High refractive index and low-birefringence polyamides containing thiazole and naphthalene units
Ali
Javadi
a,
Zahra
Najjar
b,
Saedeh
Bahadori
b,
Vahid
Vatanpour
b,
Ali
Malek
c,
Ebrahim
Abouzari-Lotf
de and
Abbas
Shockravi
*b
aDepartment of Polymer Engineering, University of Akron, Akron, Ohio 44325-0301, USA
bFaculty of Chemistry, Kharazmi University, Mofatteh Avenue 49, 15719-14911 Tehran, Iran. E-mail: abbas_shockravi@yahoo.co.uk; shockravi@khu.ac.ir; Tel: +98-912-2596174
cDepartment of Chemistry, Simon Fraser University, 8888 University Drive, Burnaby, BC V5A 1S6, Canada
dCenter of Hydrogen Energy, Institute of Future Energy, International Campus, Universiti Teknologi Malaysia, 54100 Kuala Lumpur, Malaysia
eMalaysia–Japan International Institute of Technology, Universiti Teknologi Malaysia, 54100 Kuala Lumpur, Malaysia
First published on 22nd October 2015
Abstract
Highly refractive and solution processable polyamides (PAs) were synthesized by the introduction of thiazole rings, naphthalene groups, and thioether linkages. These PAs were synthesized by the polycondensation of a new diamine monomer, 5,5′-thiobis(2-amino-4-(2-naphthyl)thiazole) (DA), with various aromatic diacids. The bulky pendant naphthyl units endowed the resulting PAs with non-coplanar structures and excellent solubilities in organic solvents. The obtained PAs showed high thermal stability, with 10% weight loss temperatures exceeding 478 °C under nitrogen and 431 °C in air atmosphere, while their glass transition temperatures were in the range of 194–229 °C. The synergic effects of the thiazole groups, naphthyl substituents, and thioether linkages provided PAs with very high refractive indices of up to 1.7701 at 632.8 nm, along with small birefringences (<0.0076) and high Abbe's numbers. The structure–property relationships of these PAs due to the presence of naphthyl substituents were also studied in detail by comparing the results with the previously reported analogous PAs.
Introduction
Polymeric materials with high refractive index (high-n), low birefringence (Δn), good optical transparency, and high Abbe's number (high-ν) have received much attention due to their wide range of applications. These materials are highly required in advanced optoelectronic applications including optical encapsulants or adhesives for antireflective coatings,1 substrates for display devices,2 microlens for complementary metal oxide semiconductor (CMOS) image sensors3 or charge coupled device (CCD), etc.4 Compared to inorganic glasses, the high-n polymers show better impact resistance, lightweight, processability, and dying ability. Although the typical refractive index values of conventional polymers are in the range of 1.30–1.65, a very high n value is mainly desired for polymeric microlenses for CMOS image sensors.5Based on the Lorentz–Lorenz equation, introducing substituents with high molar refraction/molar volume ratios ([R]/V0) can increase the n values of polymers.6,7 Hence, the incorporation of sulfur atoms, aromatic rings, heavy halogen atoms, and heteroaromatic rings containing –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C– or –N–N
N–C– or –N–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– bonds is an effective way for developing high-n polymers.4,8 In general, sulfur-containing substituents lead to a high [R] value and are hence used for the synthesis of high refractive index materials.9 Sulfur-containing polymers exhibit high n values which depend on many parameters, such as the aromatic content, sulfur content (Sc) in the repeating unit, and molecular volume.10 Quite recently, we have utilized thioether-bridged diamine monomers derived from thiazole, a heteroaromatic ring containing a sulfur atom and one –C
C– bonds is an effective way for developing high-n polymers.4,8 In general, sulfur-containing substituents lead to a high [R] value and are hence used for the synthesis of high refractive index materials.9 Sulfur-containing polymers exhibit high n values which depend on many parameters, such as the aromatic content, sulfur content (Sc) in the repeating unit, and molecular volume.10 Quite recently, we have utilized thioether-bridged diamine monomers derived from thiazole, a heteroaromatic ring containing a sulfur atom and one –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C– bond, to develop high-n polyamides (PAs) and poly(amide imide)s (PAIs).7,8,11,12 In fact, the thiazole ring was effective for enhancing the Sc while maintaining low V0 and the introduction of this heterocyclic compound as well as the thioether linkages resulted in the thermally stable and solution processable polymers with high n values exceeding 1.73, low Δn (<0.01), and high optical transparency.
N–C– bond, to develop high-n polyamides (PAs) and poly(amide imide)s (PAIs).7,8,11,12 In fact, the thiazole ring was effective for enhancing the Sc while maintaining low V0 and the introduction of this heterocyclic compound as well as the thioether linkages resulted in the thermally stable and solution processable polymers with high n values exceeding 1.73, low Δn (<0.01), and high optical transparency.
Among the high-n polymers, polyimides (PIs) are promising materials for optical applications because of their high inherent refractive indices, good thermal properties, and excellent mechanical properties.13 Over the past decade, several sulfur-containing high-n PIs have been investigated thoroughly by Ueda, Ando, and co-workers.14–17 However, in optoelectronic and microelectronic engineering, most PI films suffer from deep colors, poor optical dispersion, large birefringence, and low solubility originated from the formation of charge transfer complexes in their highly conjugated structures.18 Therefore, it seems that the development of high-n polymeric materials possessing excellent optical properties is still a challenging issue. Several studies have been conducted to modify the molecular interactions of PIs to allow processing by conventional methods, while maintaining the thermal stability. Major structural modifications such as the introduction of non-coplanar or unsymmetrical structures,19 thermally stable but flexible linkages,20 bulky polar or non-polar pendant substituents,21 and hydrogen-bonded amide groups22,23 have been already reported in the literature. On the basis of these considerations, much attention has been devoted to the high-n PAs with more flexible structures than PIs, leading to lower birefringence and higher optical transparency.24–27
Recent studies demonstrated that the introduction of naphthalene units as bulky, rigid, and heat resistant structures, into the polymer backbone can disrupt the crystal packing, reducing intermolecular interactions and increasing solubility and processability of the polymers.28–31 In 2013, we provided an extensive literature review, in which the use of binaphthyl-based systems as either bulky pendant groups or bulky main chain units for developing high performance PAs, PIs, and their copolymers was reported.32 The naphthalene group has a much higher [R]/V0 value (0.392) compared to that of benzene ring having the [R]/V0 value of 0.297.4,7,33 Hence, the incorporation of naphthyl substituents into the polymer backbone can be a promising method of increasing the refractive index of the polymeric materials. Nevertheless, the contribution of naphthalene units to high-n polymers had been neglected in the literature until recently. Ueda and coworkers34 developed high-n poly(phenylene thioether)s with good solubilities by introducing bulky binaphthyl or diphenylfluorene units. These polymers showed good transparency in the visible region, high n values of 1.776 and 1.737, and low birefringences of 0.0049 and 0.0035, respectively. In another study, Voit and coworkers35 reported the synthesis of new naphthalene-containing hyperbranched polymers with high refractive indices by a thiol–yne click reaction. Compared to their previous work,36,37 it was found that the substitution of benzene rings by naphthalene groups significantly increased the refractive index values.
Our previous investigations clarified that thiazole-containing polymers were well suited for highly refractive materials.7,8,11,12 The n values of these polymers could be increased by introducing effective substituents onto the thiazole rings. In order to obtain PAs with even higher refractive indices, we designed and synthesized a novel diamine monomer, 5,5′-thiobis(2-amino-4-(2-naphthyl)thiazole) (DA), containing naphthyl substituents and thiazole moieties. The high performance polyamides were synthesized from this DA and different aromatic diacids. The resulting polymers showed high thermal stability and excellent optical properties which significantly depended on the molecular structure of the polymer main chains. These PAs exhibited good optical transparency (>89% at 450 nm), high refractive index values (1.7570–1.7701 at 632.8 nm), and low birefringences (<0.0076). To the best of our knowledge, the n value of PA-5 (1.7701), derived from 2,2′-dithiodibenzoic acid and DA, is the highest n value reported for highly refractive polyamides. By comparing with the previously developed high-n PAs in our research group,12 the physicochemical and optical properties of the synthesized PAs were investigated in detail to reveal the effect of naphthyl substituents.
Experimental
Materials
2-Acetonaphthone (SIGMA-ALDRICH), thiourea (SIGMA-ALDRICH), iodine (SIGMA-ALDRICH), and triphenyl phosphite (TPP, MERCK) were used without further purification. N-Methyl-2-pyrrolidone (NMP, MERCK) as polymerization solvent and pyridine (Py, Fluka) were purified by distillation under reduced pressure over calcium hydride (CaH2) and stored over 4 Å molecular sieves prior to use. Lithium chloride (LiCl, MERCK) was dried at 200 °C for 24 h under vacuum before use. The aromatic dicarboxylic acids such as terephthalic acid (1; MERCK), isophthalic acid (2; MERCK), pyridine 2,5-dicarboxylic acid (3; MERCK), pyridine 3,5-dicarboxylic acid (4; MERCK), and 2,2′-dithiodibenzoic acid (5; ALDRICH) were used as received. All other chemicals were reagent grade and used as received.Characterization
The melting points (m.p.) were measured by an Electrothermal engineering Ltd 9200 digital melting point apparatus. The elemental analyses of the synthesized monomer and polymers were obtained using a Perkin Elmer 2004 (II) CHN analyzer. The Fourier transform infrared (FT-IR) spectra were measured in potassium bromide (KBr) pellets by means of a Perkin Elmer FT spectrum RX1. The inherent viscosities (ηinh = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ηr/c) were measured at a concentration of 0.5 g dL−1 by an Ubbelohde suspended-level viscometer using N,N-dimethylacetamide (DMAc) at 30 °C. The solution state 1H NMR (300 MHz) and 13C NMR (75 MHz) spectra of the resulting monomer and polymers were obtained on a Bruker DRX 300 AVANCE spectrometer using deuterated dimethyl sulfoxide (DMSO-d6) as a solvent and trimethylsilane (TMS) as the reference (0 ppm). The wide-angle X-ray diffraction (WAXD) patterns of the polymers were recorded on a Philips PW 1800 diffractometer using the graphite monochromatized Cu Kα radiation (wavelength: 0.15401 nm) with 2θ increments of 0.08° s−1 over a range of 2θ = 4–80° at room temperature. The glass transition temperatures (Tgs) were recorded on a 2010 DSC TA apparatus at a heating rate of 10 °C min−1. The thermo-gravimetric analyses (TGA) of the polymers were also obtained with a thermal analyzer (DuPont 2000) at the same heating rate under nitrogen or air atmospheres. The ultraviolet-visible (UV-vis) optical transmittance spectra of the polymers were measured using a Perkin-Elmer Lambda 25 spectrophotometer at room temperature. Before the UV-vis measurements, the polymer films were dried at 100 °C for 2 h. The refractive index values of the polymer films were determined using a prism coupler (Metricon, model PC-2010) equipped with a He–Ne laser light source (wavelength: 632.8 nm) at room temperature. The in-plane (nTE) and out-of-plane (nTM) refractive indices were measured using a linearly polarized laser light parallel and perpendicular polarizations to the polymer film plane, respectively. The average refractive indices (nAV) were calculated based on the equation: nAV = [(2nTE2 + nTM2)/3]½. The birefringences (Δn) of the samples were also calculated as a difference between nTE and nTM values. In the visible region, the wavelength dispersion of refractive indices were recorded using an Abbe refractometer (Atago, model DR-M2) equipped with a halogen lamp and interference filters at the wavelengths of 486.1, 589.3, and 656.3 nm. The Abbe's number (νD) was calculated based on the equation: νD = (nD − 1)/(nF − nC); where nD, nF, and nC are considered as the nAV values measured at 589.3, 486.1, and 656.3 nm, respectively. Moreover, the dielectric constant (ε) values of the PAs were estimated from the refractive indices according to the Maxwell equation: ε = 1.10nAV2.38
ηr/c) were measured at a concentration of 0.5 g dL−1 by an Ubbelohde suspended-level viscometer using N,N-dimethylacetamide (DMAc) at 30 °C. The solution state 1H NMR (300 MHz) and 13C NMR (75 MHz) spectra of the resulting monomer and polymers were obtained on a Bruker DRX 300 AVANCE spectrometer using deuterated dimethyl sulfoxide (DMSO-d6) as a solvent and trimethylsilane (TMS) as the reference (0 ppm). The wide-angle X-ray diffraction (WAXD) patterns of the polymers were recorded on a Philips PW 1800 diffractometer using the graphite monochromatized Cu Kα radiation (wavelength: 0.15401 nm) with 2θ increments of 0.08° s−1 over a range of 2θ = 4–80° at room temperature. The glass transition temperatures (Tgs) were recorded on a 2010 DSC TA apparatus at a heating rate of 10 °C min−1. The thermo-gravimetric analyses (TGA) of the polymers were also obtained with a thermal analyzer (DuPont 2000) at the same heating rate under nitrogen or air atmospheres. The ultraviolet-visible (UV-vis) optical transmittance spectra of the polymers were measured using a Perkin-Elmer Lambda 25 spectrophotometer at room temperature. Before the UV-vis measurements, the polymer films were dried at 100 °C for 2 h. The refractive index values of the polymer films were determined using a prism coupler (Metricon, model PC-2010) equipped with a He–Ne laser light source (wavelength: 632.8 nm) at room temperature. The in-plane (nTE) and out-of-plane (nTM) refractive indices were measured using a linearly polarized laser light parallel and perpendicular polarizations to the polymer film plane, respectively. The average refractive indices (nAV) were calculated based on the equation: nAV = [(2nTE2 + nTM2)/3]½. The birefringences (Δn) of the samples were also calculated as a difference between nTE and nTM values. In the visible region, the wavelength dispersion of refractive indices were recorded using an Abbe refractometer (Atago, model DR-M2) equipped with a halogen lamp and interference filters at the wavelengths of 486.1, 589.3, and 656.3 nm. The Abbe's number (νD) was calculated based on the equation: νD = (nD − 1)/(nF − nC); where nD, nF, and nC are considered as the nAV values measured at 589.3, 486.1, and 656.3 nm, respectively. Moreover, the dielectric constant (ε) values of the PAs were estimated from the refractive indices according to the Maxwell equation: ε = 1.10nAV2.38
Calculations
The calculations were performed by the software package of Gaussian-09 (Rev.B01).39,40 The Lorentz–Lorenz equation was used to calculate the refractive index values of models for the naphthyl-substituted PAs based on the reported method by Ando.41 The geometry optimizations under no constraints was carried out at B3LYP/6-31+G(d,p), and the calculations of linear polarizabilities was performed by the Coulomb-Attenuating Method (CAM-B3LYP) and the 6-311++G(d,p) basis set. In addition, a typical molecular packing coefficient (Kp) of 0.60 was utilized for evaluating the intrinsic V0 values of the PAs models and predicting their refractive indices.42Monomer synthesis
The new thiazole-containing diamine monomer, 5,5′-thiobis(2-amino-4-(2-naphthyl)thiazole) (DA), was synthesized using a reported procedure which was originally developed by Woodbridge and Dougherty43 and was later modified by our laboratory.7,8,11,12 In a three-necked flask equipped with a reflux condenser, 8.50 g (50 mmol) of 2-acetonaphthone, 7.61 g (100 mmol) of thiourea, and 30 mL of ethanol were added. After heating to refluxing temperature with stirring, 12.70 g (50 mmol) of diiodine was added in small portions over 15 min. After addition of diiodine, the mixture was refluxed for 24 h and then, the mixture was poured into 100 mL of cold water. The precipitate was filtered and washed with sodium thiosulfate solution and boiling water to afford 2-amino-4-(2-naphthyl)thiazole (MA) (yield: 91%; melting point: 153–157 °C). IR (KBr): 3440, 3292, 3263, 3111, 1636, 1531, 1323 and 751 cm−1. 1H NMR: 8.31 (s), 7.95–7.85 (m), 7.51–7.42 (m), 7.16 (s), and 7.13 (s, NH2) ppm. 13C NMR: 109.5, 123.4, 124.7, 126.03, 126.6, 126.9, 127.1, 128.2, 128.9, 132.3, 132.7, 148.8, 169.03 ppm. At the second step, a mixture of 2.26 g (10 mmol) of MA and 1.141 g (15 mmol) of thiourea was stirred in 15 mL of ethanol under reflux condition. After 30 min, 2.54 g (10 mmol) of diiodine was added in small portions over 15 min. The reaction mixture was stirred for 2.0 h. At the end of the reaction, the mixture was neutralized with aqueous solution of sodium hydroxide. The solid product was filtered, washed with water and hot ethanol (3 × 100 mL) to produce pure 5,5′-thiobis(2-amino-4-(2-naphthyl)thiazole) (DA) monomer (yield: 30%; melting point: 211–213 °C). IR (KBr): 3456, 3360, 3263, 3099, 1622, 1608, 1527, 1493, 1491, 1322, 1293, 1198, 819, and 748 cm−1. 1H NMR: 8.28 (s), 7.9–7.3 (m), and 7.38 (s, NH2) ppm. 13C NMR: 110.2, 126.2, 126.4, 126.9, 127.1, 127.4, 127.7, 128.2, 131.5, 132.4, 132.5, 152.2, 168.5 ppm.Polymer synthesis and film preparation
The naphthalene-containing polyamides were synthesized by the condensation reaction of DA monomer and various aromatic dicarboxylic acids (1–5) via Yamazaki–Higashi phosphorylation technique. A representative process for the preparation of PA-1 is described as follows. A mixture of 0.482 g (1 mmol) of DA, 0.166 g (1 mmol) of terephthalic acid, 0.3 g of LiCl, 0.6 mL of TPP, 0.6 mL of pyridine, and 2 mL of NMP was heated and stirred at 120 °C for 6 h, under nitrogen atmosphere. As the polycondensation proceeded, the reaction mixture became viscous gradually. After 2.0 h, the viscosity of reaction solution increased and additional amount of NMP (1 mL) was added to the reaction mixture. At the end of the polymerization reaction, the PA solution was slowly poured into 300 mL of methanol under vigorous stirring. The resulting fibrous precipitate was filtered off, washed with hot water (2 × 100 mL) and hot methanol (100 mL) thoroughly, and dried in a vacuum oven at 100 °C overnight. The yield was almost quantitative and further purification was performed by dissolving the PA in NMP, filtering the polymer solution, and reprecipitating into hot methanol. The inherent viscosity of PA-1 in DMAc was 0.93 dL g−1, measured at a concentration of 0.5 g dL−1 at 30 °C. IR (KBr): 3237, 3053, 1669, 1540, 1292, 748 cm−1. Anal. calcd for (C34H20N4O2S3)n: C, 66.65%; H, 3.29%; N, 9.14%. Found C, 66.56%; H, 3.37%; N, 9.06%. A similar method was followed in this study for the synthesis of other PAs. The polymer films were prepared using spin-coating of the purified DMAc solutions containing 5 wt% PAs on silicon wafers. The thickness was mainly controlled by regulating the spinning rate and the viscosity of the PA solution. The purification for eliminating any particulates which could affect the final quality of the thin films was carried out by prefiltration of PAs solutions through a Teflon syringe filter. The polymer thin films were then dried under vacuum at 100 °C for 2 h. The thickness of PAs films for UV measurements was controlled to be ∼10 μm.Results and discussion
Monomer synthesis and characterization
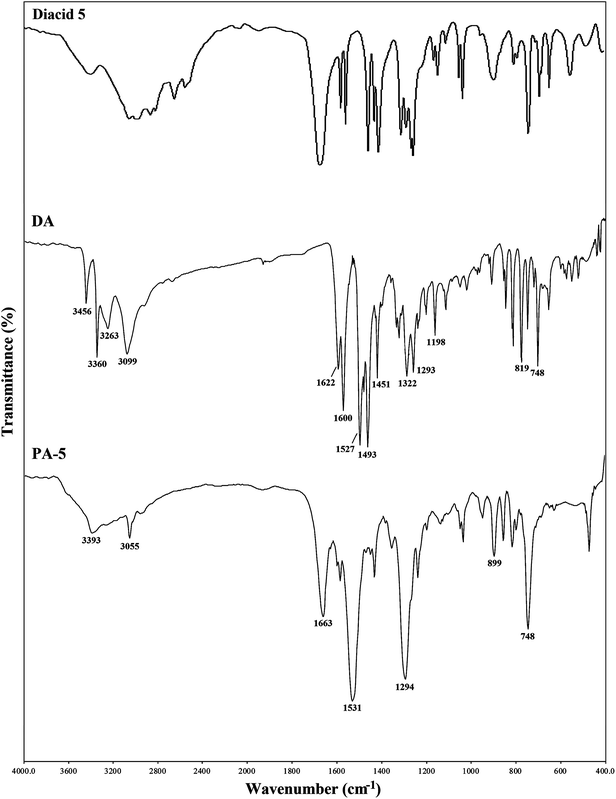
Because the refractive index of polymers is directly related to the chemical structures and electrical and magnetic properties, it is essential to acquire a good understanding of the structure–property relationships for practical design of polymers having improved properties and optical applications. We already reported that the refractive indices of thiazole-containing polymers, as highly refractive materials, could be adjusted by the introduction of effective substituents such as methyl, phenyl, or nitrophenyl groups onto the thiazole rings.7,8,11,12 In order to develop PAs with even higher refractive indices and lower birefringences, in the present study, a novel diamine monomer (DA) containing thiazole units, naphthyl substituents, and thioether linkage was synthesized by a two-step process as shown in Scheme 1. The chemical structure of this monomer was fully characterized by elemental analysis, FT-IR, and NMR spectroscopy. As shown in Fig. 1, the FT-IR spectrum of DA monomer showed the absorption bands due to the primary amino groups (N–H stretching) at 3360 and 3456 cm−1. All peaks detected in the 1H NMR spectrum of the DA monomer were carefully assigned (Fig. 2). The signal at 7.39 ppm was assigned to the amino groups (Ha), and the aromatic protons of the naphthyl substituents were observed at 8.28 (Hb), 7.95–7.73 (Hg, Hc, Hf, and Hh), and 7.52–7.42 ppm (Hd and He). As shown in Fig. 3, thirteen carbon signals were observed in the 13C NMR spectrum of the DA monomer, consistent with the expected chemical structure. The structure of this DA monomer was further confirmed using the elemental analysis, in which the measured C, H, and N compositions agreed well with the theoretical values.Polymer synthesis and characterization
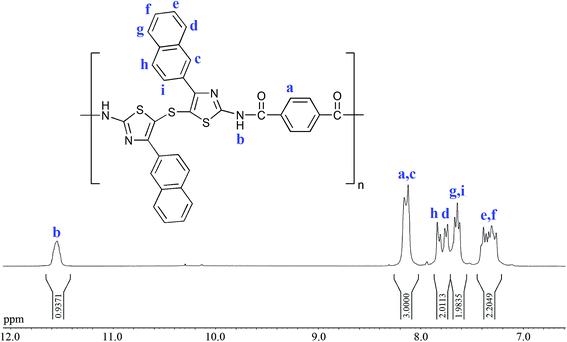
Most intrinsic high refractive index polymers have been synthesized either by radical polymerizations, or by step growth polymerizations, via Michael polyaddition or polycondensation reactions.44 The Yamazaki–Higashi phosphorylation polycondensation of aromatic diamines with aromatic dicarboxylic acids is a very useful laboratory technique to synthesize PAs of moderate to high degrees of polymerization. It avoids using moisture-sensitive acid chlorides or isocyanates.45,46 In this process, the condensing agents, such as TPP and Py and solubility promoters, such as lithium chloride (LiCl) or calcium chloride (CaCl2) are often utilized. In our previous study,12 we employed this method to develop a series of solution processable PAs using 5,5′-thiobis(2-amino-4-phenyl-thiazole) monomer and various aromatic dicarboxylic acids. As expected, the thiazole rings, flexible thioether linkages, and pendant phenyl substituents, endowed the resulting polymers with high n values (1.7414–1.7542), low Δn (0.0061–0.0087), good optical transparency, and high thermal and thermo-oxidative stability.As part of our recent efforts to investigate the effects of different functional groups on the structure–property relationships of high refractive index polymers, a series of new naphthalene-containing PAs was synthesized in this study. The direct polycondensation of DA monomer with aromatic dicarboxylic acids 1–5 was performed in a freshly dried NMP solution of LiCl using TPP and Py as condensing agents at 120 °C for 6 h. Although the polymerizations of PA-2–PA-5 proceeded in the homogeneous solutions, the reaction mixture of PA-1 became hazy and the additional amount of solvent was needed during the reaction. Table 1 displays the synthetic conditions and inherent viscosities of the PAs. These polymers had ηinh values in the range of 0.58–0.93 dL g−1, indicating the formation of high molecular weight polyamides. The chemical structures of the resulting PAs were confirmed by elemental analysis, FT-IR, and 1H NMR spectroscopy. Fig. 1 exhibits the FT-IR spectrum of PA-5 derived from DA and 2,2′-dithiodibenzoic acid as an example. This spectrum showed a broad band around 3300 cm−1 for the N–H stretching frequency. The amide band of PA-5, associated with the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, appeared at 1663 cm−1. In addition, the combined N–H bending and C–N stretching vibration appeared at 1531 cm−1. In order to acquire a more reliable structural characterization, the synthesized polymers were also investigated by 1H NMR spectroscopy. A typical 1H NMR spectrum of PA-1 is shown in Fig. 4, in which all the protons are in good agreement with the proposed chemical structure. The formation of amide linkages was supported by the resonance peak appearing in the most downfield region. As shown in Table 2, the values of the elemental analyses agreed well with the calculated ones for the proposed structures of the resulting PAs. The water uptakes of these PAs were slightly lower than those of the previously reported PAs12 due to the presence of naphthyl substituents.47 In general, these characterization techniques revealed that the new naphthalene-containing PAs developed in this study had the expected chemical structures (Scheme 2).
O group, appeared at 1663 cm−1. In addition, the combined N–H bending and C–N stretching vibration appeared at 1531 cm−1. In order to acquire a more reliable structural characterization, the synthesized polymers were also investigated by 1H NMR spectroscopy. A typical 1H NMR spectrum of PA-1 is shown in Fig. 4, in which all the protons are in good agreement with the proposed chemical structure. The formation of amide linkages was supported by the resonance peak appearing in the most downfield region. As shown in Table 2, the values of the elemental analyses agreed well with the calculated ones for the proposed structures of the resulting PAs. The water uptakes of these PAs were slightly lower than those of the previously reported PAs12 due to the presence of naphthyl substituents.47 In general, these characterization techniques revealed that the new naphthalene-containing PAs developed in this study had the expected chemical structures (Scheme 2).
| Polymer code | Amount of reagent useda | η inh c (dL g−1) | ||
|---|---|---|---|---|
| NMP (mL) | Py (mL) | LiCl (g) | ||
| a Amount of DA and diacid monomer = 1.0 mmol; TPP = 0.6 mL; reaction temperature = 120 °C; reaction time = 6 h. b ‘2 + 1’ means that an initial amount of 2 mL NMP was used and an additional 1 mL of NMP was added when the reaction solution became too viscous. c Measured at a concentration of 0.5 g dL−1 in DMAc at 30 °C. | ||||
| PA-1 | 2 + 1b | 0.6 | 0.30 | 0.93 |
| PA-2 | 2 | 0.5 | 0.25 | 0.75 |
| PA-3 | 2 | 0.6 | 0.30 | 0.90 |
| PA-4 | 2 | 0.5 | 0.25 | 0.69 |
| PA-5 | 2 | 0.5 | 0.20 | 0.58 |
| Polymer code | Formula (formula weight) | Elemental analysis (%) | Moisture uptakea (%) | |||
|---|---|---|---|---|---|---|
| C | H | N | ||||
| a Moisture uptake (%) = (W − W0/W0) × 100%; W: weight of polymer sample after standing at room temperature, W0: weight of polymer sample after being dried in vacuum at 100 °C for 12 h. | ||||||
| PA-1 | (C34H20N4O2S3)n (612.74)n | Calcd | 66.65 | 3.29 | 9.14 | 1.21 |
| Found | 66.56 | 3.37 | 9.06 | |||
| PA-2 | (C34H20N4O2S3)n (612.74)n | Calcd | 66.65 | 3.29 | 9.14 | 1.24 |
| Found | 66.55 | 3.39 | 9.03 | |||
| PA-3 | (C33H19N5O2S3)n (613.73)n | Calcd | 64.58 | 3.12 | 11.41 | 1.30 |
| Found | 64.44 | 3.28 | 11.26 | |||
| PA-4 | (C33H19N5O2S3)n (613.73)n | Calcd | 64.58 | 3.12 | 11.41 | 1.43 |
| Found | 64.40 | 3.29 | 11.21 | |||
| PA-5 | (C40H24N4O2S5)n (752.97)n | Calcd | 63.80 | 3.21 | 7.44 | 1.10 |
| Found | 63.73 | 3.28 | 7.38 | |||
Solubility and morphology of the polymers
Most PAs are very difficult to process due to their poor solubility in common solvents caused by their highly rigid backbones and the strong intermolecular interactions by hydrogen bonding. Therefore, various efforts have been made to enhance their solubilities and processabilities. For example, the introduction of bulky pendent segments results in improving the solubility because of producing chain separation, lowering of chain packing, and weakening of hydrogen bonding.48 Our recent studies28 demonstrated that the incorporation of flexible thioether linkages, bulky pendant naphthalene units, and ortho-catenated aromatic rings into the polymer chains could improve the solubility of aramids, without any significant reduction in thermal stability. In the present study, the solubility characteristics of the naphthalene-containing PAs are summarized in Table 3, and the results are compared to those of the previously reported analogous PAs containing pendant phenyl groups. The solubilities were tested by dissolving 0.05 g of the polymers in 1 mL of different solvents for 24 h at room temperature or upon heating in sealed vials. All the polymers were soluble in the selected aprotic polar solvents such as NMP, DMSO, DMAc, N,N-dimethylformamide (DMF), and pyridine at ambient temperature. In fact, such improved solubility was attributed to the disturbance of interchain packing of the polymer chains by the formation of non-coplanar conformation due to the presence of ortho-sulfide linkages and bulky pendant naphthyl substituents. In general, the solubility of the PAs depended on the dicarboxylic acid used. PA-5 was soluble in less efficient solvents such as THF, acetone, and chloroform at room temperature or upon heating. The increased solubility of PA-5 was ascribed to the highly flexible disulfide linkages and the ortho-linked 2,2′-dithiodibenzoic acid, which further enhanced disorder in the polymer chain and hindered the chain packing, thereby decreasing chain interactions. However, PA-1 was only partially soluble in acetone and swollen in chloroform, because of the presence of rigid and symmetrical 1,4-phenylene rings. In comparison with the previously reported analogous high-n PAs containing phenyl pendant groups,12 these naphthyl-substituted polymers exhibited better solubility. This observation could be attributed to the packing-disruptive naphthyl groups, which produced wider separation of polymer chains and disturbed the regularity and intermolecular interactions.| Polymer code | Solventc | |||||||
|---|---|---|---|---|---|---|---|---|
| NMP | DMSO | DMAc | DMF | THF | Pyridine | Acetone | Chloroform | |
| a (++) soluble at room temperature, (+) soluble after heating, (±) partially soluble, (S) swelling, (−) insoluble. b Solubility: measured at a polymer concentration of 0.05 g mL−1. c NMP: N-methyl-2-pyrrolidone; DMSO: dimethyl sulfoxide; DMAc: N,N-dimethylacetamide; DMF: N,N-dimethylformamide; THF: tetrahydrofuran. d Data in parentheses are the reported data of the analogous polymers (see ref. 12). | ||||||||
| PA-1 | ++ (++)d | ++ (++) | ++ (++) | ++ (++) | + (S) | ++ (±) | ± (−) | S (−) |
| PA-2 | ++ (++) | ++ (++) | ++ (++) | ++ (++) | + (+) | ++ (+) | + (±) | ± (±) |
| PA-3 | ++ (++) | ++ (++) | ++ (++) | ++ (++) | + (S) | ++ (±) | ± (±) | ± (−) |
| PA-4 | ++ (++) | ++ (++) | ++ (++) | ++ (++) | ++ (+) | ++ (+) | + (+) | + (±) |
| PA-5 | ++ (++) | ++ (++) | ++ (++) | ++ (++) | ++ (++) | ++ (++) | ++ (+) | + (+) |
Developing amorphous polymers with optical applications is valuable because the crystalline structure is considered as a defect which interrupts the guiding light and affects the birefringence and refractive index of polymeric materials.49,50 The morphological structures of the synthesized PAs were estimated by wide angle X-ray diffraction (WAXD) in a spectral window ranging from 2θ = 4–80° at room temperature. The curves of all the polymers were broad at 2θ = ∼20–30° and without obvious peak features, which indicated that they were all amorphous in nature. In general, the formation of non-coplanar conformation because of the presence of direct or indirect ortho-linkage between two naphthyl rings can easily disturb interchain packing, decrease glass transition temperature, and improve solubility.32 Similarly, the highly amorphous nature of the PAs synthesized in the present study was mainly attributed to the formation of bent structures by the bulky pendant naphthyl groups and the non-coplanar ortho-linked in the polymer backbone which decreased the backbone regularity and symmetry, subsequently causing a decrease in crystallinity. The amorphous structures of these PAs are also reflected in their excellent solubilities and good film forming abilities.
Thermal properties of the polyamides
The extremely high glass transition temperatures (Tgs) of polyamides, which lie above their decomposition temperatures, result in processing difficulties and limit their commercial applications. The Tg of high-n polymers is an important factor in optical device design and fabrication.51 In the present study, the thermal properties of the naphthyl-substituted PAs were evaluated using differential scanning calorimetry (DSC) and thermo-gravimetric analysis (TGA). As listed in Table 4, the DSC analyses of the PA samples showed no evidence of crystallization and melting processes, which obviously supported the amorphous natures of these polymers. As shown in Fig. 5, the Tg values of the PAs were in the range of 194–229 °C. In comparison with the reported phenyl-substituted PAs,12 the Tg values of these naphthyl-substituted PAs were slightly lower. In fact, the incorporation of naphthalene as either main chain or pendant groups results in the separation of polymer chains, which reduces the packing efficiency and Tgs. On the other hand, bulky naphthyl units restrict molecular mobility and the overall effect is an increase in Tg values. Hence, a balance between these properties must be considered because of this trade-off. As expected, the Tg values of these PAs increased with increasing the polymer backbone rigidity. PA-1, obtained from terephthalic acid, showed the highest Tg because of the rigid para-substituted structure. In contrast, PA-5 exhibited the lowest Tg owing to the flexible disulfide links between the ortho-catenated phenylene units. In addition, the meta-substituted PAs 2 and 4, had lower Tg values than did the analogous para-substituted PAs of 1 and 3, which was attributed to the larger degree of conformational freedom of the meta-linked polymers compared to the more symmetrical para-linked polymers.| Polymer code | DSC | TGA | ||
|---|---|---|---|---|
| Decomposition temperaturea | Char yieldb (%) | |||
| T g c (°C) | In air | In nitrogen | ||
| a Temperature of 10% weight loss determined in nitrogen and air atmospheres. b Residual weight (%) at 700 °C in nitrogen. c T g measured by DSC at scanning rate of 10 °C min−1 in flowing nitrogen. d Data in parentheses are the reported data of the analogous polymers (see ref. 12). | ||||
| PA-1 | 229 (239)d | 470 (451) | 513 (493) | 67 (59) |
| PA-2 | 212 (223) | 452 (437) | 501 (481) | 59 (52) |
| PA-3 | 228 (236) | 465 (442) | 507 (488) | 65 (56) |
| PA-4 | 203 (215) | 450 (435) | 496 (479) | 59 (51) |
| PA-5 | 194 (207) | 431 (424) | 478 (469) | 50 (45) |
Recently, optical polymers with high thermal stability have been desired due to the rapid development of optoelectronic engineering and the increasing demands for high integration, high reliability, and high signal transmission speed in optoelectronic devices. Heterocyclic rings have been widely introduced into the chains of thermally stable polymers such as polyamides, polyesters, polyazomethines, polyureas, polycyanurates, etc.48 In addition, the incorporation of naphthalene units as rigid and heat resistant groups into the polymer backbone is beneficial for increasing the thermal stability of the resulting polymers. In the present study, the effect of polymer structure on the thermal and thermo-oxidative stabilities of the thiazole-containing PAs was determined using TGA at a heating rate of 10 °C min−1 in both nitrogen and air atmospheres. The thermogravimetric curves of the synthesized PAs in nitrogen atmosphere are shown in Fig. 6. The PAs showed high heat resistance and no notable weight loss below 480 °C in nitrogen atmosphere. The temperatures for 10% weight loss (T10) of these PAs were in the range of 478–513 °C in nitrogen and 431–470 °C in air. Since the polymer degradation is because of chain scission, the lowest T10 value of PA-5 among these polymers could be a result of the tendency of the disulfide bonds to cleave. As expected, in comparison with the analogous PAs,12 the prepared naphthyl-substituted PAs exhibited slightly higher T10 values. Moreover, the anaerobic char yields at 700 °C for all PAs were in the range of 50–67 wt% under nitrogen. In particular, PA-1 showed the highest yield due to the presence of rigid para-substituted phenylene rings in the main chain. The high char yields of these PAs could be ascribed to their high aromatic content.
Optical properties of the polyamides
Optically transparent and heat resistant polymeric optical films have recently attracted a great deal of attentions from both of the engineering and academic applications. One of the most challenging issues for the molecular design of optical polymers is the balance among their optical transparency and thermal stability which usually contradict with each other. An effective technique which can improve both of these characteristics is the effective functionalization of the starting monomers through the introduction of alicyclic substituents, trifluoromethyl (–CF3) groups, or asymmetrically-substituted biphenyl or binaphthyl moieties.18 In the present study, the optical properties of the synthesized naphthyl-containing PAs are summarized in Table 5. As shown in Fig. 7, the UV-vis transmission spectra of approximately 10 μm-thick PA films were similar to one another. The cutoff wavelengths (λcutoff) of the PA films, which could be determined from the λ values corresponding to the intersection points of the lines tangent to the curves,14,52 were in the range of 352–386 nm. At 450 nm, the optical transmittances of the synthesized PA films were higher than 89%. The improved transparency of these PAs resulted from their highly twisted conformation owing to the ortho-linked structures, bulky pendant naphthyl groups, and flexible linkages, which decreased the backbone symmetry and prevented the interchain packing. Hence, the introduction of aromatic naphthalene units into the PA chains did not reduce their transparencies. In addition, a comparison of the naphthyl-substituted PAs with those of the reported PAs12 showed that the substitution of naphthyl substituents for phenyl substituents on the thiazole rings slightly increased optical transparency because of the bulkiness of the naphthalene units, which are more likely to disturb well-packed orderings than the phenyl units.| Polymer code | S c a (wt%) | T 450 b (%) | d c (μm) | Refractive indices, birefringences, Abbe numbers, and dispersion coefficients | Dielectric constantsl | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n TE d | n TM e | n AV f | Δng | n calcd h | ν D i | n ∞ j | D k | |||||
| a Sulfur content. b Transmittance at 450 nm. c Film thickness. d n TE: the in-plane refractive index at 632.8 nm. e n TM: the out-of-plane refractive index at 632.8 nm. f Average refractive index; nAV = [(2nTE2 + nTM2)/3]½. g Birefringence; Δn = nTE − nTM. h Calculated refractive index. i Abbe's number; νD = (n589 − 1)/(n486 − n656), see measurements section. j Refractive index at the infinite wavelength. k Coefficient of wavelength dispersion determined by fitting with the simplified Cauchy's formula. l Optically estimated dielectric constant; ε = 1.10nAV2. m Data in parentheses are the reported data of the analogous PAs (see ref. 12). | ||||||||||||
| PA-1 | 15.70 | 89 (85)m | 9.9 | 1.7606 | 1.7530 | 1.7581 (1.7423) | 0.0076 | 1.7580 | 29.69 | 1.7242 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 661 661 |
3.40 |
| PA-2 | 15.70 | 91 (88) | 10.1 | 1.7591 | 1.7528 | 1.7570 (1.7414) | 0.0063 | 1.7550 | 29.88 | 1.7233 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 580 580 |
3.40 |
| PA-3 | 15.67 | 90 (86) | 10.0 | 1.7638 | 1.7564 | 1.7613 (1.7455) | 0.0074 | 1.7630 | 28.62 | 1.7261 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 237 237 |
3.41 |
| PA-4 | 15.67 | 93 (90) | 9.8 | 1.7624 | 1.7564 | 1.7604 (1.7430) | 0.0060 | 1.7590 | 28.90 | 1.7256 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 031 031 |
3.41 |
| PA-5 | 21.29 | 97 (94) | 10.1 | 1.7718 | 1.7668 | 1.7701 (1.7542) | 0.0050 | 1.7720 | 26.79 | 1.7324 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 276 276 |
3.45 |
Refractive index, as one of the most important characteristics of the optical polymers, is affected by various parameters such as the Sc, [R]/V0, molecular geometry, flexibility of polymer chain, and degree of molecular packing (Kp).53 In general, the n values of polymers can be calculated using the Lorentz–Lorenz eqn (1):
 | (1) |
 | (2) |
Based on eqn (2), the incorporation of substituents with high [R]/V0 values can increase the refractive indices of polymers. In a previous study,7 we conducted a detailed study on the relation between the [R]/V0 values and the experimental and calculated refractive index values (nexp, ncalcd) of benzene and its derivatives. When the n values were plotted against [R]/V0, a very good proportional relation was obtained. It was clearly found that the substituents with higher [R]/V0 values than that of hydrogen increase the refractive index of benzene. The [R]/V0 values of these substituents, such as –OCH3, –Cl, –CN, –OH, –NO2, –Br, –C6H5, –OC6H5, –NH2, –COC6H5, and –SC6H5, were in the range of 0.302–0.345.
Recently, our research laboratory has launched a systematic program to use the thiazole-based monomers for development of highly refractive polymeric materials with low birefringence and good optical transparency. The thiazole unit could be effective in increasing Sc and [R], while keeping low V0. In order to construct the thiazole-containing polymers with tunable optical properties, we have already incorporated effective substituents such as methyl, phenyl, or nitrophenyl groups with different [R]/V0 values into a thioether-bridged bithiazole compound as a monomer. As mentioned in the Introduction, the naphthalene unit has a much higher [R]/V0 value (0.392) compared to that of benzene and its derivatives. Therefore, it is expected that the substitution of naphthyl groups for phenyl groups into this bithiazole-based monomer can be very effective for enhancing the n values of the corresponding polymers. In the present study, we designed and synthesized the naphthyl-substituted diamine monomer, 5,5′-thiobis(2-amino-4-(2-naphthyl)thiazole) (DA), to prepare a new series of high-n polyamides.
As shown in Table 5, the in-plane (nTE) and out-of-plane (nTM) refractive indices of the resulting PA films ranged from 1.7591 to 1.7718 and 1.7528 to 1.7668, respectively. The average refractive index values (nAV) of these PAs ranged between 1.7570 and 1.7701. The lower refractive indices of PA-2 and PA-4 than those of PA-1 and PA-3 showed that the meta-linked structures could inhibit molecular packing and led to decreased refractive indices. PA-5 particularly showed the highest refractive index (1.7701) among the high-n polyamides previously reported in the literature.8,10,12,24–27,55 This achievement was attributed to the higher Sc of PA-5 (21.29) due to the presence of disulfide linkages, compared to the Sc values of other PAs. Naturally, the Sc is not the only parameter affecting the refractive index of polymers. For example, because pyridine ring has a higher n and [R]/V0 values than those of benzene,7 PA-3 and PA-4 showed slightly higher refractive index despite lower Sc values than those of PA-1 and PA-2. Moreover, although the substitution of naphthyl groups for phenyl groups into the diamine monomer reduced the Sc, the prepared naphthyl-substituted PAs exhibited higher refractive indices than those of the previously reported phenyl-substituted PAs.12 Therefore, we could conclude that the incorporation of naphthyl substituents compensated for the decrease in the Sc of the resulting PAs.
At 632.8 nm, the calculated refractive index (ncalcd) of the synthesized PAs ranged from 1.7550 to 1.7720. A typical Kp value of 0.60 was assumed to estimate the intrinsic V0 values of models for the naphthyl-substituted PAs. Fig. 8 shows the relationship between the nAV and ncalcd of the resulting PAs, in which a linear relationship with the nAV was observed. These calculations successfully reproduced the average refractive indices of these naphthalene-containing PAs.
The properties of high-n polymers with low birefringence (Δn) are preferable for optical applications such as optical discs and lenses as well as plastic substrates for liquid crystal displays (LCDs). Δn values in polymeric materials depend on many parameters including the polarizability, the preferred orientations of main chain and side chains, and the van der Waals volume of repeating units.56 Hence, considerable research efforts have recently been devoted to the development of functional optical polymers that show a balance of these parameters. As seen in Table 5, all PA films showed higher nTE values than nTM values, indicating that they had positive Δn and the polymer chains preferentially aligned in the film plane. Despite their rigid molecular structures and high refractive indices, the Δn values of these PA films ranged from 0.0050 to 0.0076, which were extremely low values compared to those of the previously reported high-n PAs and PIs in the literature. The low birefringences of these naphthyl-substituted PAs could have been due to the formation of the non-coplanar ortho-linked structures by the flexible thioether links and bulky pendant naphthyl units, which effectively suppressed the in-plane orientation of the aromatic rings in the bulk. PA-5 showed the smallest Δn, which was mainly attributable to the very flexible disulfide linkages in the polymer backbone. In comparison with the previously developed PAs12 in our research group, these naphthyl-substituted PAs showed lower Δn values by ∼0.0010 owing to the bulkier structure of naphthyl than that of a phenyl substituent, which reduced the degree of orientation of the polyamides main chains.
The Abbe's number (νD), which is commonly used to estimate the wavelength dependence of the refractive indices, is quite important for optical materials used in the visible region. The Abbe's number is given by eqn (3):
 | (3) |
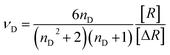 | (4) |
In this equation, νD is directly related to the refractive index, molar refraction, and molar dispersion (ΔR). In conventional optical polymers, there is a trade-off between the nD and νD values. In fact, high-n polymers generally lead to small νD values, which correspond to high dispersion in the refractive indices. In addition, large νD is often expected because the small dispersion of refractive index is desirable for applications in micro-optics and optoelectronics. Although some sulfur-based polymers with both high n and νD values have been reported,57,58 sulfur-containing PAs and PIs with refractive index values of 1.70 or higher generally show νD lower than 25.0.4,12,14 As listed in Table 5, the νD values of the synthesized naphthyl-substituted PAs were in the range of 26.79–29.88. The fact that the νD values of these PAs were slightly higher than those of our previously reported high-n PAs7,8,12 may be due to their very good transparency, though more systematic study on this point should be performed in our future work.
The plots of the wavelength-dependent nAV values measured at the wavelengths of 486, 589, 633, and 656 nm for the synthesized naphthyl-substituted PAs are shown in Fig. 9. The dispersions of nAV were well fitted by a simplified Cauchy's eqn (5):
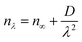 | (5) |
 | ||
| Fig. 10 Relationship between the refractive indices at the infinite wavelength and dispersion coefficients. | ||
Based on the Maxwell eqn (6), the refractive index of a material is related to the dielectric constant (ε) and relative magnetic permittivity (μ):
| 1.10nAV2 = εμ | (6) |
Conclusions
Thiazole and naphthalene units as well as thioether linkages were introduced into the polyamides structures via a newly synthesized diamine monomer. From the experimental results, it could be concluded that the synergic effects of non-coplanar structures and bulky pendant groups were effective for developing the novel thermally stable and organic soluble PAs with high refractive indices, low birefringences, and high optical transparencies. The refractive indices higher than 1.7570, birefringences lower than 0.0076, and optical transparencies higher than 89% at 450 nm were successfully achieved. In particular, PA-5 containing disulfide linkages, showed the highest refractive index for polyamides reported thus far at 1.7701. All these properties were highly desired for advanced optical applications, including the components for advanced optical device fabrications. The structure–property relationships of these PAs due to the presence of bulky naphthyl substituents were also studied in detail.Acknowledgements
This work was mainly supported by the Kharazmi University. A. J. is thankful to Prof. Shinji Ando (Tokyo Institute of Technology) and Dr Mahmood Kamali (Kharazmi University) for helpful discussions.References
- K. C. Krogman, T. Druffel and M. K. Sunkara, Nanotechnology, 2005, 16, S338 CrossRef PubMed.
- T. Higashihara and M. Ueda, Macromolecules, 2015, 48, 1915–1929 CrossRef CAS.
- J. L. Regolini, D. Benoit and P. Morin, Microelectron. Reliab., 2007, 47, 739–742 CrossRef CAS PubMed.
- J.-g. Liu and M. Ueda, J. Mater. Chem., 2009, 19, 8907–8919 RSC.
- Y. Suzuki, K. Murakami, S. Ando, T. Higashihara and M. Ueda, J. Mater. Chem., 2011, 21, 15727–15731 RSC.
- C.-J. Yang and S. A. Jenekhe, Chem. Mater., 1995, 7, 1276–1285 CrossRef CAS.
- A. Javadi, A. Shockravi, A. Rafieimanesh, A. Malek and S. Ando, Polym. Int., 2015, 64, 486–495 CrossRef CAS PubMed.
- A. Javadi, A. Shockravi, M. Koohgard, A. Malek, F. A. Shourkaei and S. Ando, Eur. Polym. J., 2015, 66, 328–341 CrossRef CAS PubMed.
- J. Lim, J. Pyun and K. Char, Angew. Chem., Int. Ed., 2015, 54, 3249–3258 CrossRef CAS PubMed.
- G. Zhang, D.-s. Li, G.-s. Huang, X.-j. Wang, S.-r. Long and J. Yang, React. Funct. Polym., 2011, 71, 775–781 CrossRef CAS PubMed.
- A. Shockravi, A. Javadi, M. Kamali and S. Hajavi, J. Appl. Polym. Sci., 2012, 125, 1521–1529 CrossRef CAS PubMed.
- A. Javadi, A. Shockravi, M. Kamali, A. Rafieimanesh and A. M. Malek, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 3505–3515 CrossRef CAS PubMed.
- D.-J. Liaw, K.-L. Wang, Y.-C. Huang, K.-R. Lee, J.-Y. Lai and C.-S. Ha, Prog. Polym. Sci., 2012, 37, 907–974 CrossRef CAS PubMed.
- J.-g. Liu, Y. Nakamura, Y. Suzuki, Y. Shibasaki, S. Ando and M. Ueda, Macromolecules, 2007, 40, 7902–7909 CrossRef CAS.
- N.-H. You, Y. Suzuki, D. Yorifuji, S. Ando and M. Ueda, Macromolecules, 2008, 41, 6361–6366 CrossRef CAS.
- N.-H. You, N. Fukuzaki, Y. Suzuki, Y. Nakamura, T. Higashihara, S. Ando and M. Ueda, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 4428–4434 CrossRef CAS PubMed.
- N.-H. You, Y. Nakamura, Y. Suzuki, T. Higashihara, S. Ando and M. Ueda, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 4886–4894 CrossRef CAS PubMed.
- H.-j. Ni, J.-g. Liu, Z.-h. Wang and S.-y. Yang, J. Ind. Eng. Chem., 2015, 28, 16–27 CrossRef CAS PubMed.
- Y. Shao, Y.-F. Li, X. Zhao, X.-L. Wang, T. Ma and F.-C. Yang, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 6836–6846 CrossRef CAS PubMed.
- S. Mehdipour-Ataei, S. Babanzadeh and E. Abouzari-Lotf, Des. Monomers Polym., 2015, 18, 451–459 CrossRef CAS PubMed.
- M. G. Dhara and S. Banerjee, Prog. Polym. Sci., 2010, 35, 1022–1077 CrossRef CAS PubMed.
- A. Shockravi, E. Abouzari-Lotf, A. Javadi and F. Atabaki, Eur. Polym. J., 2009, 45, 1599–1606 CrossRef CAS PubMed.
- E. Abouzari-Lotf, A. Shockravi and A. Javadi, Polym. Degrad. Stab., 2011, 96, 1022–1028 CrossRef CAS PubMed.
- G. Zhang, G.-s. Huang, X.-j. Wang, S.-r. Long and J. Yang, J. Polym. Res., 2011, 18, 1261–1268 CrossRef CAS.
- G. Zhang, D.-t. Bai, D.-s. Li, S.-r. Long, X.-j. Wang and J. Yang, Polym. Int., 2013, 62, 1358–1367 CrossRef CAS PubMed.
- H. Kiani, M. Nasef, A. Javadi, E. Abouzari-Lotf and F. Nemati, J. Polym. Res., 2013, 20, 1–12 CAS.
- A. Javadi, E. Abouzari-Lotf, S. Mehdipour-Ataei, M. Zakeri, M. M. Nasef, A. Ahmad and A. Ripin, J. Polym. Sci., Part A: Polym. Chem., 2015 DOI:10.1002/pola.27764.
- A. Shockravi, A. Javadi and E. Abouzari-Lotf, Polym. J., 2011, 43, 816–825 CrossRef CAS PubMed.
- S.-H. Hsiao, C.-P. Yang, C.-Y. Tsai and G.-S. Liou, Eur. Polym. J., 2004, 40, 1081–1094 CrossRef CAS PubMed.
- D.-J. Liaw, F.-C. Chang, M.-k. Leung, M.-Y. Chou and K. Muellen, Macromolecules, 2005, 38, 4024–4029 CrossRef CAS.
- S. Mehdipour-Ataei, Eur. Polym. J., 2005, 41, 91–96 CrossRef CAS PubMed.
- A. Shockravi, A. Javadi and E. Abouzari-Lotf, RSC Adv., 2013, 3, 6717–6746 RSC.
- K. Binran, Handbook of Chemistry, Pure Chemistry, CSJ Publications, 5 edn, Japan, 2004 Search PubMed.
- Y. Nakagawa, Y. Suzuki, T. Higashihara, S. Ando and M. Ueda, Polym. Chem., 2012, 3, 2531–2536 RSC.
- Q. Wei, R. Pötzsch, H. Komber, D. Pospiech and B. Voit, Polymer, 2014, 55, 5600–5607 CrossRef CAS PubMed.
- R. Potzsch, B. C. Stahl, H. Komber, C. J. Hawker and B. I. Voit, Polym. Chem., 2014, 5, 2911–2921 RSC.
- R. Pötzsch, H. Komber, B. C. Stahl, C. J. Hawker and B. I. Voit, Macromol. Rapid Commun., 2013, 34, 1772–1778 CrossRef PubMed.
- D. Boese, H. Lee, D. Y. Yoon, J. D. Swalen and J. F. Rabolt, J. Polym. Sci., Part B: Polym. Phys., 1992, 30, 1321–1327 CrossRef CAS PubMed.
- T. Yanai, D. P. Tew and N. C. Handy, Chem. Phys. Lett., 2004, 393, 51–57 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Journal, 2009, 9096580 Search PubMed.
- S. Ando, J. Photopolym. Sci. Technol., 2006, 19, 351–360 CrossRef CAS.
- Y. Terui and S. Ando, J. Photopolym. Sci. Technol., 2005, 18, 337–340 CrossRef CAS.
- R. G. Woodbridge and G. Dougherty, J. Am. Chem. Soc., 1949, 71, 1744–1745 CrossRef CAS.
- E. K. Macdonald and M. P. Shaver, Polym. Int., 2015, 64, 6–14 CrossRef CAS PubMed.
- N. Yamazaki, M. Matsumoto and F. Higashi, J. Polym. Sci., Polym. Chem. Ed., 1975, 13, 1373–1380 CrossRef CAS PubMed.
- F. Higashi, S.-I. Ogata and Y. Aoki, J. Polym. Sci., Polym. Chem. Ed., 1982, 20, 2081–2087 CrossRef CAS PubMed.
- C. Berti, A. Celli, E. Marianucci and M. Vannini, Eur. Polym. J., 2007, 43, 2453–2461 CrossRef CAS PubMed.
- J. M. García, F. C. García, F. Serna and J. L. de la Peña, Prog. Polym. Sci., 2010, 35, 623–686 CrossRef PubMed.
- H. Ma, A. K. Y. Jen and L. R. Dalton, Adv. Mater., 2002, 14, 1339–1365 CrossRef CAS.
- M. Zhou, Opt. Eng., 2002, 41, 1631–1643 CrossRef CAS PubMed.
- G. Maier, Prog. Polym. Sci., 2001, 26, 3–65 CrossRef CAS.
- Y. Suzuki, J.-g. Liu, Y. Nakamura, Y. Shibasaki, S. Ando and M. Ueda, Polym. J., 2008, 40, 414–420 CrossRef CAS.
- S. Ando, T. Matsuura and S. Sasaki, Polym. J., 1997, 29, 69–76 CrossRef CAS.
- M. Born and E. Wolf, Principles of Optics: Electromagnetic Theory of Propagation, Interference and Diffraction of Light, Cambridge University Press, 7th edn, 1999 Search PubMed.
- G. Zhang, M.-L. Zhang, X.-J. Wang, S.-R. Long and J. Yang, J. Macromol. Sci., Part A: Pure Appl.Chem., 2010, 47, 892–902 CrossRef CAS PubMed.
- Y. Song, J. Wang, G. Li, Q. Sun, X. Jian, J. Teng and H. Zhang, Polymer, 2008, 49, 4995–5001 CrossRef CAS PubMed.
- R. Okutsu, S. Ando and M. Ueda, Chem. Mater., 2008, 20, 4017–4023 CrossRef CAS.
- R. Okutsu, Y. Suzuki, S. Ando and M. Ueda, Macromolecules, 2008, 41, 6165–6168 CrossRef CAS.
- N.-H. You, Y. Suzuki, T. Higashihara, S. Ando and M. Ueda, Polymer, 2009, 50, 789–795 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |