Microwave-assisted synthesis of asymmetric disulfides†
Abstract
Thiourea is very useful in the synthesis of asymmetric and symmetric disulfides. In this paper, we report an efficient, odorless, and one-pot procedure for the synthesis of benzyl alkyl disulfides by using the mixtures of thiourea, alkyl halides, and benzyl thiocyanates. The reaction was carried out in water with the assistance of microwaves, enabling a time-saving process. Optimal conditions were obtained as follows: benzyl thiocyanate (1.0 equiv.), thiourea (2.0 equiv.), and alkyl halide (1.2 equiv.) were reacted under microwave irradiation using K3PO4 as the base in water at 90 °C for 15 min. In most cases, the yields are higher than 80%. In this protocol, the substrates are readily available and their synthesis is much easier than the corresponding thiols. Moreover, it is environmentally and economically friendly.
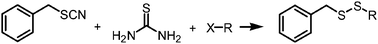

 Please wait while we load your content...
Please wait while we load your content...