Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ozone, hydrogen peroxide, and peroxymonosulfate disinfection of MS2 coliphage in water†
Zi-Chen
Yang
a,
Wen-Long
Wang
a,
Zi-Bo
Jing
a,
Yi-Qing
Jiang
a,
He-Qing
Zhang
b,
Min-Yong
Lee
c,
Lu
Peng
 *a and
Qian-Yuan
Wu
*a and
Qian-Yuan
Wu
 a
a
aKey Laboratory of Microorganism Application and Risk Control of Shenzhen, Guangdong Provincial Engineering Research Center for Urban Water Recycling and Environmental Safety, Institute of Environment and Ecology, Tsinghua Shenzhen International Graduate School, Tsinghua University, Shenzhen 518055, China. E-mail: penglu@sz.tsinghua.edu.cn
bCSCEC Scimee Sci.&Tech. Co., Ltd., Beijing 100084, PR China
cNational Institute of Environment Research, Ministry of Environment, Incheon 22689, Republic of Korea
First published on 7th February 2024
Abstract
The control of viruses in water is critical to preventing the spread of infectious viral diseases. Many oxidants can inactivate viruses, and this study aims to systematically compare the disinfection effects of ozone (O3), peroxymonosulfate (PMS), and hydrogen peroxide (H2O2) on MS2 coliphage. The effects of oxidant dose and contact time on disinfection were explored, as were the disinfection effects of three oxidizing agents in secondary effluent. The 4-log inactivation of MS2 coliphage required 0.05 mM O3, 0.5 mM PMS, or 25 mM H2O2 with a contact time of 30 min. All three oxidants achieved at least 4-log disinfection within 30 min, and O3 required only 0.5 min. In secondary effluent, all three oxidants also achieved 4-log inactivation of MS2 coliphage. Excitation–emission matrix (EEM) results indicate that all three oxidants removed dissolved organic matter synchronously and O3 oxidized dissolved organic matter more thoroughly while maintaining disinfection efficacy. Considering the criteria of oxidant dose, contact time, and disinfection efficacy in secondary effluent, O3 is the best choice for MS2 coliphage disinfection among the three oxidants.
Environmental significanceDisinfection is considered to be a critical step in controlling the spread of viruses in water. Oxidants are effective viral disinfectants. However, conclusive studies are lacking in the relative efficacy of oxidants for viral inactivation, and the disinfection performance in real water samples is not fully clear. In this study, the disinfection effects of ozone (O3), hydrogen peroxide (H2O2), and peroxymonosulfate (PMS) against MS2 coliphage with different doses and contact times were evaluated. The results showed that O3 inactivated MS2 coliphage at lower doses with the shortest contact time. To achieve 4-log disinfection of MS2 coliphage, the oxidant doses required were ranked as O3 < PMS < H2O2 and the contact times required were ranked as O3 < H2O2 < PMS. Besides, the disinfection performance of the three oxidants in deionized water and secondary effluent was comprehensively compared. All three oxidants achieved 4-log inactivation of MS2 coliphage. Excitation–emission matrix (EEM) results indicated that all three oxidants removed dissolved organic matter synchronously and O3 oxidized dissolved organic matter more thoroughly while maintaining disinfection efficacy. To sum up, O3 is the best choice for MS2 coliphage disinfection among these three oxidants. The results enriched the research of virus disinfection in water and provided a theoretical basis for further studies of the dosage of oxidants in industrial practice. |
1 Introduction
Viral infectious diseases pose worldwide risks to public health, and the control of viral pathogens in water is a priority. There are various viruses in water that can affect human health and cause a variety of diseases,1 including polioviruses, adenoviruses, rotaviruses, noroviruses, and others. Human adenoviruses (Ads) can infect the respiratory system, intestines, and eyes,2 and cause various diseases including conjunctivitis, haemorrhagic cystitis, meningoencephalitis, and gastroenteritis.3 Rotaviruses of serogroups A–C can infect the gastrointestinal tract, and almost 128![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 children (age < 5 years) worldwide died from childhood diarrhoea caused by rotavirus in 2016.4 Noroviruses are widespread waterborne pathogens transmitted via the faecal-oral route and are a major cause of gastroenteritis,5 which cause long-term symptoms in immunocompromised people.6 Moreover, viruses can persist widely in the environment for a long time in surface water, wastewater, and even in treated effluents or drinking water.6 Iaconelli et al. detected enteroviruses, human adenoviruses, hepatitis A virus, and hepatitis E virus in raw sewage water,7 and astroviruses and rotaviruses have also been found in surface waters throughout the world.8,9 Furthermore, according to Rodríguez-Lázaro et al., hepatitis A virus can survive for almost 60 days in tap water and can subsist for more than 6 weeks in surface water.10 Mouse hepatitis virus and transmissible gastroenteritis virus can survive for 4–25 days in 3 °C wastewater after pasteurization.11 Controlling viruses in water is therefore crucial.
500 children (age < 5 years) worldwide died from childhood diarrhoea caused by rotavirus in 2016.4 Noroviruses are widespread waterborne pathogens transmitted via the faecal-oral route and are a major cause of gastroenteritis,5 which cause long-term symptoms in immunocompromised people.6 Moreover, viruses can persist widely in the environment for a long time in surface water, wastewater, and even in treated effluents or drinking water.6 Iaconelli et al. detected enteroviruses, human adenoviruses, hepatitis A virus, and hepatitis E virus in raw sewage water,7 and astroviruses and rotaviruses have also been found in surface waters throughout the world.8,9 Furthermore, according to Rodríguez-Lázaro et al., hepatitis A virus can survive for almost 60 days in tap water and can subsist for more than 6 weeks in surface water.10 Mouse hepatitis virus and transmissible gastroenteritis virus can survive for 4–25 days in 3 °C wastewater after pasteurization.11 Controlling viruses in water is therefore crucial.
Bacteriophages are common surrogates for virus disinfection because of their safety of cultivation,12 and they are similar to mammalian viral pathogens in many characteristics such as size and morphology.13 MS2 coliphage, which is from Leviviridae, is an icosahedral virus with positive-sense single-stranded RNA.14 The host of MS2 is Escherichia coli, and it appears frequently in sewage. In previous environmental virology studies, MS2 coliphage is used as a surrogate for many human enteric viruses,15 such as hepatitis A virus16 and norovirus.13 Therefore, it is feasible to use MS2 coliphage as a surrogate to study virus inactivation in water.
Several oxidants are effective viral disinfectants. Currently, chlorination is commonly used for disinfection in practical applications, but the potential health risks of disinfection by-products cannot be ignored,17,18 and other oxidants must be identified. Ozone (O3) is an extensively used oxidant to which most viruses are sensitive.19 O3 is effective in removing enteroviruses including astrovirus, hepatitis A virus, hepatitis E virus, norovirus, mengovirus, and rotavirus. Ozone-based inactivation exceeds 4-log disinfection (achieved by conventional treatment) by an additional 1–2 logs, and most enteroviruses are reduced to undetectable levels following ozonation.20 This shows that O3 disinfection is effective in preventing the spread of viruses in water. Peroxymonosulfate (PMS) is a broad-spectrum oxidant that can inactivate viruses. The concentration and contact time of PMS affect the inactivation performance against viruses, and Tulalamba et al. found that 93.7% of SARS-CoV-2 virions can be inactivated with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 w/v potassium peroxymonosulfate solution.21 Hydrogen peroxide (H2O2) is a widely used oxidizing agent that is common in oral therapy because it quickly inactivates pathogenic microorganisms.22 H2O2 is also used for the sterilization of surfaces and surgical tools because it is effective against various pathogenic microorganisms.
100 w/v potassium peroxymonosulfate solution.21 Hydrogen peroxide (H2O2) is a widely used oxidizing agent that is common in oral therapy because it quickly inactivates pathogenic microorganisms.22 H2O2 is also used for the sterilization of surfaces and surgical tools because it is effective against various pathogenic microorganisms.
Although O3, H2O2, and PMS are all proven viral disinfectants, conclusive studies are lacking on the relative efficacy of these oxidants for viral inactivation. Previous studies have used different conditions so it is difficult to make comparisons. More research is also required on the performance of these three oxidants in actual water disinfection. In this study, O3, H2O2, and PMS were used to disinfect MS2 coliphage under uniform experimental conditions while exploring the effects of dose and contact time, which were the common parameters of water disinfection.23 In addition, the potential of these oxidants in practical applications was investigated by testing disinfection performance against MS2 coliphage in deionized (DI) water and secondary effluents as a reference for actual water treatment. This study aimed at comparing the potential of O3, PMS, and H2O2, in virus disinfection in water, and providing a theoretical basis for the selection and dosage of oxidants in industrial practice.
2 Materials and methods
2.1 Materials
Coliphage MS2 liquid medium, coliphage MS2 semisolid medium, and Luria–Bertani (LB) nutrient agar were supplied by Qingdao Hope Bio-Technology Co., Ltd. (Qingdao, China). PMS, potassium iodide, and potassium phthalate monobasic were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Ammonium molybdate was purchased from Tianjin Damao Chemical Reagent Factory (Tianjin, China). H2O2 (30%) was purchased from Guangdong Guanghua Sci-Tech Co., Ltd. (Guangdong, China). Sodium thiosulfate, disodium phosphate, and sodium dihydrogen phosphate were purchased from Macklin Biochemical Co., Ltd. (Shanghai, China). O3 was produced by using an ozone generator (Absolute Ozone ATLAS 30, Absolute Ozone, Canada). Deionized (DI) water was produced by using a Milli-Q Water System (Millipore, USA) and sterilized using a high-pressure sterilizer (Panasonic MLS-3751L). All the solutions were prepared using DI water unless otherwise stated.2.2 Experimental methods
O3 was provided by saturated ozonated ultrapure water, which was generated by using an ozone generator (Absolute Ozone ATLAS 30, Absolute Ozone, Canada), and the concentration of ozone was determined with a PTH 043 ozone analyzer (Palintest, UK). H2O2 and PMS were prepared in a volumetric flask, and the concentration of H2O2 was measured by the KI method.24 PMS was determined by the DPD colorimetric method with a Hach Pocket Colorimeter II (Loveland, CO).
For disinfection, O3, H2O2, or PMS was added in conical flasks, with the beginning of contact time. In oxidant dose experiments, 0.001, 0.005, 0.01, 0.03, 0.05, 0.10, 0.25, 0.50, and 1 mM O3, 0.01, 0.1, 0.25, 0.50, 0.75, 1.00, 2.50, and 5.00 mM PMS, and 0.01, 0.05, 0.10, 0.50, 1.00, 2.50, 5.00, 10.00, 25.00, 50.00, 100.00, and 200.00 mM H2O2 were added. And 1 mL sample was taken from the system at 30 minutes. In the contact time experiments, 1 mL sample was taken at 0.5, 1, 1.5, 3, 5, 10, and 30 minutes.
In the secondary effluent disinfection experiment, the secondary effluent was obtained from a municipal sewage treatment plant in Shenzhen, China. The secondary effluent was first filtered through a 0.22 μm PES microporous membrane and stored in a refrigerator at 2–4 °C. Before the disinfection experiment, the secondary effluent was filtered through a 0.22 μm PES microporous membrane, and its water quality was determined. And 1 mL MS2 coliphage stock (109 PFU mL−1) was added into 1 L secondary effluent, and 50 mL aliquots (106 PFU per mL MS2 coliphage) were added into the conical flasks. 0.1 mM O3, 1 mM PMS, and 50 mM H2O2 were added into the conical flasks, and 1 mL sample was taken at 30 minutes.
The residual oxidant was quenched with sodium thiosulfate at 1.5 times the stoichiometric ratio. Experiments were performed in triplicate.
To evaluate each disinfection process, the logarithmic inactivation efficiency of MS2 coliphage was calculated according to eqn (1):
| Logarithmic inactivation efficiency = log10(N0/Nt) | (1) |
The CT of PMS and H2O2 was calculated by multiplying the geometric mean of the initial and final residual concentration and the contact time, and the CT of O3 was calculated from the integrated area under the ozone decay curve. The excitation–emission matrix (EEM) of water samples was tested by using an F-7000 fluorescence spectrophotometer (Hitachi Ltd. of Japan) and analyzed by the fluorescence regional integration (FRI) method.
2.3 Experimental quality control
The drugs, tools, and equipment used in the experiments were all sterilized using a high-pressure sterilizer (Panasonic MLS-3751L), and 75% ethanol was used to disinfect hands and prevent environmental contamination. Following the experiment, contaminated items were disposed of to avoid environmental impacts, ensure test quality, and minimize deviations.3 Results and discussion
3.1 Effect of oxidant dose
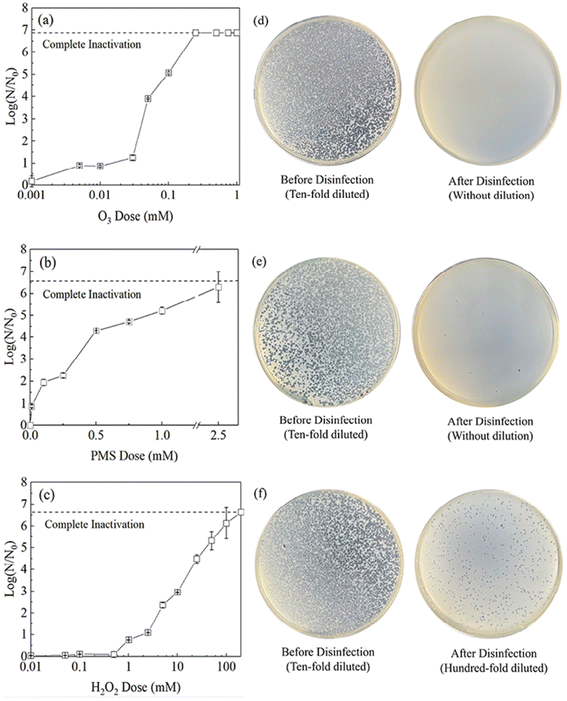
As shown in Fig. 1, O3 inactivates MS2 coliphage at lower doses than are required for the other oxidants, with the contact time limited to 30 min. To achieve the conventional requirement of 4-log (99.99%) inactivation of MS2 coliphage, the oxidants were required at varying doses for O3 (0.05 mM), PMS (0.5 mM), and H2O2 (25 mM). Fig. 1d–f show disinfection performance for each of the three oxidants at 1 mM; the number of plaques on each plate was reduced significantly after disinfection, and O3 outperformed the other oxidants at this dose.At low concentrations, O3 and PMS can inactivate MS2 coliphage. As shown in Fig. 1a–c, O3 and PMS reach 0.9-log inactivation of MS2 coliphage at concentrations of 0.005 mM and 0.01 mM, while H2O2 has no obvious effect at this dose. Fang et al. found 0.1 mg per L O3 achieved around 1-log inactivation of MS2 coliphage,25 which is similar to our result. O3 may inactivate MS2 coliphage at low doses because O3 can degrade amino acids at low doses. Methionine on the MS2 coliphage surface was preferentially targeted.26 H2O2 is only effective (reaching 0.8-log inactivation) at concentrations of 1 mM and above.
The inactivation potential of O3 increases at higher concentrations, achieving complete inactivation (6.9-log) of MS2 coliphage at 0.25 mM, while higher doses of PMS (2.5 mM) and H2O2 (100 mM) are required to achieve the same effect. Thus, O3 is more potent than PMS and H2O2 by a factor of 10× and 400×, respectively, possibly because many viruses are sensitive to O3. Kong et al. found that 2 mg min per L O3 can achieve 4-log inactivation of most viruses.19 Shin et al. found that 0.37 mg per L O3 can achieve 3-log inactivation of poliovirus 1 virus and >3-log inactivation of Norwalk virus within 10 s.5 The protein folding and higher-order structures of capsid proteins are more vulnerable to O3 at tyrosine, histidine, cysteine, and methionine residues.27
3.2 Effect of contact time
Because of the large differences in disinfection potency among the three oxidants, a single concentration was chosen for each oxidant that achieves 5-log inactivation so that the influence of contact time on disinfection could be studied. We used 0.1 mM O3, 1 mM PMS, and 50 mM H2O2. The decay of 0.1 mM O3, 1 mM PMS, and 50 mM H2O2 within 30 min is shown in Fig. S1.† The decay of O3 was fast, and those of PMS and H2O2 were not obvious.As shown in Fig. 2, disinfection by O3 is more effective and with a very brief contact time; O3 achieves 4.6-log disinfection within 0.5 min, and this increases to 5.4-log disinfection in 30 min. According to previous studies, O3 disinfection mainly relies on ozone molecules,5,28 and residual O3 decreases rapidly in 30 s.23,29 These results demonstrate that O3 can achieve 4-log inactivation of MS2 coliphage in 0.5 min (CT: 2.28 mg min L−1).
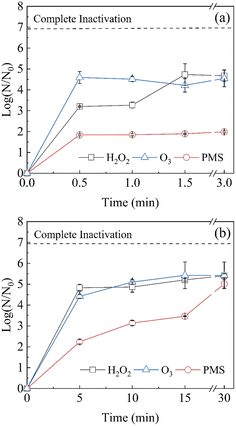 | ||
| Fig. 2 Inactivation efficiency of MS2 coliphage using O3 (0.1 mM), PMS (1 mM), and H2O2 (50 mM) at different contact times (a) 0.5–3 min, and (b) 5–30 min. | ||
H2O2 requires a relatively short contact time, achieving 3.2-log disinfection within 0.5 min (CT: 836.72 mg min L−1), and 4.7-log disinfection can be achieved after 1.5 min (CT: 2513.28 mg min L−1) and then remaining stable. H2O2 mainly disinfects via hydroxyl radicals (˙OH),30 and the slow diffusion of hydroxyl radicals in viruses may limit disinfection by H2O2.31
MS2 coliphage inactivation by PMS reaches only 1.8-log at 0.5 min (CT: 155.44 mg min L−1), and disinfection slowly increases to 5.0-log at 30 min (CT: 8495.22 mg min L−1). Rhee et al. found that 0.3 g per L PMS achieves 4-log inactivation of MS2 coliphage within 30 min,32 which is consistent with the result. Disinfection by PMS occurs via the production of ˙OH, sulfate radicals (SO4˙−), and 1O2.33 Both H2O2 and PMS require free radical production to disinfect MS2 coliphage, and the disinfection rates of these oxidants are significantly slower than that of O3. Extended contact times may allow lower concentrations of PMS and H2O2 to disinfect to the same degree. In some studies, 25 ppm H2O2 vapor achieves around 3-log inactivation of MS2 coliphage in 2 h.34
3.3 Disinfection in secondary effluent
All three oxidants are effective in secondary effluent treatment. The disinfection performance of all the conditions is summarized in Table S4.† Wastewater properties may vary greatly, and in this study, the secondary effluent was obtained from a sewage treatment plant in the Guangdong region. The water quality parameters are shown in Table S2.† 106 PFU per mL MS2 coliphage was added to secondary effluent, and disinfection was studied using the oxidant doses required for 5-log inactivation of MS2 coliphage in DI water. The decay of 0.1 mM O3, 1 mM PMS, and 50 mM H2O2 within 30 min is shown in Fig. S2.† The decay of the three oxidants was obvious. As shown in Fig. 3, in the secondary effluent system, disinfection by the three oxidants achieved 4-log inactivation, which is only 1-log lower than the effects achieved in DI water. The secondary effluent contains a small amount of dissolved organic matter and some suspended solids that may influence disinfection efficacy.35 These organic compounds consume oxidants, though the suspended solids may adsorb viruses such that oxidation is more difficult. However, the three oxidants still achieve the 4-log inactivation that is conventionally required for practical applications, indicating that the three oxidants have the potential for practical water disinfection applications.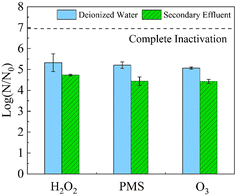 | ||
| Fig. 3 Inactivation efficiency of MS2 coliphage using O3 (0.1 mM), PMS (1 mM), and H2O2 (50 mM) with a contact time of 30 min in DI water and secondary effluent. | ||
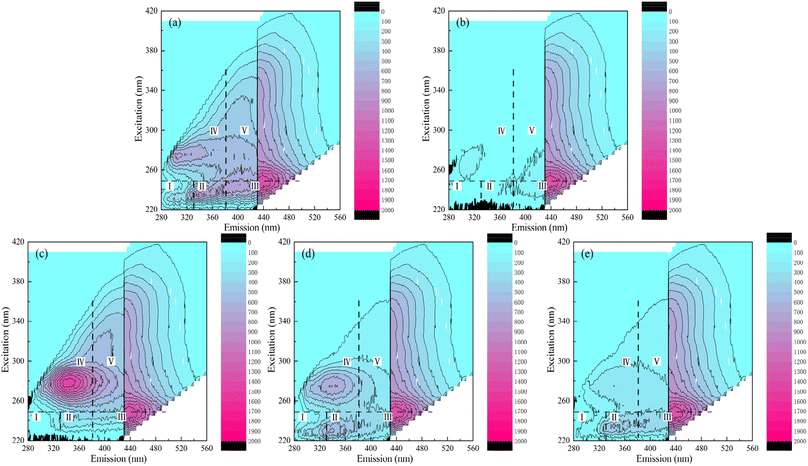
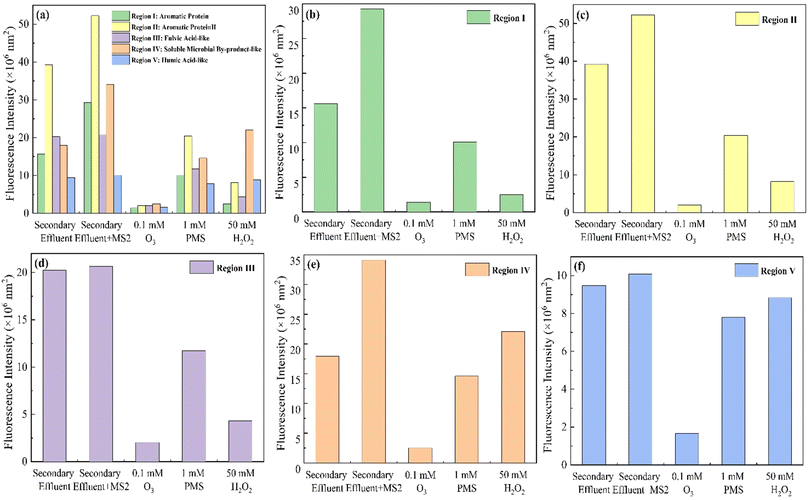
The EEM diagram of water samples before and after disinfection is shown in Fig. 4, and the horizontal and vertical coordinates of Fig. 4 were the emission and excitation wavelengths respectively. The color reflected the peak intensity of fluorescence at a specific excitation/emission wavelength. The integrated fluorescence intensity of 5 FRI regions of water samples is shown in Fig. 5. According to the excitation/emission range, the EEM can be divided into five regions, representing five types of organic matter shown in Table S3.†36 The EEM diagram of secondary effluent (3-fold diluted) is shown in Fig. 4a. The maximum fluorescence of the secondary effluent samples was observed at λex/λem = 230/340 nm. As shown in Fig. 5, the secondary effluent has obvious integrated fluorescence intensity in region I–V, indicating the presence of aromatic protein fragments, soluble microbial by-products, and substances related to humic acid and fulvic acid.37 After adding MS2 to the secondary effluent (Fig. 4b), the fluorescence peak was also observed at λex/λem = 275/340 nm, and the integrated fluorescence intensity in region II and region IV increased significantly, indicating that the absorption peaks in this region correspond to the MS2 coliphage.
For O3 disinfection, the synchronous removal of viruses and dissolved pollutants in water was obvious. Fig. 4c shows the EEM diagram of the water sample after disinfection with O3. The peak intensity of the water sample after disinfection with O3 was obviously weak, while that after disinfection with PMS and H2O2 still remained. And in Fig. 5, the fluorescence intensity of the water sample after O3 disinfection decreased significantly across all regions. In 5 regions, the fluorescence intensity decreased by 95.16%, 96.13%, 90.19%, 92.64%, and 83.59%. This indicates that O3 synchronously removes pollutants in secondary effluent more strongly and with the same MS2 coliphage disinfection effect. Some studies report that O3 can degrade some macromolecular organic substances into small molecules, thereby reducing the amount of dissolved organic carbon in the water.38 Protein-like components and fulvic acid-like substances (regions I–III) were preferentially oxidized by O3 because of their lower molecular weights and the presence of amide and phenolic groups.39 Therefore, O3 has a synchronous removal effect on viruses and dissolved organic pollutants, and the oxidation of MS2 coliphage and other dissolved organic carbon is more thorough, indicating that decolourization and flavour removal are more effective.
PMS and H2O2 also remove organic matter in secondary effluents with similar disinfection effects (4.0-log and 4.4-log). As shown in Fig. 5, after PMS disinfection, the fluorescence intensity of 5 regions after PMS disinfection decreased by 65.46%, 60.92%, 43.23%, 57.23%, and 22.62%, and it decreased by 91.53%, 84.35%, 79.10%, 35.28%, and 12.54% after H2O2 disinfection. The fluorescence intensity of region IV and region V after PMS treatment was lower than that after H2O2 treatment, indicating that the ability of PMS to synchronously oxidize soluble microbial by-products and humic acid-like components is greater than that of H2O2. And H2O2 oxidized more aromatic protein and fulvic acid-like components (regions I–III) than PMS. That was possibly because hydroxyl radicals remove protein-like components and fulvic acid-like substances more thoroughly.40 In addition, the fluorescence peak in region IV shifted to λex/λem = 275/300 nm after PMS treatment (Fig. 4d). That was possibly because of the formation of tryptophan-like substances.
4 Conclusions
This study systematically investigated the effects of dose, contact time, and secondary effluent on the disinfection of MS2 coliphage using O3, PMS, and H2O2. This paper draws the following main conclusions:(1) The doses of the three oxidants required to achieve 4-log disinfection of MS2 coliphage are ranked as O3 < PMS < H2O2. O3 is more effective in inactivating MS2 coliphage at low doses; 0.05 mM O3 achieves 4-log inactivation within 30 min, while 0.1 mM O3 achieves 5-log inactivation within 30 s and 0.25 mM O3 achieves complete 6-log inactivation within 30 min.
(2) The oxidant contact time required to achieve 4-log disinfection of MS2 coliphage is ranked as O3 < H2O2 < PMS. The contact time required for O3 is briefer, and 4-log MS2 coliphage inactivation can be achieved within 30 s.
(3) O3, PMS, and H2O2 also disinfect MS2 coliphage in secondary effluent with an efficacy only 1-log lower than that in DI water, as treatment with O3 (0.1 mM), PMS (1 mM), and H2O2 (25 mM) still achieves 4-log inactivation of MS2 coliphage within 30 min.
(4) O3, PMS, and H2O2 can simultaneously remove some organic pollutants while achieving 4-log disinfection of MS2 coliphage in secondary effluent, and O3 outperforms the other oxidants in synchronous removal.
The results enriched the research of virus disinfection in water and provided a theoretical basis for further studies of disinfection by oxidizing agents. In addition, it showed the significance of the dosage of oxidants in the actual wastewater treatment toward virus disinfection.
Author contributions
Zi-Chen Yang: conceptualization, formal analysis, data curation, writing – original draft. Wen-Long Wang: methodology, supervision. Zi-Bo Jing: data curation, formal analysis. Yi-Qing Jiang: data curation, visualization. He-Qing Zhang: investigation, formal analysis. Min-Yong Lee: validation. Lu Peng: conceptualization, methodology, writing – review & editing, project administration. Qian-Yuan Wu: funding acquisition, writing – review & editing, supervision, resources, project administration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the National Key R&D Program of China (No. 2022YFE0104900), the Shenzhen Science, Technology and Innovation Commission (No. RCJC20221008092758099/JCYJ20220818101010022), and China Postdoctoral Science Foundation (2023M741929).References
- N. S. Upfold, G. A. Luke and C. Knox, Occurrence of Human Enteric Viruses in Water Sources and Shellfish: A Focus on Africa, Food Environ. Virol., 2021, 13(1), 1–31 CrossRef PubMed.
- N. Hassou, R. Boussettine, N. Abouchoaib and M. M. Ennaji, Chapter 39 - Enteric Adenoviruses: Emerging of a Public Health Threat, in Emerging and Reemerging Viral Pathogens, ed. Ennaji M. M., Academic Press, 2020, pp. 879–905 Search PubMed.
- J. O. Akello, R. Kamgang, M. T. Barbani, F. Suter-Riniker, S. L. Leib and A. Ramette, Epidemiology of Human Adenoviruses: A 20-Year Retrospective Observational Study in Hospitalized Patients in Bern, Switzerland, Clin. Epidemiol., 2020, 12, 353–366 CrossRef PubMed.
- C. Troeger, I. A. Khalil, P. C. Rao, S. Cao, B. F. Blacker and T. Ahmed, et al., Rotavirus Vaccination and the Global Burden of Rotavirus Diarrhea Among Children Younger Than 5 Years, JAMA Pediatr., 2018, 172(10), 958–965 CrossRef PubMed.
- G.-A. Shin and D. Sobsey Mark, Reduction of Norwalk Virus, Poliovirus 1, and Bacteriophage MS2 by Ozone Disinfection of Water, Appl. Environ. Microbiol., 2003, 69(7), 3975–3978 CrossRef CAS PubMed.
- A. A. Lanrewaju, A. M. Enitan-Folami, S. Sabiu, J. N. Edokpayi and F. M. Swalaha, Global public health implications of human exposure to viral contaminated water, Front. Microbiol., 2022, 13, 981896 CrossRef PubMed.
- M. Iaconelli, B. Valdazo-González, M. Equestre, A. R. Ciccaglione, C. Marcantonio and S. Della Libera, et al., Molecular characterization of human adenoviruses in urban wastewaters using next generation and Sanger sequencing, Water Res., 2017, 121, 240–247 CrossRef CAS PubMed.
- A. Hata, M. Kitajima, E. Haramoto, S. Lee, M. Ihara and C. P. Gerba, et al., Next-generation amplicon sequencing identifies genetically diverse human astroviruses, including recombinant strains, in environmental waters, Sci. Rep., 2018, 8(1), 11837 CrossRef PubMed.
- M. B. Taylor, N. Cox, M. A. Vrey and W. O. K. Grabow, The occurrence of hepatitis A and astroviruses in selected river and dam waters in South Africa, Water Res., 2001, 35(11), 2653–2660 CrossRef CAS PubMed.
- D. Rodríguez-Lázaro, N. Cook, F. M. Ruggeri, J. Sellwood, A. Nasser and M. S. J. Nascimento, et al., Virus hazards from food, water and other contaminated environments, FEMS Microbiol. Rev., 2012, 36(4), 786–814 CrossRef PubMed.
- L. Casanova, W. A. Rutala, D. J. Weber and M. D. Sobsey, Survival of surrogate coronaviruses in water, Water Res., 2009, 43(7), 1893–1898 CrossRef CAS PubMed.
- V. C. da Silva, M. Elois, B. P. Savi, M. Miotto, J. De Dea Lindner and G. Fongaro, et al., Bioaccumulation Dynamic by Crassostrea gigas Oysters of Viruses That Are Proposed as Surrogates for Enteric Virus Contamination in Environmental Samples, Food Environ. Virol., 2023, 15(1), 1–7 CrossRef CAS PubMed.
- D. J. Dawson, A. Paish, L. M. Staffell, I. J. Seymour and H. Appleton, Survival of viruses on fresh produce, using MS2 as a surrogate for norovirus, J. Appl. Microbiol., 2005, 98(1), 203–209 CrossRef CAS PubMed.
- S. H. E. van den Worm, R. I. Koning, H. J. Warmenhoven, H. K. Koerten and J. van Duin, Cryo Electron Microscopy Reconstructions of the Leviviridae Unveil the Densest Icosahedral RNA Packing Possible, J. Mol. Biol., 2006, 363(4), 858–865 CrossRef CAS PubMed.
- R. Yin, C. E. Anderson, J. Zhao, A. B. Boehm and W. A. Mitch, Controlling contaminants using a far-UVC-based advanced oxidation process for potable reuse, Nature Water, 2023, 1(6), 555–562 CrossRef.
- M. J. Casteel, C. E. Schmidt and M. D. Sobsey, Chlorine disinfection of produce to inactivate hepatitis A virus and coliphage MS2, Int. J. Food Microbiol., 2008, 125(3), 267–273 CrossRef CAS PubMed.
- L. Peng, H. Zhu, H. Wang, Z. Guo, Q. Wu and C. Yang, et al., Hydrodynamic tearing of bacteria on nanotips for sustainable water disinfection, Nat. Commun., 2023, 14(1), 5734 CrossRef CAS PubMed.
- S. D. Richardson, M. J. Plewa, E. D. Wagner, R. Schoeny and D. M. DeMarini, Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research, Mutat. Res., Rev. Mutat. Res., 2007, 636(1), 178–242 CrossRef CAS PubMed.
- J. Kong, Y. Lu, Y. Ren, Z. Chen and M. Chen, The virus removal in UV irradiation, ozonation and chlorination, Water Cycle, 2021, 2, 23–31 CrossRef.
- H. Wang, P. Sikora, C. Rutgersson, M. Lindh, T. Brodin and B. Björlenius, et al., Differential removal of human pathogenic viruses from sewage by conventional and ozone treatments, Int. J. Hyg. Environ. Health, 2018, 221(3), 479–488 CrossRef CAS PubMed.
- W. Tulalamba, A. Assawamakin, A. Thayananuphat and V. Viprakasit, Evaluation of potassium peroxymonosulfate (MPS) efficacy against SARS-CoV-2 virus using RT-qPCR-based method, Int. J. Infect. Dis., 2021, 110, 162–164 CrossRef CAS PubMed.
- M. V. Marshall, L. P. Cancro and S. L. Fischman, Hydrogen Peroxide: A Review of Its Use in Dentistry, J. Periodontol., 1995, 66(9), 786–796 CrossRef CAS PubMed.
- S. A. Tyrrell, S. R. Rippey and W. D. Watkins, Inactivation of bacterial and viral indicators in secondary sewage effluents, using chlorine and ozone, Water Res., 1995, 29(11), 2483–2490 CrossRef CAS.
- N. V. Klassen, D. Marchington and H. C. E. McGowan, H2O2 Determination by the I3- Method and by KMnO4 Titration, Anal. Chem., 1994, 66(18), 2921–2925 CrossRef CAS.
- J. Fang, H. Liu, C. Shang, M. Zeng, M. Ni and W. E. Liu, coli and bacteriophage MS2 disinfection by UV, ozone and the combined UV and ozone processes, Front. Environ. Sci. Eng., 2014, 8(4), 547–552 CrossRef CAS.
- J. K. Choe, D. H. Richards, C. J. Wilson and W. A. Mitch, Degradation of Amino Acids and Structure in Model Proteins and Bacteriophage MS2 by Chlorine, Bromine, and Ozone, Environ. Sci. Technol., 2015, 49(22), 13331–13339 CrossRef CAS PubMed.
- V. K. Sharma and N. J. D. Graham, Oxidation of Amino Acids, Peptides and Proteins by Ozone: A Review, Ozone: Sci. Eng., 2010, 32(2), 81–90 CrossRef CAS.
- J. Edwards-Brandt, H. Shorney-Darby, J. Neemann, J. Hesby and C. Tona, Use of Ozone for Disinfection and Taste and Odor Control at Proposed Membrane Facility, Ozone: Sci. Eng., 2007, 29(4), 281–286 CrossRef CAS.
- G. Wen, Z. Liang, X. Xu, R. Cao, Q. Wan and G. Ji, et al., Inactivation of fungal spores in water using ozone: Kinetics, influencing factors and mechanisms, Water Res., 2020, 185, 116218 CrossRef CAS PubMed.
- P. Sun, C. Tyree and C.-H. Huang, Inactivation of Escherichia coli, Bacteriophage MS2, and Bacillus Spores under UV/H2O2 and UV/Peroxydisulfate Advanced Disinfection Conditions, Environ. Sci. Technol., 2016, 50(8), 4448–4458 CrossRef CAS PubMed.
- H. Mamane, H. Shemer and K. G. Linden, Inactivation of E. coli, B. subtilis spores, and MS2, T4, and T7 phage using UV/H2O2 advanced oxidation, J. Hazard. Mater., 2007, 146(3), 479–486 CrossRef CAS PubMed.
- C. H. Rhee, S.-C. Park, M. Her and W. Jeong, Surrogate Selection for Foot-and-Mouth Disease Virus in Disinfectant Efficacy Tests by Simultaneous Comparison of Bacteriophage MS2 and Bovine Enterovirus Type 1, Viruses, 2022, 14(12), 2590 CrossRef CAS PubMed.
- Y. Jiang, Z. Wang, J. Huang, F. Yan, Y. Du and C. He, et al., A singlet oxygen dominated process through photocatalysis of CuS-modified MIL-101(Fe) assisted by peroxymonosulfate for efficient water disinfection, Chem. Eng. J., 2022, 439, 135788 CrossRef CAS.
- J. P. Wood, W. Richter, M. Sunderman, M. W. Calfee, S. Serre and L. Mickelsen, Evaluating the Environmental Persistence and Inactivation of MS2 Bacteriophage and the Presumed Ebola Virus Surrogate Phi6 Using Low Concentration Hydrogen Peroxide Vapor, Environ. Sci. Technol., 2020, 54(6), 3581–3590 CrossRef CAS PubMed.
- L. S. Pérez-Mora, L. D. Mejia-da-Silva, E. D. Cezare-Gomes, É. D. Santo, A. K. Gohara-Beirigo and M. C. Matsudo, et al., Phycoremediation Processes for Secondary Effluent from Sewage Treatment Plants Using Photosynthetic Microorganisms: A Review, Appl. Microbiol., 2023, 3(2), 400–416 CrossRef.
- W. Chen, P. Westerhoff, J. A. Leenheer and K. Booksh, Fluorescence Excitation−Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter, Environ. Sci. Technol., 2003, 37(24), 5701–5710 CrossRef CAS PubMed.
- R. J. C. Go, H.-L. Yang, C.-C. Kan, D. C. Ong, S. Garcia-Segura and M. D. G. de Luna, Natural Organic Matter Removal from Raw Surface Water: Benchmarking Performance of Chemical Coagulants through Excitation-Emission Fluorescence Matrix Spectroscopy Analysis, Water, 2021, 13(2), 146 CrossRef CAS.
- C. Lei, Y. Chen, A. Li, R. Gao, Z. Zhang and J. Chen, et al., A new process to further remove dissolved organic matter and disinfection by-product formation potential during drinking water treatment, Environ. Sci. Pollut. Res., 2023, 30(8), 20959–20969 CrossRef CAS PubMed.
- H. Yu, F. Qu, X. Zhang, S. Shao, H. Rong and H. Liang, et al., Development of correlation spectroscopy (COS) method for analyzing fluorescence excitation emission matrix (EEM): A case study of effluent organic matter (EfOM) ozonation, Chemosphere, 2019, 228, 35–43 CrossRef CAS PubMed.
- P.-F. Yan, S. Yuan, W. Wang, Z.-H. Hu, Y. Mu and H.-Q. Yu, Efficiency of sequential UV/H2O2 and biofilm process for the treatment of secondary effluent, Environ. Sci. Pollut. Res., 2019, 26(1), 577–585 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3em00527e |
| This journal is © The Royal Society of Chemistry 2024 |