Near-infrared organic light-emitting materials, devices and applications
Mengxin
Xu
,
Xinyi
Li
,
Shihao
Liu
 ,
Letian
Zhang
,
Letian
Zhang
 * and
Wenfa
Xie
* and
Wenfa
Xie
 *
*
State Key Laboratory of Integrated Optoelectronics, College of Electronics Science and Engineering, Jilin University, Changchun, 130012, People's Republic of China. E-mail: zlt@jlu.edu.cn; xiewf@jlu.edu.cn
First published on 4th July 2023
Abstract
Near-infrared (NIR) light-emitting devices have recently garnered significant interest for integration in various applications, including photodynamic therapy and component determination, with emission wavelengths ranging from 700 nm to 2500 nm. In this review, we first introduce molecular design strategies for NIR materials, with a focus on fluorescent, phosphorescent, and TADF materials, and summarize recent advancements in highly luminescent NIR materials. Secondly, we provide an overview of high-performing NIR organic light-emitting devices (OLEDs), examining their structural designs and underlying device physics. Thirdly, we highlight practical applications of NIR materials and OLEDs in areas such as biomedicine and component determination. Finally, we discuss current limitations and prospects for the development of NIR materials, devices, and applications.
1. Introduction
Near-infrared (NIR) light refers to electromagnetic radiation with a wavelength ranging from visible light to middle infrared light, typically between 700 nm and 2500 nm.1 NIR light can easily penetrate human tissue and matches the absorption regions of certain organic molecules, making it a promising application in various fields, including biomedicine and component determination.2–4 For instance, in photodynamic therapy, NIR luminescent materials can be utilized as photosensitizers and can effectively image tissues due to their high penetration and low autofluorescence interference.5–8 Furthermore, due to the ability of NIR light to be absorbed by C–H, N–H, O–H, and S–H bonds in a sample, NIR spectroscopy offers notable benefits in monitoring food components. Compared to traditional methods, NIR spectroscopy is non-invasive, rapid, and requires minimal sample preparation.9,10 As a result, research on NIR materials and light-emitting devices has become a highly sought-after topic in recent years.11–13Compared to inorganic materials, organic NIR-emitting materials provide advantages on low cost, lightweight, mechanical flexibility, and biocompatibility, thus endowing them with broader potential applications and higher research value.14–17 Organic NIR-emitting materials can be categorized into fluorescent, phosphorescent, and thermally activated delayed fluorescent (TADF) materials, each utilizing different luminescence mechanisms.18 Radiation resulting from the transition from the lowest singlet state (S1) and the lowest triplet state (T1) to the ground state (S0) is defined as fluorescence and phosphorescence respectively. TADF emitters, on the other hand, allow reverse intersystem crossing (RISC) processes at ambient temperature, leading to radiation luminescence because of the narrow energy split (ΔEST) between S1 and T1. Triplet excitons can transition to S1 through RISC, and then to S0, resulting in radiation luminescence. As both phosphorescent and TADF materials can utilize both singlet and triplet excitons, organic light-emitting devices (OLEDs) made with these materials theoretically have the potential to achieve an internal quantum efficiency (IQE) of 100%.19,20 However, due to the small band gap of NIR materials, non-radiative decay can be significant according to the energy gap law, resulting in a photoluminescence quantum yield (PLQY) that is often below unity. Therefore, many efforts have been made to reduce the probability of non-radiative decay and greatly improve the PLQY of NIR emitters.21,22 Currently, NIR emitter with an emission peak at 732 nm can show a PLQY over 24%.23
To fully harness the benefits of organic NIR emitters, the development of efficient NIR OLEDs requires precise structural design and detailed investigations into device physics. A well-designed device configuration can enhance carrier injection and transport, thereby increasing the likelihood of exciton formation.24–27 Elaborate choice of host materials and compositional adjustment can affect the exciton distribution profile, and thus bimolecular quenching. Moreover, outcoupling efficiency (OCE) of NIR OLEDs is determined by the refractive indexes and the thicknesses of multilayer structure, light extraction units, and so on. With these efforts, the art-to-state NIR OLED have achieved a peak external quantum efficiency (EQE) of 13.4% with an emission peak at 734 nm.28–31
However, there are still certain limitations and challenges in NIR organic luminescent materials and devices. For NIR materials, in addition to the lower PLQY, efficiency degradation or luminescence attenuation in high-temperature environments will also occur. This is because high temperatures can cause adverse effects such as structural disorder or changes in energy levels;32 For NIR OLEDs, it is necessary to search for the optimal device structure and process parameters based on the energy level of the material; In addition, the application of NIR OLEDs usually requires integration with other devices or packaging in special environments, which will significantly increase the preparation cost of devices.33 Therefore, research on molecular design strategies and device optimization techniques are important for further application of NIR materials and OLEDs.
Considering that current reviews have mainly focused on NIR materials, their PL properties, and their applications in photodynamic therapy or NIR spectroscopy,34,35 this article aims to cover recent advancements in organic NIR materials, specifically their electroluminescent (EL) properties (Fig. 1). We firstly summarize the three types of EL mechanisms of NIR materials, including fluorescent, phosphorescent, and TADF materials. We then summarize advancements in the design strategies and device physics of NIR OLEDs, and examine the practical applications of NIR materials and OLEDs. Finally, we discuss the prospects of NIR materials, devices, and applications.
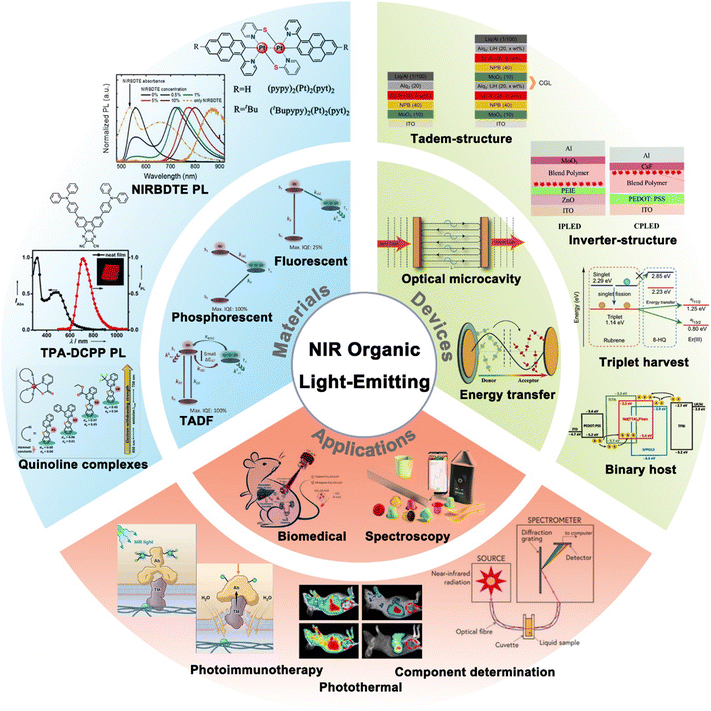 | ||
| Fig. 1 General concept of NIR organic light-emitting materials, devices and applications. Reproduced with permission from ref. 26. Copyright 2020 Journal of Materials Chemistry C. Reproduced with permission from ref. 27. Copyright 2017 Angewandte Chemie International Edition. | ||
2. Organic near-infrared materials
2.1. Fluorescent materials
Fluorescent materials can only harvest singlet excitons, thus their maximum IQE cannot exceed 25%. Besides, the energy gap law states that the rate of non-radiative transitions in the excited state of the semiconductor will increase exponentially with a redshift in the excitation wavelength, leading to a significantly decrease in the quantum yield.36 As a result, the maximum EQE of NIR fluorescent OLEDs reported so far is less than 2%. Despite this, fluorescent materials have the advantages of lower cost, higher biocompatibility and great environmental friendliness compared to phosphorescent materials because they don’t contain heavy metal elements. Furthermore, the exciton lifetime of fluorescent materials is 100 times shorter than that of TADF and phosphorescent materials, which provides advantages in high-speed transmission fields such as Li-Fi applications.37 In this section, we will review the most efficient NIR fluorescent materials based on donor–accepter–donor (D–A–D) and accepter–donor–accepter (A–D–A) oligomers, polymer materials, and some unusual but promising NIR fluorescent materials.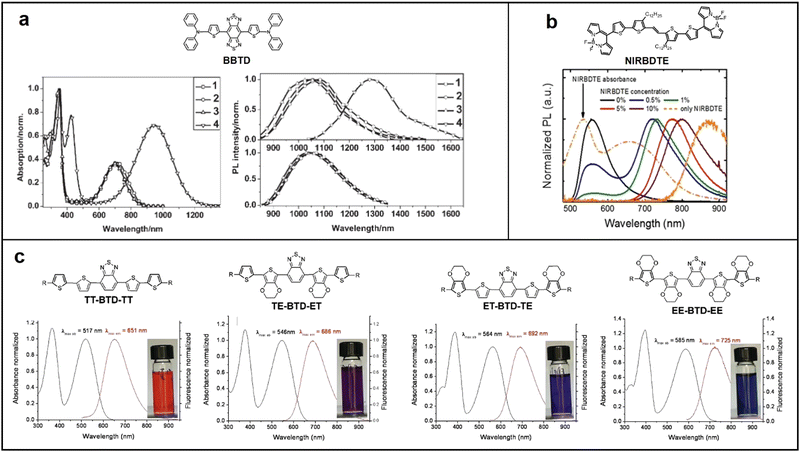 | ||
| Fig. 2 Chemical structure of BBTD, absorption and emission spectra of (a) BBTD-based compounds; reproduced with permission from ref. 38. Copyright 2009 Advanced Materials. (b) NIRBDTE; reproduced with permission from ref. 39. Copyright 2011 Chemistry of Materials. (c) TT-BTD-TT, ET-BTD-TE, TE-BTD-ET, and EE-BTD-EE; reproduced with permission from ref. 40. Copyright 2017 Scientific Reports. | ||
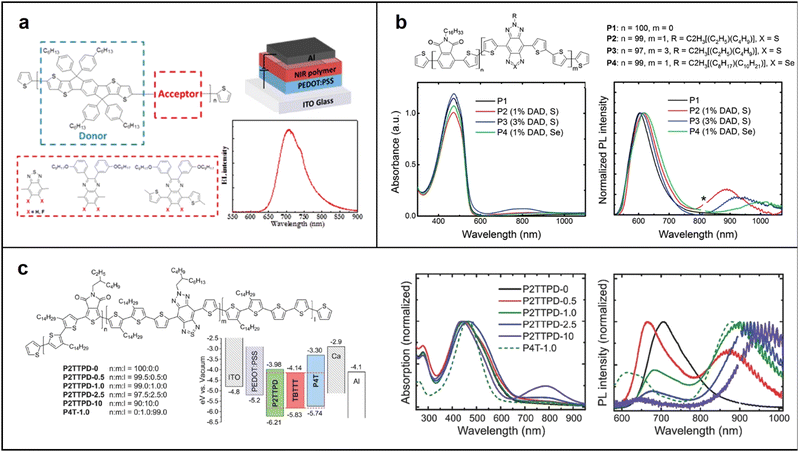 | ||
| Fig. 3 (a) Chemical structure of polymer NIR emitter with IDTT as donor, and EL spectrum of PLED based on PIDTT-TQF; chemical structure, absorption and emission spectra of (b) wide-gap host polymer (P1) and the copolymers (P2, P3 and P4); Reproduced with permission from ref. 42. Copyright 2017 Chemistry of Materials. Reproduced with permission from ref. 43. Copyright 2016 Advanced Optical Materials. (c) Phthalimide–thiophene host-based copolymers. Reproduced with permission from ref. 44. Copyright 2015 Journal of Materials Chemistry C. | ||
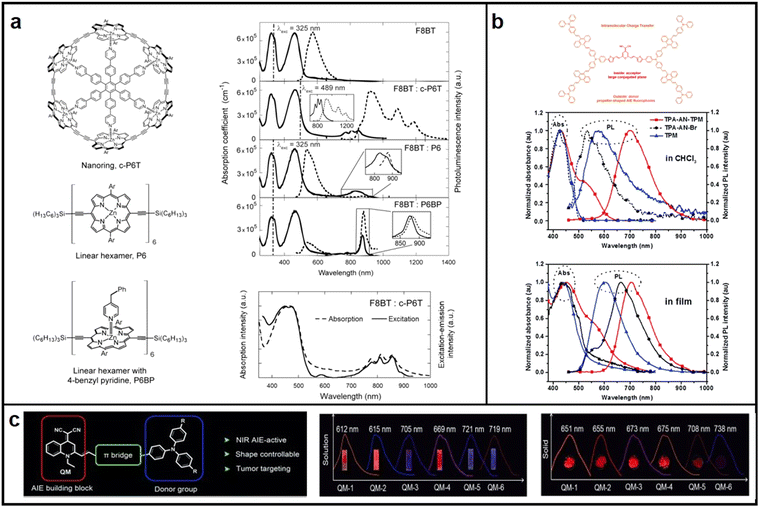 | ||
| Fig. 4 Chemical structures, absorption and emission spectra of (a) porphyrin hexamers and the complex of the linear hexamer; Reproduced with permission from ref. 45. Copyright 2011 Nano Letters. (b) TPA-AN-TPM and TPA-AN-Br in CHCl3; reproduced with permission from ref. 47. Copyright 2014 Polymer Chemistry. (c) QM derivative. reproduced with permission from ref. 48. Copyright 2015 Angewandte Chemie International Edition. | ||
Aggregation-induced emission (AIE) emitters have been regarded as one of the most promising materials for manufacturing high-performance OLEDs with a high EQE and low efficiency roll-off, as they emit strong light in their aggregated state and avoid the undesired ACQ effect.46 In 2014, Wang introduced a novel approach to develop molecules with both red emission and AIE characteristics. This involved enveloping disc-shaped fluorophores with AIE fluorophores shaped like propellers, which segregated the inner large conjugated planes, thereby preventing quenching of fluorescence (Fig. 4b).47 In 2015, Zhu conducted a synthesis of a range of new AIE-active molecules utilizing quinoline-malononitrile (QM) as the fundamental building block.48 Variation of electron donor groups and thiophene π-bridges led to the creation of AIE-active QM derivatives exhibiting red to NIR emissions. Of these derivatives, QM-5 exhibited a considerable red shift in its emission spectra, from 668 nm in pure THF solution to 721 nm in a mixed THF/H2O solution (Fig. 4c).
2.2. Phosphorescent materials
Typically, transitions between multiple states, such as T1 to S0, are forbidden due to spin prohibition. However, when the energy levels of the singlet and triplet orbitals in an organic molecule are closely spaced and located near each other, spin–orbit coupling (SOC) can occur. This phenomenon results in the display of some singlet properties by the triplet electron, leading to intersystem crossing (ISC) and phosphorescence emission. This effect is particularly notable in metal complexes containing heavy metals such as iridium (Ir), platinum (Pt), osmium (Os), palladium (Pd), europium (Eu), and terbium (Tb), etc. By exploiting all excitons, the IQE of materials can reach a maximum of 100%.49–51 Therefore, phosphorescent materials have emerged as the research focus of NIR luminescent materials.NIR-emitting phosphorescent materials are primarily classified into two groups: rare earth metal complexes and transition metal complexes. Presently, rare earth metal complexes, such as lanthanum (La), neodymium (Nd), and erbium (Er) are often inadequate for practical applications due to their low brightness.52 In this section, we will examine recent developments in Pt and Ir NIR phosphorescent materials.
Bidentate, tridentate, and tetradentate ligand complexes are the three types of mononuclear Pt complexes based on the type of ligands. Among these, bidentate ligand complexes are effective in regulating energy levels through the design of D–A structure. In 2021, Kidanu introduced AtFOND, a novel NIR material that utilized a potent donor ligand, 4-hydroxy-8-phenoxy-1,5-naphthyridine (OPhNDH), in 2-(4-methoxyphenyl)-5-(trifluoromethyl)pyridine (AtF). The introduction of OPhNDH improved the metal–metal-to-ligand charge transfer (MMLCT), leading to extremely long emission wavelengths up to maximum λEL 774 nm, but also decreased the EQE. In contrast, PBSNND, another NIR material, was created by incorporating another donor ligand, 4-hydroxy-8-dimethylamino-1,5-naphthyridine (dmaNDH), into 2-phenylbenzodthiazole (PBS), which exhibited a relatively high EQE (about 10.1%) with a maximum emission wavelength of λEL 704 nm. The high EQE was attributed to the optimal molecular arrangement of PBSNND, which increased the light OCE to 32%, a value 1.6 times higher than most conventional OLEDs (Fig. 5a).56
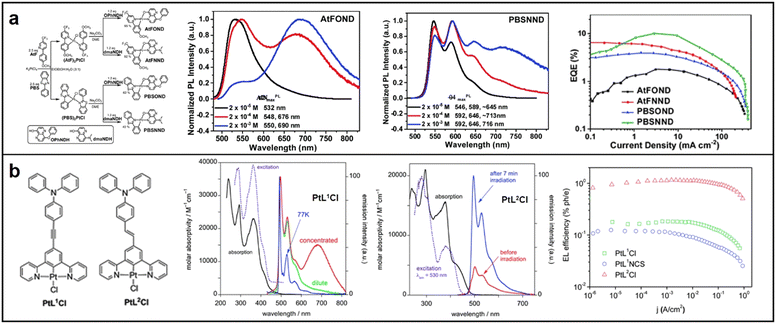 | ||
| Fig. 5 Chemical structures, absorption and emission spectra, devices EQE of (a) AtFOND and PBSNND; reproduced with permission from ref. 56. Copyright 2021 Materials Advances. (b) PtL1Cl and PtL2Cl; reproduced with permission from ref. 58. Copyright 2014 Journal of Materials Chemistry C. | ||
In contrast to bidentate ligand complexes, tridentate ligand complexes have potential for higher luminous efficiency due to their more rigid structures, which minimize nonradiative decay. However, ligand bond activity can lead to excimer emission and greater intermolecular interactions, resulting in challenges for molecular modification and device fabrication.57 In 2013, Filippo synthesized and characterized two highly luminous N^C^N pincer ligands and their corresponding Pt(II) complexes, PtL1Cl and PtL2Cl. PtL1Cl is suitable for bioimaging and sensing applications owing to its high quantum yield. On the other hand, PtL2Cl complexes are excellent candidates for developing NIR-OLEDs due to their high EL quantum efficiencies (Fig. 5b).58 In 2017, Yang and colleagues reported on a group of new N^C^N-type tridentate ligands and their Pt(II) complexes with pyrimidine, which showed a unique rod-coil shape, enhanced intermolecular π–π stacking, and excellent self-assembly performance in organic solvents. Varying the concentration of the material resulted in a redshift of the maximum emission wavelength, with the emission band covering the range of 600–850 nm (Fig. 6a).59
 | ||
| Fig. 6 (a) N^C^N-type tridentate ligand-based Pt(II) complexes; reproduced with permission from ref. 59. Copyright 2017 Chinese Journal of Organic Chemistry (b) PtNTBP and cis-PtN2TBP; reproduced with permission from ref. 61. Copyright 2016 Applied Physics Letters. (c) TPA-2q-PtCl2 and 3TPA-2q-PtCl2. Reproduced with permission from ref. 62. Copyright 2017 Dyes and Pigments. | ||
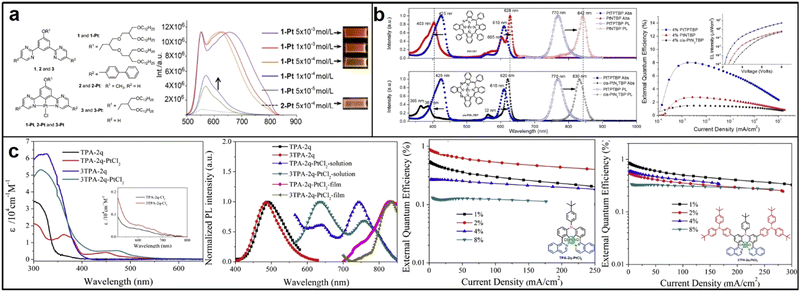
Tetradentate ligand complexes are more stable than bidentate or tridentate ligand complexes due to the additional coordination sites provided by the extra donor atom. The resulting stronger metal–ligand bonds and steric hindrance provided by the arrangement of four donor atoms can prevent unwanted interactions and improve efficiency. Despite these advantages, the synthesis of tetradentate ligand complexes remains challenging, and their production can be more expensive.60 In 2016, Huang discovered that two platinum(II) complexes, aza-triphenyltetrabenzoporphyrin (PtNTBP) and cis-diaza-diphenyltetrabenzoporphyrin (cis-PtN2TBP), exhibited EL emission peaks at 848 nm and 846 nm, respectively, with maximum EQEs of 2.8% and 1.5%. These results indicated that color tuning could be achieved by replacing meso-carbon groups with nitrogen groups in a porphyrin ring, causing the EL emission peak to shift nearly 80 nm towards the infrared region, (Fig. 6b).61 In 2017, Zhang developed two tetradentate bis-cyclometalated platinum(IV) complexes, TPA-2q-PtCl2 and 3TPA-2q-PtCl2, using tetradentate C^N*N^C ligands with triphenylamine units. These highly thermally stable complexes emitted EL peaks at 733 nm with an EQE of 0.87% and 750 nm with an EQE of 0.85% in their doped devices, demonstrating that tetradentate bis-cyclometalated platinum(IV) complexes with d6 octahedral structures are also potential materials for PLED applications in organic NIR EL devices (Fig. 6c).62
Mononuclear platinum complexes have a localized excited state, resulting in emissions based on a single metal center, with a narrow absorption and emission spectrum and visible region emissions. Conversely, dinuclear or polynuclear complexes with bridging ligands enable more efficient electronic communication between metal centers. Excitation of one metal center transfers energy to neighboring metal centers, producing a delocalized excited state spread over multiple metal centers. The result is a broadened absorption and emission spectrum and NIR emissions at longer wavelengths.63 In 2017, Su synthesized two binuclear platinum complexes, (pypy)2Pt2(pyt)2 and (tBupypy)2Pt2(pyt)2, in which pypy, tBupypy and pyt refer to 2-(1-pyrenyl)pyridine, 2-(7-tert-butyl-1-pyrenyl)pyridine (tBupypy) and pyridyl-2-thiolate, respectively. The unique three-dimensional chemical structure formed by the pyridyl-2-thiolate bridging units helps prevents π–π stacking and concentration quenching. Both complexes exhibited efficient NIR emission via metal–metal-to-ligand charge transfer (MMLCT) transition, with doped PLEDs displaying a NIR emission peak at 692 nm and a shoulder at 753 nm. The (tBupypy)2Pt2(pyt)2-doped devices showed the best EL performance, with a EQEmax of 0.74% and a maximum radiance (Rmax) of 123.4 μW cm−2 at a doping concentration of 2 wt% (Fig. 7a).64 In 2021, Marsel discovered a novel dinuclear platinum(II) complex, Pt2(bis-dthpym)(dpm)2, with a symmetric structure. This was achieved through a double N^C cyclometallation of 4,6-bis(dithienyl)-pyrimidine (H2bis-dthpym). The coordination sphere of each Pt centre was completed by O^O-coordinating dipivaloylmethane (dpm). This structure generates low-energy unimolecular emission through twice the number of higher-lying singlet states suitable for spin–orbit coupling (SOC) with the emitting triplet state T1, resulting in predominantly NIR emission.65 This approach complements the increasingly popular method of exploiting bimolecular aggregates or excimers (Fig. 7b).
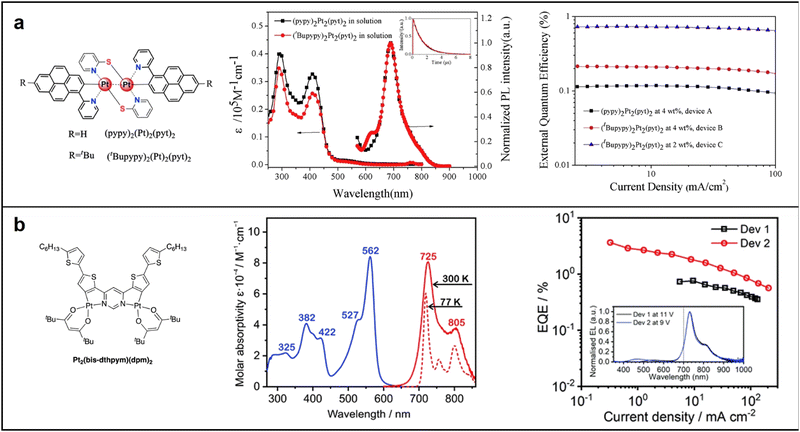 | ||
| Fig. 7 Chemical structures, absorption and emission spectra, devices EQE of (a) (pypy)2Pt2(pyt)2 and (tBupypy)2Pt2(pyt)2; reproduced with permission from ref. 64. Copyright 2017 Dyes and Pigments. (b) Pt2(bis-dthpym)(dpm)2. reproduced with permission from ref. 65. Copyright 2021 Journal of Materials Chemistry C. | ||
A study by Qiao et al. in 2017 demonstrated the use of benzo[g]phthalazine derivatives as cyclometalated ligands to synthesize homoleptic facial NIR-emitting Ir(III) complexes tris[1,4-di(thiophen-2-yl)benzo[g]phthalazine]iridium(III) (Ir(dtbpa)3) and tris[1-(2,4-bis(trifluoromethyl)phenyl)-4-(thiophen-2-yl)benzo[g]phthalazine]iridium(III) (Ir(Ftbpa)3). These complexes exhibit NIR-emission with high PLQY of up to 5.2% at 824 nm and 17.3% at 765 nm, respectively. It is worth mentioning that the noble metal content percentages of their complexes are around 10% Ir, which is significantly less compared to the typical green-emitting tris(2-phenylpyridine)iridium with about 30% Ir.67 These results propose a fresh method to produce strong and long-lasting NIR emitters with high efficiency, minimal roll-off, and low cost for practical applications in NIR-OLEDs (Fig. 8a). In 2018, Liu successfully synthesized and characterized a new π-extended iridium complex (t-BuPyrPyTPA)2Ir(acac) that exhibited stable NIR emission at a concentration of 4 wt%. The complex had a peak emission at 697 nm with a shoulder at 764 nm. It demonstrated a maximum EQE of 0.56% and a highest radiant emittance of 54.3 μW cm−2 at 119.3 mA cm−2.68 The study suggests that increasing the conjugation length of the ligand is an effective strategy to achieve stable NIR emission in PLEDs (Fig. 8b). In 2020, Kim synthesized a range of iridium(III) phosphorescent dopants by introducing substituents to the quinoline moiety of the cyclometalating ligand. The phosphorescence emission wavelength and quantum efficiencies were significantly affected by the phenyl, ethyl ester, and trifluoromethyl substituents. A high PLQY of 0.66 was recorded for Ir2 with a phenyl substituent, while Ir4 with a strong electron-withdrawing –CF3 substituent showed decent NIR emission at 716 nm with a quantum efficiency of 0.14. (Fig. 8c). Remarkably, Ir2-based OLEDs exhibited high deep-red emission with an excellent EQE of 7.29% at high doping ratios, suggesting its potential as a candidate for solution-processable PhOLEDs.69
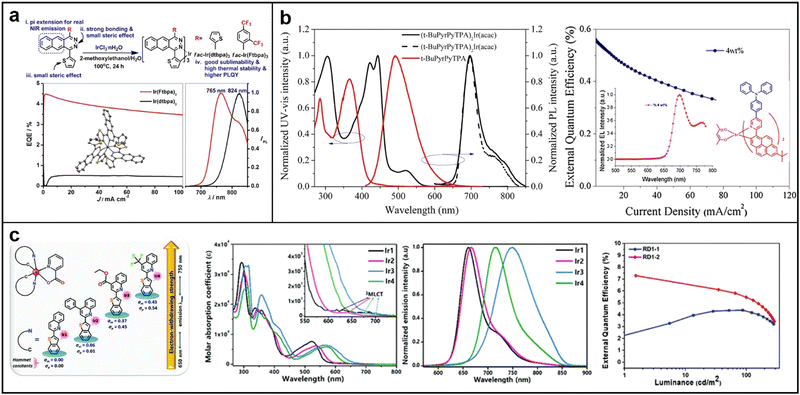 | ||
| Fig. 8 Chemical structures, absorption and emission spectra, devices EQE of (a) Ir(dtbpa)3 and Ir(Ftbpa)3; Reproduced with permission from ref. 67. Copyright 2017 Chemistry of Materials. (b) (t-BuPyrPyTPA)2Ir(acac); reproduced with permission from ref. 68. Copyright 2018 Chemical Physics Letters. (c) Quinoline-based Iridium(III) complexes Ir1–Ir4. Reproduced with permission from ref. 69. Copyright 2020 Journal of Materials Chemistry C. | ||
2.3. TADF materials
The RISC process enables electrons in the triplet state to reach the singlet state at room temperature (298 K) when the energy of the triplet state approaches that of the singlet state. This process, along with radiation transition, allows for TADF to occur. TADF materials that undergo efficient RISC, can achieve a 100% IQE.70–72 At present, visible emitting TADF-based OLEDs have been able to compete with phosphorescent OLEDs in terms of EQE. However, NIR TADF-based OLEDs have a low EQE, generally less than 5%, which is mainly due to the nonradiative transition process and concentration quenching effect induced by the strong D–A structure.73 This review section will discuss recent developments in NIR TADF materials, including molecular design strategies and photophysical properties.NIR TADF emitters typically have D–A structures, with the choice of donor and acceptor groups significantly impacting the charge transfer (CT) characteristics and photophysical properties of the emitter.74 Commonly used electron-donating groups include pyridine, triarylamine, carbazole, and thiophene, while pyrazine derivatives, phenanthroline, benzimidazole, and tetracyanoethylene are some widely used electron-withdrawing groups.75–77 This section will focus on NIR TADF materials that incorporate pyrazine derivatives as electron acceptors and various electron donors, as well as other NIR TADF materials that exhibit exceptional properties.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 707 mW Sr−1 m−2 was achieved.79 This work presents a simple and efficient molecular design strategy for developing NIR-emitting materials. In 2021, Balijapalli conducted a study introducing a new electron donor D–A TADF molecule, 11,12-bis(4-(diphenylamino)phenyl)dibenzo[a,c]phenazine-2,3,6,7-tetracarbonitrile (TPA-PZTCN), which displays strong NIR EL luminescence (EQEmax: 13.4% ±0.8%) at 734 nm with excellent EQE roll-off suppression (EQE > 10% at 1 mA cm−2). The efficient TADF can be attributed to the rigid and strong electron-withdrawing core of PZTCN, which facilitates the harvesting of triplets and sensitizing of deeper-NIR-fluorophores. Furthermore, the TADF-sensitized NIR-OLED utilizing TPA-PZTCN demonstrates excellent device durability, with a lifetime exceeding 600 hours at an LT95 threshold (Fig. 10a).80 In 2022, Wang reported the discovery of a novel NIR TADF molecule named CNPP-TPA. This molecule contains triphenylamine (TPA) as the donor and 2,3-dicyanopyrazino phenanthroline (CNPP) as the acceptor. The strong electron-withdrawing ability and the intramolecular hydrogen bond present in CNPP-TPA result in NIR photoluminescence with a peak at 701 nm, with a PLQY of 96.5% (Fig. 10b). This is attributed to the rapid singlet radiation and effective RISC. Furthermore, the doped film of CNPP-TPA possesses a rigid structure, with a balanced enhancement of intermolecular charge transfer and quenching suppression, leading to a PLQY of 96.5% and a near-zero ΔEST of 0.08 eV.81 In 2022, Fan’ group developed a novel D–A1–A2–A3 configuration NIR TADF material named TPA-CN-N4-2PY. This material incorporated three distinct sub-acceptor units, cyano (CN), dipyrido-3,2-a:2′,3′-c-phenazine (N4), and pyridine (PY), into the chemical structure to enhance its electron-accepting capability. By introducing two pyridine units to TPA-CN-N4, the resulting TPA-CN-N4-2PY had an extended π-backbone, which shifted the EL emission to the NIR region and simultaneously improved the horizontal ratio of emitting dipole orientation. When applied in OLEDs, TPA-CN-N4-2PY achieved a record-breaking EQE of 21.9% with an emission peak at 712 nm at a doping ratio of 9.0 wt%. This approach of utilizing multiple sub-acceptors could offer a new direction for creating NIR-TADF materials (Fig. 10c).82
707 mW Sr−1 m−2 was achieved.79 This work presents a simple and efficient molecular design strategy for developing NIR-emitting materials. In 2021, Balijapalli conducted a study introducing a new electron donor D–A TADF molecule, 11,12-bis(4-(diphenylamino)phenyl)dibenzo[a,c]phenazine-2,3,6,7-tetracarbonitrile (TPA-PZTCN), which displays strong NIR EL luminescence (EQEmax: 13.4% ±0.8%) at 734 nm with excellent EQE roll-off suppression (EQE > 10% at 1 mA cm−2). The efficient TADF can be attributed to the rigid and strong electron-withdrawing core of PZTCN, which facilitates the harvesting of triplets and sensitizing of deeper-NIR-fluorophores. Furthermore, the TADF-sensitized NIR-OLED utilizing TPA-PZTCN demonstrates excellent device durability, with a lifetime exceeding 600 hours at an LT95 threshold (Fig. 10a).80 In 2022, Wang reported the discovery of a novel NIR TADF molecule named CNPP-TPA. This molecule contains triphenylamine (TPA) as the donor and 2,3-dicyanopyrazino phenanthroline (CNPP) as the acceptor. The strong electron-withdrawing ability and the intramolecular hydrogen bond present in CNPP-TPA result in NIR photoluminescence with a peak at 701 nm, with a PLQY of 96.5% (Fig. 10b). This is attributed to the rapid singlet radiation and effective RISC. Furthermore, the doped film of CNPP-TPA possesses a rigid structure, with a balanced enhancement of intermolecular charge transfer and quenching suppression, leading to a PLQY of 96.5% and a near-zero ΔEST of 0.08 eV.81 In 2022, Fan’ group developed a novel D–A1–A2–A3 configuration NIR TADF material named TPA-CN-N4-2PY. This material incorporated three distinct sub-acceptor units, cyano (CN), dipyrido-3,2-a:2′,3′-c-phenazine (N4), and pyridine (PY), into the chemical structure to enhance its electron-accepting capability. By introducing two pyridine units to TPA-CN-N4, the resulting TPA-CN-N4-2PY had an extended π-backbone, which shifted the EL emission to the NIR region and simultaneously improved the horizontal ratio of emitting dipole orientation. When applied in OLEDs, TPA-CN-N4-2PY achieved a record-breaking EQE of 21.9% with an emission peak at 712 nm at a doping ratio of 9.0 wt%. This approach of utilizing multiple sub-acceptors could offer a new direction for creating NIR-TADF materials (Fig. 10c).82
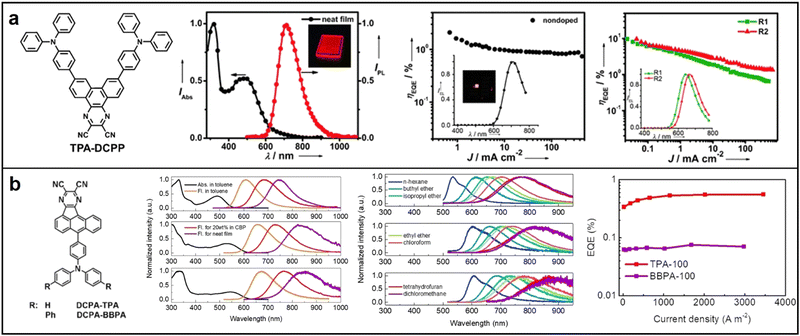 | ||
| Fig. 9 Chemical structures, absorption and emission spectra, devices EQE of (a) TPA-DCPP; Reproduced with permission from ref. 78. Copyright 2015 Angewandte Chemie International Edition. (b) DCPA-TPA and DCPA-BBPA; Reproduced with permission from ref. 79. Copyright 2020 Angewandte Chemie International Edition. | ||
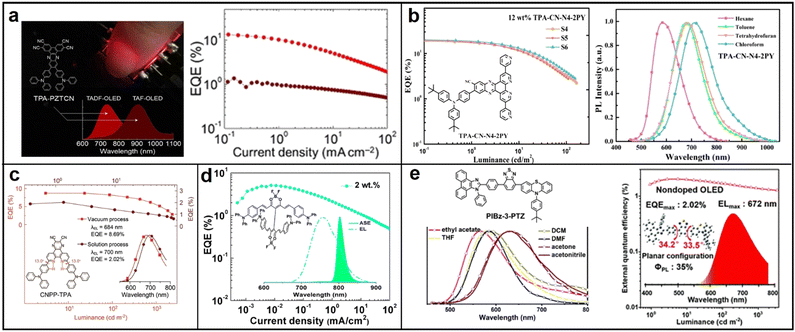 | ||
| Fig. 10 (a) TPA-PZTCN; reproduced with permission from ref. 80. Copyright 2021 Angewandte Chemie International Edition. (b) CNPP-TPA; reproduced with permission from ref. 81. Copyright 2022 Chemical Engineering Journal. (c) TPA-CN-N4-2PY; reproduced with permission from ref. 82. Copyright 2022 Materials Horizons. (d) TADF-ASE emitter; reproduced with permission from ref. 83. Copyright 2018 Chemistry of Materials. (e) PIBz-3-PTZ. Reproduced with permission from ref. 84. Copyright 2020 Journal of Materials Chemistry C. | ||
The NIR luminescent materials and their properties mentioned above are summarized in Table 1.
| Class | Material | λ max,PL (nm) | Φ PL (%) | λ max,EL (nm) | EQEmax (%) | Ref. | |
|---|---|---|---|---|---|---|---|
| Fluorescent material | D–A–D oligomer | BBTD | 1255 | 0.5 | 1080 | 0.28 | 38 |
| EE-BTD-EE | 725 | 0.07 | — | 0.87 | 39 | ||
| A–D–A oligomer | BODIPY derivative | — | 20 | 720 | 1.1 | 40 | |
| Polymer | IDTT | — | 19 | 705 | 0.1 | 42 | |
| P2TTPD | 924 | — | 909 | 0.01 | 43 | ||
| Phthalimide–thiophene polymer | 890 | 31 | 895 | 0.09 | 44 | ||
| Porphyrin derivative | c-P6T | 920 | 7.7 | 960 | 0.024 | 45 | |
| AIE emitter | TPA-AN-Br | 706 | 12.9 | — | — | 47 | |
| QM | 721 | — | — | — | 48 | ||
| Phosphorescent material | Platinum(II) | PBSNND | 732 | 33 | 704 | 10.1 | 56 |
| PtL2Cl | 702 | 0.003 | 685 | 1.2 | 58 | ||
| N^C^N-type complex | 685 | — | — | — | 59 | ||
| PtNTBP | 842 | — | 848 | 2.8 | 61 | ||
| cis-PtN2TBP | 830 | — | 846 | 1.5 | 61 | ||
| TPA-2q-PtCl2 | 742 | — | 733 | 0.87 | 62 | ||
| 3TPA-2q-PtCl2 | 758 | — | 750 | 0.85 | 62 | ||
| (pypy)2Pt2(pyt)2 | 690 | 2.89 | 692 | 0.22 | 64 | ||
| (tBupypy)2Pt2(pyt)2 | 690 | 6.80 | 692 | 0.74 | 64 | ||
| Pt2(bis-dthpym)(dpm)2 | 725 | 0.17 | 731 | 3.6 | 65 | ||
| Iridium(II) | Ir(dtbpa)3 | 824 | 5.2 | 811 | 0.5 | 67 | |
| Ir(Ftbpa)3 | 765 | 17.3 | 760 | 4.5 | 67 | ||
| (t-BuPyrPyTPA)2Ir(acac) | 697 | 1.92 | 697 | 0.56 | 68 | ||
| Ir4 | 716 | 0.12 | 716 | 1.07 | 69 | ||
| TADF material | Pyrazine derivative | TPA-DCPP | 708 | 14 | 710 | 2.1 | 78 |
| DCPA-TPA | 824 | 75 | 838 | 0.58 | 79 | ||
| DCPA-BBPA | 854 | 73 | 916 | 0.07 | 79 | ||
| TPA-PZTCN | 729 | 19.1 | 734 | 13.4 | 80 | ||
| CNPP-TPA | 701 | 96.5 | 700 | 2.02 | 81 | ||
| TPA-CN-N4-2PY | 653 | 68 | 712 | 21.9 | 82 | ||
| Others | Borondifluoride derivative | 760 | 45 | 758 | 5.1 | 83 | |
| PIBz-3-PTZ | 643 | 58 | 672 | 2.02 | 84 | ||
3. Near-infrared organic light-emitting devices
Since its inception by Tang and VanSlyke in 1987,85 OLED technology has made remarkable progress. Today, OLED has become one of the most popular display technologies due to its ultrathin, lightweight, flexible, foldable, and biocompatible properties.86–88 These features make OLEDs ideal for applications in fields such as NIR biomedicine, NIR spectroscopy, and others. In this section, the mechanism and luminescent characterization of OLEDs will be introduced first, followed by a review of recent research on high-efficiency NIR OLEDs, with an emphasis on device structure design and physics.3.1. Operating mechanism and luminescent characterization
The light-emission process in OLEDs follows a sequence of four steps. Initially, electrons and holes are injected into the organic layers between the cathode and anode, respectively. Secondly, the carriers are transported towards the emitting layer (EML) under the influence of an electric field. Thirdly, the combination of electrons and holes results in the formation of excitons. Finally, radiation transitions occur as the excitons return to the ground state, resulting in light emission.89 Each of the processes mentioned above has a significant impact on the luminescent properties of the device. The quality of OLEDs is usually evaluated by the luminous efficiency (LE), which is a measure of the combined effects of the above processes. There are three ways to express LE. The first is known as current efficiency (CE), which represents the percentage of the device's emission brightness to the injection current density and is expressed in cd A−1. The second is power efficiency, which represents the percentage of output optical power to input power and is expressed in lm W−1. The third is quantum efficiency, which represents the percentage of the device's emission brightness to injection current density and is expressed as a percentage.90,91 Notably, there are two types of quantum efficiency: IQE and EQE. IQE represents the percentage of the total number of photons generated by the device as a percentage of injected carriers, while EQE represents the percentage of photons emitted from the device as a percentage of injected carriers. EQE is the most commonly used efficiency term as it is independent of the photopic weighting factor and takes into account all observation directions. They can be expressed as follows:92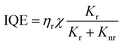 | (1) |
| EQE = ηeIQE | (2) |
In the equation, ηr is the ratio of the number of recombined carriers to the number of injected carriers, χ is the ratio of excitons used to generate light emission to the total number of excitons, Kr and Knr represent the rates of the optical and non-optical radiation processes generated by the excited state, respectively, and ηe is the OCE.
At present, OLEDs based on high-efficiency phosphorescent and TADF materials can achieve almost 100% IQE, while the EQE is generally less than 30%.92 This is primarily because the energy generated by the dipole emitter is transferred to various modes, causing significant energy loss. To address this issue, we will use the normalized in-plane wavevector (u) to analyze the light propagation and coupling in OLEDs, as it is conserved across all boundaries and dielectric media. It is expressed as follows:93
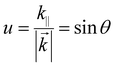 | (3) |
![[k with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_006b_20d1.gif) is the wavevector, k‖ is the horizontal component of
is the wavevector, k‖ is the horizontal component of ![[k with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_006b_20d1.gif) , and θ is the angle between the direction of light propagation and the normal direction. As θ changes, light displays four distinct modes: the air mode, substrate mode, waveguide mode, and surface plasmon polariton (SPP) mode. The air mode refers to the portion of light transmitted outside the OLED, and the proportion of this mode to the total is the OCE. The substrate mode refers to the portion of light confined within the substrate due to total internal reflection (TIR) at the interface between the substrate and air. The waveguide mode indicates the portion of light confined within the optical cavity of an OLED due to TIR at the electrode surface. The SPP mode refers to the electromagnetic waves generated by the coupling of photons and metal surface plasmons. Table 2 illustrates the relationship between the value of θ and the different modes. The OCE of an OLED with an optimized structure is typically between 20–30%, resulting in a relatively low EQE value.94 Currently, strategies to improve the EQE of NIR OLEDs are centered on two aspects: optical microcavity design and exciton energy transfer optimization.95–101
, and θ is the angle between the direction of light propagation and the normal direction. As θ changes, light displays four distinct modes: the air mode, substrate mode, waveguide mode, and surface plasmon polariton (SPP) mode. The air mode refers to the portion of light transmitted outside the OLED, and the proportion of this mode to the total is the OCE. The substrate mode refers to the portion of light confined within the substrate due to total internal reflection (TIR) at the interface between the substrate and air. The waveguide mode indicates the portion of light confined within the optical cavity of an OLED due to TIR at the electrode surface. The SPP mode refers to the electromagnetic waves generated by the coupling of photons and metal surface plasmons. Table 2 illustrates the relationship between the value of θ and the different modes. The OCE of an OLED with an optimized structure is typically between 20–30%, resulting in a relatively low EQE value.94 Currently, strategies to improve the EQE of NIR OLEDs are centered on two aspects: optical microcavity design and exciton energy transfer optimization.95–101
| Channels | Modes | θ (rad) |
|---|---|---|
| Outcoupling | Air mode | 0 < θ < θc1 = sin−1(1/norg) |
| Substrate mode | θ c1 < θ < θc2 = sin−1(nsub/norg) | |
| Loss | Waveguide mode | θ c2 < θ < π/2 |
| SPP mode | Undefined |
3.2. High efficiency NIR OLEDs
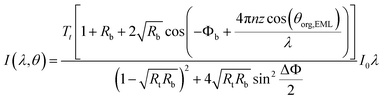 | (4) |
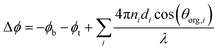 | (5) |
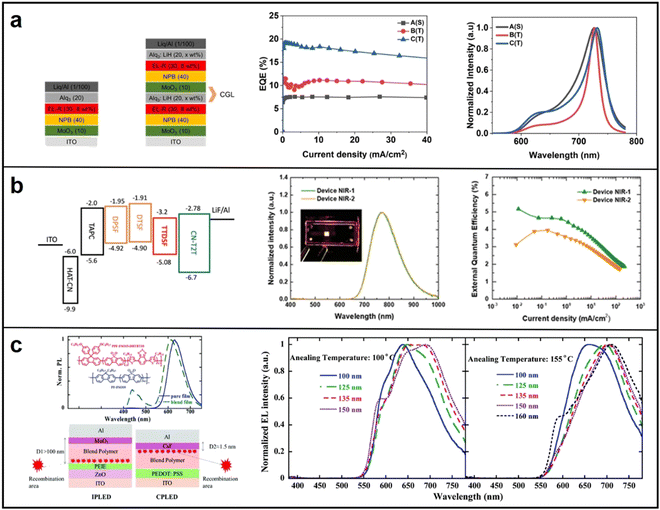 | ||
| Fig. 11 (a) Device structure of single and tandem OLEDs, EQE of single OLED, tandem OLEDs with Ag (20 nm) and Ag(30 nm), respectivity. Reproduced with permission from ref. 96. Copyright 2022 Scientific Reports. (b) Energy levels, EL Spectra and EQE of DPSF:CN-T2T and DPSF:CN-T2T:TTDSF-based OLEDs, respectivity. Reproduced with permission from ref. 97. Copyright 2021 Advanced Optical Materials. (c) Device Structure and EQE of inverted microcavity devices. Reproduced with permission from ref. 98. Copyright 2019 Journal of Materials Chemistry C. | ||
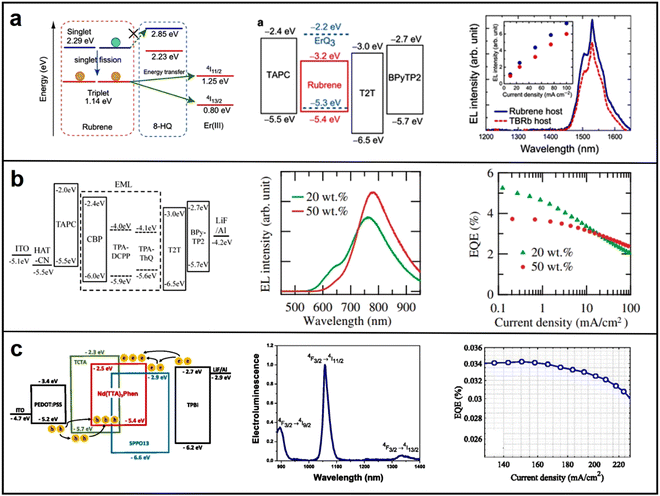 | ||
| Fig. 12 (a) Jablonski diagram for the harvesting of triplets of ErQ3 (left), energy level (middle) and EL spectrum (right) of OLEDs with a host of rubrene or TBRb; energy level, EL spectrum and EQE of (b) TADF-sensitized OLEDs and (c) Nd3+ ions-based binary ambipolar host OLEDs. Reproduced with permission from ref. 99. Copyright 2018 Advanced Materials. Reproduced with permission from ref. 100. Copyright 2008 Applied Physics Express. Reproduced with permission from ref. 101. Copyright 2019 Applied Physics Letters. | ||
The NIR OLEDs and their properties mentioned above are summarized in Table 3.
| Optimization strategy | Emitter | Host | Device structure | λ max,EL (nm) | EQEmax (%) | Ref. |
|---|---|---|---|---|---|---|
| Optical microcavity design | Ir(piq)3 | Bebq2 | ITO/MoO3 (10 nm)/NPB (40 nm)/Bebq2:Ir(piq)3 (30 nm, 8 wt %)/Alq3:LiH (20 nm, 5 wt%)/MoO3 (10 nm)/NPB (40 nm)/Bebq2:Ir(piq)3 (30 nm, 8 wt%)/Alq3:LiH (20 nm, 2 wt%)/Liq (1 nm)/Al (100 nm) | 730 | 19.17 | 96 |
| DTSF | CN-T2T | ITO/HAT-CN (30 nm)/TAPC (70 nm)/DTSF (15 nm)/DTSF:CN-T2T (50 wt%, 30 nm)/CN-T2T (50 nm)/LiF (1 nm)/Al (100 nm) | 746 | 5.3 | 97 | |
| PPF-FSO15-DHTBT10 | PF-FSO10 | ITO/ZnO/PEIE/PF-FSO10:PPF-FSO15-DHTBT10/MoO3 (10 nm)/Al (120 nm) (No thickness provided) | 700 | 0.54 | 98 | |
| Exciton energy transfer optimization | ErQ3 | Rubrene | ITO/TAPC (50 nm)/2 wt% ErQ3:rubrene (30 nm)/T2T (10 nm)/BPy-TP2 (55 nm)/LiQ (2 nm)/Mg:Ag (15 nm) | 1530 | — | 99 |
| TPA-ThQ | CBP | ITO (100 nm)/HAT-CN (10 nm)/TAPC(50 nm)/CBP, 5 wt% TPA-DCPP (30 nm)/T2T (20 nm)/Bpy-TP2 (60 nm)/LiF (0.8 nm)/Al (100 nm) | 780 | 3.8 | 100 | |
| Nd(TTA)3Phen | SPPO13 and TCTA | ITO/PEDOT:PSS (35 nm)/SPPO13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TCTA TCTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Nd(TTA)3Phen (6 Nd(TTA)3Phen (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (20 nm)/TPBi (40 nm)/LiF (0.8 nm)/Al (100 nm) 1) (20 nm)/TPBi (40 nm)/LiF (0.8 nm)/Al (100 nm) |
890 | 0.034 | 101 | |
4. Application areas of NIR materials and OLEDs
4.1. Photothermal and photoimmunotherapy
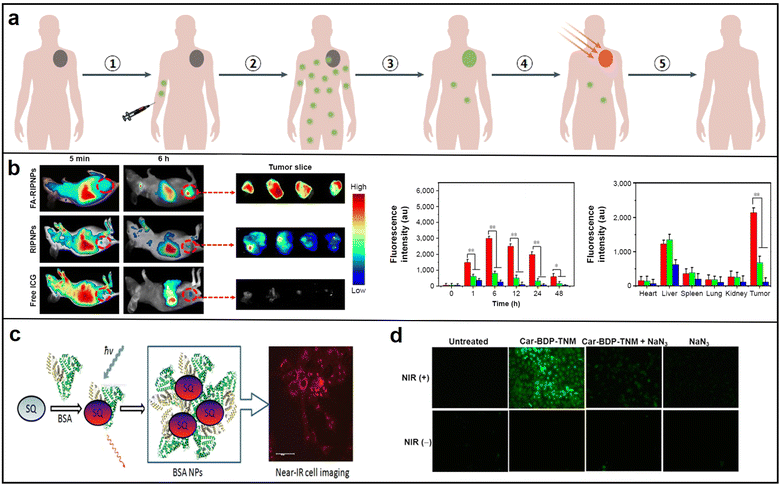 | ||
| Fig. 13 (a) Schematic diagram of NIR photothermal treatment process. Reproduced with permission from ref. 104. Copyright 2020 Nature Reviews Clinical Oncology. (b) Representative fluorescence images of mice after tail vein injection with free ICG, RIPNPs and FA-RIPNPs (left), and quantitative in vivo analysis of the fluorescence signals of the tumor regions (right); reproduced with permission from ref. 110. Copyright 2016 Dove press. (c) BSA NPs and NIR cell imaging; reproduced with permission from ref. 111. Copyright 2013 ACS Applied Materials & Interfaces. (d) Fluorescence microscopy of singlet-oxygen generation in Car-BDP-TNM. Reproduced with permission from ref. 112. Copyright 2016 Journal of the American Chemical Society. | ||
To ensure optimal efficacy in therapy and imaging, photosensitizing agents must exhibit certain key properties, such as high absorption at the treatment wavelength, safety in the absence of light, stability during the treatment process, favorable biocompatibility, and efficient accumulation in the targeted tissue.108 These agents can be broadly classified into several categories, including organic molecules, nanoparticles, carbon-based materials, and other inorganic materials. Our discussion will mainly focus on the potential application of NIR organic molecules and their corresponding nanoparticles.109 In 2016, Yu and colleagues prepared nanoparticles FA-RSV/ICG-PLGA-lipid NPs (FA-RIPNPs) by encapsulating indocyanine green (ICG) and poly(D,L-lactide-co-glycolide) (PLGA). This method was found to exhibit remarkable stability and biocompatibility features, and to provide high-quality fluorescence imaging. Furthermore, the treatment produced a greater rate of tumor cell inhibition (81.4% ± 2.1% U87 cell) via apoptosis. Subsequently, the researchers identified square derivative dyes that contain an oxocyclobutenate core, which exhibit a high molar absorption and robust absorption bands (Fig. 13b).110 In 2013, Zhang conducted a research project that explored the noncovalent binding of squaraine (SQ) dyes with bovine serum albumin (BSA). The findings of this study demonstrated a considerable enhancement in fluorescence intensity, approximately ten times higher than the baseline, as well as a ten-fold increase in fluorescence lifetime. Additionally, high-quality confocal fluorescent images were obtained for HCT 116 cells landscape as potential photodynamic therapy (PDT) agents due to their remarkable attributes, including high luminescence purity, exceptional thermal and photochemical stability, and high quantum yield (Fig. 13c).111 In 2016, Huang's research team introduced a biocompatible and highly efficient carbazole-substituted BODIPY (Car-BDP) molecule as a photosensitizer for PDT, which can penetrate deep into tissues with imaging guidance. These molecules exhibit exceptional NIR fluorescence, which enables their in vivo tracking and demonstrated potent tumor volume inhibition (∼80%) at deep tissue levels (Fig. 13d).112
Similar to PDT, PIT can also use NIR OLED as an excitation light source.96 For photosensitizing agents, most clinical and preclinical research activities focus on administering a conjugate comprised of IRdye700DX (IR700), a NIR, water-soluble, silicon-phthalocyanine derivative, and a monoclonal antibody (mAb) that recognizes an expressed antigen on the surface of cancer cells. Recently, the mechanisms underlying cell death by these conjugate complexes have been elucidated. Initially, the mAb binds to transmembrane proteins situated on the cancer cell's surface. Subsequently, exposure to NIR light triggers photochemical ligand reactions in IR700, releasing its hydrophilic side chains and rendering the rest of the molecule highly hydrophobic. This chemical transformation results in the formation of a Z-stack multimer of silicon-phthalocyanine IR700 rings or water-insoluble aggregates of antigen-presenting cells (APCs) or APC–antigen complexes, culminating in the quenching of IR700 fluorescence. The photo-chemical ligand release reaction instigates physicochemical changes within the APC–antigen complex, compromising the transmembrane target proteins and weakening the cell membrane's integrity. This leads to an influx of water into the cell, causing cell swelling and content release. Consequently, stress markers such as heat shock proteins 70 and 90, or dying signals such as calreticulin, ATP, and HMGB1 are activated promptly, promoting the maturation of immature dendritic cells and stimulating a host immune response against the antigens released from the dying cancer cells (Fig. 14a).113,114 Clinical trials of NIR-PIT for head and neck cancers were initiated in the United States in 2015, with the administration of RM-1929, a conjugate of IR700 and cetuximab, which targets the overexpression of EGFR in HNSCC. The results of the trials indicated that RM-1929 was safe and effective in treating locally recurrent HNSCC that cannot be surgically removed, demonstrated a superior therapeutic effect in athymic nude mouse models compared to cetuximab-based NIR-PIT, which can be attributed to its longer half-life (Fig. 14b).115
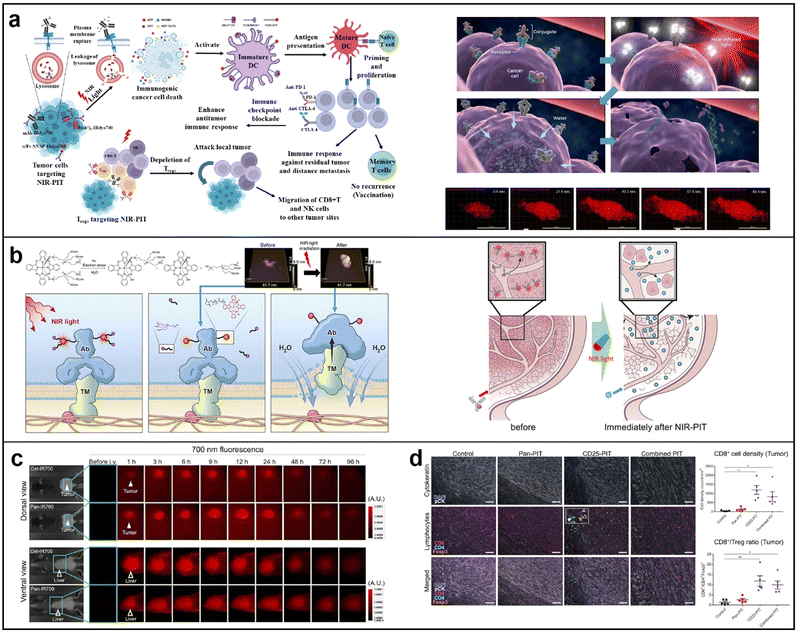 | ||
| Fig. 14 (a) The principle of NIR photoimmunotherapy; reproduced with permission from ref. 113. Copyright 2023 International Journal of Molecular Sciences. (b) Process of RM-1929 destroying target cells (left), mechanism of super enhanced permeability and retention (SUPR) effects induced by NIR-PIT (right); reproduced with permission from ref. 115. Copyright 2021 Head & Neck. (c) fluorescence microscopy of tissue and liver treated with Cet-IR700 and Pan-IR700, respectively; reproduced with permission from ref. 116. Copyright 2022 Cancer Immunology, Immunotherapy. (d) Multiplex immunohistochemical staining of the tumors 7 days. Reproduced with permission from ref. 117. Copyright 2021 eBioMedicine. | ||
Panitumumab is an antibody that targets the human epidermal growth factor receptor (hEGFR) and is employed for therapeutic purposes. This study demonstrated that panitumumab-based NIR-PIT displayed enhanced therapeutic effectiveness compared to cetuximab-based NIR-PIT in athymic nude mouse models. This advantage was attributed to panitumumab's longer half-life (Fig. 14c).116 Besides, combining two antibodies has been shown to be more effective than a single antibody–IRdye700 conjugate, resulting in improved homogeneous microdistribution. In 2021, Okada conducted research that demonstrated the superior inhibition of tumor growth through the use of a combination of two NIR-PIT reagents: panitumumab-IRdye700 and anti-CD25-F(ab0)2-IRdye700. CD8+ cells showed a significantly higher density in the CD25-PIT group and in the combined PIT group compared to the control group (Fig. 14d).117
In conclusion, PDT and PIT have shown promise in treating specific tumors. Nevertheless, there are still challenges to overcome, such as the insufficient specificity of photosensitive materials resulting in their accumulation in normal tissues and limited efficacy for tumor cells located deeper due to the NIR light's limited penetration depth. Addressing these issues will be crucial for future research in this field.
4.2. Component determination in food industry
As the demand for nutritious foods with bioactive compounds or phytonutrients increases, researchers are exploring functional food development techniques. Effective analysis of a food's chemical components is crucial in this process. Commonly used methods such as UV spectroscopy can be costly, slow, and require complex sample preparation.118 In contrast, NIR spectroscopy has emerged as a popular technique in the food and agriculture industry due to its intrinsic benefits such as being non-invasive, rapid, and requiring minimal sample preparation. NIR spectroscopy works based on the principle that chemical bonds such as C–H, N–H, O–H, and S–H in a sample will absorb a portion of photon energy when NIR light radiation passes through it, causing a change in the energy and frequency of the photons. The absorbed and scattered light can then be analyzed to determine the type and quantity of substances present in the sample, and to derive the sample's components and content.119,120 This section will specifically focus on the potential applications, challenges, and opportunities of NIR spectroscopy in measuring and quantifying the functional properties of cereal and dairy food products.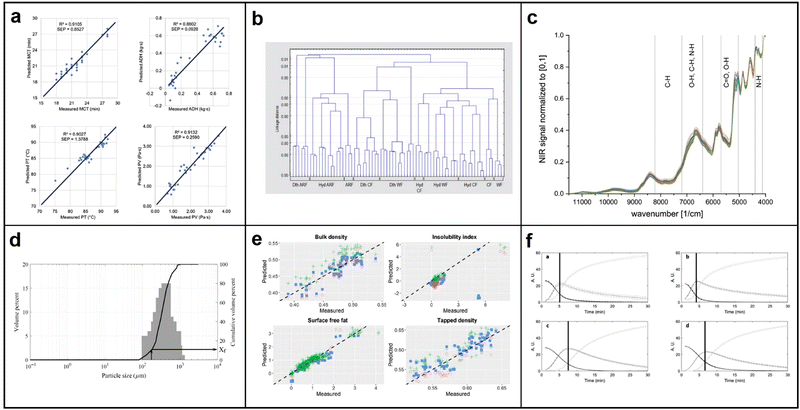 | ||
| Fig. 15 (a) Comparison between NIR predicted values and measured values for selected quality parameters of validation samples; MCT = minimum cooking time; ADH = adhesiveness; PT = pasting temperature; PV = peak viscosity; reproduced with permission from ref. 122. Copyright 2016 Journal of Cereal Science. (b) Cluster analysis dendrogram in the case of Hungarian wheat flour (WF), Hungarian cake flour (CF) and aleurone-rich wheat flour (ARF) samples in reference to the untreated, dry-thermal treated (Dth) and hydrothermal treated (Hyd) samples in the range of 1100-2500 nm; reproduced with permission from ref. 123. Copyright 2016 Quality Assurance and Safety of Crops & Foods. (c) NIR spectra of vital gluten samples G1–G39; reproduced with permission from ref. 124. Copyright 2021 European Food Research and Technology. (d) Particle size distributions of a milk powder sample with fine particle fraction estimation from cumulative distribution curve; reproduced with permission from ref. 126. Copyright 2020 International Journal of Dairy Technology. (e) Scatter plots for predicted against expected values for the external validation dataset; reproduced with permission from ref. 127. Copyright 2021 International Dairy Journal. (f) MCR-ALS concentration profiles of coagulation trials performed with the mixtures of pasteurized skimmed milk and reconstituted milk samples: EPI 40, EPI 60, SCA 40 and SCA 60. reproduced with permission from ref. 128. Copyright 2021 Food Control. | ||
To summarize, NIR spectroscopy is widely utilized in the food industry because of its non-destructive, rapid, and environmentally friendly characteristics. It can effectively determine the proximate composition of food products, such as protein, moisture, dry matter, fat, and starch. Due to the generally low efficiency of NIR OLEDs, there are few reports on using them as light sources for spectrometers. However, NIR OLED has the potential to be applied in NIR spectrometer light sources due to its advantages such as lightweight, flexibility, and fast response speed.14,21 However, further development and training are necessary to improve its ability to predict or quantify the functional properties of foods. Additionally, calibration models must be systematically established, incorporating samples from various sources and environmental conditions.129 The integration of NIR spectroscopy with other sensing technologies, data analysis techniques, and the Internet of Things is expected to contribute significantly to the advancement of the agricultural and food industries. It should be pointed out that NIR spectroscopy can not only be applied in agricultural industry, but also in fields such as drug analysis,130 environmental monitoring,131 and process analytical technology.132 With the development and innovation of technology, the application of NIR spectroscopy is constantly expanding and deepening.
5. Summary and outlook
Our review explores recent developments in organic NIR materials, devices, and applications. Specifically, we focus on molecular design strategies for efficient fluorescent, phosphorescent, and TADF emitters. These strategies involve optimizing the HOMO–LUMO energy levels, increasing conjugation length, and incorporating heavy atoms to enhance spin–orbit coupling. Notably, the design strategies vary for each type of material. For instance, efficient fluorescent materials require high quantum yields and effective energy transfer pathways, while heavy atom incorporation is essential for facilitating intersystem crossing in phosphorescent materials. In contrast, TADF materials necessitate a small energy gap between the singlet and triplet excited states to enable efficient RISC.Design strategies for high-performing NIR OLEDs can be summarized in two aspects. On the one hand, the micro-cavity effect in OLEDs enhances OCE, resulting in a narrower emission spectrum and a potential shift in emitted light wavelength. The constructive interference of light waves at specific wavelengths boosts the amount of emitted light, leading to improved color purity and accuracy. What's even more remarkable is that controlling the microcavity effect can be achieved simply by adjusting the device thickness, which is a highly efficient and low-cost device development strategy. On the other hand, optimizing charge balance and carrier injection/transport properties can reduce energy loss due to non-radiative processes, which can further improve device efficiency and stability.
NIR materials and OLEDs have a wide range of potential applications in biomedical and component determination. NIR fluorescence imaging, for instance, has the ability to detect and monitor tumors non-invasively in biological tissues. Furthermore, NIR spectroscopy can be used for the chemical analysis of components in various fields, such as food quality control and environmental monitoring. Nonetheless, there are limitations to these applications. For example, the accuracy and resolution of imaging may be affected by the limited penetration depth of NIR light in biological tissues due to absorption and scattering. Additionally, the stability and toxicity of NIR materials must be thoroughly considered for biomedical applications.
Despite the limitations, the future prospects for the development of NIR materials, devices, and applications are exciting. Advances in nanotechnology and biocompatible materials offer the potential for improved imaging resolution and sensitivity. Furthermore, the development of flexible OLEDs holds promise for new applications in wearable devices and displays.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 62174067, 62175085), Science and Technology Development Planning of Jilin Province (Project No. 20230101061JC) and Open Project of Key Lab of Special Functional Materials of Ministry of Education, Henan University (KFKT-2022-01).References
- J. Zhang, H. R. Ye, Y. X. Jin and D. M. Han, Recent progress in near-infrared organic electroluminescent materials, Top. Curr. Chem., 2022, 380, 6 CrossRef CAS PubMed.
- D. Liu, G. Li, P. Dang, Q. Zhang, Y. Wei, L. Qiu, M. S. Molokeev, H. Lian, M. Shang and J. Lin, Highly efficient Fe3+-doped A2BB′O6 (A = Sr2+, Ca2+; B, B′ = In3+, Sb5+, Sn4+) broadband near-infrared-emitting phosphors for spectroscopic analysis, Light Sci. Appl., 2022, 11, 112 CrossRef CAS PubMed.
- J. Ackermann, J. T. Metternich, S. Herbertz and S. Kruss, Biosensing with fluorescent carbon nanotubes, Angew. Chem., Int. Ed., 2022, 61, e202112372 CrossRef CAS PubMed.
- A. A. Ansari, A. K. Parchur and G. Chen, Surface modified lanthanide upconversion nanoparticles for drug delivery, cellular uptake mechanism, and current challenges in NIR-driven therapies, Coord. Chem. Rev., 2022, 457, 214423 CrossRef CAS.
- F. Fang, Y. Yuan, Y. Wan, J. Li, Y. Song, W. C. Chen, D. Zhao, Y. Chi, M. Li, C. S. Lee and J. Zhang, Near-infrared thermally activated delayed fluorescence nanoparticle: a metal-free photosensitizer for two-photon-activated photodynamic therapy at the cell and small animal levels, Small, 2022, 18, 2106215 CrossRef CAS PubMed.
- M. Y. Zhao, B. H. Li, Y. F. Wu, H. S. He, X. Y. Zhu, H. X. Zhang, C. R. Dou, L. S. Feng, Y. Fan and F. Zhang, A tumor-microenvironment-responsive lanthanide-cyanine FRET sensor for NIR-II luminescence-lifetime in situ imaging of hepatocellular carcinoma, Adv. Mater., 2020, 32, 2001172 CrossRef CAS PubMed.
- L. Feng, C. Li, L. Liu, Z. Wang, Z. Chen, J. Yu, W. Ji, G. Jiang, P. Zhang, J. Wang and B. Z. Tang, Acceptor planarization and donor rotation: a facile strategy for realizing synergistic cancer phototherapy via type I PDT and PTT, ACS Nano, 2022, 16, 4162–4174 CrossRef CAS PubMed.
- H. Li, H. Kim, F. Xu, J. Han, Q. Yao, J. Wang, K. Pu, X. Peng and J. Yoon, Activity-based NIR fluorescent probes based on the versatile hemicyanine scaffold: design strategy, biomedical applications, and outlook, Chem. Soc. Rev., 2022, 51, 1795–1835 RSC.
- T. Basile, A. D. Marsico and R. Perniola, Use of artificial neural networks and NIR spectroscopy for non-destructive grape texture prediction, Foods, 2022, 11, 281 CrossRef PubMed.
- F. Zhao, Z. Song and Q. Liu, Advances in chromium-activated phosphors for near-infrared light sources, Laser Photonics Rev., 2022, 16, 2200380 CrossRef CAS.
- Y. C. Wei, S. F. Wang, Y. Hu, L. S. Liao, D. G. Chen, K. H. Chang, C. W. Wang, S. H. Liu, W. H. Chan, J. L. Liao, W. Y. Hung, T. H. Wang, P. T. Chen, H. F. Hsu, Y. Chi and P. T. Chou, Overcoming the energy gap law in near-infrared OLEDs by exciton-vibration decoupling, Nat. Photonics, 2020, 14, 570–577 CrossRef CAS.
- D. M. Xi, M. Xiao, J. F. Cao, L. Y. Zhao, N. Xu, S. R. Long, J. L. Fan, K. Shao, W. Sun and X. H. Yan, NIR Light-Driving Barrier-Free Group Rotation in Nanoparticles with an 88.3% Photothermal Conversion Efficiency for Photothermal Therapy, Adv. Mater., 2020, 32, 1907855 CrossRef CAS PubMed.
- Q. X. Dang, Y. Y. Jiang, J. F. Wang, J. Q. Wang, Q. H. Zhang, M. K. Zhang, S. M. Luo, Y. J. Xie, K. Y. Pu and Q. Q. Li, Room-temperature phosphorescence resonance energy transfer for construction of near-infrared afterglow imaging agents, Adv. Mater., 2020, 32, 2006752 CrossRef PubMed.
- Q. Y. Zhang, M. X. Xu, L. M. Zhou, S. H. Liu, W. Wang, L. T. Zhang, W. F. Xie and C. J. Yu, A flexible organic mechanoluminophore device, Nat. Commun., 2023, 14, 1257 CrossRef CAS PubMed.
- T. Pan, S. H. Liu, L. T. Zhang, W. F. Xie and C. J. Yu, A flexible, multifunctional, optoelectronic anticounterfeiting device from high-performance organic light-emitting paper, Light: Sci. Appl., 2022, 11, 59 CrossRef CAS PubMed.
- S. H. Liu, J. M. Zhang, C. X. Zang, L. T. Zhang, W. F. Xie and C. S. Lee, Centimeter-scale hole diffusion and its application in organic light-emitting diodes, Sci. Adv., 1999, 8, eabm1999 CrossRef PubMed.
- C. X. Zang, S. H. Liu, M. X. Xu, R. F. Wang, C. Cao, Z. L. Zhu, J. M. Zhang, H. Wang, L. T. Zhang, W. F. Xie and C. S. Lee, Top-emitting thermally activated delayed fluorescence organic light-emitting devices with weak light-matter coupling, Light: Sci. Appl., 2021, 10, 116 CrossRef CAS PubMed.
- M. Y. Wong and E. Zysman-Colman, Purely organic thermally activated delayed fluorescence materials for organic light-emitting diodes, Adv. Mater., 2017, 29, 1605444 CrossRef PubMed.
- Z. Yu, Q. Y. Li, Q. Ma, W. P. Ye, Z. F. An and H. L. Ma, Excited-state descriptors for high-throughput screening of efficient electro-fluorescent materials, Chem. Mater., 2023, 35, 1827–1833 CrossRef CAS.
- Y. Z. Shi, H. Wu, K. Wang, J. Yu, X. M. Ou and X. H. Zhang, Recent progress in thermally activated delayed fluorescence emitters for nondoped organic light-emitting diodes, Chem. Sci., 2022, 13, 3625–3651 RSC.
- G. Y. Zhang, D. Y. Wang, B. B. Lou, C. G. Ma and Y. H. Wang, Efficient broadband near-infrared emission from lead-free halide double perovskite single crystal, Angew. Chem. Int. Ed., 2022, 61, e202207454 CAS.
- Q. Wang, W. X. Dai, Y. L. Xie, Q. Q. Ke, C. H. Zhao, B. Zhang, Z. B. Zeng, Z. M. Wang and B. Z. Tang, AIE-active deep red/near-infrared electroluminescent emitters with fine regulation of excited state, Chem. Eng. J., 2022, 451, 138529 Search PubMed.
- C. You, D. Liu, M. Zhu, J. Yu, B. Zhang, Y. Liu, Y. Wang and W. Zhu, Σ–π and p–π conjugation induced NIR-emitting iridium(III) complexes anchored by flexible side chains in a rigid dibenzo[a,c]phenazine moiety and their application in highly efficient solution-processable NIR-emitting devices, J. Mater. Chem. C, 2020, 8, 7079–7088 RSC.
- X. Zhong, T. Chervy, L. Zhang, A. Thomas, J. George, C. Genet, J. A. Hutchison and T. W. Ebbesen, Energy transfer between spatially separated entangled molecules, Angew. Chem., Int. Ed., 2017, 56, 9034–9038 CrossRef CAS PubMed.
- S. H. Liu, C. X. Zang, J. M. Zhang, S. Tian, Y. Wu, D. Shen, L. T. Zhang, W. F. Xie and C. S. Lee, Air-stable ultrabright inverted organic light-emitting devices with metal ion-chelated polymer injection layer, Nanomicro Lett., 2021, 14, 14 CrossRef PubMed.
- C. X. Zang, M. X. Xu, L. T. Zhang, S. H. Liu and W. F. Xie, Organic–inorganic hybrid thin film light-emitting devices: Interfacial engineering and device physics, J. Mater. Chem. C, 2021, 9, 1484–1519 RSC.
- J. M. Zhang, S. A. Liu, Y. F. Chen, L. T. Zhang and W. F. Xie, Simple-structure color-tunable fluorescent organic light-emitting devices with chromaticity difference beyond five-step mcadam ellipses, J. Phys. D, 2021, 54, 505103 CrossRef CAS.
- U. Balijapalli, R. Nagata, N. Yamada, H. Nakanotani, M. Tanaka, A. D'Aleo, V. Placide, M. Mamada, Y. Tsuchiya and C. Adachi, Highly efficient near-infrared electrofluorescence from a thermally activated delayed fluorescence molecule, Angew. Chem., Int. Ed., 2021, 60, 8477–8482 CrossRef CAS PubMed.
- C. F. You, D. H. Liu, J. T. Yu, H. Tan, M. B. Zhu, B. Zhang, Y. Liu, Y. F. Wang and W. G. Zhu, Boosting efficiency of near-infrared emitting iridium(III) phosphors by administrating their pi-pi conjugation effect of core-shell structure in solution-processed OLEDs, Adv. Opt. Mater., 2020, 8, 2000154 CrossRef CAS.
- S. F. Wang, Y. Yuan, Y. C. Wei, W. H. Chan, L. W. Fu, B. K. Su, I. Y. Chen, K. J. Chou, P. T. Chen, H. F. Hsu, C. L. Ko, W. Y. Hung, C. S. Lee, P. T. Chou and Y. Chi, Highly efficient near-infrared electroluminescence up to 800 nm using platinum(II) phosphors, Adv. Funct. Mater., 2020, 30, 2002173 CrossRef CAS.
- A. Shahalizad, A. Malinge, L. Hu, G. Laflamme, L. Haeberle, D. M. Myers, J. Mao, W. G. Skene and S. Kena-Cohen, Efficient solution-processed hyperfluorescent OLEDs with spectrally narrow emission at 840 nm, Adv. Funct. Mater., 2021, 31, 2007119 CrossRef CAS.
- C. Y. Sun, X. Z. Wang, Y. M. Xu, W. B. Lu, X. Y. Teng, G. S. Fu and W. Yu, Physical origins of high photoluminescence quantum yield in alpha-CsPbI3 nanocrystals and their stability, Appl. Surf. Sci., 2020, 508, 145188 CrossRef CAS.
- Y. Y. Wang, T. E. Hsieh, I. C. Chen and C. H. Chen, Direct encapsulation of organic light-emitting devices (OLEDs) using photo-curable co-polyacrylate/silica nanocomposite resin, IEEE Trans. Adv. Packag., 2007, 30, 421–427 Search PubMed.
- H. U. Kim, T. Kim, C. Kim, M. Kim and T. Park, Recent advances in structural design of efficient near-infrared light-emitting organic small molecules, Adv. Funct. Mater., 2022, 33, 202208082 Search PubMed.
- C. T. Jackson, S. Jeong, G. F. Dorlhiac and M. P. Landry, Advances in engineering near-infrared luminescent materials, Iscience, 2021, 24, 102156 CrossRef CAS PubMed.
- S. T. Le, T. Kanesan, F. Bausi, P. A. Haigh, S. Rajbhandari, Z. Ghassemlooy, I. Papakonstantinou, W. O. Popoola, A. Burton, H. Le Minh, F. Cacialli and A. D. Ellis, 10 Mb/s visible light transmission system using a polymer light-emitting diode with orthogonal frequency division multiplexing, Opt. Lett., 2014, 39, 3876–3879 CrossRef PubMed.
- P. A. Haigh, F. Bausi, Z. Ghassemlooy, I. Papakonstantinou, H. Le Minh, C. Fléchon and F. Cacialli, Visible light communications: Real time 10 Mb/s link with a low bandwidth polymer light-emitting diode, Opt. Express, 2014, 22, 2830–2838 CrossRef PubMed.
- G. Qian, Z. Zhong, M. Luo, D. Yu, Z. Zhang, Z. Y. Wang and D. Ma, Simple and efficient near-infrared organic chromophores for light-emitting diodes with single electroluminescent emission above 1000 nm, Adv. Mater., 2009, 21, 111–116 CrossRef CAS.
- S. Ellinger, K. R. Graham, P. Shi, R. T. Farley, T. T. Steckler, R. N. Brookins, P. Taranekar, J. Mei, L. A. Padilha, T. R. Ensley, H. Hu, S. Webster, D. J. Hagan, E. W. Van Stryland, K. S. Schanze and J. R. Reynolds, Donor–acceptor–donor-based π-conjugated oligomers for nonlinear optics and near-IR emission, Chem. Mater., 2011, 23, 3805–3817 CrossRef CAS.
- A. Zampetti, A. Minotto, B. M. Squeo, V. G. Gregoriou, S. Allard, U. Scherf, C. L. Chochos and F. Cacialli, Highly efficient solid-state near-infrared organic light-emitting diodes incorporating A–D–A dyes based on α,β-unsubstituted “bodipy” moieties, Sci. Rep., 2017, 7, 1611 CrossRef PubMed.
- J. Zhang, L. Xu, C. L. Ho and W. Y. Wong, Functional organometallic poly(arylene ethynylene)s: from synthesis to applications, Top. Curr. Chem., 2017, 375, 17 CrossRef PubMed.
- S. Tang, P. Murto, X. Xu, C. Larsen, E. Wang and L. Edman, Intense and stable near-infrared emission from light-emitting electrochemical cells comprising a metal-free indacenodithieno [3,2-b]thiophene-based copolymer as the single emitter, Chem. Mater., 2017, 29, 7750–7759 CrossRef CAS.
- P. Murto, A. Minotto, A. Zampetti, X. Xu, M. R. Andersson, F. Cacialli and E. Wang, Triazolobenzothiadiazole-based copolymers for polymer light-emitting diodes: pure near-infrared emission via optimized energy and charge transfer, Adv. Opt. Mater., 2016, 4, 2068–2076 CrossRef CAS.
- G. Tregnago, T. T. Steckler, O. Fenwick, M. R. Andersson and F. Cacialli, Thia- and selena-diazole containing polymers for near-infrared light-emitting diodes, J. Mater. Chem. C, 2015, 3, 2792–2797 RSC.
- O. Fenwick, J. K. Sprafke, J. Binas, D. V. Kondratuk, F. Di Stasio, H. L. Anderson and F. Cacialli, Linear and cyclic porphyrin hexamers as near-infrared emitters in organic light-emitting diodes, Nano Lett., 2011, 11, 2451–2456 CrossRef CAS PubMed.
- Z. Zhao, H. K. Zhang, J. W. Y. Lam and B. Z. Tang, Aggregation-induced emission: new vistas at the aggregate level, Angew. Chem., Int. Ed., 2020, 59, 9888–9907 CrossRef CAS PubMed.
- Z. Wang, L. Yan, L. Zhang, Y. Chen, H. Li, J. Zhang, Y. Zhang, X. Li, B. Xu, X. Fu, Z. Sun and W. Tian, Ultra bright red AIE dots for cytoplasm and nuclear imaging, Polym. Chem., 2014, 5, 7013–7020 RSC.
- A. D. Shao, Y. S. Xie, S. J. Zhu, Z. Q. Guo, S. Q. Zhu, J. Guo, P. Shi, T. D. James, H. Tian and W. H. Zhu, Far-red and near-IR AIE-active fluorescent organic nanoprobes with enhanced tumor-targeting efficacy: shape-specific effects, Angew. Chem., Int. Ed., 2015, 54, 7275–7280 CrossRef CAS PubMed.
- J. Luo, X. F. Rong, Y. Y. Ye, W. Z. Li, X. Q. Wang and W. J. Wang, Research progress on triarylmethyl radical-based high-efficiency OLED, Molecules, 2022, 27, 1632 CrossRef CAS PubMed.
- F. C. Zhao, Y. Wei, H. Xu, D. Chen, T. Ahamad, S. Alshehri, Q. B. Pei and D. G. Ma, Spatial exciton allocation strategy with reduced energy loss for high-efficiency fluorescent/phosphorescent hybrid white organic light-emitting diodes, Mater. Horiz., 2017, 4, 641–648 RSC.
- H. T. Kim, K. Lee, W. Jin, H. D. Um, M. Lee, E. Hwang, T. H. Kwon and K. Seo, Phosphorescent energy downshifting for diminishing surface recombination in silicon nanowire solar cells, Sci. Rep., 2018, 8, 16974 CrossRef PubMed.
- J. Zhang, H. Ye, Y. Jin and D. Han, Recent progress in near-infrared organic electroluminescent materials, Top. Curr. Chem., 2021, 380, 6 CrossRef PubMed.
- X. L. Yang, G. J. Zhou and W. Y. Wong, Functionalization of phosphorescent emitters and their host materials by main-group elements for phosphorescent organic light-emitting devices, Chem. Soc. Rev., 2015, 44, 8484–8575 RSC.
- S. Y. Yang, F. Y. Meng, X. G. Wu, Z. Yin, X. Z. Liu, C. F. You, Y. F. Wang, S. J. Su and W. G. Zhu, Dinuclear platinum(II) complex dominated by a zig-zag-type cyclometalated ligand: a new approach to realize high-efficiency near infrared emission, J. Mater. Chem. C, 2018, 6, 5769–5777 RSC.
- X. L. Yang, B. Jiao, J. S. Dang, Y. H. Sun, Y. Wu, G. J. Zhou and W. Y. Wong, Achieving high-performance solution-processed orange OLEDs with the phosphorescent cyclometalated trinuclear Pt(II) complex, ACS Appl. Mater. Interfaces, 2018, 10, 10227–10235 CrossRef CAS PubMed.
- H. T. Kidanu, J. H. Lee and C.-T. Chen, Photoluminescence and electroluminescence characterization of high-performance near-infrared emitters based on 1,5-naphthyridin-4-ol-containing heteroleptic platinum(II) complexes, Mater. Adv., 2021, 2, 3589–3599 RSC.
- Y. M. Zhang, F. Y. Meng, J. H. Tang, Y. F. Wang, C. F. You, H. Tan, Y. Liu, Y. W. Zhong, S. J. Su and W. G. Zhu, Achieving near-infrared emission in platinum(II) complexes by using an extended donor-acceptor-type ligand, Dalton Trans., 2016, 45, 5071–5080 RSC.
- F. Nisic, A. Colombo, C. Dragonetti, D. Roberto, A. Valore, J. M. Malicka, M. Cocchi, G. R. Freeman and J. A. G. Williams, Platinum(II) complexes with cyclometallated 5-π-delocalized-donor-1,3-di(2-pyridyl)benzene ligands as efficient phosphors for NIR-OLEDs, J. Mater. Chem. C, 2014, 2, 1791–1800 RSC.
- L. Yang, J. Hu, W. Zeng, Y. Wu, X. Li, D. Zhang and W. Jin, Synthesis, self-assemble and fluorescence of pyrimidine-contained novel rod-coil structured n^c^n-type divalent platium complexes, Chinese J. Org. Chem., 2017, 37, 2647–2654 CAS.
- G. Y. Pan, H. R. Jia, Y. X. Zhu and F. G. Wu, Turning double hydrophilic into amphiphilic: Ir825-conjugated polymeric nanomicelles for near-infrared fluorescence imaging-guided photothermal cancer therapy, Nanoscale, 2018, 10, 2115–2127 RSC.
- L. Huang, C. D. Park, T. Fleetham and J. Li, Platinum(II) azatetrabenzoporphyrins for near-infrared organic light emitting diodes, Appl. Phys. Lett., 2016, 109, 233302 CrossRef.
- Y. Zhang, F. Meng, C. You, S. Yang, L. Xiong, W. Xiong, W. Zhu, Y. Wang, Y. Pei and S. Su, Efficient near-infrared emitting tetradentate bis-cyclometalated platinum(IV) complexes for solution-processed polymer light-emitting diodes, Dyes Pigm., 2017, 142, 457–464 CrossRef CAS.
- B. Blondel, A. Colin, M. Lopes, F. Alary, G. Zissis, I. Sasaki and C. Renaud, Application of [Pt(II)(tetra-tert-butylsalophen)] complex within organic devices: deep red emission, bistable light-emitting diodes and operational stability, Appl. Sci., 2018, 8, 762 CrossRef.
- N. Su, F. Meng, P. Wang, X. Liu, M. Zhu, W. Zhu, S. Su and J. Yu, Near-infrared emission from binuclear platinum (II) complexes containing pyrenylpyridine and pyridylthiolate units: synthesis, photo-physical and electroluminescent properties, Dyes Pigm., 2017, 138, 162–168 CrossRef CAS.
- M. Z. Shafikov, P. Pander, A. V. Zaytsev, R. Daniels, R. Martinscroft, F. B. Dias, J. A. G. Williams and V. N. Kozhevnikov, Extended ligand conjugation and dinuclearity as a route to efficient platinum-based near-infrared (NIR) triplet emitters and solution-processed NIR-OLEDs, J. Mater. Chem. C, 2021, 9, 127–135 RSC.
- C. L. Ho, W. Y. Wong, G. J. Zhou, B. Yao, Z. Xie and L. Wang, Solution-processible multi-component cyclometalated Iridium phosphors for high-efficiency orange-emitting OLEDs and their potential use as white light sources, Adv. Funct. Mater., 2007, 17, 2925–2936 CrossRef CAS.
- J. Xue, L. Xin, J. Hou, L. Duan, R. Wang, Y. Wei and J. Qiao, Homoleptic facial ir(III) complexes via facile synthesis for high-efficiency and low-roll-off near-infrared organic light-emitting diodes over 750 nm, Chem. Mater., 2017, 29, 4775–4782 CrossRef CAS.
- Y. Liu, Z. Hao, F. Meng, P. Wang, L. Yang, Y. Wang, Y. Pei and S. Su, Efficient near-infrared emission of π-extended cyclometalated iridium complexes based on pyrene in solution-processed polymer light-emitting diode, Chem. Phys. Lett., 2018, 699, 99–106 CrossRef CAS.
- H. U. Kim, H. J. Jang, W. Choi, S. Park, T. Park, J. Y. Lee and K. S. Bejoymohandas, Aggregation-induced phosphorescence enhancement in deep-red and near-infrared emissive iridium(III) complexes for solution-processable OLEDs, J. Mater. Chem. C, 2020, 8, 4789–4800 RSC.
- X. K. Chen, D. Kim and J. L. Bredas, Thermally activated delayed fluorescence (TADF) path toward efficient electroluminescence in purely organic materials: molecular level insight, Acc. Chem. Res., 2018, 51, 2215–2224 CrossRef CAS PubMed.
- X. K. Liu, Z. Chen, J. Qing, W. J. Zhang, B. Wu, H. L. Tam, F. R. Zhu, X. H. Zhang and C. S. Lee, Remanagement of singlet and triplet excitons in single-emissive-layer hybrid white organic light-emitting devices using thermally activated delayed fluorescent blue exciplex, Adv. Mater., 2015, 27, 7079 CrossRef CAS PubMed.
- K. H. Kim, S. J. Yoo and J. J. Kim, Boosting triplet harvest by reducing nonradiative transition of exciplex toward fluorescent organic light-emitting diodes with 100% internal quantum efficiency, Chem. Mater., 2016, 28, 1936–1941 CrossRef CAS.
- C. Li, L. Duan, D. D. Zhang and Y. Qiu, Thermally activated delayed fluorescence sensitized phosphorescence: a strategy to break the trade-off between efficiency and efficiency roll-off, ACS Appl. Mater. Interfaces, 2015, 7, 15154–15159 CrossRef CAS PubMed.
- Y. Z. Shi, H. Wu, K. Wang, J. Yu, X. M. Ou and X. H. Zhang, Recent progress in thermally activated delayed fluorescence emitters for nondoped organic light-emitting diodes, Chem. Sci., 2022, 13, 3625–3651 RSC.
- B. Zhao, H. Wang, C. Han, P. Ma, Z. Li, P. Chang and H. Xu, Highly efficient deep-red non-doped diodes based on a t-shape thermally activated delayed fluorescence emitter, Angew. Chem., Int. Ed., 2020, 59, 19042–19047 CrossRef CAS PubMed.
- Z. Y. Cai, X. Wu, H. Liu, J. J. Guo, D. Z. Yang, D. G. Ma, Z. J. Zhao and B. Z. Tang, Realizing record-high electroluminescence efficiency of 31.5% for red thermally activated delayed fluorescence molecules, Angew. Chem., Int. Ed., 2021, 60, 23635–23640 CrossRef CAS PubMed.
- Y. G. Zhang, D. D. Zhang, M. H. Cai, Y. L. Li, D. Q. Zhang, Y. Qiu and L. Duan, Towards highly efficient red thermally activated delayed fluorescence materials by the control of intra-molecular π–π stacking interactions, Nanotechnology, 2016, 27, 094001 CrossRef PubMed.
- S. Wang, X. Yan, Z. Cheng, H. Zhang, Y. Liu and Y. Wang, Highly efficient near-infrared delayed fluorescence organic light emitting diodes using a phenanthrene-based charge-transfer compound, Angew. Chem., Int. Ed., 2015, 54, 13068–13072 CrossRef CAS PubMed.
- Y. J. Yu, Y. Hu, S. Y. Yang, W. Luo, Y. Yuan, C. C. Peng, J. F. Liu, A. Khan, Z. Q. Jiang and L. S. Liao, Near-infrared electroluminescence beyond 800 nm with high efficiency and radiance from anthracene cored emitters, Angew. Chem., Int. Ed., 2020, 59, 21578–21584 CrossRef CAS PubMed.
- U. Balijapalli, R. Nagata, N. Yamada, H. Nakanotani, M. Tanaka, A. D'Aléo, V. Placide, M. Mamada, Y. Tsuchiya and C. Adachi, Highly efficient near-infrared electrofluorescence from a thermally activated delayed fluorescence molecule, Angew. Chem., Int. Ed., 2021, 60, 8477–8482 CrossRef CAS PubMed.
- H. Wang, B. Zhao, C. Qu, C. Duan, Z. Li, P. Ma, P. Chang, C. Han and H. Xu, 2,3-dicyanopyrazino phenanthroline enhanced charge transfer for efficient near-infrared thermally activated delayed fluorescent diodes, Chem. Eng. J., 2022, 436, 135080 CrossRef CAS.
- J. L. He, Y. Tang, K. Zhang, Y. Zhao, Y. C. Lin, C. K. Hsu, C. H. Chen, T. L. Chiu, J. H. Lee, C. K. Wang, C. C. Wu and J. Fan, An extended π-backbone for highly efficient near-infrared thermally activated delayed fluorescence with enhanced horizontal molecular orientation, Mater. Horiz., 2022, 9, 772–779 RSC.
- H. Ye, D. H. Kim, X. Chen, A. S. D. Sandanayaka, J. U. Kim, E. Zaborova, G. Canard, Y. Tsuchiya, E. Y. Choi, J. W. Wu, F. Fages, J. L. Bredas, A. D’Aléo, J. C. Ribierre and C. Adachi, Near-infrared electroluminescence and low threshold amplified spontaneous emission above 800 nm from a thermally activated delayed fluorescent emitter, Chem. Mater., 2018, 30, 6702–6710 CrossRef CAS.
- F. Liu, Y. Tan, H. Liu, X. Tang, L. Gao, C. Du, J. Min, H. Jin and P. Lu, High-efficiency near-infrared fluorescent organic light-emitting diodes with small efficiency roll-off based on AIE-active phenanthro[9,10-d]imidazole derivatives, J. Mater. Chem. C, 2020, 8, 6883–6890 RSC.
- C. W. Tang and S. A. VanSlyke, Organic electroluminescent diodes, Appl. Phys. Lett., 1987, 51, 913–915 CrossRef CAS.
- H. Wang, H. Y. Zhao, C. X. Zang, S. H. Liu, L. T. Zhang and W. F. Xie, Stable and efficient phosphorescent organic light-emitting device utilizing a δ-carboline-containing host displaying thermally activated delayed fluorescence, J. Mater. Chem. C, 2020, 8, 3800–3806 RSC.
- S. H. Liu, Y. C. Wang, C. M. Chang, T. Yasuda, N. Fukui, H. Maeda, P. Long, K. Nakazato, W. B. Jian, W. F. Xie, K. Tsukagoshi and H. Nishihara, Solution-processed organometallic quasi-two-dimensional nanosheets as a hole buffer layer for organic light-emitting devices, Nanoscale, 2020, 12, 6983–6990 RSC.
- C. X. Zang, H. Wang, S. H. Liu, W. B. Guo, L. T. Zhang and W. F. Xie, Efficiency enhancement in an inverted organic light-emitting device with a TiO2 electron injection layer through interfacial engineering, J. Mater. Chem. C, 2020, 8, 8206–8212 RSC.
- C. X. Zang, X. M. Peng, H. Wang, Z. W. Yu, L. T. Zhang, W. F. Xie and H. Y. Zhao, Efficient multilayer and single layer phosphorescent organic lightemitting devices using a host with balanced bipolar transporting properties and appropriate energy level, Org. Electron., 2017, 50, 106–114 CrossRef CAS.
- H. W. Yu, J. M. Zhang, T. Long, M. X. Xu, H. W. Feng, L. T. Zhang, S. H. Liu and W. F. Xie, Efficient all-blade-coated quantum dot light-emitting diodes through solvent engineering, J. Phys. Chem. Lett., 2020, 11, 9019–9025 CrossRef CAS PubMed.
- S. H. Liu, X. Zhang, M. J. Yin, H. W. Feng, J. M. Zhang, L. T. Zhang and W. F. Xie, Coffee-ring-free ultrasonic spray coating single-emission layers for white organic light-emitting devices and their energy-transfer mechanism, ACS Appl. Energy Mater., 2018, 1, 103–112 CrossRef CAS.
- C. Murawski, K. Leo and M. C. Gather, Efficiency roll-off in organic light-emitting diodes, Adv. Mater., 2013, 25, 6801–6827 CrossRef CAS PubMed.
- A. Salehi, X. Fu, D.-H. Shin and F. So, Recent advances in OLED optical design, Adv. Funct. Mater., 2019, 29, 1808803 CrossRef.
- Z. W. Yu, J. M. Zhang, S. H. Liu, L. T. Zhang, Y. Zhao, H. W. Zhao and W. F. Xie, High-efficiency blue phosphorescent organic light-emitting devices with low efficiency roll-off at ultrahigh luminance by the reduction of triplet-polaron quenching, ACS Appl. Mater. Interfaces, 2019, 11, 6292–6301 CrossRef CAS PubMed.
- X. Gong, C. H. Lu, W. K. Lee, P. Li, Y. H. Huang, Z. X. Chen, L. S. Zhan, C. C. Wu, S. L. Gong and C. L. Yang, High-efficiency red thermally activated delayed fluorescence emitters based on benzothiophene-fused spiro-acridine donor, Chem. Eng. J., 2021, 405, 126663 CrossRef CAS.
- Y. Park, H. R. Choi, Y. Jeon, H. Kim, J. W. Shin, C. H. Huh, K. C. Park and K. C. Choi, Cell proliferation effect of deep-penetrating microcavity tandem NIR OLEDs with therapeutic trend analysis, Sci. Rep., 2022, 12, 10935 CrossRef CAS PubMed.
- Y. S. Chen, D. Luo, W. C. Wei, B. L. Chen, T. H. Yeh, S. W. Liu and K. T. Wong, New exciplex-forming co-host system and thienothiadazole-based fluorescent emitter for high-efficiency and promising stability near-infrared OLED, Adv. Opt. Mater., 2022, 10, 2101952 CrossRef CAS.
- J. Xu, F. Peng, Z. Sun, L. Yu, W. Yang and Y. Cao, Near-infrared polymer light-emitting diodes based on an inverted device structure, J. Mater. Chem. C, 2019, 7, 12114–12120 RSC.
- R. Nagata, H. Nakanotani, W. J. Potscavage Jr and C. Adachi, Exploiting singlet fission in organic light-emitting diodes, Adv. Mater., 2018, 30, 1801484 CrossRef PubMed.
- T. Yamanaka, H. Nakanotani, S. Hara, T. Hirohata and C. Adachi, Near-infrared organic light-emitting diodes for biosensing with high operating stability, Appl. Phys. Express, 2017, 10, 074101 CrossRef.
- A. Shahalizad, D. H. Kim, S. Rao Bobbara, Y. Tsuchiya, A. D'Aléo, C. Andraud, J. C. Ribierre, J. M. Nunzi and C. Adachi, Enhanced near-infrared electroluminescence from a Neodymium complex in organic light-emitting diodes with a solution-processed exciplex host, Appl. Phys. Lett., 2019, 114, 033301 CrossRef.
- S. Hofmann, M. Thomschke, B. Luessem and K. Leo, Top-emitting organic light-emitting diodes, Opt. Express, 2011, 19, A1250–A1264 CrossRef CAS PubMed.
- Y. C. Wei, S. F. Wang, Y. Hu, L. S. Liao, D. G. Chen, K. H. Chang, C. W. Wang, S. H. Liu, W. H. Chan and J. L. Liao, Overcoming the energy gap law in near-infrared OLEDs by exciton-vibration decoupling, Nat. Photonics, 2022, 14, 570–577 CrossRef.
- X. S. Li, J. F. Lovell, J. Yoon and X. Y. Chen, Clinical development and potential of photothermal and photodynamic therapies for cancer, Nat. Rev. Clin. Oncol., 2020, 17, 657–674 CrossRef PubMed.
- X. Jiang, B. Du, Y. Huang, M. Yu and J. Zheng, Cancer photothermal therapy with ICG-conjugated gold nanoclusters, Bioconjugate Chem., 2020, 31, 1522–1528 CrossRef CAS PubMed.
- D. E. J. G. J. Dolmans, D. Fukumura and R. K. Jain, Photodynamic therapy for cancer, Nat. Rev. Cancer, 2003, 3, 380–387 CrossRef CAS PubMed.
- R. Ackroyd, C. Kelty, N. Brown and M. Reed, The history of photodetection and photodynamic therapy, Photochem. Photobiol., 2001, 74, 656–669 CrossRef CAS PubMed.
- Y. Liu, P. Bhattarai, Z. Dai and X. Chen, Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer, Chem. Soc. Rev., 2019, 48, 2053–2108 RSC.
- X. Li, J. F. Lovell, J. Yoon and X. Chen, Clinical development and potential of photothermal and photodynamic therapies for cancer, Nat. Rev. Clin. Oncol., 2020, 17, 657–674 CrossRef PubMed.
- Y. Xin, T. Liu and C. L. Yang, Development of PLGA-lipid nanoparticles with covalently conjugated indocyanine green as a versatile nanoplatform for tumor-targeted imaging and drug delivery, Int. J. Nanomedicine, 2016, 11, 5807–5820 CrossRef CAS PubMed.
- Y. Zhang, X. Yue, B. Kim, S. Yao, M. V. Bondar and K. D. Belfield, Bovine serum albumin nanoparticles with fluorogenic near-IR-emitting squaraine dyes, ACS Appl. Mater. Interfaces, 2013, 5, 8710–8717 CrossRef CAS PubMed.
- L. Huang, Z. Li, Y. Zhao, Y. Zhang, S. Wu, J. Zhao and G. Han, Ultralow-power near infrared lamp light operable targeted organic nanoparticle photodynamic therapy, J. Am. Chem. Soc., 2016, 138, 14586–14591 CrossRef CAS PubMed.
- T. M. Mohiuddin, C. Zhang, W. Sheng, M. Al-Rawe, F. Zeppernick, I. Meinhold-Heerlein and A. F. Hussain, Near infrared photoimmunotherapy: a review of recent progress and their target molecules for cancer therapy, Int. J. Mol. Sci., 2023, 24, 2655 CrossRef CAS PubMed.
- H. Kobayashi and P. L. Choyke, Near-infrared photoimmunotherapy of cancer, Acc. Chem. Res., 2019, 52, 2332–2339 CrossRef CAS PubMed.
- D. M. Cognetti, J. M. Johnson, J. M. Curry, S. T. Kochuparambil, D. McDonald, F. Mott, M. J. Fidler, K. Stenson, N. R. Vasan, M. A. Razaq, J. Campana, P. Ha, G. Mann, K. Ishida, M. Garcia-Guzman, M. Biel and A. M. Gillenwater, Phase 1/2a, open-label, multicenter study of RM-1929 photoimmunotherapy in patients with locoregional, recurrent head and neck squamous cell carcinoma, Head Neck, 2021, 43, 3875–3887 CrossRef PubMed.
- R. Okada, T. Kato, A. Furusawa, F. Inagaki, H. Wakiyama, D. Fujimura, S. Okuyama, H. Furumoto, H. Fukushima, P. L. Choyke and H. Kobayashi, Selection of antibody and light exposure regimens alters therapeutic effects of EGRF-targeted near-infrared photoimmunotherapy, Cancer Immunol. Immunother., 2022, 71, 1877–1887 CrossRef CAS PubMed.
- R. Okada, A. Furusawa, D. W. Vermeer, F. Inagaki, H. Wakiyama, T. Kato, T. Nagaya, P. L. Choyke, W. C. Spanos, C. T. Allen and H. Kobayashi, Near-infrared photoimmunotherapy targeting human-EGRF in a mouse tumor model simulating current and future clinical trials, EBioMedicine, 2021, 67, 103345 CrossRef CAS PubMed.
- L. K. Bittner, S. A. Schonbichler, G. K. Bonn and C. W. Huck, Near infrared spectroscopy (NIRs) as a tool to analyze phenolic compounds in plants, Curr. Anal. Chem., 2013, 9, 417–423 CrossRef CAS.
- R. Kaavya, R. Pandiselvam, M. Mohammed, R. Dakshayani, A. Kothakota, S. V. Ramesh, D. Cozzolino and C. Ashokkumar, Application of infrared spectroscopy techniques for the assessment of quality and safety in spices: a review, Appl. Spectrosc. Rev., 2020, 55, 593–611 CrossRef CAS.
- D. Cozzolino, The sample, the spectra and the maths—the critical pillars in the development of robust and sound applications of vibrational spectroscopy, Molecules, 2020, 25, 3674 CrossRef CAS PubMed.
- S. Balet, A. Guelpa, G. Fox and M. Manley, Rapid visco analyser (RVA) as a tool for measuring starch-related physiochemical properties in cereals: a review, Food Anal. Methods, 2019, 12, 2344–2360 CrossRef.
- N. Thanathornvarakul, J. Anuntagool and K. Tananuwong, Aging of low and high amylose rice at elevated temperature: mechanism and predictive modeling, J. Cereal Sci., 2016, 70, 155–163 CrossRef CAS.
- E. Izsó, M. Bartalné-Berceli, A. Salgó and S. Gergely, Monitoring of heat-treated wheat milling fractions by near infrared spectroscopic method, Qual. Assur. Saf. Crop. Foods, 2018, 10, 93–102 CrossRef.
- M. Schopf, M. C. Wehrli, T. Becker, M. Jekle and K. A. Scherf, Fundamental characterization of wheat gluten, Eur. Food Res. Technol., 2021, 247, 985–997 CrossRef CAS.
- R. Kaavya, R. Pandiselvam, M. Mohammed, R. Dakshayani, A. Kothakota, S. V. Ramesh, D. Cozzolino and C. Ashokkumar, Application of infrared spectroscopy techniques for the assessment of quality and safety in spices: a review, Appl. Spectrosc. Rev., 2020, 55, 593–611 CrossRef CAS.
- A. Khan, M. T. Munir, W. Yu and B. R. Young, Near-infrared spectroscopy and data analysis for predicting milk powder quality attributes, Int. J. Dairy Technol., 2021, 74, 235–245 CrossRef CAS.
- C. Wang, M. G. Reis, G. I. N. Waterhouse, Y. Hemar and M. M. Reis, Prediction of dairy powder functionality attributes using diffuse reflectance in the visible and near infrared (VIS-NIR) region, Int. Dairy J., 2021, 117, 104981 CrossRef CAS.
- L. Strani, S. Grassi, C. Alamprese, E. Casiraghi, R. Ghiglietti, F. Locci, N. Pricca and A. D. Juan, Effect of physicochemical factors and use of milk powder on milk rennet-coagulation: Process understanding by near infrared spectroscopy and chemometrics, Food Control, 2021, 119, 107494 CrossRef CAS.
- D. Cozzolino, The ability of near infrared (NIR) spectroscopy to predict functional properties in foods: Challenges and opportunities, Molecules, 2021, 26, 6981 CrossRef CAS PubMed.
- O. Y. Rodionova, L. P. Houmoller, A. L. Pomerantsev, P. Geladi, J. Burger, V. L. Dorofeyev and A. P. Arzamastsev, NIR spectrometry for counterfeit drug detection - A feasibility study, Anal. Chim. Acta, 2005, 549, 151–158 CrossRef CAS.
- A. Paul, L. Wander, R. Becker, C. Goedecke and U. Braun, High-throughput NIR spectroscopic (NIRS) detection of microplastics in soil, Environ. Sci. Pollut. Res., 2019, 26, 7364–7374 CrossRef CAS PubMed.
- S. Sacher, J. Poms, J. Rehrl and J. G. Khinast, PAT implementation for advanced process control in solid dosage manufacturing-A practical guide, Int. J. Pharm., 2021, 613, 121408 CrossRef PubMed.
| This journal is © the Partner Organisations 2023 |
