K–O2 electrochemistry at the Au/DMSO interface probed by in situ spectroscopy and theoretical calculations
Abstract
The reaction mechanism underpinning the operation of K–O2 batteries, particularly the O2 reactions at the positive electrode, is still not completely understood. In this work, by combining in situ Raman spectroelectrochemistry and density functional theory calculations, we report on a fundamental study of K–O2 electrochemistry at a model interface of Au electrode/DMSO electrolyte. The key products and intermediates (O2−, KO2 and K2O2) are identified and their dependency on the electrode potential is revealed. At high potentials, the first reduction intermediate of O2−* radical anions (* denotes the adsorbed state) can desorb from the Au electrode surface and combine with K+ cations in the electrolyte producing KO2via a solution-mediated pathway. At low potentials, O2 can be directly reduced to 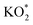 on the Au electrode surface, which can be further reduced to
on the Au electrode surface, which can be further reduced to 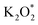 at extremely low potentials. The fact that K2O2 has only been detected in the very high overpotential regime indicates a lack of KO2 disproportionation reaction both on the Au electrode surface and in the electrolyte solution. This work addresses the fundamental mechanism and origin of the high reversibility of the aprotic K–O2 batteries.
at extremely low potentials. The fact that K2O2 has only been detected in the very high overpotential regime indicates a lack of KO2 disproportionation reaction both on the Au electrode surface and in the electrolyte solution. This work addresses the fundamental mechanism and origin of the high reversibility of the aprotic K–O2 batteries.

- This article is part of the themed collection: Rechargeable non-aqueous metal-oxygen batteries


 Please wait while we load your content...
Please wait while we load your content...