Safety of lithium battery materials chemistry
Xuning
Feng†
ab,
Dongsheng
Ren†
c and
Minggao
Ouyang
 *ab
*ab
aState Key Laboratory of Intelligent Green Vehicle and Mobility, Tsinghua University, Beijing, 100084, China. E-mail: ouymg@mail.tsinghua.edu.cn
bSchool of Vehicle and Mobility, Tsinghua University, Beijing, 100084, China
cInstitute of Nuclear and New Energy Technology, Tsinghua University, Beijing, 100084, China
First published on 21st October 2023
Abstract
Safety problems hinder the utilization of high-energy lithium and lithium-ion batteries, although some electrochemical materials chemistries look promising. This study discusses the opinions of the authors on the predominant battery safety issues. Statistical results indicate that there are three major kinds of safety studies: intrinsic, active, and passive safety. Among these, intrinsic safety covers approximately 80% of the total studies, suggesting that searching for safety solutions in materials chemistry is of high-priority. The most investigated research area is the electrolyte that directly links to the battery fire hazard. Therefore, the major part of this study discusses the safety of lithium-ion batteries with liquid electrolytes and solid-state batteries. To begin with, a reaction zone model was first proposed to depict the dual problem of battery fire and thermal runaway. The problem was further quantified by a diagram with the lowest flammable limit and maximum temperature during battery thermal failure as the two axes. As validated by experimental data from commercial lithium-ion batteries, the diagram helped predict the combustion behavior of lithium and lithium-ion batteries with new materials chemistries. Regarding the safety of solid-state batteries, this perspective discusses five major concerns that are critical but unsolved: (1) the thermal instability of components used in solid-state batteries, (2) the interfacial reactions at the cathode/anode and solid electrolyte interfaces, (3) chemical crosstalk between cathode and anode, (4) lithium dendrite formation and internal short circuit, and (5) the environmental hazards related to the evolved gases and molten lithium. This information suggests that not only should the manufacturing problem be solved before all-solid-state batteries are commercialized, but also safety problems may be the bottleneck that is obstructing the massive production. Safety modelling that may facilitate the development of new materials chemistry is discussed. This perspective may provide new insights into improving the safety of high-energy lithium and lithium-ion batteries, accelerating the research and development of new battery materials chemistry.
Introduction
Batteries are the hope of mankind to store high-quality electric energy for powering transportation and leveraging renewable energy. The pursuit of batteries with higher energy and power density through innovations in materials chemistry still persists.1,2 However, the high energy density always accompanies low safety, which threatens the lives and properties of human beings; thus, several promising electrochemical technologies cannot be commercialized, wasting the efforts of many researchers. Safety is the highest priority for a battery with a new electrochemical system before its wide applications.3People may forget the lessons learnt from the fire and explosion caused by lithium batteries in 1989, but the fatal failure of lithium batteries that resulted in the destruction of ten thousand cell-phones shattered our dreams of using high-performance cell-phones and laptops in the early 1990s.4,5 The major safety issue for lithium batteries is the reactive lithium metal. During cycling, the lithium metal deteriorates into a powder that has a much larger area for failure reactions with electrolytes. Moreover, dendrites forming on the lithium anode lead to an internal short circuit (ISC) of the cell. The invention of lithium-ion batteries resolved the dilemma of an unstable lithium anode by storing lithium in layered materials.6 Furthermore, the invention of more stable lithium-ion batteries reshaped our world by powering the commercialization of electric vehicles, smartphones, laptops, and electric energy storage stations and was awarded the Nobel Prize in 2019.7
However, lithium-ion batteries also burn. The failure rate of lithium-ion batteries is approximately 1 in 1 million cells and 1 in 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 electric cars.8 The fire is usually caused by the combustion of flammable electrolytes, especially the carbonate solvents and gases from their pyrolysis during thermal failure.9 To tackle lithium-ion battery fire, researchers are making great efforts to find countermeasures.
000 electric cars.8 The fire is usually caused by the combustion of flammable electrolytes, especially the carbonate solvents and gases from their pyrolysis during thermal failure.9 To tackle lithium-ion battery fire, researchers are making great efforts to find countermeasures.
When “Battery Safety” is searched on the Web of Science, we can see that the number of papers has increased exponentially since 1990, as shown in Fig. 1. As we first proposed in 2018,10 battery safety research includes three major terms: intrinsic safety, active safety, and passive safety.11
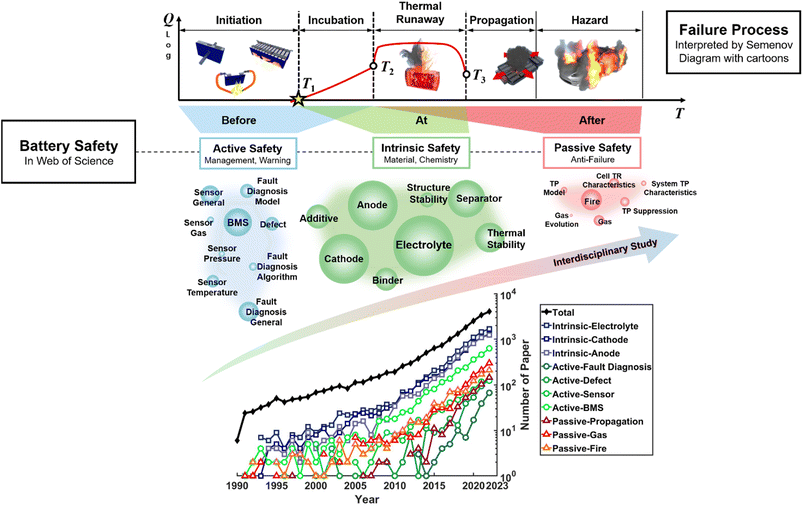 | ||
| Fig. 1 Search results for “battery safety” in the Web of Science. Three categories of countermeasures named “intrinsic safety”, “active safety”, and “passive safety” are proposed using ref. 10 and 11. Each category contains 7–9 trending key words in the recent literature. The size of the circles with the key words corresponds to the number of the representative papers. The number of papers with the corresponding key words is illustrated chronologically in the sub-figure located at the bottom. BMS = battery management system. The relationship among the three safety technologies is described at the top using the Semenov diagram. The y axis is the heat generation Q during failure and the x axis is the temperature T. Three characteristic temperatures have been widely used to quantify the battery thermal runaway.12T1 is the onset temperature, T2 is the triggering temperature, and T3 is the maximum temperature. The three characteristic temperatures divide the plane into five regions: initiation, incubation, thermal runaway, propagation, and hazard. The active safety technology function before thermal runaway is triggered at T2. Intrinsic safety technologies are made to improve the thermal stability of the battery and manipulate the T1–T2–T3 curve. Passive safety technologies are essential to avoid failure propagation and further hazards that may affect human beings and damage properties. | ||
The intrinsic safety research confronts the moment “At” battery thermal runaway. Three characteristic temperatures {T1, T2, T3} set the criterion for judging the intrinsic safety of lithium-ion batteries.12 A battery that has higher T1 and T2 (onset and triggering temperature), and lower T3 (maximum temperature) is regarded as safer. The clear target for intrinsic safety technology is to increase T1 and T2, and to decrease T3. Intrinsic safety cares about the thermal stability in the materials chemistry level and includes novel safety design of the single cell.
Active safety tries to inspect the battery during operation and detect and warn the possible fault “Before” it evolves into a thermal runaway. The T1–T2 segment is the overlap between the active safety and intrinsic safety, and it is the last chance for active safety countermeasures to take action. Active safety mainly includes research on battery management system (BMS), for which safety monitoring is the bottom line. For BMS research, it relates to sensors, models and algorithms, all of which support the core function of fault diagnosis and early warning. Nowadays, cooling systems and fire extinguishing systems function to suppress abnormal thermal events when a warning signal is triggered.
Passive safety protects human life “After” battery thermal runaway is triggered. As the number of batteries is huge in commercial applications, the probability of failure will never be zero. During safety design, passive safety refers to the “fail-safe” design against a pre-set failure scenario. Hence, correlated studies are concerned with fire and propagation after thermal failure occurs.
According to the search results, intrinsic safety issues occupy most (more than 80%) of battery safety publications, while active safety and passive safety have similar shares. The “Safety of Lithium Battery Materials Chemistry” is the most important issue in battery safety research based on statistics. The hottest keywords belonging to the three kinds of safety papers are illustrated by coloured circles, as shown in Fig. 1. There is a trend of interdisciplinary study on battery safety, as each paper may cover two or more key words, as shown in Fig. 1. The most mentioned research is on electrolytes, which directly link with the battery fire hazard. Researchers are trying to develop a non-flammable electrolyte13 or a solid electrolyte (SE),14 substituting for the current carbonate electrolyte. Therefore, the first part of this perspective discusses the “Safety of Liquid Lithium-ion Batteries”, whereas the second part discusses the “Safety of Solid-State Batteries”. Moreover, one of the most difficult aspects of battery safety design is the modelling tool, which requires more knowledge of thermal science than materials chemistry. Hence, the “Modelling and Prediction of Battery Safety” is discussed in the last section.
Safety of liquid lithium-ion batteries
For the safety of liquid lithium-ion batteries, here we focus on the relationship between battery fire and thermal runaway (TR), which is first discussed in the literature to the best knowledge of the authors. Owing to space limitations, interested readers are recommended to ref. 10, 11, 15 and 16 for more details on other safety solutions that have been reviewed. The left side of Fig. 2 shows that battery failure is a dual problem: fire (outside) and TR (inside). The heat and fuel within the fire triangle (the third one is oxygen) come from inside the cell. Extinguishing the fire outside the battery might be futile, while suppressing the TR from inside the battery may hit the point.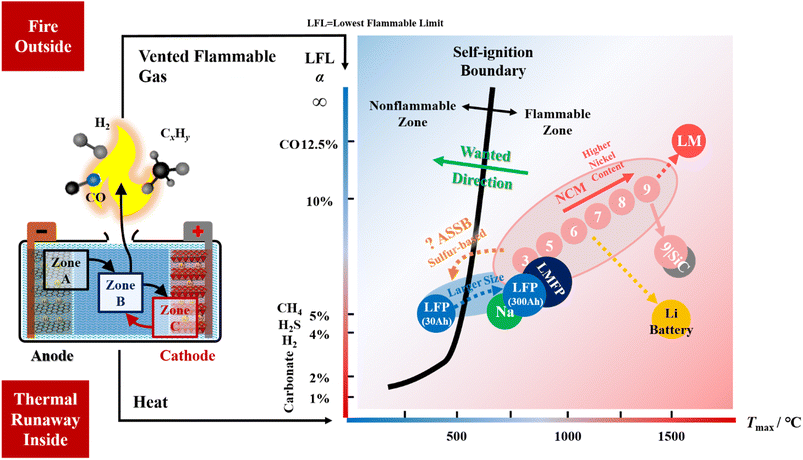 | ||
| Fig. 2 The dual problem in battery safety design: fire (outside) and thermal runaway (inside). The fire is caused by flammable gases vented from inside the cell. Flammable gases are the product of thermal runaway reactions. The self-ignition energy comes from the thermal runaway inside the cell. There are three reaction zones inside the lithium-ion battery: Zone A contains the anode and the interface between the anode and electrolyte; Zone C contains the cathode and the interface between the cathode and electrolyte; and Zone B is for the reaction region between the cathode and anode, where the energetic (reductive and oxidative) species meet and the flammable gases are generated. The right-side figure has the maximum temperature (Tmax) on the x axis and the lowest flammable limit (LFL) of the vent gas on the y axis. The LFL yields Le Chatelier's equation and mostly depends on the major components of the vented gas mixture.20 The solid black line divides the plane into the “non-flammable zone” and “flammable zone”. The blue circles denote the test result for the battery with LFP (LiFePO4) cathode, while the pink circles denote the batteries with the NCM (LiNixCoyMnzO2) cathode and graphite anode. The number marked in the pink circles is for the stoichiometric proportion of the nickel content. For instance, 3 is for x = 0.333, 5 is for x = 0.5, …, and 9 is for x = 0.9. The values of Tmax and LFL, LFP and NCM batteries mostly comes from ref. 21. “Na” refers to the sodium-ion battery with layered cathode and hard carbon anode; “LMFP” refers to the lithium-ion battery with LiMnxFe1−xPO4 cathode and graphite anode; the “9|SiC” refers to the lithium-ion battery with LiNixCoyMnzO2 (x = 0.9) cathode and graphite anode with approximately 10% addition of SiOx. The above data for the three kinds of batteries were collected by us. Further predictions (with dotted arrows) are made based on our research experience. The LM is for a battery with a lithium-rich manganese-based cathode. The location of the all-solid-state-battery (ASSB) and the battery with lithium as the anode (Li battery) are predicted according to our recent test data for Tmax and the compositions of vented gas. The reference data for ASSB can be found in ref. 22. The typical LFLs for pure gases, which appear in the vented mixture, are listed beside the y axis, including carbonate, H2, H2S, CH4, and CO. | ||
The left side of Fig. 2 illustrates the “Reaction Zone Model” that interprets the mechanisms of battery TR, which is first proposed. The complex reactions are incorporated into three reaction zones (A, B and C), indicating the location where the reaction occurs. A is for the Anode, C is for the Cathode, and B is for the reaction zone “Between” the anode and cathode. The existence of Zone B can be inferred from in operando TR tests.17 The reactions within Zone A, at the full region of the anode electrode, including the interface between the anode and electrolyte, emit reductive gases,18 while the reactions within Zone C generate oxidative species.19 The products from Zones A and C meet together and further react in Zone B. The characterization methods for studying the failure mechanisms in Zone A or C are much easier to perform than those for Zone B. The reaction mechanism in Zone B is too complex for the current experimental instruments. Our recent opinion is that after the products from Zones A and C neutralize in Zone B, the residuals flush the counter zone and then burst out of the battery. As the venting gas is reductive (flammable), the crosstalk model of cathode oxygen release19 is insufficient to conclude the TR mechanisms. More attention should be paid to the origins of flammable (reductive) gases at Zone A if one wants to solve the fire extinguishing problem caused by battery TR. The reaction zone model reminds us that regarding the view of the electrochemical stoichiometric ratio, the battery materials chemistry might be balanced. However, because of chemical reactions during TR, the anode is excessive for most current lithium-ion batteries. This might be the root cause of battery fire, which has never been achieved before. There might be some corollaries for the reaction zone model when the battery chemistry is changed. The first one is that if the solvent vaporizes at lower temperatures before the reactions are activated in Zones A and C, the vent gas might be the oxidative species emitted from the cathode if there are residuals after the oxidant reacts with the anode. Oxidative vent gases, although hot, might be ideal for battery safety design because they are usually non-flammable. The second one is that if the electrical insulation distance between the cathode and anode is quite small, it might be hard for Zone B to form during battery TR.
The right side of Fig. 2 tries to quantify the dual problem of battery fire and TR. As the oxygen condition in the fire triangle is always satisfied with the vent gas, the temperature and flammability of the vent gas become critical in fire extinguishing. Therefore, the x axis is set for the maximum temperature (Tmax) that the battery TR can reach, while the y axis is set for the lowest flammability limit (LFL) of the vent gas mixture. Tmax represents the total chemical energy that is released in heat during TR, and it is usually determined by the chemical potential gap between the cathode and anode.12Tmax may change if we manipulate the reaction sequences as in ref. 11 and 18. The LFL refers to the lower limit of the gaseous fuel's concentration exposed in the air, which quantifies the boundary of fire ignition. Gas with a higher LFL is regarded as safer than those with a lower LFL. When the gas concentration in the air is lower than the LFL, the gas mixture is regarded as non-flammable. Generally, regardless of the pressure effect, we summarize a rough self-ignition boundary (the thick black solid line on the right side in Fig. 2) for commercial lithium-ion batteries that we have tested.20,21 The vent gas of a cell chemistry that has a coordinate (Tmax, LFL) located at the left of the self-ignition boundary is regarded as non-flammable; otherwise, it is regarded as flammable. When we perform a safety design that fights against fire at the materials chemistry level, we want the points to move towards the left of the self-ignition boundary and better to the top-left corner in the figure.
The right side of Fig. 2 further explains the fire ignition mechanisms of the current lithium-ion batteries. For the battery with an NCM (LiNixCoyMnzO2) cathode, both the cathode and anode participate in the thermal runaway reactions. Therefore, the Tmax is high, and the points are always located in the flammable zone on the right side. As the nickel content increases, the oxidizability of the cathode increases, leading to increases in both the LFL and Tmax of the vent gas. The point moves to the top right in the figure, approaching the properties of carbon monoxide. Fortunately, the battery with the LFP (LiFePO4) cathode has a coordinate of vent gas located in the non-flammable zone on the left side of the self-ignition boundary not because of the high LFL but because of the low Tmax. Here, we remind the readers that insufficient concerns were paid to the potential hazard introduced by those large format LFP batteries (up to 300 A h). The large format LFP batteries are observed to have high Tmax (higher than 800 °C) because the low heat dissipation is introduced by the small area-to-volume ratio that causes undesired heat accumulation. Once the Tmax exceeds 600 °C, the LFP cathode may also release oxygen and join in the TR reactions, pushing the Tmax to 800 °C or higher. We observe that many of the electrical energy storage stations (up to 100 MW h to 1 GW h) use large format LFP batteries, but their safety design did not consider this dangerous factor. Here, we seriously warn the customers and firefighters that if there is a fire in an electrical energy storage station using large-format LFP batteries, please stay away at a sufficiently safe distance before the failure event is under control. This is because the designers and engineers who built the site may not know the self-ignition problem caused by the scaling up of battery sizes. The high-voltage electric system generates an arc to ignite the vent gas (though at low temperatures during venting).
We also add some (Tmax, LFL) data for the sodium-ion battery (marked as Na in green), battery with LiMnxFe1−xPO4 (marked as LMFP in dark blue) cathode, and high-energy battery with LiNix=0.9CoyMnzO2 cathode and silicon/graphite mixed anode (marked as 9|SiC in pink-grey dot). All the three cell chemistries are under consideration by industry to make cheaper or higher energy-contained batteries. However, the recent test results show that all of them have lower LFLs, indicating that they are more dangerous than LFP and NCM batteries. There are still chances for them to improve their intrinsic safety because the test samples are just raw for trial, and very few publications have discussed how to improve their intrinsic safety.
Fig. 2 depicts predictions of the combustion behaviours of new cell chemistries. First, the battery with lithium-rich manganese-based cathodes may have its point located at the top-right side of the NCM chemistries, as its oxidizability further increases. Moreover, we predict that lithium metal batteries that have organic electrolytes may be located at the bottom-right corner of the figure, indicating that they have higher Tmax and are more prone to combustion and explosion. For the all-solid-state batteries (ASSB), unfortunately, the sulfide-based SE generates H2S,22 which has an LFL near 4%, similar to H2 and CH4. In this sense, the ASSB burns. Those who believe that the ASSB ultimately has intrinsic safety should be reconsidered. However, the SE in ASSB may be promising for isolating the cathode and anode to avoid the formation of Zone B during thermal runaway, thereby decreasing the Tmax. If Tmax can be designed to be lower than 600 °C, the ASSB and lithium-ion batteries will be intrinsically safe against fire.
Safety of solid-state batteries
Solid-state batteries (SSBs) are one of the promising solutions for solving the safety problems of high-energy lithium batteries if non-flammable SEs can replace flammable organic liquid electrolytes.23 Several groups of SEs have been developed for SSBs, including polymer SEs, oxide SEs, sulfide SEs and halide SEs. The polymer SEs usually consist of the polymer matrix (poly(ethylene oxide)-PEO, poly(vinylidene fluoride)-PVDF, etc.) and lithium salts (LiPF6, Li[N(SO2F)2]-LiFSI, etc.). The ionic transport inside the polymer SEs relates to the segmental motion of polymer chains, which create free volumes for the hopping of lithium ions.24 The ion hopping is slow at ambient temperature, resulting in low ionic conductivities (10−6 to 10−4 S cm−1) that limit further commercialization. The oxide SEs, sulfide SEs and halide SEs are all inorganic electrolytes with crystal or amorphous structures. The lithium ions diffuse along favourable pathways and act like hopping between stable and intermediate sites of the solid framework, following three migration mechanisms, i.e., vacancy diffusion mechanism, direct interstitial mechanism and interstitial–substitutional exchange mechanism.25 The oxide SEs include perovskite-type, NASICON-type and garnet-type, with ionic conductivities of 10−5 to 10−3 S cm−1. The sulfide SEs are divided into Li2S–P2S5 binary system, argyrodite Li6PS5X (X = Cl, Br and I)-type, thio-LISICON type and LixGePySz-type, exhibiting high ionic conductivities of 10−3 to 10−2 S cm−1 that are comparable to those of the liquid electrolytes.26 The halide SEs share similar chemical formulas-LiaMXb (M = metal element, X = F, Cl, Br, I), and they achieve relatively high ionic conductivities of around 10−3 S cm−1.27 Halide SEs can be divided into three categories according to the metal elements: (1) group 3 elements (Sc, Y, La); (2) group 13 elements (Al, Ga, In); and (3) divalent elements (Zn, V, Fe, etc.). Among various SEs, the sulfide SEs have the highest ionic conductivities and are regarded as the most promising SEs for power batteries, while oxide and halide SEs show high stabilities toward high-voltage cathodes and are suitable as catholytes.Apart from developing high-performance SEs, numerous research efforts have been devoted to improving the electrochemical performance of SSBs through interfacial and electrode engineering. However, the safety performance of SSBs has not yet been thoroughly investigated because SSBs are usually presupposed to be safe. Recent studies have reported intense exothermic reactions between SEs and electrode materials (NCM cathode and lithium metal anode),22,28–30 indicating that the safety of SSBs may be over-expected. Therefore, a comprehensive investigation of the safety of SSBs is urgently needed.
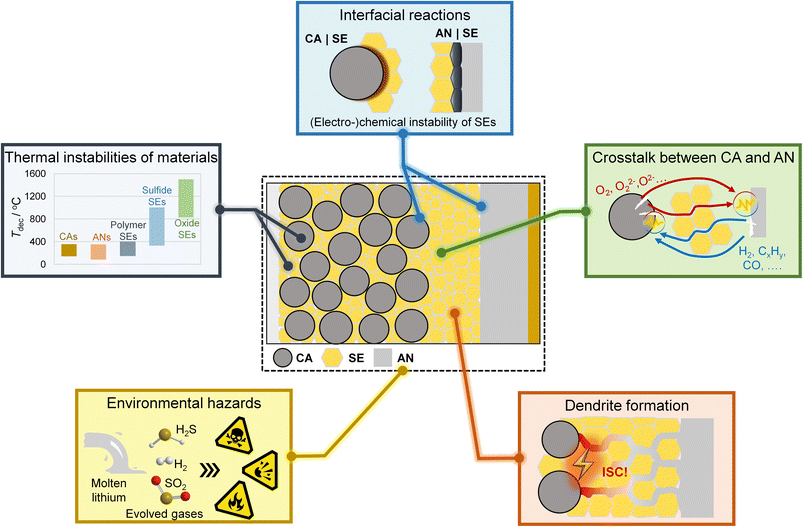
Based on our understanding of the TR of lithium-ion batteries, we mention several potential safety concerns about SSBs, as shown in Fig. 3.
I. Thermal instabilities of SSB components
The cathode and anode materials decompose at 100 –300 °C, generating significant heat and gases. Decomposition of SE materials is unavoidable at elevated temperatures. Polymer SEs have relatively lower decomposition temperatures, which further decrease in the presence of lithium salts.31 Polymer SEs can decompose into polymer monomers, which can burn and lead to complex thermal reactions. Sulfide SEs exhibit better thermal stability than polymer SEs but still suffer from sulphur precipitation at high temperatures (>300 °C).31 Oxide SEs are the most thermally stable SEs and usually show high decomposition temperatures of over 1000 °C.31 However, Chen et al.30 found that oxide SEs release oxygen and react violently with molten metallic Li at elevated temperatures. These results raise concerns about the thermal instabilities of SEs when in contact with electrode materials.II. Interfacial reactions at CA|SE and AN|SE interfaces
Interfacial reactions occur inside SSBs owing to the (electro-) chemical instability of SEs and may deteriorate the safety of SSBs. The polymer SEs are prone to be oxidized at the interface on high-voltage cathodes, and the lithium salts in polymer SEs can react violently with metallic lithium.32 Similarly, sulfide SEs can be oxidized at the cathode surface and reduced at the anode surface.33 Recently, vigorous exothermic reactions accompanied by intense heat generation were observed in composite cathodes containing oxide cathodes and sulfide SEs.22,28,29 Two distinct thermal reaction pathways were found to be responsible for intense heat generation, i.e., the gas–solid reaction pathway (the reaction between sulfide SEs and the O2 released from cathodes) and solid–solid reaction pathway (the reaction between sulfide SEs and solid decomposition products of the cathodes).22 Oxide SEs are usually electrochemically stable at cathode interfaces, while reduction reactions occur at the anode interfaces.33 Moreover, as mentioned before, intense exothermic reactions between oxide SEs and molten metallic Li were observed,30 demonstrating the critical role of interfacial reactions in SSB safety. However, the interfacial reactions inside SSBs under abuse conditions and their contribution to battery safety remain elusive.III. Chemical crosstalk between cathode and anode
The chemical crosstalk phenomenon, i.e., the diffusion of chemical species and the consequent reactions between these species and electrodes, significantly affects battery safety.34 The cathodes can produce oxidative species (such as O2), which react with the anodes and trigger battery TR.19 Reductive gases (such as H2) are also released from the anodes and can accelerate cathode phase transformation and battery TR.18 A chemically inert and dense SE layer can help retard the chemical crosstalk between the cathode and anode. However, softening the polymer SEs at a temperature above their melting points still leads to direct exposure of the electrodes and sequential chemical crosstalk. The cracking of oxide SE pellets induced by the reactions with lithium metal was also observed at around 330 °C,35 facilitating the chemical crosstalk between electrodes. Therefore, whether the SE layers can inhibit chemical crosstalk between electrodes at elevated temperatures is still questionable.IV. Lithium dendrite growth
Dendrite-induced ISC is the most detrimental problem for the application of lithium metal anode. SE layers with high mechanical strength are expected to suppress lithium dendrite growth in SSBs. However, recent studies have demonstrated that lithium dendrite still grows and propagates in SSBs, even faster than in liquid lithium batteries. Once lithium dendrite forms, the polymer SEs may be too soft to suppress the lithium dendrite penetration.36 For inorganic SEs, lithium dendrite can grow along the cracks, defects and grain boundaries, and even form inside SEs.36 Compared to liquid lithium batteries, the lithium dendrite growth mechanisms in SSBs are much more complicated. The underlying mechanism of the dendrite-induced ISC and its influence on SSB safety is unclear. Practical strategies for dendrite suppression are urgently needed to promote the development of solid-state lithium batteries.V. Environmental hazards
Lithium-ion batteries may release a significant number of hazardous species under abuse conditions, leading to potential threats to human health, fire and explosion.21 Fire and explosion hazards related to the vented gas from liquid lithium-ion batteries have been characterized.21 However, the potential environmental hazards associated with SSBs have rarely been studied. SO2 gas was detected in sulfide SE-based SSBs at high temperature and voltage,22,29 and H2S is released once the sulfide SEs were exposed to air moisture.37 These toxic, flammable and explosive gases threaten the health of passengers and first aid responders and thus require much attention. The environmental hazards of polymer SE and oxide SE-based SSBs may be lower compared to sulfide SE-based SSBs. However, flammable and explosive gases may also be released from these SSBs at high temperatures and thus should be comprehensively evaluated. Another safety concern of SSBs that requires special consideration is the environmental hazards of molten lithium.38 Once broken, the molten lithium can flow out of the battery case and react violently with air moisture, leading to fire and even explosion.Overall, although several academic and industrial researchers have demonstrated the relatively high safety of SSBs under specific abuse tests, safety concerns about SSBs still exist according to the physicochemical properties of battery components. With the acceleration of the mass production of SSBs, mechanistic understanding, comprehensive evaluation and potential new regulations of the safety concern about SSBs are urgently needed.
Thermal runaway modelling of lithium-ion batteries
In parallel with experiments, computational modelling is becoming increasingly important in battery TR investigations. Fig. 4(a) illustrates the fundamental theory of battery TR modelling, where the heat generation and dissipation determine the battery temperature increase. Heat dissipation is composed of heat conduction, convection and radiation. Heat generation originates from exothermic chemical reactions and ISC. After parameterization, the TR models can be applied to predict the battery thermal/electrical behaviours, clarify battery TR mechanisms, and guide the battery safety design.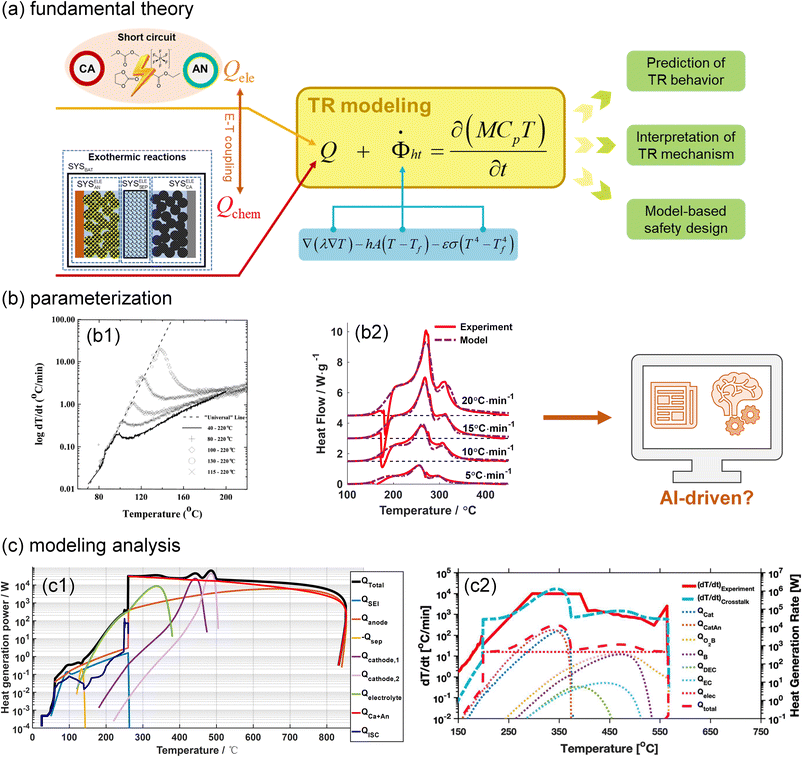 | ||
| Fig. 4 Thermal runaway modelling of lithium(-ion) batteries. (a) Fundamental theory for battery TR modelling. (b) Parameterization of the exothermic reactions based on the (b1) ARC and (b2) DSC test data. Reproduced with permission from ref. 39 and 40. Copyright 1999, IOP Publishing, and Copyright 2018, Elsevier. (c) Model-based analysis of the battery TR mechanism. Modified with permission from ref. 41 (c1) and 42 (c2). Copyright 2018, IOP Publishing, and Copyright 2022, Elsevier. | ||
Parameterization, i.e., determining the critical parameters of heat generation and dissipation processes, is usually the first step to establish a reliable TR model for lithium-ion batteries. The physical and heat dissipation-related parameters can be directly measured from calibration experiments. In contrast, identifying the kinetics parameters of heat generation terms is challenging. The Arrhenius equation-based reaction kinetics, 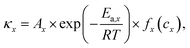 is widely used to simulate the exothermic reaction rate, where κx denotes the reaction rate and cx denotes the normalized concentration of the reactant x. The pre-exponential factor Ax, activation energy Ea,x and mechanism function fx are the kinetics triplets. The heat generation from the reaction can then be calculated by multiplying the reaction rate κx by the enthalpy ΔHx. The kinetics parameters [Ax, Ea,x, fx, ΔHx] determine the heat generation of a reaction and are usually identified from the accelerating rate calorimetry (ARC) and differential scanning calorimetry (DSC) test results,39,40 as illustrated in Fig. 4(b). However, oversimplified assumptions, such as unity reactions and single-step reactions, are usually adopted in reaction kinetics analysis, leading to the low fidelity of the reaction model. In the future, artificial intelligence (AI)-driven approaches may help to identify the kinetics parameters without analytical solutions. Furthermore, compared to the kinetics analysis of exothermic chemical reactions, it is more challenging to characterize heat generation from the ISC. The heat generation from ISC is usually simulated by the near-joule heating equation (QISC = ηU2/RISC), with the ISC resistance RISC estimated to fit the experimental results, and η is defined for the efficiency of heat conversion from electricity.41 However, the battery ISC resistance varies with different types of short circuits, pressure and temperature, making it difficult to measure. Finally, the electrical and chemical heat generation processes inside the battery usually exhibit significant interactions during the TR process. Few studies have addressed the parameterization of electrochemical-thermal coupled TR processes, and progress in this issue is expected to improve the capability of battery TR models.
is widely used to simulate the exothermic reaction rate, where κx denotes the reaction rate and cx denotes the normalized concentration of the reactant x. The pre-exponential factor Ax, activation energy Ea,x and mechanism function fx are the kinetics triplets. The heat generation from the reaction can then be calculated by multiplying the reaction rate κx by the enthalpy ΔHx. The kinetics parameters [Ax, Ea,x, fx, ΔHx] determine the heat generation of a reaction and are usually identified from the accelerating rate calorimetry (ARC) and differential scanning calorimetry (DSC) test results,39,40 as illustrated in Fig. 4(b). However, oversimplified assumptions, such as unity reactions and single-step reactions, are usually adopted in reaction kinetics analysis, leading to the low fidelity of the reaction model. In the future, artificial intelligence (AI)-driven approaches may help to identify the kinetics parameters without analytical solutions. Furthermore, compared to the kinetics analysis of exothermic chemical reactions, it is more challenging to characterize heat generation from the ISC. The heat generation from ISC is usually simulated by the near-joule heating equation (QISC = ηU2/RISC), with the ISC resistance RISC estimated to fit the experimental results, and η is defined for the efficiency of heat conversion from electricity.41 However, the battery ISC resistance varies with different types of short circuits, pressure and temperature, making it difficult to measure. Finally, the electrical and chemical heat generation processes inside the battery usually exhibit significant interactions during the TR process. Few studies have addressed the parameterization of electrochemical-thermal coupled TR processes, and progress in this issue is expected to improve the capability of battery TR models.
TR models can be used to predict battery performance under abuse conditions. Hatchard et al.43 and Kim et al.44 built lumped and three-dimensional models to predict the TR behaviour of lithium-ion batteries. Battery TR models were further developed to predict the venting and jet fire processes45 because TR is usually accompanied by smoke and fire outside the battery. In the future, battery TR models incorporated with more multi-physical processes are strongly encouraged to achieve high fidelity in predicting the multi-physical battery TR process.
TR modelling can also help to interpret battery failure mechanisms that are difficult to characterize. One example is to clarify the contribution of different reactions to total heat generation. As presented in Fig. 4(c1), our group quantified the proportion of the ISC in the total heat generation using an electrochemical-thermal TR Model and found that ISC was not the major heat source during battery TR.41 More importantly, battery TR modelling and tests can work together to gain insights into battery TR mechanisms. Our group revealed that battery TR can be triggered by chemical crosstalk between electrodes.18,19 As shown in Fig. 4(c2), Zhou et al.42 quantified the exothermic effects of crosstalk on battery TR through modelling, quantifying complicated interactions among battery components. With a more profound understanding of the TR mechanisms, battery TR modelling will also become more accurate and effective in predicting and analysing the TR of batteries with different formats under different scenarios.
Finally, TR modelling can accelerate battery safety design by reducing “trial-and-error” costs. However, although TR modelling has been widely applied in battery module/pack design,46,47 model-based optimization of battery materials chemistry has rarely been reported. Several challenges still hinder the utilization of TR models in battery materials chemistry design. First, there is a lack of databases that contain the reaction kinetics of various battery components. Standard test and analysis methods that can quantify the exothermic reactions between different electrode materials, electrolytes and their interactions are required to promote the establishment of the material databases. Second, the accuracy of the TR model requires improvement, but the exothermic reactions, electrode crosstalk, and multi-physical coupling mechanisms are not fully understood. Parameterization of these processes also requires further elaborate tests and advanced characterization tools. Third, the battery safety design should balance the electrochemical and safety performance. Any key performance indicator we want to optimize must be simulated simultaneously by mathematical equations, which is an arduous task. The fast-growing AI technique may facilitate the rapid prediction of battery performance by reducing the mathematical modelling and parameterization processes. The AI technique can further enable multi-objective optimization of battery performances, thereby paving the way to the model-based safety design of batteries.
Summary and outlook
This study proposes some current opinions on the safety of lithium/lithium-ion battery materials chemistry. The literature on battery safety research has been categorized into three kinds of countermeasures: intrinsic safety, active safety and passive safety. Owing to limited spaces and considering the scope of this renowned journal, only key insights into intrinsic safety were discussed. For the solutions of active safety and passive safety, interested readers are referred to ref. 11 for more information. When we focus on the intrinsic safety of lithium and lithium-ion batteries, based on statistics, the hottest research issue is on the electrolyte that determines whether a battery burns under a failure scenario. Therefore, regarding electrolytes, this perspective paper discusses the safety of liquid lithium-ion batteries and that of solid-state batteries.A “Reaction Zone Model” is first proposed to depict the dual problem of fire and thermal runaway for all kinds of lithium-ion batteries with liquid organic electrolytes. The relationship between battery fire and thermal runaway is not only qualitatively described but also quantitatively defined through a diagram with the lowest-flammable-limit and maximum temperature during thermal runaway as the two axes. A self-ignition boundary slices the plane into non-flammable and flammable zones. Because the diagram has high fidelity in interpreting the safety of commercial lithium-ion batteries, predictions are made for scaled-up batteries, batteries with lithium-rich manganese-based cathodes, batteries with lithium metal anodes, and all-solid-state batteries.
For solid-state batteries, this perspective reminds us of five emerging safety concerns that may hinder further research, development and applications. The first one is the thermal instability of the components of solid-state batteries. New materials chemistry may bring unexpected side reactions. The second one is the reactions at the interface between the cathode/anode and solid electrolyte, which were reported in a few papers with negative influences on the battery safety performance. The third one is the chemical crosstalk that exists between the cathode and anode. As long as the chemical crosstalk occurs, the thermal energy stored in the cathode and anode is fully released, resulting in a disastrous event. The solid electrolyte must continue separating the two electrodes against crosstalk. The fourth one is to prevent lithium dendrite growth and further induction of ISC. The last one is to take care of the potential environmental hazards of sulphur-contained gas and molten lithium.
The model prediction of battery safety for materials chemistry development is discussed in brief, as the simulation tools can link with artificial intelligence that may largely accelerate the battery safety design process. Relying on a deep learning algorithm, our team is making significant progress in predicting battery thermal runaway behaviour based on materials calorimetric data. Moreover, the digital twin model, which contains information on scales from material (nm) to system (m), is in demand.
Based on the 200 year history of the battery industry, we conclude with a three-step law for a new battery chemistry to be developed. The first step is to have a promising electrochemical system based on materials chemistry, and it should have high energy density, long cycle life and low cost. Then, the second step is to improve its intrinsic safety to avoid fire and explosion in use. The third step is to monitor the status of the battery and make it work wisely.48 The lithium-ion batteries now seem to be at the third step, working with artificial intelligence to better serve the electrified world. LFP batteries with the help of scaling up, such as the blade battery and cell-to-pack battery structure, have balanced properties of energy, power, longevity and cost, thereby occupying the largest market share. However, the fire propagation of a huge LFP battery system at the MW h to GW h level should be investigated because the cell number is large with a high failure rate. Self-poisoned strategies may help destruct the failure cell to avoid propagation towards its neighbours.49 The NCM batteries are struggling in the second step to compete with the LFP batteries in vehicle application scenarios. However, NCM batteries, if intrinsically safe, still have broad chances to beat the LFP batteries because the latter have reached their limit of energy density. The intrinsic safety of NCM batteries can be improved by electrolyte design,50 electrode modification,51 separator enhancement,34 and cell design52 guided by the reaction sequence map proposed in ref. 11. The solid-state batteries and batteries with other cell chemistries must overcome the problems at the first step; afterwards, safety issues may emerge. We still cannot obtain clear information on how new cell chemistry will behave under safety tests before it is achieved at full scale. Predictions of fire and explosion hazards are beneficial, relying on prototype cell tests and modelling analysis.
We hope that this perspective will provide new insights and ideas for tackling the bothering failure problems of high-energy lithium and lithium-ion batteries. Most of the opinions are proposed based on our group's recent viewpoints, which might have flaws and limitations. The authors welcome comments, suggestions and corrections.
Author contributions
Feng X., writing: abstract, introduction, safety of liquid lithium-ion batteries, summary; conception; revision. Ren D., writing: safety of solid-state batteries, safety modelling; revision. Ouyang M, supervision, conception, correspondence.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Key R&D Program-Strategic Scientific and Technological Innovation Cooperation [Grant No. 2022YFE0207900]; The National Natural Science Foundation of China [Grant No. 52076121, 52007099]. We thank Dr Yue Pan for collecting the raw data in Fig. 1.References
- V. Viswanathan, A. H. Epstein, Y. M. Chiang, E. Takeuchi, M. Bradley, J. Langford and M. Winter, Nature, 2022, 601, 519–525 CrossRef CAS PubMed.
- H. Kim, J.-Y. Hwang, S. Bang, H.-G. Jung and Y.-K. Sun, J. Mater. Chem. A, 2022, 10, 10844–10853 RSC.
- Z. Zhu, T. Jiang, M. Ali, Y. Meng, Y. Jin, Y. Cui and W. Chen, Chem. Rev., 2022, 122(22), 16610–16751 CrossRef CAS PubMed.
- K. Nakajima, Conversation too hot to handle. Mainichi Daily News, October 15, 1989 Search PubMed.
- S. Pennington, Moving in on Moli, Vancouver Sun, Business Section, September 28, 1991 Search PubMed.
- M. Winter, B. Barnett and K. Xu, Chem. Rev., 2018, 118(23), 11433–11456 CrossRef CAS PubMed.
- A. Yoshino, Brief history and future of the lithium-ion battery, The Nobel Prizes Lecture, 2019 Search PubMed.
- W. Huang, X. Feng, X. Han, W. Zhang and F. Jiang, Cell Rep. Phys. Sci., 2021, 2(1), 100285 CrossRef CAS.
- J. Lee, A.-R. Jeon, H. J. Lee, U. Shin, Y. Yoo, H.-D. Lim, C. Han, H. Lee, Y. J. Kim, J. Baek, D.-H. Seo and M. Lee, Energy Environ. Sci., 2023, 16, 2924–2933 RSC.
- X. Feng, M. Ouyang, X. Liu, L. Lu, Y. Xia and X. He, Energy Storage Mater., 2018, 10, 246–267 CrossRef.
- X. Feng, D. Ren, X. He and M. Ouyang, Joule, 2020, 4(4), 743–770 CrossRef CAS.
- X. Feng, S. Zheng, D. Ren, X. He, L. Wang, H. Cui, X. Liu, C. Jin, F. Zhang, C. Xu, H. Hsu, S. Gao, T. Chen, Y. Li, T. Wang, H. Wang, M. Li and M. Ouyang, Appl. Energy, 2019, 246, 53–64 CrossRef CAS.
- J. Xu, X. Ji, J. Zhang, C. Yang, P. Wang, S. Liu, K. Ludwig, F. Chen, P. Kofinas and C. Wang, Nat. Energy, 2022, 7, 186–193 CrossRef CAS.
- Y. Li, S. Song, H. Kim, K. Nomoto, H. Kim, X. Sun, S. Hori, K. Suzuki, N. Matsui, M. Hirayama, T. Mizoguchi, T. Saito, T. Kamiyama and R. Kanno, Science, 2023, 381, 50–53 CrossRef CAS PubMed.
- K. Liu, Y. Liu, D. Lin, A. Pei and Y. Cui, Sci. Adv., 2018, 4, eaas9820 CrossRef PubMed.
- Q. Wang, B. Mao, S. I. Stoliarov and J. Sun, Prog. Energy Combust. Sci., 2019, 73, 95–131 CrossRef.
- D. P. Finegan, E. Darcy, M. Keyser, B. Tjaden, T. M. M. Heenan, R. Jervis, J. J. Bailey, R. Malik, N. T. Vo, O. V. Magdysyuk, R. Atwood, M. Drakopoulos, M. DiMichiel, A. Rack, G. Hinds, D. J. L. Brett and P. R. Shearing, Energy Environ. Sci., 2017, 10, 1377 RSC.
- Y. Wang, X. Feng, Y. Peng, F. Zhang, D. Ren, X. Liu, L. Lu, Y. Nitta, L. Wang and M. Ouyang, Joule, 2022, 6(12), 2810–2820 CrossRef CAS.
- X. Liu, D. Ren, H. Hsu, X. Feng, G.-L. Xu, M. Zhuang, H. Gao, L. Lu, X. Han, Z. Chu, J. Li, X. He, K. Amine and M. Ouyang, Joule, 2018, 2(10), 2047–2064 CrossRef CAS.
- W. Li, H. Wang, Y. Zhang and M. Ouyang, J. Energy Storage, 2019, 24, 100775 CrossRef.
- H. Wang, H. Xu, Z. Zhang, Q. Wang, C. Jin, C. Wu, C. Xu, J. Hao, L. Sun, Z. Du, Y. Li, J. Sun and X. Feng, eTransportation, 2022, 13, 100190 CrossRef.
- X. Rui, D. Ren, X. Liu, X. Wang, K. Wang, Y. Lu, L. Li, P. Wang, G. Zhu, Y. Mao, X. Feng, L. Lu, H. Wang and M. Ouyang, Energy Environ. Sci., 2023, 16, 3552–3563 RSC.
- D. Wu and F. Wu, eTransportation, 2023, 16, 100224 CrossRef.
- Q. Zhao, S. Stalin, C. Zhao and L. A. Archer, Nat. Rev. Mater., 2020, 5(3), 229–252 CrossRef CAS.
- T. Famprikis, P. Canepa, J. A. Dawson, M. S. Islam and C. Masquelier, Nat. Mater., 2019, 18(12), 1278–1291 CrossRef CAS PubMed.
- Q. Zhang, D. Cao, Y. Ma, A. Natan, P. Aurora and H. Zhu, Adv. Mater., 2019, 31(44), 1901131 CrossRef CAS PubMed.
- X. Li, J. Liang, X. Yang, K. R. Adair, C. Wang, F. Zhao and X. Sun, Energy Environ. Sci., 2020, 13(5), 1429–1461 RSC.
- S. Wang, Y. Wu, T. Ma, L. Chen, H. Li and F. Wu, ACS Nano, 2022, 16, 16158–16176 CrossRef CAS.
- T. Kim, K. Kim, S. Lee, G. Song, M. S. Jung and K. T. Lee, Chem. Mater., 2022, 34, 9159–9171 CrossRef CAS.
- R. Chen, A. M. Nolan, J. Lu, J. Wang, X. Yu, Y. Mo, L. Chen, X. Huang and H. Li, Joule, 2020, 4, 812–821 CrossRef CAS.
- Y. Wu, S. Wang, H. Li, L. Chen and F. Wu, InfoMat, 2021, 3(8), 827–853 CrossRef CAS.
- R. Chen, Q. Li, X. Yu, L. Chen and H. Li, Chem. Rev., 2020, 120, 6820–6877 CrossRef CAS PubMed.
- Y. Zhu, X. He and Y. Mo, J. Mater. Chem. A, 2016, 4, 3253–3266 RSC.
- Y. Song, L. Wang, L. Sheng, D. Ren, H. Liang, Y. Li, A. Wang, H. Zhang, H. Xu and X. He, Energy Environ. Sci., 2023, 16(5), 1943–1963 RSC.
- S. Kaboli, G. Girard, W. Zhu, A. G. Nita, A. Vijh, C. George, M. L. Trudeau and A. Paolella, Chem. Commun., 2021, 57(84), 11076–11079 RSC.
- D. Cao, X. Sun, Q. Li, A. Natan, P. Xiang and H. Zhu, Matter, 2020, 3(1), 57–94 CrossRef.
- P. Lu, D. Wu, L. Chen, H. Li and F. Wu, Electrochem. Energy Rev., 2022, 5, 3 CrossRef CAS.
- L. Wang, Z. Chen, Y. Liu, Y. Li, H. Zhang and X. He, eTransportation, 2023, 16, 100239 CrossRef.
- M. N. Richard and J. R. Dahn, J. Electrochem. Soc., 1999, 146(6), 2068–2077 CrossRef CAS.
- D. Ren, X. Liu, X. Feng, L. Lu, M. Ouyang, J. Li and X. He, Appl. Energy, 2018, 228, 633–644 CrossRef CAS.
- X. Feng, X. He, M. Ouyang, L. Wang, L. Lu, D. Ren and S. Santhanagopalan, J. Electrochem. Soc., 2018, 165(16), A3748–A3765 CrossRef CAS.
- H. Zhou, M. Parmananda, K. R. Crompton, M. P. Hladky, M. A. Dann, J. K. Ostanek and P. P. Mukherjee, Energy Storage Mater., 2022, 44, 326–341 CrossRef.
- T. D. Hatchard, D. D. MacNeil, A. Basu and J. R. Dahn, J. Electrochem. Soc., 2001, 148(7), A755–A761 CrossRef CAS.
- G. H. Kim, A. Pesaran and R. Spotnitz, J. Power Sources, 2007, 170, 476–489 CrossRef CAS.
- D. Kong, G. Wang, P. Ping and J. Wen, eTransportation, 2022, 12, 100157 CrossRef.
- X. Feng, L. Lu, M. Ouyang, J. Li and X. He, Energy, 2016, 115, 194–208 CrossRef CAS.
- C. Jin, Y. Sun, J. Yao, X. Feng, X. Lai, K. Shen, H. Wang, X. Rui, C. Xu, Y. Zheng, L. Lu, H. Wang and M. Ouyang, eTransportation, 2022, 14, 100199 CrossRef.
- J. Zhang, Y. Wang, B. Jiang, H. He, S. Huang, C. Wang, Y. Zhang, X. Han, D. Guo, G. He and M. Ouyang, Nat. Commun., 2023, 14, 5940 CrossRef CAS PubMed.
- X. Lai, Z. Meng, F. Zhang, Y. Peng, W. Zhang, L. Sun, L. Wang, F. Gao, J. Sheng, S. Su, Y. Zheng and X. Feng, J. Energy Chem., 2023, 83, 3–15 CrossRef CAS.
- Y. Wu, D. Ren, X. Liu, G. L. Xu, X. N. Feng, Y. J. Zheng, Y. L. Li, M. Yang, Y. Peng, X. B. Han, L. Wang, Z. H. Chen, Y. Ren, L. G. Lu, X. M. He, J. T. Chen, K. Amine and M. G. Ouyang, Adv. Energy Mater., 2021, 11(47), 2102299 CrossRef CAS.
- X. Liu, G. L. Xu, V. S. C. Kolluru, C. Zhao, Q. T. Li, X. W. Zhou, Y. Z. Liu, L. Yin, Z. Q. Zhuo, A. Daali, J. J. Fan, W. J. Liu, Y. Ren, W. Xu, J. J. Deng, I. Hwang, D. S. Ren, X. N. Feng, C. J. Sun, L. Huang, T. Zhou, M. Du, Z. H. Chen, S. G. Sun, M. K. Y. Chan, W. L. Yang, M. G. Ouyang and K. Amine, Nat. Energy, 7(9), 808–817 CrossRef CAS.
- Y. Hong, Y. J. Zhang, C. Li, F. M. Zhang, F. Gao, J. Sheng, S. F. Su, S. Q. Chen, C. S. Xu, C. Y. Jin, H. B. Wang, Y. J. Zheng, H. W. Wang, X. N. Feng and M. G. Ouyang, J. Energy Storage, 2023, 64, 107133 CrossRef.
Footnote |
| † These authors contributed equally to this perspective. |
| This journal is © The Royal Society of Chemistry 2023 |