Ring opening polymerization of β-acetoxy-δ-methylvalerolactone, a triacetic acid lactone derivative†
Abstract
We report here the synthesis and polymerization of a novel disubstituted valerolactone, β-acetoxy-δ-methylvalerolactone, derived from the renewable feedstock triacetic acid lactone (TAL). The bulk polymerization proceeds to 45% equilibrium monomer conversion at room temperature using diphenyl phosphate as the organic catalyst. The resultant amorphous material displays a glass transition temperature of 25 °C. The ring opening polymerization (ROP) behavior of the disubstituted valerolactone was examined, and the enthalpy (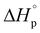 ) and entropy *(
) and entropy *(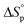 ) of polymerization were calculated to be −25 ± 2 kJ mol−1 and −81 ± 5 mol−1 K−1, respectively. The polymerization kinetics were also measured and compared to those of other substituted valerolactones reported in the literature. This report is the first to demonstrate the successful ROP of a disubstituted valerolactone as well as the first to establish the ROP of a derivative of TAL.
) of polymerization were calculated to be −25 ± 2 kJ mol−1 and −81 ± 5 mol−1 K−1, respectively. The polymerization kinetics were also measured and compared to those of other substituted valerolactones reported in the literature. This report is the first to demonstrate the successful ROP of a disubstituted valerolactone as well as the first to establish the ROP of a derivative of TAL.

- This article is part of the themed collection: Sustainable Polymers


 Please wait while we load your content...
Please wait while we load your content...