Conceptualization: A. Gloria, T. Russo, M. Alfè and G. Ausanio. Data curation: T. Russo, A. Gloria and M. Alfè. Formal analysis: T. Russo, A. Gloria, V. Gargiulo and M. Alfè. Funding acquisition: G. Ausanio, A. Gloria and M. Alfè. Investigation: T. Russo, V. Peluso, and G. Ausanio. Methodology: T. Russo, V. Peluso, G. Ausanio, A. Gloria and M. Alfè. Supervision: G. Ausanio, A. Gloria and M. Alfè. Validation: T. Russo, V. Peluso, A. Gloria and M. Alfè. Writing – original draft and review & editing: T. Russo, V. Peluso, A. Gloria, V. Gargiulo and M. Alfè.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The technical assistance of Mr Luciano Cortese for SEM analyses and Mr Rodolfo Morra for mechanical testing is gratefully acknowledged. The technical assistance of Dr Stefania Zeppetelli for biological analyses is also acknowledged. The research funding program of the University of Naples “Federico II” (Project title: CATe-GRAF, Fondo Ricerca di Ateneo—Linea B) is acknowledged for the financial support.References
- R. Pecci, S. Baiguera, P. Ioppolo, R. Bedini and C. Del Gaudio, J. Mech. Behav. Biomed. Mater., 2020, 103, 103583 CrossRef CAS PubMed
.
- H. Qu, Mater. Today Commun., 2020, 24, 101024 CrossRef CAS
.
- S. Cometa, M. A. Bonifacio, E. Tranquillo, A. Gloria, M. Domingos and E. De Giglio, Polymers, 2021, 13, 150 CrossRef CAS PubMed
.
- D. W. Hutmacher, Biomaterials, 2000, 21, 2529–2543 CrossRef CAS PubMed
.
- A. Gloria, B. Frydman, M. L. Lamas, A. C. Serra, M. Martorelli, J. F. Coelho, A. C. Fonseca and M. Domingos, Mater. Sci. Eng., C, 2019, 98, 994–1004 CrossRef CAS PubMed
.
- J. Groll, T. Boland, T. Blunk, J. A. Burdick, D.-W. Cho, P. D. Dalton, B. Derby, G. Forgacs, Q. Li and V. A. Mironov, Biofabrication, 2016, 8, 013001 CrossRef PubMed
.
- R. Dhandapani, P. D. Krishnan, A. Zennifer, V. Kannan, A. Manigandan, M. R. Arul, D. Jaiswal, A. Subramanian, S. G. Kumbar and S. Sethuraman, Bioact. Mater., 2020, 5, 458–467 Search PubMed
.
- T. Russo, V. Peluso, A. Gloria, O. Oliviero, L. Rinaldi, G. Improta, R. De Santis and V. D'Antò, Nanomaterials, 2020, 10, 577 CrossRef CAS PubMed
.
- A. Tampieri, T. D'Alessandro, M. Sandri, S. Sprio, E. Landi, L. Bertinetti, S. Panseri, G. Pepponi, J. Goettlicher and M. Bañobre-López, Acta Biomater., 2012, 8, 843–851 CrossRef CAS PubMed
.
- N. Lewinski, V. Colvin and R. Drezek, Small, 2008, 4, 26–49 CrossRef CAS PubMed
.
- N. Singh, G. J. Jenkins, R. Asadi and S. H. Doak, Nano Rev., 2010, 1, 5358 CrossRef PubMed
.
- C. C. Berry and A. S. Curtis, J. Phys. D: Appl. Phys., 2003, 36, R198 CrossRef CAS
.
- A. Gloria, T. Russo, U. d'Amora, S. Zeppetelli, T. d'Alessandro, M. Sandri, M. Bañobre-López, Y. Piñeiro-Redondo, M. Uhlarz and A. Tampieri, J. R. Soc., Interface, 2013, 10, 20120833 CrossRef CAS PubMed
.
- D. Ronca, F. Langella, M. Chierchia, U. D'Amora, T. Russo, M. Domingos, A. Gloria, P. Bartolo and L. Ambrosio, Procedia CIRP, 2016, 49, 51–54 CrossRef
.
- R. De Santis, A. Gloria, T. Russo, U. d'Amora, S. Zeppetelli, C. Dionigi, A. Sytcheva, T. Herrmannsdörfer, V. Dediu and L. Ambrosio, J. Appl. Polym. Sci., 2011, 122, 3599–3605 CrossRef CAS
.
- R. De Santis, A. Russo, A. Gloria, U. D'Amora, T. Russo, S. Panseri, M. Sandri, A. Tampieri, M. Marcacci and V. A. Dediu, J. Biomed. Nanotechnol., 2015, 11, 1236–1246 CrossRef CAS PubMed
.
- U. D'Amora, T. Russo, A. Gloria, V. Rivieccio, V. D'Antò, G. Negri, L. Ambrosio and R. De Santis, Bioact. Mater., 2017, 2, 138–145 Search PubMed
.
- C. Backes, A. Abdelkader, C. Alonso, A. Andrieux-Ledier, R. Arenal, J. Azpeitia, N. Balakrishnan, L. Banszerus, J. Barjon and R. Bartali, 2D Mater., 2020, 7, 022001 CrossRef CAS
.
- S. Saha, P. Lakhe, M. J. Mason, B. J. Coleman, K. Arole, X. Zhao, S. Yakovlev, S. Uppili, M. J. Green and R. A. Hule, npj 2D Mater. Appl., 2021, 5, 75 CrossRef CAS
.
- U. Sierra, P. Álvarez, C. Blanco, M. Granda, R. Santamaría and R. Menéndez, Carbon, 2015, 93, 812–818 CrossRef CAS
.
- U. Sierra, P. Álvarez, C. Blanco, M. Granda, R. Santamaría and R. Menéndez, Fuel, 2016, 166, 400–403 CrossRef CAS
.
- U. Sierra, A. Mercado, E. Cuara, E. D. Barriga-Castro, A. Cortes, C. Gallardo-Vega and S. Fernandez, Fuel, 2020, 262, 116455 CrossRef CAS
.
- O. Vieira, R. S. Ribeiro, J. L. D. de Tuesta, H. T. Gomes and A. M. Silva, Chem. Eng. J., 2022, 428, 131399 CrossRef CAS
.
- X. Kong, Y. Zhu, H. Lei, C. Wang, Y. Zhao, E. Huo, X. Lin, Q. Zhang, M. Qian and W. Mateo, Chem. Eng. J., 2020, 399, 125808 CrossRef CAS
.
- N. Raghavan, S. Thangavel and G. Venugopal, Appl. Mater. Today, 2017, 7, 246–254 CrossRef
.
- A. Poorna, R. Saravanathamizhan and N. Balasubramanian, Electrochem. Sci. Adv., 2021, 1, e2000028 CrossRef CAS
.
- M. T.-u. Safian, U. S. Haron and M. M. Ibrahim, BioResources, 2020, 15, 9756 Search PubMed
.
- M. Olivi, M. Alfè, V. Gargiulo, F. Valle, F. Mura, M. Di Giosia, S. Rapino, C. Palleschi, D. Uccelletti and S. Fiorito, J. Nanopart. Res., 2016, 18, 1–10 CrossRef CAS
.
- M. d'Amora, M. Alfe, V. Gargiulo and S. Giordani, Nanomaterials, 2020, 10, 1472 CrossRef PubMed
.
- M. Alfe, G. Minopoli, M. Tartaglia, V. Gargiulo, U. Caruso, G. P. Pepe and G. Ausanio, Nanomaterials, 2022, 12, 3663 CrossRef CAS PubMed
.
- V. Gargiulo, M. Alfè, R. Di Capua, A. R. Togna, V. Cammisotto, S. Fiorito, A. Musto, A. Navarra, S. Parisi and A. Pezzella, J. Mater. Chem. B, 2015, 3, 5070–5079 RSC
.
- F. Amantea, G. Antignani, G. Pota, E. Cascone, S. Parisi, M. Alfè, V. Gargiulo, G. Luciani, A. Pezzella and G. D'Errico, Appl. Surf. Sci., 2023, 157608 CrossRef CAS
.
- H.-S. Hung, A. Y.-H. Yu, S.-C. Hsieh, M.-L. Kung, H.-Y. Huang, R.-H. Fu, C.-A. Yeh and S.-H. Hsu, ACS Appl. Mater. Interfaces, 2020, 12, 44393–44406 CrossRef CAS PubMed
.
- S. Pandit, K. Gaska, R. Kádár and I. Mijakovic, ChemPhysChem, 2021, 22, 250–263 CrossRef CAS PubMed
.
- S. Noimark, C. W. Dunnill, M. Wilson and I. P. Parkin, Chem. Soc. Rev., 2009, 38, 3435–3448 RSC
.
- A. D. Stiff-Roberts and W. Ge, Appl. Phys. Rev., 2017, 4, 041303 Search PubMed
.
- D. B. Chrisey, A. Piqué, R. A. McGill, J. S. Horwitz, B. R. Ringeisen, D. M. Bubb and P. K. Wu, Chem. Rev., 2003, 103, 553–576 CrossRef CAS PubMed
.
- M. Alfè, V. Gargiulo, R. Di Capua, F. Chiarella, J. N. Rouzaud, A. Vergara and A. Ciajolo, ACS Appl. Mater. Interfaces, 2012, 4, 4491–4498 CrossRef PubMed
.
- V. Gargiulo, B. Alfano, R. Di Capua, M. Alfé, M. Vorokhta, T. Polichetti, E. Massera, M. L. Miglietta, C. Schiattarella and G. Di Francia, J. Appl. Phys., 2018, 123, 024503 CrossRef
.
- M. A. Steiner and J. M. Fitz-Gerald, Appl. Phys. A: Mater. Sci. Process., 2015, 119, 629–638 CrossRef CAS
.
- X. F. Wu and Y. A. Dzenis, Acta Mech., 2006, 185, 215–225 CrossRef
.
- H. Chen, H. Cheng, Z. Jiang, D. Qin, Y. Yu, G. Tian, F. Lu, B. Fei and G. Wang, BioResources, 2013, 8, 2827–2838 Search PubMed
.
- S. R. Moxon, M. J. S. Ferreira, P. Santos, B. Popa, A. Gloria, R. Katsarava, D. Tugushi, A. C. Serra, N. M. Hooper and S. J. Kimber,
et al.
, Polymers, 2020, 12(7), 1478 CrossRef CAS PubMed
.
- L. Ferroni, C. Gardin, F. Rigoni, E. Balliana, F. Zanotti, M. Scatto, P. Riello and B. Zavan, Coatings, 2022, 12, 799 CrossRef CAS
.
- M. Tabetah, A. Matei, C. Constantinescu, N. P. Mortensen, M. Dinescu, J. Schou and L. V. Zhigilei, J. Phys. Chem. B, 2014, 118, 13290–13299 CrossRef CAS PubMed
.
- M. Alfe, V. Gargiulo and R. Di Capua, Appl. Surf. Sci., 2015, 353, 628–635 CrossRef CAS
.
- A. Kozbial, Z. Li, C. Conaway, R. McGinley, S. Dhingra, V. Vahdat, F. Zhou, B. D'Urso, H. Liu and L. Li, Langmuir, 2014, 30(28), 8598–8606 CrossRef CAS PubMed
.
- C. Shuxiang, W. Chuanxiang, Y. Wenguang, L. Wenfeng, Y. Haibo and L. Lianqing, Nanotechnol. Rev., 2020, 9(1), 971–989 CrossRef
.
- V. M. Villapun Puzas, L. N. C. Arter, C. Schröder, P. E. Colavita, D. A. Hoey, M. A. Webber, O. Addison, D. E. T. Shepherd, M. M. Attallah, L. M. Grover and S. C. Cox, ACS Biomater. Sci. Eng., 2022, 8(10), 4311–4326 CrossRef CAS PubMed
.
- S. Bailey, G. Karsenty, C. Gundberg and D. Vashishth, Ann. N. Y. Acad. Sci., 2017, 1409, 79–84 CrossRef CAS PubMed
.
- S. Chen, J. Gluhak-Heinrich, Y. Wang, Y. Wu, H. H. Chuang, L. Chen, G. Yuan, J. Dong, I. Gay and M. MacDougall, J. Dent. Res., 2009, 88, 904–909 CrossRef CAS PubMed
.
- F. Lin, F. Du, J. Huang, A. Chau, Y. Zhou, H. Duan, J. Wang and C. Xiong, Colloids Surf., B, 2016, 146, 785–793 CrossRef CAS PubMed
.
- Q. Wang, Y. Chu, J. He, W. Shao, Y. Zhou, K. Qi, L. Wang and S. Cui, Mater. Sci. Eng., C, 2017, 80, 232–242 CrossRef CAS PubMed
.
- P. Ginestra, J. Mech. Behav. Biomed. Mater., 2019, 100, 103387 CrossRef CAS PubMed
.
- J. Guo, G. Cao, S. Wei, Y. Han and P. Xu, Heliyon, 2023, 9(11), e21872 CrossRef CAS PubMed
.
Footnote |
| † Both first authors. |
| This journal is © The Royal Society of Chemistry 2024 |