Comparison of microporous/mesoporous and microporous HZSM-5 as catalysts for Friedel–Crafts alkylation of toluene with ethene
Zebastian Bohström*a,
Hanna Härelindb,
Börje Geverta,
Sven-Ingvar Anderssona and
Krister Holmberg*a
aDepartment of Chemical and Biological Engineering, Applied Surface Chemistry, Chalmers University of Technology, SE-412 96 Göteborg, Sweden. E-mail: kh@chalmers.se; zebastian.bostrom@chalmers.se; Fax: +46 31 16 00 62; Tel: +46 31 772 29 69
bDepartment of Chemical and Biological Engineering and Competence Centre for Catalysis, Chalmers University of Technology, SE-412 96 Göteborg, Sweden
First published on 11th June 2014
Abstract
In this work we investigated the effect of mesopores in a standard zeolite used as a catalyst for Friedel–Crafts alkylation of toluene with ethene. A cationic polymer was used for templating mesopores in a microporous ZSM-5 framework. The mesopore-containing zeolite was compared with a regular zeolite with only micropores with respect to conversion, yield and selectivity. The two NaZSM-5 materials were prepared with the same Si/Al molar ratio and diffuse reflection infrared Fourier transform spectroscopy (DRIFT-FTIR) confirmed that the acidity of the ion-exchanged forms (HZSM-5) were identical. Scanning electron microscopy (SEM) and dynamic light scattering (DLS) were used to determine the particle size of the zeolites, which was similar for the two HZSM-5 materials and nitrogen sorption was used to determine the surface area and pore size distribution. X-ray diffraction (XRD) analysis displayed typical crystalline diffraction patterns for the ZSM-5 framework for both the microporous/mesoporous and the microporous ZSM-5 materials. The results from catalytic testing show an increase in the overall conversion of toluene for the zeolite that contains mesopores. Furthermore, a higher product yield (C9) is obtained for this catalyst. The increase in yield and conversion is most likely due to the mesopores; however, incorporation of mesopores in the microporous ZSM-5 framework gives only minor effects on selectivity with respect to mono- vs. dialkylation, and ortho![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) meta
meta![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) para ratio. Consequently, this work shows that the presence of mesopores in a microporous ZSM-5 framework is beneficial for the reaction in terms of conversion of starting material and reaction yield but does not markedly affect the product composition.
para ratio. Consequently, this work shows that the presence of mesopores in a microporous ZSM-5 framework is beneficial for the reaction in terms of conversion of starting material and reaction yield but does not markedly affect the product composition.
Introduction
Microporous zeolites are extensively used as catalysts in industry.1 However, microporous catalysts are often associated with efficiency problems such as short lifetime and poor mass transport.2 The small pores are easily clogged by carbonaceous material, in particular when the reaction temperature is high. Mesoporous catalysts do not exhibit the same mass transport and lifetime limitations as microporous catalysts.3 On the other hand, the use of mesoporous catalysts in industrial processes is often restricted by the low hydrothermal stability of the material.4 Furthermore, large pore catalysts sometimes lack the isomer selectivity that can sometimes be obtained with microporous catalysts.5Thus, both microporous and mesoporous catalysts have specific desirable properties, as well as specific drawbacks associated with the character of the pores. It would therefore be advantageous to combine, in one material, the favorable properties of microporous catalysts with those of mesoporous catalysts, thereby obtaining a uniquely versatile catalyst. Efforts have recently been made to prepare mesoporous zeolites.6 It has been shown that depending on the preparation route and the synthesis conditions zeolites with different populations of micro- and mesopores can be obtained. In this work we prepared microporous zeolites with mesopores penetrating the structure. The crystalline micropore structure is of MFI-type (ZSM-5). The performance of this microporous/mesoporous ZSM-5 zeolite has been studied in the Friedel–Crafts alkylation of toluene with ethene. In addition, we prepared a conventional microporous ZSM-5 zeolite and compared its catalytic activity with that of the microporous/mesoporous ZSM-5. The two ZSM-5 zeolites were prepared with the same Si/Al weight ratio in order to ensure that differences in acidity and activity of the catalysts are due to differences in structure, not in composition. The optimum conversion of toluene into ethyltoluene over zeolitic catalysts is reported to lie in the temperature range from 300 °C to 350 °C.7
Experimental
Materials and reagents
Sodium aluminate (Sigma-Aldrich, technical), tetrapropylammonium hydroxide solution (Sigma-Aldrich, 1 M in H2O), tetraethyl orthosilicate (Sigma-Aldrich) and poly(diallyldimethylammonium chloride)(Aldrich, 65 wt% in H2O) were used to prepare the NaZSM-5 zeolites. Ammonium nitrate (A.C.S. reagent ≥ 98%), LiBO2·H2O (Aldrich) and nitric acid (Sigma-Aldrich, A.C.S. 70%) were used to ion-exchange the zeolites from Na-form to H-form. Toluene (Sigma-Aldrich, 99.9%) and ethene gas (AGA, 95%, containing 3 ± 2 mol% nitrogen) were used for the alkylation reaction. 2-Ethyltoluene (purum, ≥98%, FLUKA), 3-ethyltoluene (purum, ≥98%, FLUKA) and 4-ethyltoluene (≥95%, FLUKA) were used for identification of relative amounts of ethyltoluene isomers in the liquid samples produced from the continuous reactor.Preparation of ZSM-5 zeolites
The microporous and microporous/mesoporous ZSM-5 zeolites were prepared by essentially following the procedure described by Wang et al.8 Sodium aluminate (1.12 g), tetraethyl orthosilicate (39.76 g) and tetrapropylammonium hydroxide (56.0 g) were added to distilled water (140.0 g) and aged at 100 °C under stirring for 3 h. Then the polymer poly(diallyldimethylammonium chloride) (10 wt%, 42.0 g) was added to the mixture. For the preparation of the microporous ZSM-5 no polymer was added. After stirring at room temperature for 48 h, the reaction mixture was transferred into an autoclave and crystallised at 180 °C for 144 h. The product was collected by centrifugation and calcined at 550 °C for 5 h with 1 h ramping.Preparation of the zeolites in H-form
The Na-zeolites were converted into H-zeolite following the procedure described by Punyapalakul et al.9 Ammonium nitrate solution (1.0 M) was mixed with the zeolite at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, solid
20, solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid. The mixture was stirred at 60 °C for 2 h, filtered and washed with Milli-Q water (18.2 MΩ cm). This exchange procedure was repeated. The solid was collected and dried at 100 °C for 12 h and then calcined at 400 °C for 3 h with a 6 h ramping. The degree of ion-exchange was determined with atomic absorption spectroscopy. The first ion-exchange cycle resulted in a 42% exchange of the sodium content in the sample. After two cycles 87% of the sodium had been exchanged.
liquid. The mixture was stirred at 60 °C for 2 h, filtered and washed with Milli-Q water (18.2 MΩ cm). This exchange procedure was repeated. The solid was collected and dried at 100 °C for 12 h and then calcined at 400 °C for 3 h with a 6 h ramping. The degree of ion-exchange was determined with atomic absorption spectroscopy. The first ion-exchange cycle resulted in a 42% exchange of the sodium content in the sample. After two cycles 87% of the sodium had been exchanged.
Friedel–Crafts alkylation reactor setup
The Friedel–Crafts alkylation reactions were performed in a fixed bed down-flow reactor with an internal diameter of 10 mm and a length of 175 mm. The reactor was equipped with a heating jacket and a high sensitivity temperature controller. The setup was designed to carry out a vapour phase reaction under atmospheric pressure. Before the experiments the fixed bed reactor was packed with quartz wool, followed by a layer of glass pellets and another layer of quartz wool. Next, the catalyst powder was added to the reactor and packed tightly. The catalyst powder (4.0 g) consisted of a mixture of zeolite powder (1.25 g) and silicon powder (2.75 g). Subsequently, quartz wool was added on top of the catalyst powder and finally a layer of glass pellets was applied (Table 1).| Zeolite | Nitrogen sorption | Average particle size (nm) | ||||||
|---|---|---|---|---|---|---|---|---|
| as,BETa (m2 g−1) | Calculatedb (r, nm) | Measuredc (r, nm) | Pvolc (cm3 g−1) | Microvold (cm3 g−1) | Vtota (cm3 g−1) | DLSe | SEMf | |
| a Calculated from nitrogen sorption using the BET method.b Calculated 2Vtot/SBET.c Calculated from the adsorption branch of the nitrogen sorption isotherm using the BJH method.d Calculated from t-plot.e Particle size determined from DLS analysis presented as means of 4 independent runs.f Particle size determined with SEM. The values reported are means of 50 particles. | ||||||||
| Micro/mesoporous ZSM5 | 380 | 1.32 | 3.61 | 0.10 | 0.13 | 0.25 | 312 ± 26 | 350 ± 80 |
| Microporous ZSM5 | 372 | 0.91 | — | — | 0.11 | 0.17 | 328 ± 38 | 340 ± 110 |
The reactor was heated to the reaction temperature and ethene was introduced into the system over heated liquid toluene. The ethene–toluene gas mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was fed into the reactor and then separated in a cooling trap with cold glycerol (for flow rates, see Table 2). The gas leaving the cooling trap was periodically analysed and directly fed into a Perkin Elmer Clarus 500 GC equipped with a flame ionization detector (FID-GC) and a thermal conductivity detector (TCD). The condensed products collected in the cooling trap were analysed qualitatively with a GC-MS setup and quantitatively with a GC-FID setup.
1) was fed into the reactor and then separated in a cooling trap with cold glycerol (for flow rates, see Table 2). The gas leaving the cooling trap was periodically analysed and directly fed into a Perkin Elmer Clarus 500 GC equipped with a flame ionization detector (FID-GC) and a thermal conductivity detector (TCD). The condensed products collected in the cooling trap were analysed qualitatively with a GC-MS setup and quantitatively with a GC-FID setup.
| Catalyst | Microporous/mesoporous HZSM-5 | Microporous HZSM-5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| a Calculated from C7, C9 and C11 molar fraction in the liquid samples.b Calculated from in situ GC with TCD detector.c Mol ratio. n.d. = not detected. | ||||||||||
| Temperature (°C) | 325 | 350 | 375 | 400 | 375 | 375 | 325 | 350 | 375 | 400 |
| Flow (ml min−1) | 30 | 30 | 30 | 30 | 15 | 60 | 30 | 30 | 30 | 30 |
| Toluene conversiona (%) | 32 | 61 | 70 | 65 | 70 | 68 | 46 | 49 | 49 | 41 |
| Ethene conversionb (%) | >99 | >99 | >99 | >99 | >99 | 97 | 93 | 95 | >99 | >99 |
| Products (wt%) | ||||||||||
| C5 | n.d. | 5.9 | 5.7 | 5.2 | 4.4 | 3.9 | 4.0 | 1.2 | 0.4 | 0.2 |
| C6 | n.d. | 0.4 | 0.4 | 0.1 | 0.3 | 0.2 | 0.3 | 0.3 | 0.1 | n.d. |
| C7 | 73 | 38 | 30 | 32 | 28 | 30 | 54 | 56 | 57 | 66 |
| C8 | n.d. | 3.5 | 3.8 | 12 | 5.7 | 10 | 0.5 | 0.2 | n.d. | n.d. |
| C9 | 25 | 40 | 46 | 37 | 44 | 38 | 27 | 34 | 34 | 30 |
| C10 | 1 | 5.6 | 8.0 | 5.7 | 9.8 | 7.7 | 6.7 | 2.4 | 2.0 | 0.1 |
| C11 | 1 | 5.1 | 6.0 | 5.9 | 6.2 | 6.7 | 6.5 | 5.9 | 6.5 | 3.7 |
| C12 | n.d. | 0.5 | 0.1 | 2.0 | 1.6 | 3.4 | n.d. | n.d. | n.d. | n.d. |
| C13 | n.d. | n.d. | n.d. | 0.1 | n.d. | 0.1 | n.d. | n.d. | n.d. | n.d. |
| Ethyltoluene C9 | 24 | 38 | 45 | 34 | 42 | 37 | 23 | 33 | 34 | 29 |
| Ortho | — | 3 | 6 | 9 | 9 | 8 | 1 | 6 | 6 | 7 |
| Meta | 48 | 55 | 58 | 59 | 61 | 62 | 54 | 56 | 57 | 56 |
| Para | 52 | 42 | 36 | 32 | 30 | 30 | 45 | 38 | 37 | 37 |
| Mono/di ratioc | 20.3 | 6.4 | 6.2 | 4.8 | 5.8 | 4.6 | 3.4 | 4.7 | 4.2 | 6.6 |
Analysis techniques
Determination of the specific surface area was performed on an ASAP 2010 instrument, using nitrogen adsorption and calculated with the BET (Brunauer–Emmett–Teller) method.10 The pore size distribution was calculated from the isotherms using the BJH (Barett–Joyner–Halenda) procedure.11 All samples were dried at 225 °C in a vacuum oven for approximately 3 h before the measurement.Samples for scanning electron microscopy (SEM) were run on a LEO, Ultra, 55 FEG, SEM equipped with an Oxford Inca EDX system operated at 1–2 kV. A secondary electron detector was used for detection. Samples for SEM were prepared by dispersing a small amount of zeolite in ethanol and then grinding the mixture and placing a droplet onto the metallic sample holder.
Samples for transmission electron microscopy (TEM), run on a JEOL 1200 EX II instrument at 120 kV, were prepared by placing a drop of an ethanol dispersion of the zeolitic material onto a copper Holey grid.
In situ FTIR (Fourier Transform Infrared) spectroscopy measurements were carried out using a BioRad FTS 6000 spectrometer equipped with a Harrick Praying Mantis DRIFT (Diffuse Reflection Infrared Fourier Transform) reaction cell.12 The sample was put in the DRIFT cell and the gases; Ar, NH3 and O2 were introduced via mass flow controllers (Bronkhorst Hi-Tech) to the cell. The samples were initially pre-treated in O2 (8%) at 500 °C for 30 minutes and then evacuated in Ar for 15 minutes (keeping the total flow rate constant at 200 ml min−1). Adsorption of NH3 (1000 ppm) was performed at 25 °C during 30 minutes, followed by evacuation in Ar (6 scans per min, 1 cm−1 resolution). Background spectra were collected in Ar (6 scans per min, 1 cm−1 resolution).
X-ray powder diffraction (XRD) was performed on a LynxEye AXS D8 ADVANCE θ/2θ diffractometer, equipped with a linear detector. The runs were performed at 40 kV and 40 mA, in monochromatic mode with G(111) CuKα1 radiation (λ = 1.5406 Å, step size 0.050, step time 366 s and primary slit width 0.2 mm).
Atomic absorption spectroscopy was performed on a Thermo Scientific ICE 3000 Series AA spectrometer. The solid catalyst (100 mg) was mixed with LiBO2·H2O (1.0 g) in a platinum crucible and the mixture was heated at 1000 °C for 13 minutes. When the crucible had cooled to room temperature, aqueous nitric acid (3 wt%) was added (1.5–3.0 ml) and the mixture in the platinum crucible was stirred at 300 rpm for 6 h. The content in the crucible was added to a 25 ml flask and aqueous nitric acid (3 wt%) was added to the 25 ml mark. A flame was used to atomize the sample and a sodium hollow cathode lamp was used for the irradiation.
Dynamic light scattering (DLS) was performed on a BI-90 Brookhaven Instruments particle sizer. Samples were prepared by taking a small fraction of the solid material and dispersing it into a glass beaker containing 5.0 ml filtered (0.2 μm filter) Milli-Q water (18.2 MΩ cm). The beaker was immersed in a sonic bath for 1 minute. 3.0 ml of this solution was then transferred into another glass beaker containing 3.0 ml filtered (0.2 μm filter) Milli-Q water. This beaker was then immersed into a sonic bath for 1 minute and 2.5 ml of this solution was transferred into the quarts cuvette with a syringe fitted with a 1.2 μm filter and analysed.
Flame ionization and thermal conductivity gas chromatography (FID-GC and TCD-GC, respectively) was performed on a PE Clarus 500 GC and used to monitor the gaseous products formed during the reaction. The instrument was fitted with a standard RGA Model 1115 Analyser GC column set. The injector temperature was 50 °C, helium was used as carrier gas and nitrogen was the actuator gas. Both gases were kept at a pressure of 6.2 bars. The gases were used at a flow rate of 0.91 ml min−1 and the splitflow was 50 ml min−1. The TCD detection temperature was 200 °C and the current used for the detector was 40 mA. The FID detection temperature was 250 °C. Samples from the continuous reactor (5.0 μl) were introduced from a 0.125 and a 1.0 cm3 gas loop valve into the GC. The analysis was performed during 15 minutes at a temperature of 60 °C. The liquid product formed during the reaction was analysed qualitatively with GC-MS and quantitatively with a GC-FID setup. The GC-MS analysis was performed with a HP5890 gas chromatograph coupled to a thermo trace mass spectrometer and the GC was fitted with an Agilent J&W DB-5MS, 30 m × 0.25 mm × 1.0 μm column. The analysis was performed with an injector temperature of 200 °C, helium was used as the carrier gas at a flow of 1.2 ml min−1, the splitflow was 40 ml min−1 and the scans performed at a mode of 40–300 amu at an ion source temperature of 200 °C. The temperature program used started at 40 °C and was maintained at that temperature for 3 min, then ramped to 125 °C at a rate of 4 °C min−1, then increased from 125 °C to 150 °C at a rate of 2.5 °C min−1, and finally went from 150 °C to 200 °C at a rate of 5.0 °C min−1. For the quantitative GC-FID analysis the same GC column and temperature program was used for the qualitative GC-MS analysis. The injector temperature was 275 °C, helium was used as carrier gas at a flow of 0.92 ml min−1 and the splitflow was 40 ml min−1. The FID detection temperature was 275 °C. Toluene, para-ethyltoluene, meta-ethyltoluene and ortho-ethyltoluene were injected into the GC-MS setup to identify the specific retention times of each of these compounds.
Results and discussion
The microporous and the microporous/mesoporous ZSM-5 zeolites
Two different types of zeolites were prepared, microporous NaZSM-5 and microporous/mesoporous NaZSM-5. The zeolites were prepared with the same Si/Al molar ratio. In Fig. 1 the diffractograms of the two NaZSM-5 zeolites are shown. The crystalline structure displayed is typical for the ZSM-5 zeolite type.13 In addition, no difference in intensities was observed between the two ZSM-5 zeolites. The microporous NaZSM-5 displayed a nitrogen sorption isotherm of Type I with a H4 hysteresis characteristic for microporous materials,14 see Fig. 2. The microporous/mesoporous NaZSM-5 displayed a Type IV sorption isotherm and a H3 hysteresis. The Type IV sorption isotherm is typical for mesoporous materials.14 H3 hysteresis has been interpreted as an intermediate between H1 and H4 hysteresis curves.14 The hysteresis ranging from 0.8 to 0.95 seen for the microporous/mesoporous NaZSM-5 zeolite is indicative of hierarchical mesopores.8 In situ DRIFT experiments with ammonia adsorption followed by argon flushing were performed in order to characterize the surface acidity of the samples and the results are shown in Fig. 3. When ammonia is adsorbed on the microporous HZSM-5 sample multiple peaks at 3395, 3275 and 1288 cm−1 evolve. These peaks can, according to Wang et al. and Tsyganenko et al., be ascribed to NH3 adsorbed on Lewis acid sites.15 Another group of peaks at 1572 and 1510 cm−1 can be attributed to NH2 surface species formed concurrently with OH groups during ammonia dissociation.15 Furthermore, the negative peaks at 3689 and 3610 cm−1 are indicative of blocking of surface OH species by adsorbed NH3.15 Similar peaks evolve upon addition of ammonia to the microporous/mesoporous HZSM-5. No significant differences are observed for the two samples, indicating that the acidic sites are similar. This is not surprising since the composition of the two zeolites is the same. Fig. 4 shows scanning electron microscopy (SEM) images of the microporous and the microporous/mesoporous NaZSM-5 zeolites, as well as transmission electron microscopy (TEM) micrograph of the microporous/mesoporous NaZSM-5. The SEM images show that the particle size is similar for the two zeolites. The morphology of the particles is very different, however. Whereas the microporous zeolite particles have a smooth surface and round edges, the microporous/mesoporous zeolite particles are very rough. They appear to consist of granules glued together. The TEM image analysis indicates that the granules are porous or hollow. From the data presented above one can conclude that the microporous and the microporous/mesoporous ZSM-5 zeolites are very similar in terms of particle size, specific surface area and chemical composition, including acidic surface sites. However, as indicated by both the nitrogen sorption analysis and the electron microscopy images the ZSM-5 prepared with a polymer template contains pores not only in the micro size but also in the meso size range. Thus, any difference in yield, rate or selectivity of an organic reaction where these materials are used as catalyst can be attributed to the presence of mesopores in one of the zeolites.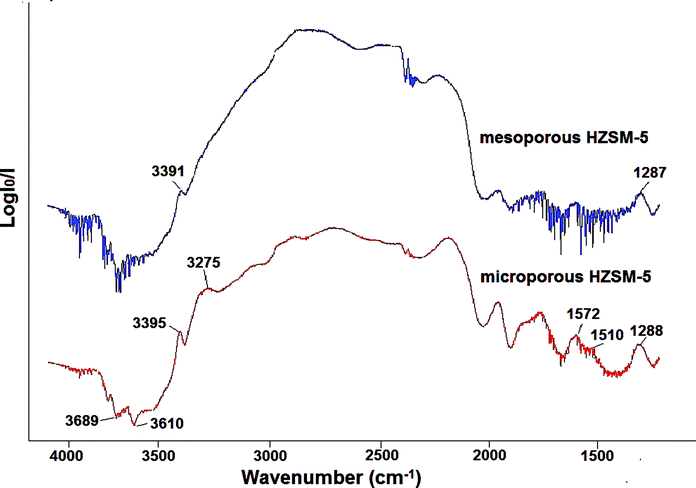 | ||
| Fig. 3 DRIFT spectra showing surface NH3 and NH4+ species after adsorption of ammonia during 30 minutes followed by argon flushing during 30 minutes. | ||
 | ||
| Fig. 4 (a and b) are SEM images of the microporous and the microporous/mesoporous NaZSM-5, respectively. (c) is a TEM image of the microporous/mesoporous NaZSM-5. | ||
The Friedel–Crafts alkylation reaction
Alkylation of toluene over zeolitic catalysts has been the topic of many studies. The activity and selectivity of conventional microporous zeolites, in particular HZSM-5 zeolites, have been investigated indepth.16 In this study we have investigated and compared the conversion, yield and regioselectivity of the microporous and the microporous/mesoporous HZSM-5 zeolites as catalysts for the Friedel–Crafts alkylation of toluene with ethene. However, before any catalytic testing were performed the mesoporous/microporous and microporous ZSM-5 zeolites thermal and hydrothermal stability was investigated. It was found that the mesoporous/microporous ZSM-5 was stable under temperatures and conditions used for catalytic testing.Alkylations at high temperatures in continuous flow-bed reactors usually give rise to several side reactions that occur in parallel to the target alkylation reaction. Transalkylation, disproportionation, dealkylation and catalytic cracking are examples of such side reactions17 and they are illustrated in Scheme 1 for the toluene–ethene system. Furthermore, previous studies at our laboratory have shown that ethene starts to undergo zeolite-catalyzed oligomerization at temperatures around 320 °C. Catalytic cracking over HZSM-5 zeolites is generally performed at temperatures ranging from 550 °C to 650 °C.18 However, catalytic cracking occurs also at lower temperatures.19 Suza et al. recently reported that at 350 °C there was 10–13% cracking conversion of natural gasoline in a continuous flow reactor.19b All these side reactions give rise to other products than the target product of the Friedel–Crafts reaction, i.e., ethyltoluene (and possibly some diethyltoluene) and these products will all appear in the liquid fraction from the reaction. GC analysis showed that the yield of C5–C11 products in the gaseous fraction from the reaction was negligible. Whereas the conversion of ethene is quantitative already at 325 °C with the microporous/mesoporous catalyst, reaction over the conventional microporous zeolite did not reach full ethene conversion until the temperature reached 375 °C. This indicates a slightly higher activity of the catalyst that contains mesopores. The figures for toluene conversion give the same picture; the catalyst with mesopores seems to be more active. The conversion of toluene is clearly temperature dependent, in particular for the microporous/mesoporous catalyst, but it never reaches above 70%. The obvious interpretation of these results is that ethene is a very reactive reactant, which participates in reactions other than the Friedel–Crafts alkylation, seet he discussion above. When it comes to product distribution, the C7 and the C9 fractions dominate, for both catalysts and at all temperatures. The C7 fraction is unreacted toluene and the C9 fraction is mainly, but not exclusively, ethyltoluene. The relative amount of the C9 fraction, and also of ethyltoluene specifically, seems to reach a maximum at around 375 °C for both the catalysts. The fact that the yields of the C9 and the C11 fractions are lower at 400 °C than at 375 °C for both the catalysts indicates that catalytic cracking of monoethyl- and diethyltoluene becomes important at the higher temperature. The drop in yield when going from 375 °C to 400 °C is particularly pronounced for the mesopore-containing catalyst, which suggests that this material is a more active cracking catalyst. However, the yields are generally higher for the microporous/mesoporous catalyst than for the microporous catalyst, which is in line with the values for conversion of toluene. As can be seen from Table 2, several other fractions, in particular C8, C10 and C11, are generated in non-negligible amounts.
The C11 fraction can be attributed to dialkylation, i.e., to formation of diethyltoluene and the ratio of monoalkylation to dialkylation has been calculated and is also given in Table 2. One may have anticipated that the larger pores of the mesopores-containing catalyst would have resulted in more dialkylation but that was evidently not the case. On the contrary, reaction over microporous/mesoporous HZSM-5 at the lowest temperature (325 °C) gives a very high ratio of mono- to dialkylation and reaction over microporous HZSM-5 at the same temperature gives the lowest ratio.
No attempts have been made to derive the formation mechanism for the C8 and C10 fractions. Several of the pathways shown in Scheme 1, as well as ethene oligomerization, may lead to such products. The fact that the difference in yield for the two catalysts is larger at 375 °C than at 325 °C indicates that pore clogging is a deactivation mechanism. It seems reasonable that the catalyst that contains mesopores is more resistant to clogging by high molecular weight carbonaceous materials than the catalyst with only micropores. The drop in reaction yield with both the catalysts when the temperature is raised to 400 °C is probably due to catalytic cracking of the reaction product becoming important, as was discussed above. Table 2 gives the relative amounts of the three regioisomers of ethyltoluene obtained with the two catalysts at the different temperatures. As can be seen, both the microporous/mesoporous and the microporous HZSM-5 give very little alkylation in ortho position. This is in agreement with previous studies on ethylation of toluene with ethene over HZSM-5 zeolite.16a Whereas the thermodynamic equilibrium for ethylation of toluene is para![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) meta
meta![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ortho 30–35
ortho 30–35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15–20 (values are temperature dependent), alkylation with ethene over HZSM-5 gives a much lower yield of the ortho isomer.16e Unmodified HZSM-5 has been reported to give a higher relative amount of the meta isomer and a somewhat lower relative amount of the para isomer. The regioselectivity also depends on the reaction temperature and on the acidity of the catalyst. A decrease in temperature results in an increase in the relative amount of the para isomer.7a The para selectivity also increases when the Brönsted acidity is reduced.16c By a proper choice of catalyst and reaction conditions a para selectivity well above 90% can be achieved. These deviations from the thermodynamical isomer ratio are caused by the fact that ortho-ethyltoluene has the largest and para-ethyltoluene the smallest minimum dimension. The product ratio will depend on the relative rates of diffusion of the regioisomers. It has been demonstrated that the diffusion of the para isomer of ethyltoluene in the pores of a zeolite can be several orders of magnitude higher than that of the bulkier ortho and meta isomers.20 The more slowly moving ortho and meta isomers remain within the zeolite and once the para isomer is generated as a result of the random isomerization that occurs under the influence of the acidic catalyst it rapidly diffuses out. The net result is that the relative amount of para-ethyltoluene can become very high and the relative amount of ortho-ethyltoluene, which is the most voluminous and therefore the most slowly diffusing isomer, virtually zero. Table 2 show that the ortho to meta to para ratio did not differ dramatically for the two types of catalysts. Thus, the large pores in the microporous/mesoporous material did not result in a higher relative yield of the more bulky ortho and meta isomers. For the catalyst with mesopores there was a substantial increase in the meta to para ratio with an increase in temperature. This is in agreement with previously reported trends.7a Reactions over the regular microporous catalyst gave almost the same meta to para ratio at the different temperatures, however. The effect of the ethene flow rate was investigated for microporous/mesoporous HZSM-5 at 375 °C. As can be seen in Table 2, neither the conversion of toluene, nor the product composition was much influenced by this parameter under the conditions studied.
15–20 (values are temperature dependent), alkylation with ethene over HZSM-5 gives a much lower yield of the ortho isomer.16e Unmodified HZSM-5 has been reported to give a higher relative amount of the meta isomer and a somewhat lower relative amount of the para isomer. The regioselectivity also depends on the reaction temperature and on the acidity of the catalyst. A decrease in temperature results in an increase in the relative amount of the para isomer.7a The para selectivity also increases when the Brönsted acidity is reduced.16c By a proper choice of catalyst and reaction conditions a para selectivity well above 90% can be achieved. These deviations from the thermodynamical isomer ratio are caused by the fact that ortho-ethyltoluene has the largest and para-ethyltoluene the smallest minimum dimension. The product ratio will depend on the relative rates of diffusion of the regioisomers. It has been demonstrated that the diffusion of the para isomer of ethyltoluene in the pores of a zeolite can be several orders of magnitude higher than that of the bulkier ortho and meta isomers.20 The more slowly moving ortho and meta isomers remain within the zeolite and once the para isomer is generated as a result of the random isomerization that occurs under the influence of the acidic catalyst it rapidly diffuses out. The net result is that the relative amount of para-ethyltoluene can become very high and the relative amount of ortho-ethyltoluene, which is the most voluminous and therefore the most slowly diffusing isomer, virtually zero. Table 2 show that the ortho to meta to para ratio did not differ dramatically for the two types of catalysts. Thus, the large pores in the microporous/mesoporous material did not result in a higher relative yield of the more bulky ortho and meta isomers. For the catalyst with mesopores there was a substantial increase in the meta to para ratio with an increase in temperature. This is in agreement with previously reported trends.7a Reactions over the regular microporous catalyst gave almost the same meta to para ratio at the different temperatures, however. The effect of the ethene flow rate was investigated for microporous/mesoporous HZSM-5 at 375 °C. As can be seen in Table 2, neither the conversion of toluene, nor the product composition was much influenced by this parameter under the conditions studied.
Conclusion
The microporous/mesoporous HZSM-5 catalyst gave a slightly higher conversion than the conventional microporous zeolite in the Friedel–Crafts alkylation of toluene with ethene. The yield of ethyltoluene was also somewhat higher with the former catalyst. For both catalysts the yield went through a maximum around 375 °C. The difference in yield obtained with the two catalysts increased with increasing temperature. This indicates that clogging of the pores with carbonaceous material is a deactivation mechanism at higher temperature. A catalyst that contains mesopores is likely to be more resistant to clogging than a catalyst that only contains micropores. Catalytic cracking of the products formed, i.e. ethyl- and diethyltoluene, is another possible reason for the decrease in yield at higher temperature. One might have expected the catalyst with mesopores to give a higher ratio of dialkylation to monoalkylation but this was not the case. On the contrary, the mesopore-containing catalyst gave a slightly higher selectivity for monoalkylation than the catalyst with only micropores. There was an interesting difference in regioselectivity for the two catalysts. Whereas the microporous zeolite gave a relatively constant meta to para ratio over the temperature interval studied, the meta to para ratio went from 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 52 at 325 °C to 59
52 at 325 °C to 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 at 400 °C for the microporous/mesoporous zeolite. Both catalysts gave very small yield of the ortho isomer.
32 at 400 °C for the microporous/mesoporous zeolite. Both catalysts gave very small yield of the ortho isomer.
Acknowledgements
Cecilia Thorstensson at AkzoNobel Surface Chemistry is acknowledged for help with the GC-MS analysis. The Swedish Research Council is acknowledged for financial support.Notes and references
- S. Kulprathipanja, Microporous Zeolites in Industrial Catalysis and Separation, Wiley-VCH Verlag GmbH, Weinheim, 2010 Search PubMed.
- (a) M. Guidotti, C. Canaff, J. M. Coustard, P. Magnoux and M. Guisnet, J. Catal., 2005, 230, 375 CrossRef CAS PubMed; (b) E. G. Derouane, C. J. Dillon, D. Bethell and S. B. Derouane-Abd Hamid, J. Catal., 1999, 187, 209 CrossRef CAS; (c) M. E. Davis, Nature, 2002, 417, 813 CrossRef CAS PubMed.
- (a) R. Srivastava, M. Choi and R. Ryoo, Chem. Commun., 2006, 4489 RSC; (b) A. Taguchi and F. Schüth, Microporous Mesoporous Mater., 2005, 77, 1 CrossRef CAS PubMed; (c) Z. Bohström, I. Rico-Lattes and K. Holmberg, Green Chem., 2010, 12, 1861 RSC; (d) Z. Bohström, J. Porous Mater., 2012, 19, 921 CrossRef; (e) Z. Bohström and K. Holmberg, J. Mol. Catal. A: Chem., 2013, 366, 64 CrossRef PubMed.
- Y. Han, N. Li, L. Zhao, D. Li, X. Xu, S. Wu, Y. Di, C. Li, Y. Zou, Y. Yu and F.-S. Xiao, J. Phys. Chem. B, 2003, 197, 7551 CrossRef.
- F.-S. Xiao, L. Wang, C. Yin, K. Lin, Y. Di, J. Li, R. Xu, D. Su, R. Schlögl, T. Yokoi and T. Tatsumi, Angew. Chem., Int. Ed., 2006, 45, 3090 CrossRef CAS PubMed.
- (a) Z. Yang, Y. Xia and R. Mokaya, Adv. Mater., 2004, 16, 727 CrossRef CAS; (b) H. C. Li, Y. Sakamoto, Z. Liu, T. Ohsuna, O. Terasaki, M. Thommes and S. Che, Microporous Mesoporous Mater., 2007, 106, 174 CrossRef CAS PubMed; (c) Y. M. Fang and H. Q. Hu, J. Am. Chem. Soc., 2006, 128, 10636 CrossRef CAS PubMed; (d) W.-C. Li, A.-H. Lu, R. Palkovits, W. Schmidt, B. Spliethoff and F. Schüth, J. Am. Chem. Soc., 2005, 127, 12595 CrossRef CAS PubMed; (e) R. Srivastava, N. Iwasa, S.-I. Fujita and M. Arai, Chem.–Eur. J., 2008, 14, 9507 CrossRef CAS PubMed; (f) H. Wang and T. J. Pinnavaia, Angew. Chem., Int. Ed., 2006, 45, 7603 CrossRef CAS PubMed; (g) M. Choi, H. S. Cho, R. Srivastava, C. Venkatesan, D. Choi and R. Ryoo, Nat. Mater., 2006, 5, 718 CrossRef CAS PubMed; (h) K. Egeblad, M. Kustova, S. K. Klitgaard, K. Zhu and C. H. Christensen, Microporous Mesoporous Mater., 2007, 101, 214 CrossRef CAS PubMed.
- (a) J. Cejka, B. Wichterlov and S. Bednarova, Appl. Catal., A, 1991, 79, 215 CrossRef CAS; (b) S. Rummel, S. M. Yunusov, H. Langguth and V. B. Shuth, Russ. Chem. Bull., 1999, 48, 2083 CrossRef CAS.
- L. Wang, Z. Zhang, C. Yin, Z. Shan and F.-S. Xiao, Microporous Mesoporous Mater., 2010, 131, 58 CrossRef CAS PubMed.
- P. Punyapalakul, S. Soonglerdsongpha, C. Kanlayaprasit, C. Ngamcharussrivichai and S. Khaodhiar, J. Hazard. Mater., 2009, 171, 491 CrossRef CAS PubMed.
- S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 60, 309 CrossRef CAS.
- E. P. Barrett, L. G. Joyner and P. P. Halenda, J. Am. Chem. Soc., 1951, 73, 373 CrossRef CAS.
- (a) A. Hinz, M. Skoglundh, E. Fridell and A. Andersson, J. Catal., 2001, 201, 247 CrossRef CAS; (b) H. H. Ingelsten, Å. Hildesson, E. Fridell and M. Skoglundh, J. Mol. Catal. A: Chem., 2004, 209, 199 CrossRef CAS PubMed.
- S. Z. Chen, K. Huddersman, D. Keir and L. V. C. Rees, Zeolites, 1988, 8, 106 CrossRef CAS.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603 CrossRef CAS.
- (a) Z.-M. Wang, M. Yamaguchi, I. Goto and M. Kumagai, Phys. Chem. Chem. Phys., 2002, 2, 3007 RSC; (b) A. A. Tsyganenko, D. V. Pozdnyakov and V. N. Filimonov, J. Mol. Struct., 1975, 29, 299 CrossRef CAS; (c) A. A. Tsyganenko and V. N. Filimonov, J. Mol. Struct., 1973, 19, 579 CrossRef CAS; (d) A. A. Tsyganenko and P. P. Mardilovich, J. Chem. Soc., Faraday Trans., 1996, 92, 4843 RSC.
- (a) B. S. Kwak and T. J. Kim, Appl. Catal., A, 1999, 188, 99 CrossRef CAS; (b) L. Z. Chen and Y. Q. Feng, Zeolites, 1992, 11, 347 CrossRef; (c) F. Lónyi, J. Engelhardt and D. Kalló, Zeolites, 1991, 11, 169 CrossRef; (d) S. Sealy and Y. Traa, Appl. Catal., A, 2005, 294, 273 CrossRef CAS PubMed; (e) W. W. Kaeding, L. B. Young and C.-C. Chu, J. Catal., 1984, 89, 267 CrossRef CAS; (f) V. Mavrodinova, Ch. Minchev, V. Penchev and H. Lechert, Zeolites, 1985, 4, 217 CrossRef.
- (a) N. Rahimi and R. Karimzadeh, Appl. Catal., A, 2011, 398, 1 CrossRef CAS PubMed; (b) T.-C. Tsai, S.-B. Liu and I. Wang, Appl. Catal., A, 1999, 181, 355 CrossRef CAS.
- C. Song, J. Garces and Y. Sugi, Shape Selective Catalysis - Chemical Synthesis and Hydrocarbon Processing, ACS Symposium Series, Washington DC, 1999 Search PubMed.
- (a) A. Marcilla, A. Gómez-Siurana and F. J. Valdés, Microporous Mesoporous Mater., 2008, 109, 420 CrossRef CAS PubMed; (b) M. J. B. Souza, F. A. N. Fernandes, A. M. G. Pedrosa and A. S. Araujo, Fuel Process. Technol., 2008, 89, 819 CrossRef CAS PubMed.
- N. Y. Chen, W. W. Kaeding and F. G. Dwyer, J. Am. Chem. Soc., 1979, 101, 6783 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |



