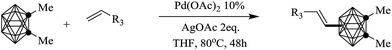Palladium catalyzed regioselective mono-alkenylation of o-carboranes via Heck type coupling reaction of a cage B–H bond†
Ji
Wu
,
Ke
Cao
*,
Tao-Tao
Xu
,
Xiao-Juan
Zhang
,
Linhai
Jiang
,
Junxiao
Yang
and
Yawen
Huang
State Key Laboratory Cultivation Base for Nonmetal Composite and Functional Materials, School of Materials Science and Engineering, Southwest University of Science and Technology, Mianyang, P. R. China. E-mail: caoke@swust.edu.cn
First published on 22nd October 2015
Abstract
A regioselective mono-alkenylation of o-carboranes on B(8) and B(9) via palladium catalyzed Heck type coupling of a cage B–H bond has been developed, and kinds of B(8)/B(9)-alkenyl-o-carboranes decorated with active groups have been synthesized with moderate yield as well as good regioselectivity. A PdII catalyzed electrophilic B–H activation mechanism was also proposed.
Carboranes with icosahedron and three-dimensional aromaticity are an important construction unit featuring in medicinal chemistry,1 supramolecular2 and coordination chemistry.3 Synthesis of carborane derivatives with diverse functional groups would offer a platform to examine their coveted properties and applications.4 In particular, introducing aromatic or conjugated groups into the cage carbon of carboranes to extend the π-conjugated system to examine their light-emitting or nonlinear optical properties has received much attention.5 In contrast to the well-established methodologies for carbon functionalization of carboranes,6 methods for regioselective boron functionalization have been difficult as the slight difference in reactivity of the not equally full 10 cage B–H bonds had led their functionalization being more complicated.7 Therefore, developing new methods for the regioselective boron derivatization is eagerly desired. Except the typical methods involving boron-halogenation,8 recently, using transition metal catalyzed B–H activation for regioselective functionalization of o-carborane has been a prominent strategy but a challenging subject.9,7b
For o-carboranes, molecular orbitals calculation demonstrate B(9,12) possess slight higher negative charge density than that of B(8,10), which led the electrophilic reaction of B(9,12) prior to that of B(8,10).10 Base on this characteristic, we consider that distinguishing the slight difference in reactivity might reliable by transition metal catalyzed electrophilic B–H activation, and the congested environment of o-carborane would restraint the forming of poly-substituted o-carboranes to achieve its regioselective mono-functionalization. With this assumption, we recently disclosed a regioselective mono-arylation at B(8) and B(9) of o-carboranes with iodobenzenes.9e With our continued research interest in boron cluster chemistry,11 herein, we present a regioselective mono-alkenylation of o-carborane via Heck type coupling of cage B–H bond.
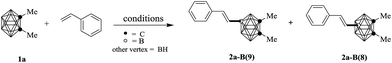
In the beginning of our research, 1,2-Me2-o-carborane (1a) and styrene were selected to examine conditions. To our delight, when the reaction was conducted with 10 mol% of Pd(OAc)2 and 2 equivalent of AgOAc in 1,2-dichloroethane at 80 °C for 48 h, the desired coupling product was obtained with 39% yield (Table 1, entry 1). Further investigation demonstrated THF was a superior medium for this coupling (entries 2–4), and the expected product was afforded in 63% yield with 77% conversion ratio of 1a, and the regioselectivity at B(8) and B(9) with a ratio of about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, meanwhile, the mono-alkenylation at B(8) and B(9) position were confirmed by the chemical shift of boron in 11B NMR. Other silver and copper salts were then examined and displayed lower efficiency than that of AgOAc (entries 5–10). Additionally, palladium source were also screened, which behaved lower catalytic efficiency (entries 11–13).
1, meanwhile, the mono-alkenylation at B(8) and B(9) position were confirmed by the chemical shift of boron in 11B NMR. Other silver and copper salts were then examined and displayed lower efficiency than that of AgOAc (entries 5–10). Additionally, palladium source were also screened, which behaved lower catalytic efficiency (entries 11–13).
| Entry | Catalyst | Additive | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a All reactions were carried on 0.250 mmol 1a, 0.025 mmol catalyst, 0.500 mmol styrene, 0.500 mmol additive and 1 mL solvent at 80 °C for 48 h under argon atmosphere. b Isolated yield, and the ratio of B(8) and B(9) isomers was determined based on 1H NMR. c DCE: 1,2-dichloroethane. d The conversion ratio was calculated based on the recovered 1a. | ||||
| 1 | Pd(OAc)2 | AgOAc | DCEc | 39 |
| 2 | Pd(OAc)2 | AgOAc | Toluene | 51 |
| 3 | Pd(OAc)2 | AgOAc | CH3CN | 28 |
| 4 | Pd(OAc)2 | AgOAc | THF | 63d |
| 5 | Pd(OAc)2 | AgBF4 | THF | 6 |
| 6 | Pd(OAc)2 | AgOTf | THF | 7 |
| 7 | Pd(OAc)2 | Ag3PO4 | THF | 10 |
| 8 | Pd(OAc)2 | Ag2O | THF | — |
| 9 | Pd(OAc)2 | Cu(OAc)2 | THF | 16 |
| 10 | Pd(OAc)2 | CuO | THF | — |
| 11 | Pd(PPh3)2Cl2 | AgOAc | THF | 54 |
| 12 | Pd2(dba)3 | AgOAc | THF | — |
| 13 | Pd(dppf)2Cl2 | AgOAc | THF | 29 |
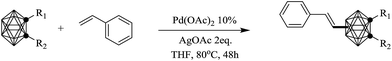
Based on the optimized conditions (Table 1, entry 4), the scope of this transformation was explored and the results were summarized in Table 2. We can see that both Ccage-mono-substituted and Ccage-disubstituted o-carborane were compatible with this reaction, and generated the expected products with moderate yields as well as good regioselectivity. However, unsubstituted o-carborane created an intricate result. The regioselectivity was uncontrollable and gave a complicated alkenylation products including the desired B(8) and B(9) isomers. This result demonstrated the substituents at Ccage play an important role in control the regioselectivity of electrophilic reaction of B–H bonds, which might ascribed to the changed charge distribution among boron atoms induced by the substituents on cage carbons.12
| a All reactions were carried on 0.25 mmol scale in 1 mL THF at 80 °C for 48 h under argon atmosphere, and the conversion ratio was calculated based on the recovered o-carboranes. b Isolated yield, the ratio of B(8) and B(9) isomers was determined based on 1H NMR and the conversion ratio of o-carborane are given in parentheses. |
|---|

|
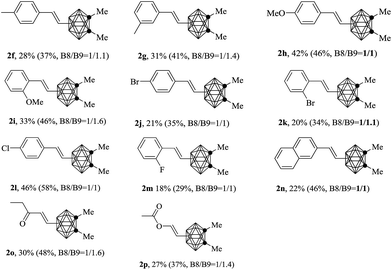
Further investigation indicated this regioselective mono-alkenylation has a good applicability to styrenes decorated with kinds of functional groups. However, styrenes either with electron donating groups or electron withdrawing groups were all not active enough for this transformation, and only attained moderate conversion ratio and gave the corresponding product with lower to moderate yields (2f–2n, Table 3). On the other hand, aliphatic alkenes such as ethyl vinyl ketone and vinyl acetate were also compatible with this reaction, and gave the desired products with moderate yields (2o–2p). Although the efficiency of the reaction is not well enough, this is the first direct approach to regioselective mono-alkenylation of o-carobranes at B(8) and B(9).13
Based on the experiment results, a possible mechanism was proposed as shown in Scheme 1. The electrophilic reaction of B–H bond to Pd(II) to form intermediate I, and the regioselectivity was determined in this step as the electrophilic reaction of B(9,12) showed slight prior to that of B(8,10). Then, double bond of olefin inserted into B–Pd bond to give II, after β-H elimination to generate alkenylation product and release Pd(0). At last, oxidation of Pd(0) to Pd(II) by Ag(I) regenerate the active catalyst. The mono-alkenylation might be ascribed to the steric hindrance of alkenyl on boron to hinder secondary alkenylation by forming transition state II as we previously discussed.14,9e
Conclusions
In conclusion, a direct regioselective mono-alkenylation of o-carboranes via palladium catalyzed electrophilic B–H activation/Heck coupling process was developed. A series of B(8)/B(9)-alkenyl-o-carboranes anchored with diverse functional groups were synthesized with moderate yields as well as good regioselectivity. Additionally, the crucial role of substituents on cage carbon in controlling the regioselectivity was disclosed, which would be useful for designation of other coupling reactions selective on B(8) and B(9).Acknowledgements
This work is supported by Scientific Research Fund of Sichuan Provincial Education Department (No. 13TD0022), Open Project of State Key Laboratory Cultivation Base for Nonmetal Composites and Functional Materials (No. 11zxfk26, 13zxfk06), and Doctoral Research Foundation of Southwest University of Science and Technology (13zx7134).Notes and references
- (a) M. F. Hawthorne, Angew. Chem., Int. Ed. Engl., 1993, 32, 950 CrossRef PubMed; (b) A. H. Soloway, W. Tjarks and J. G. Barnum, Chem. Rev., 1998, 98, 1515 CrossRef CAS PubMed; (c) J. F. Valliant, K. J. Guenther, A. S. King, P. Morel, P. Schaffer, O. O. Sogbein and K. A. Stephenson, Coord. Chem. Rev., 2002, 232, 173 CrossRef CAS; (d) A. F. Armstrong and J. F. Valliant, Dalton Trans., 2007, 4240 RSC; (e) F. Issa, M. Kassiou and L. M. Rendina, Chem. Rev., 2011, 111, 5701 CrossRef CAS PubMed.
- (a) X. Yang, W. Jiang, C. B. Knobler and M. F. Hawthorne, J. Am. Chem. Soc., 1992, 114, 9719 CrossRef CAS; (b) H. M. Colquhoun, P. L. Herbertson, K. Wade, I. Baxter and D. J. Williams, Macromolecules, 1998, 31, 1694 CrossRef CAS; (c) H. Jude, H. Disteldorf, S. Fischer, T. Wedge, A. M. Hawkridge, A. M. Arif, M. F. Hawthorne, D. C. Muddiman and P. J. Stang, J. Am. Chem. Soc., 2005, 127, 12131 CrossRef CAS PubMed; (d) B. P. Dash, R. Satapathy, E. R. Gaillard, J. A. Maguire and N. S. Hosmane, J. Am. Chem. Soc., 2010, 132, 6578 CrossRef CAS PubMed.
- (a) Z. Xie, Acc. Chem. Res., 2003, 36, 1 CrossRef CAS PubMed; (b) L. Deng and Z. Xie, Coord. Chem. Rev., 2007, 251, 2452 CrossRef CAS PubMed; (c) Z.-J. Yao and G.-X. Jin, Coord. Chem. Rev., 2013, 257, 2522 CrossRef CAS PubMed.
- (a) R. N. Grimes, Carboranes, Elsevier, 2nd edn, 2011 Search PubMed; (b) N. S. Hosmane, Boron Science: New Technologies and Applications, Taylor & Francis Group, 2012 Search PubMed; (c) N. S. Hosmane, J. A. Maguire, Comprehensive Organometallic Chemistry III, 2007, vol. 3, pp. 175–264 Search PubMed.
- (a) K. R. Wee, W. S. Han, D. W. Cho, S. Kwon, C. Pac and S. O. Kang, Angew. Chem., Int. Ed., 2012, 51, 2677 CrossRef CAS PubMed; (b) K.-R. Wee, Y.-J. Cho, J. K. Song and S. O. Kang, Angew. Chem., Int. Ed., 2013, 52, 9682 CrossRef CAS PubMed; (c) C. Shi, H. Sun, X. Tang, H. Lv, H. Yan, Q. Zhao, J. Wang and W. Huang, Angew. Chem., Int. Ed., 2013, 52, 13434 CrossRef CAS PubMed; (d) K. R. Wee, Y. J. Cho, S. Jeong, S. Kwon, J. D. Lee, I. H. Suh and S. O. Kang, J. Am. Chem. Soc., 2012, 134, 17982 CrossRef CAS PubMed.
- (a) V. I. Bregadze, Chem. Rev., 1992, 92, 209 CrossRef CAS; (b) Z. Qiu, S. Ren and Z. Xie, Acc. Chem. Res., 2011, 44, 299 CrossRef CAS PubMed.
- (a) D. Olid, R. Núñez, C. Viñas and F. Teixidor, Chem. Soc. Rev., 2013, 42, 3318 RSC; (b) Z. Qiu, Tetrahedron Lett., 2015, 56, 963 CrossRef CAS PubMed.
- (a) J. S. Andrews, J. Zayas and M. Jones Jr, Inorg. Chem., 1985, 24, 3715 CrossRef CAS; (b) Z. Zheng, W. Jiang, A. A. Zinn, C. B. Knobler and M. F. Hawthorne, Inorg. Chem., 1995, 34, 2095 CrossRef CAS.
- (a) M. G. L. Mirabelli and L. G. Sneddon, J. Am. Chem. Soc., 1988, 110, 449 CrossRef CAS; (b) Z. Qiu, Y. Quan and Z. Xie, J. Am. Chem. Soc., 2013, 135, 12192 CrossRef CAS PubMed; (c) Y. Quan and Z. Xie, J. Am. Chem. Soc., 2014, 136, 15513 CrossRef CAS PubMed; (d) Y. Quan and Z. Xie, J. Am. Chem. Soc., 2015, 137, 3502 CrossRef CAS PubMed; (e) K. Cao, Y. Huang, J. Yang and J. Wu, Chem. Commun., 2015, 51, 7257 RSC.
- (a) J. A. Potenza, W. N. Lipscomb, G. D. Vickers and H. A. Schroeder, J. Am. Chem. Soc., 1966, 88, 628 CrossRef CAS; (b) T. F. Koetzle and W. N. Lipscomb, Inorg. Chem., 1970, 9, 2743 CrossRef; (c) Z. Zheng, W. Jiang, A. A. Zinn, C. B. Knobler and M. F. Hawthorne, Inorg. Chem., 1995, 34, 2095 CrossRef CAS; (d) Z. Zheng, C. B. Knobler, M. D. Mortimer, G. Kong and M. F. Hawthorne, Inorg. Chem., 1996, 35, 1235 CrossRef CAS PubMed.
- X.-H. Yu, K. Cao, Y. Huang, J. Yang, J. Li and G. Chang, Chem. Commun., 2014, 50, 4585 RSC.
- F. Teixidor, G. Barberà, A. Vaca, R. Kivekäs, R. Sillanpää, J. Oliva and C. Viñas, J. Am. Chem. Soc., 2005, 127, 10158 CrossRef CAS PubMed.
- To the best of our knowledge, only two examples for the alkenylation of o-carboranes on B(3) and B(4) via iridium catalyzed hydroboration of alkynes developed by Sneddon and Xie, respectively, see ref. 9a and c.
- A. V. Puga, F. Teixidor, R. Sillanpää, R. Kivekäs and C. Viñas, Chem. Commun., 2011, 47, 2252 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Complete characterization data. See DOI: 10.1039/c5ra18555f |
| This journal is © The Royal Society of Chemistry 2015 |