Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Tale of the Breslow intermediate, a central player in N-heterocyclic carbene organocatalysis: then and now
Monika
Pareek†
,
Yernaidu
Reddi†
 and
Raghavan B.
Sunoj
and
Raghavan B.
Sunoj
 *
*
Department of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai 400076, India. E-mail: sunoj@chem.iitb.ac.in
First published on 11th May 2021
Abstract
N-Heterocyclic carbenes (NHCs) belong to the popular family of organocatalysts used in a wide range of reactions, including that for the synthesis of complex natural products and biologically active compounds. In their organocatalytic manifestation, NHCs are known to impart umpolung reactivity to aldehydes and ketones, which are then exploited in the generation of homoenolate, acyl anion, and enolate equivalents suitable for a plethora of reactions such as annulation, benzoin, Stetter, Claisen rearrangement, cycloaddition, and C–C and C–H bond functionalization reactions and so on. A common thread that runs through these NHC catalyzed reactions is the proposed involvement of an enaminol, also known as the Breslow intermediate, formed by the nucleophilic addition of an NHC to a carbonyl group of a suitable electrophile. In the emerging years of NHC catalysis, enaminol remained elusive and was largely considered a putative intermediate owing to the difficulties encountered in its isolation and characterization. However, in the last decade, synergistic efforts utilizing an array of computational and experimental techniques have helped in gaining important insights into the formation and characterization of Breslow intermediates. Computational studies have suggested that a direct 1,2-proton transfer within the initial zwitterionic intermediate, generated by the action of an NHC on the carbonyl carbon, is energetically prohibitive and hence the participation of other species capable of promoting an assisted proton transfer is more likely. The proton transfer assisted by additives (such as acids, bases, other species, or even a solvent) was found to ease the kinetics of formation of Breslow intermediates. These important details on the formation, in situ detection, isolation, and characterization of the Breslow intermediate are scattered over a series of reports spanning well over a decade, and we intend to consolidate them in this review and provide a critical assessment of these developments. Given the central role of the Breslow intermediate in organocatalytic reactions, this treatise is expected to serve as a valuable source of knowledge on the same.
Introduction
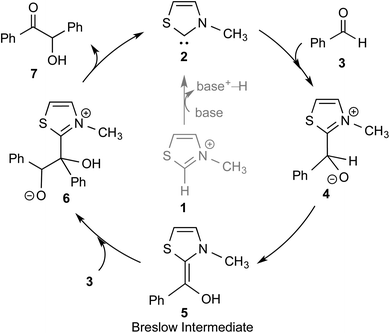
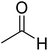
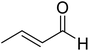
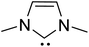
The topic of N-heterocyclic carbenes (NHCs) has been richly reviewed in recent years, with most of those reviews narrating their translational impact from a fleeting intermediate to a versatile organocatalyst for an unprecedented array of reactions.1 The key premise in NHC catalysis is the formation and the involvement of an enaminol intermediate, known as the Breslow intermediate. Albeit being scattered, many pieces of experimental evidence and a good number of computational studies on the generation of Breslow intermediates in NHC catalyzed transformations can be found in the literature. The lacuna in the burgeoning area of NHCs is the lack of a compendium on Breslow intermediates, focusing on their formation, detection, and isolation. It is therefore quite timely to give these aspects of NHCs their deserving attention in the form of a review article.The textbook concept of umpolung,2 which signifies reversal of polarity, is utilized in several organic reactions. The carbonyl carbon of an aldehyde is an electrophilic site, which upon reaction with a cyanide ion can be made nucleophilic, effectively leading to a reversal of polarity. More than a century ago, Wöhler and Liebig discovered the homodimerization of aldehydes in the presence of cyanide ions that leads to benzoin products.3 The Lapworth mechanism for such cyanide catalyzed benzoin reaction contributed to the early rationalization of umpolung reactivity.4 Decades later, Ukai and co-workers observed that thiazolium salts are also capable of catalyzing the benzoin reaction.5 In a landmark article in the year 1958, Breslow proposed a mechanism, as shown in Scheme 1, for thiazolium catalyzed benzoin reaction.6 The thiazolium catalyst precursor 1 is deprotonated at the most acidic position by the action of a base to generate carbene 2. The nucleophilic carbene 2 can then add to the electrophilic benzaldehyde 3 to form a zwitterionic intermediate, 4. A critical proton transfer from the aldehydic carbon to the geminal oxygen atom can then provide the diamino enol intermediate 5, which in more recent years came to be known as the Breslow intermediate. The nucleophilic Breslow intermediate can then combine with a second molecule of benzaldehyde to form the benzoin product (7) with the expulsion of the free carbene. While the formation of the diamino enol intermediate was suggested quite a few years ago, renewed attention to such intermediates was triggered due to the emergence of NHCs as promising organocatalysts in recent times.1,9
It is interesting to note that even decades before the transformative phase of NHCs, from an esoteric species to a ubiquitous catalyst, efforts on their isolation by Wanzlick and others led to dimerized products. The ability of such dimers to dissociate and to react with different electrophiles offered some early signs of promise toward developing the reactivity of NHCs.7 In a seminal report back in 1991, Arduengo and co-workers disclosed the crystal structure of an isolable N-adamantyl substituted NHC. Conspicuously, a chemist's ability to ‘see’ the molecular structure of a free NHC, which otherwise remained an elusive intermediate back then, served as an impetus to subsequent developments in this domain.8 In particular, the ensuing years witnessed an unprecedented growth in the use of NHCs as organocatalysts for a gamut of organic transformations. Especially, in such NHC catalyzed reactions, there has been the implicit assumption of participation of the Breslow intermediate. As one reads this article, it will become increasingly clear how systematic efforts helped researchers in taming this fleeting intermediate and eventually to make it amenable to detection and characterization.
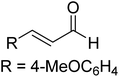
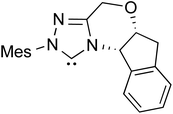
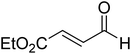
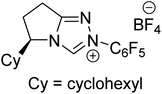
The formation of the Breslow intermediate, in transient or detectable forms, is vital to the catalytic applications of NHCs. Different reaction conditions have been employed in NHC catalysis, particularly due to their higher-level utilization such as that in asymmetric transformations. Computational studies were effectively employed in gaining valuable molecular understanding on the origin of stereoselectivities in such reactions, as evident from a representative set of examples shown in Scheme 2.9 These NHC catalyzed asymmetric transformations not only demonstrate their versatile role in diverse range of reactions, but also convey the synergism of density functional theory computations in faithfully reproducing the experimental enantioselectivities. One of the vital details encompassing the whole domain of NHC organocatalysis that demands attention is the mechanism of formation of Breslow intermediates. In the following section, an overview of the current understanding on the formation of Breslow intermediates under different catalytic conditions that vary in terms of the presence/nature of certain types of additive is provided.
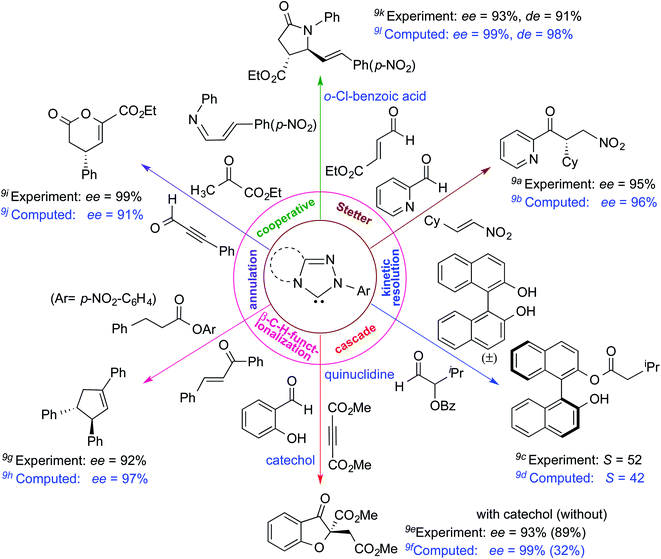 | ||
| Scheme 2 A representative set of chiral NHC catalyzed asymmetric transformations and a succinct comparison of the experimental enantioselectivities with those obtained using density functional theory (M06-2X) computations (ref. 9). | ||
Mechanistic insights on the formation of the Breslow intermediate
In the generally accepted mechanism, nucleophilic addition of the NHC to an electrophilic reaction partner (e.g., aldehyde, enal, etc.) generates a zwitterionic intermediate (8) first (Scheme 3). A critical proton transfer in this intermediate, through some of the pathways described below, can then lead to the Breslow intermediate. A large body of literature remains surprisingly silent about the feasibility of such 1,2-proton transfer whilst making implicitly general assumptions that a Breslow intermediate is readily formed. The first possibility is a direct proton transfer in the zwitterionic intermediate (8) via a highly strained three-membered transition state. In the second alternative, the zwitterionic intermediate undergoes a 1,2-hydride transfer to form the keto intermediate (11) followed by a tautomerization to the Breslow intermediate (10), again via a strained four-membered transition state. Along the expected lines, the computed barriers for the 1,2-direct proton transfer in 8 to form 10 are generally found to be very high, indicating that this pathway is not feasible (column 5, Table 1).10 To circumvent the strained geometries of the proton transfer transition state, explicit participation of other available molecules from the immediate environment has been proposed.11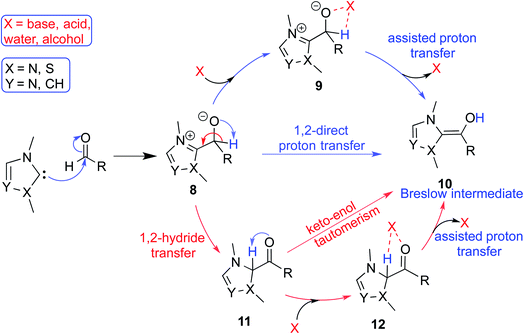 | ||
| Scheme 3 A general mechanism for the formation of the Breslow intermediate under different likely reaction conditions. | ||
An assisted proton transfer invokes a more direct role for (i) the base present in the reaction mixture (e.g., DBU, iPr2NEt, K2CO3), (ii) Brønsted acids, or (iii) other protic additives (e.g., H2O, MeOH, PhOH, catechol). There have been interesting density functional theory studies demonstrating the role of different additives in the formation of Breslow intermediates. In this section, we provide the energetic details on the formation of Breslow intermediates using a few representative examples as listed in Table 1.10 Note that most of our discussions in the latter sections make use of the reactions listed in this table.
(A) Intermolecular proton transfer pathway
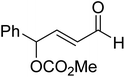
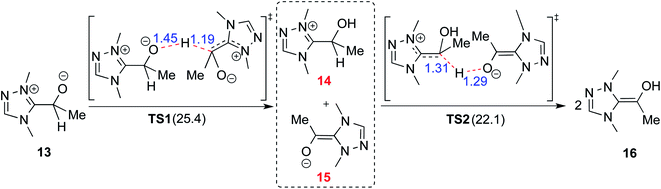
In 2008, Yates and co-workers employed density functional theory computations to study the mechanism of a Stetter reaction between acetaldehyde and a Michael acceptor (R1, Table 1).10a The proposed mechanism for the formation of the Breslow intermediate (16) is shown in Scheme 4. The most vital aspect that demands careful attention is the proton transfer in the initially formed zwitterionic adduct (13) between the NHC and acetaldehyde. A likely two-step bimolecular proton transfer between intermediates 14 and 15 was invoked.10a,b An overall activation barrier of 25.4 kcal mol−1 was found at the B3LYP/6-311+G(2d,p) level of theory, which is 13.7 kcal mol−1 lower than that for the unassisted 1,2-proton transfer in 13 (39.1 kcal mol−1).10a While this pathway appears energetically reasonable, it is instructive to consider that an encounter between two activated species (such as the NHC–aldehyde adduct) under the reaction conditions is less likely than that between the zwitterionic intermediate and any other molecule abundantly available in the medium. Hence, alternative routes for the generation of the Breslow intermediate need to be considered (vide infra).(B) Alcohol assisted pathway
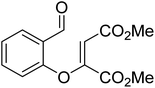
The presence of protic additives is known to be beneficial in certain reactions as they can facilitate easier proton transfer.10c,12 Interesting examples were reported wherein methanol, catechol, etc. assisted the critical proton transfer leading to the formation of the Breslow intermediate.9f,10c As part of their mechanistic investigation on NHC catalyzed ring expansion of 4-formyl-β-lactam (R2, Table 1), Domingo and co-workers reported a barrier of 44.0 kcal mol−1 for 1,2-direct proton transfer viaTS3 at the PCM(DCM)/B3LYP/6-31G** level of theory (Fig. 1).10c On the other hand, a methanol assisted proton transfer viaTS4 was noted to exhibit a much lower barrier. In the proton transfer transition state, the explicitly included methanol donates its proton to the alkoxy oxygen of the zwitterionic intermediate and concomitantly abstracts the aldehydic C–H proton. The activation barrier for this concerted methanol-assisted proton transfer (TS4) was 24.7 kcal mol−1 lower than that for the unassisted pathway viaTS3.10c Diminished strain in the transition state geometry as well as the additional specific interaction with the methanol can be considered as the origin of the lower barrier in the methanol assisted pathway.In another recent study, Sunoj and co-workers reported the mechanism and origin of enantioselectivity in an asymmetric Michael–Stetter cascade reaction catalyzed by a chiral NHC and quinuclidine leading to the formation of benzofuran (R3, Table 1).9f The energetically preferred pathway obtained at the SMD(toluene)/M06-2X/6-31+G** level of theory was suggested to begin with a nucleophilic addition of quinuclidine to DMAD as shown in Scheme 5. A few more ensuing steps (not of immediate relevance to the current discussion) furnish a DMAD–salicylaldehyde Michael adduct (17).
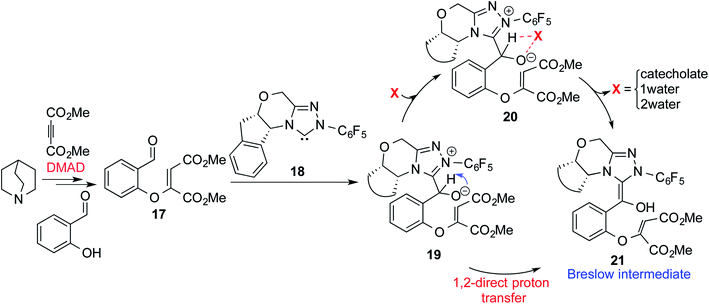 | ||
| Scheme 5 Key steps involved in the formation of the Breslow intermediate in a dual-catalytic Michael–Stetter cascade reaction. | ||
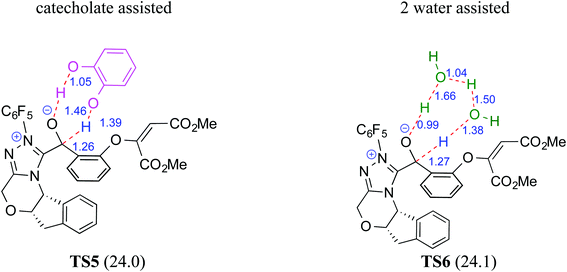
The participation of an NHC was invoked in the following step wherein the NHC reacts with the aldehydic end of the salicylate intermediate 17 to form a zwitterionic species 19. As with previous cases, a crucial proton transfer would convert 19 to the desired Breslow intermediate 21. Here, a 1,2-direct proton transfer was predicted to encounter a barrier of 48.0 kcal mol−1 prompting the authors to consider two types of assisted pathway, one with the involvement of the additive catechol and the other with an explicitly included water molecule. It was noted that catechol assists the proton transfer through a stepwise process involving the catecholate as the key species (formed by the action of quinuclidine on catechol). Shown in Fig. 2 is a qualitative depiction of a concerted transition state (TS5), wherein the alkoxy oxygen abstracts the phenolic proton of the catecholate while the proton on the alkoxy carbon is simultaneously abstracted by the phenoxide end of the catecholate. The predicted barrier was 24.0 kcal mol−1 for the catecholate assisted proton transfer, half of that for the 1,2-direct proton transfer. They also evaluated the efficacy of water assisted proton transfer en route to the Breslow intermediate. The energy of relay proton transfer, assisted by two explicit water molecules, as shown in TS6, was incidentally the same as that of the catechol assisted pathway. The barrier for the assisted proton transfer, mediated by one water molecule, was found to be 32.1 kcal mol−1 (not shown in Fig. 2 for brevity and easier comprehension of the most likely alternatives). Therefore, the mechanism of formation of the Breslow intermediate in this example is more likely to involve an assisted proton transfer pathway, facilitated by the explicit participation of one catechol or two water molecules.
(C) Base assisted pathway
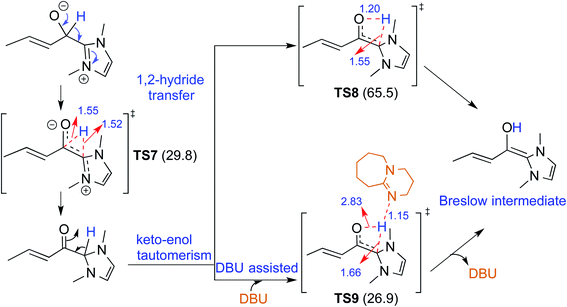
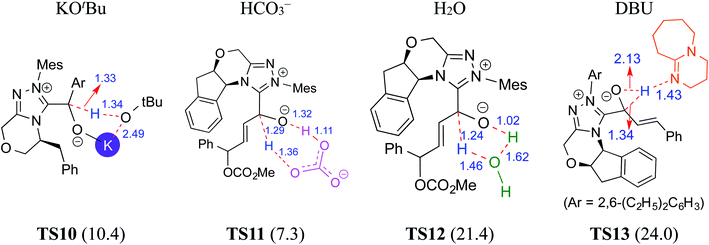
There have been reports that invoked the participation of bases such as DBU, KOtBu, K2CO3, Na2CO3, Cs2CO3, and DABCO in the mechanism of formation of the Breslow intermediate.10d–i,13 A recent computational study from our laboratory has demonstrated the importance of a critical 1,2-hydride transfer under basic reaction conditions (R4, Table 1).10d The barrier for the 1,2-hydride transfer viaTS7, as shown in Fig. 3, to form a keto-type intermediate was found to be about 30.0 kcal mol−1 at the PCM(THF)/M06-2X/6-311+G**//M06-2X/6-31+G** level of theory. A subsequent keto–enol tautomerization viaTS8 was suggested toward the formation of the desired Breslow intermediate. A high barrier for this unassisted keto–enol tautomerization prompted the authors to consider a DBU assisted relay proton transfer viaTS9. Interestingly, the barrier was found to be about 27 kcal mol−1 for the DBU assisted pathway wherein DBU abstracts the proton from the NHC carbon and delivers it to the enolate oxygen. As would become more apparent in the ensuing sections of this article, both the keto and enol variants of the Breslow intermediate are inherently appealing and thus deserve additional attention, primarily owing to the ease of detection of the former (vide infra).In another density functional theory study on a chiral NHC catalyzed intermolecular Stetter reaction (R5, Table 1),10e the Sunoj group has identified that KOtBu played a pivotal role in the formation of the Breslow intermediate. As with the previous examples, once the initial adduct between the aldehyde and NHC is generated, a proton transfer in the zwitterionic intermediate can take place through (a) a 1,2-direct process, or (b) a KOtBu assisted pathway (Scheme 6). The authors noted an explicit participation of the base, where the potassium ion is bound to the alkoxide oxygen while the butoxide counterion is hydrogen bonded to the benzylic proton (Fig. 4), as kinetically beneficial. The barrier associated with this kind of assisted proton transfer transition state TS10 was more than 40 kcal mol−1 lower than the unassisted direct proton transfer transition state at the SMD(toluene)/M06-2X/6-31G** level of theory. Additional analysis of the geometric features by using the intrinsic reaction coordinate computations on TS10 indicated that the t-butoxide first abstracts the proton from the enolate carbon to generate tBuOH and a potassium ion, thus leading to a potassium bound Breslow intermediate.
In 2015, Tang and co-workers reported the mechanism and enantioselectivity of an NHC catalyzed [4 + 2] annulation reaction between enals and azodicarboxylates in the presence of K2CO3 as the base (R6, Table 1).10f,14 The generation of the active catalyst from the pre-catalyst was suggested to take place by the action of the base. Similar to the earlier studies, three likely pathways for the 1,2-proton transfer, through unassisted and assisted modes (bicarbonate or H2O), were examined (Fig. 4). In the HCO3− assisted pathway, one end of the HCO3− abstracts the enolate C–H proton and transfers its proton to the alkoxide oxygen at the other end as depicted in TS11, in a concerted asynchronous manner. The Gibbs free energy barrier for TS11 in the HCO3− assisted proton transfer was 7.3 kcal mol−1 at the IEF-PCM(THF)/M06-2X/6-31G** level of theory, which is obviously far more favorable than the direct proton transfer process (42.4 kcal mol−1).10f Similarly, the barrier for the water assisted proton transfer viaTS12 was found to be 21.4 kcal mol−1, higher than that for the HCO3− assisted pathway. These data suggest that the kinetics of Breslow intermediate formation is likely to be sensitive to the nature of base employed during the course of its generation.
In another computational study on a cooperative NHC–LiCl catalyzed reaction toward the synthesis of spirooxindole lactone (R7, Table 1),10g Sunoj and co-workers found that a DBU-assisted 1,2 proton transfer in the zwitterionic intermediate (derived from cinnamaldehyde by the addition of an NHC) is critical to the formation of the Breslow intermediate. In this process, the basic nitrogen of DBU abstracts the C–H proton and subsequently transfers it to the alkoxide oxygen in a concerted manner (TS13 in Fig. 4). As in the earlier examples presented in this review, the DBU assisted proton transfer (24.0 kcal mol−1) was energetically more preferred over the 1,2-direct proton transfer with a prohibitively large barrier (46.4 kcal mol−1) at the SMD(THF)/B3LYP-D3/6-31G** level of theory. Through all the above-mentioned examples, we hope to have conveyed the significance of the base assisted proton transfer in the formation of the Breslow intermediate.
(D) Acid assisted pathway
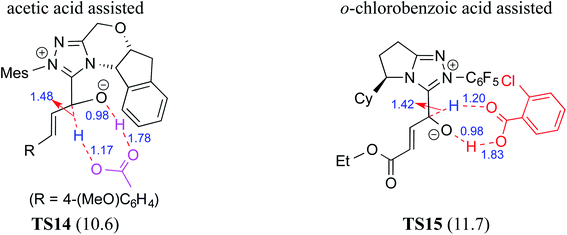
Acids such as acetic acid, benzoic acid, and other Brønsted acids have been employed as additives in various NHC catalyzed asymmetric reactions.9k,15 Acid additives have generally been considered as being capable of lowering the barrier for the formation of Breslow intermediates.9l,10j,k,16 Recently, Wei and co-workers have examined the mechanistic features and stereoinduction of a chiral NHC catalyzed [4 + 2] cycloaddition reaction at the IEF-PCM(THF)/B3LYP/6-31G** level of theory (R8, Table 1).10j They noted that a seven-membered transition state for the acid assisted proton transfer (TS14, Fig. 5) had a lower barrier (10.6 kcal mol−1) than the corresponding five-membered alternative (16.7 kcal mol−1, not shown in the figure) as well as that in a direct proton transfer pathway (36.1 kcal mol−1).17 In the acid assisted proton transfer transition states, a stronger O–H⋯O hydrogen bonding interaction was noted in the seven-membered case than in the five-membered analogue.Very recently, Sunoj and co-workers have examined the mechanism and origin of stereoinduction in cooperative asymmetric catalysis involving an NHC and Brønsted acid yielding γ-lactams by using SMD(CH3CN)/M06-2X/6-31G** computations (R9, Table 1).9l In the initial step, sodium benzoate abstracts the proton from the triazolium salt to generate a free carbene and benzoic acid. The role of the in situ generated benzoic acid in the formation of the Breslow intermediate was disclosed. The Gibbs free energy barrier for the seven-membered benzoic acid assisted proton transfer transition state (TS15) was 11.7 kcal mol−1 (Fig. 5). The benzoic acid assisted proton transfer had a much lower barrier than the unassisted 1,2-direct proton transfer (38.7) as well as that involving an alternative five-membered transition state geometry (15.5 kcal mol−1, not shown). Benzoic acid assisted proton transfer was found to be a concerted process as confirmed through IRC calculations. In another interesting study, Tang and co-workers probed the mechanism of a cooperative catalytic protocol involving an NHC and a Brønsted acid (TMEDA-H+) in a [3 + 2] annulation between enals and α-ketoamides (R10, Table 1).10k On the basis of the Gibbs free energy barriers obtained at the IEF-PCM(DCM)/M06-2X/6-31G(d,p) level of theory (Fig. 6), the Brønsted acid assisted proton transfer mechanism (TS16, 13.4 kcal mol−1) was suggested to be far more favorable than the TMEDA assisted (TS17, 20.3 kcal mol−1) as well as an unassisted proton transfer (44.3 kcal mol−1) route to the Breslow intermediate. It was noted that in TS16 the proton transfer from TMEDA-H+ is almost complete (O2–H3 = 0.99 Å, N6–H3 is 1.71 Å) as compared to the removal of C1–H4 (1.51 Å) by the amino end of the protonated base. Importantly, the O2–H3⋯N6 hydrogen bonding interaction stabilizes both the intermediate and transition states (TS16). The overall process was highly exergonic and irreversible. Furthermore, the barrier for the abstraction of the H4 proton by N5 of TMEDA viaTS16 was 13.4 kcal mol−1, which in turn leads to the Breslow intermediate and TMEDA-H+.
A summary of the activation barriers for the formation of Breslow intermediates, under different reaction conditions, is compiled in Table 2 and Fig. 7. It can readily be gleaned that the unassisted proton transfer generally encounters a prohibitively higher barrier than the alternative assisted pathway. Assisted pathways involving different acids (R8 and R9), bases (R4, R5, R6 and R7), water (R3 and R6), alcohols (R2 and R3), or other species (R1) were reported to facilitate easier proton transfer in the initially formed zwitterionic adduct between the NHC and substrate such as an aldehyde. Various two-step mechanisms, such as a bimolecular pathway, were also suggested for the proton transfer between the initially formed zwitterionic intermediate as a way to lower the barrier for the 1,2-direct proton transfer (in respect of R1).10a The barrier for the first proton transfer between two zwitterionic intermediates was 25.4 kcal mol−1 while that for the second proton transfer was 22.1 kcal mol−1. A two-step process involving the initial formation of a zwitterionic intermediate and a subsequent 1,2-hydride transfer to a keto-type intermediate was also noted in reaction R4. The barrier for DBU-assisted proton transfer in the keto–enol tautomerization was found to be much lower (27.0 kcal mol−1) as compared to that for the corresponding unassisted alternative (65.5 kcal mol−1).10d Although this two-step mechanism of formation of Breslow intermediates was proposed in certain systems, it should be noted that the single step pathways are energetically more feasible (e.g., R2, R3, R5, R6, R7, R8, and R9). Proton transfer assisted by K2CO3 was reported as energetically more favorable than other bases, acids, water, or alcohol. These findings collectively indicate that the choice of additive is likely to have a direct bearing on the efficiency of formation of the Breslow intermediate and hence on its subsequent catalytic abilities as well. Apart from the environmental influences exerted by various additives on the energetics of formation of Breslow intermediates, it would also be important to consider intrinsic factors such as the nature of the NHC and the substituents therein. In the following section, we wish to present one such interesting example for specific attention and more in the latter part of this thesis.
| Reactiona | Acid/base/water/other species | Notation | ΔG‡ (kcal mol−1) |
|---|---|---|---|
| a See Table 1 for details about reactions R1, R2, …, R10. b ΔE‡. | |||
| R1 | Zwitterionic intermediate (step 1) | im1 | 25.4 |
| Zwitterionic intermediate (step 2) | im2 | 22.1 | |
| R2 | MeOH | met | 19.3 |
| R3 | H2O | wat | 24.0 |
| Catechol | cat | 24.0 | |
| R4 | DBU | dbu | 26.9 |
| R5 | KOtBu | but | 10.4 |
| R6 | K2CO3 | car | 7.3 |
| R7 | DBU | dbu | 24.0 |
| R8 | AcOH | ace | 10.6b |
| R9 | o-Chlorobenzoic acid | ben | 11.7 |
| R10 | TMEDA | tme | 13.4 |
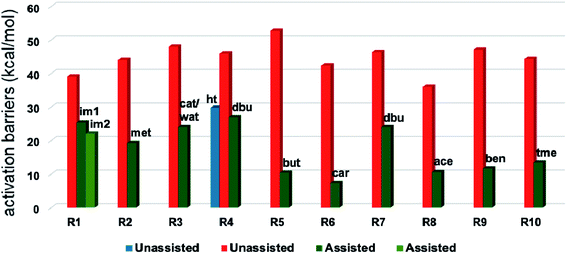 | ||
| Fig. 7 Graphical representation of Gibbs free energy barriers for the formation of the Breslow intermediate under different reaction conditions listed in Table 2. The red color in the bar diagram represents direct proton transfer and green represents acid/base/water/other species assisted proton transfer. Here, ‘ht’ represents 1,2-hydride transfer in reaction R4. | ||
Effect of N-aryl substituent on Breslow intermediate formation
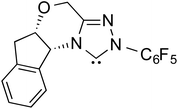
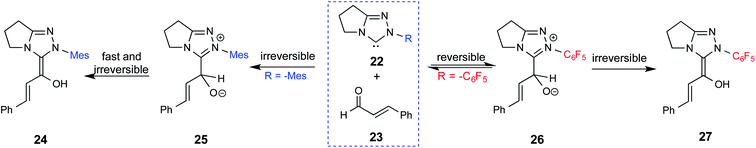
Since the early years of their development, it has widely been recognized that the substituents on the imidazolidine nitrogen play a vital role in the stability as well as electronic properties of NHCs. Hence, it is conspicuously important to the present discussion to establish the role of N-substituents in the formation of Breslow intermediates. The geometric proximity and the ability of such substituents in altering the electron donating ability of the nitrogen to the carbenic carbon are of significance to the energetics of formation of the Breslow intermediate. One such study that sheds light on the effect of N-aryl substituent in NHC catalyzed reactions was from the Bode group. Noteworthy conclusions gathered from their control experiments and kinetic measurements conveyed that the Breslow intermediate formation is irreversible with N-mesityl carbene while with the N–C6F5 analogue it is reversible (Scheme 7).18 Furthermore, the nature of the N-aryl substituent was suggested to impact the overall kinetic features of the catalytic transformation. For instance, the Breslow intermediate formation was noted as the rate determining step in the case of N-mesityl NHC while the subsequent nucleophilic addition of the Breslow intermediate to the substrate enone was found to be the rate determining step with N–C6F5 substituted NHC.For many years, the participation of the Breslow intermediate in NHC catalyzed reactions remained a mechanistic hypothesis with a long status of being a putative species. With the ever-increasing popularity of NHC catalyzed reactions, interest toward the isolation and characterization of Breslow intermediates became a thriving area of activity. A repertoire of experimental tools, such as X-ray crystallography, IR, NMR, and UV-Vis spectroscopy, have been employed as applicable in the solid state and in solution as well as in the gas phase to gather information on important features of Breslow intermediates. In the following section we describe some of the quintessential efforts made in this front.
Characterization of the Breslow intermediate
One of the earliest reports on the characterization of motifs similar to the Breslow intermediates comes from a seemingly different domain of enzymatic catalysis. In the late 80s, Jordan and co-workers synthesized alkyl thiazolium species (29 and 30) through the reaction between alkyl halides and thiazolium salts, which are a close analogue of the Breslow intermediate (Fig. 8). They also reported the structural analogues of enamine intermediates involved in enzymatic pathways and their characterization using 1H NMR spectroscopy.19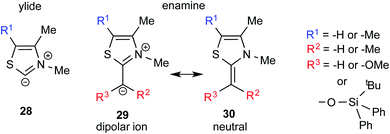 | ||
| Fig. 8 Key intermediates involved in thiamine diphosphate dependent enzymatic pathways, which can be regarded as a structural analogue of Breslow intermediates. | ||
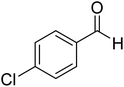
More direct efforts toward the realm of characterization of Breslow intermediates began to appear in the later years by way of engaging carefully chosen substrates such as α,β-unsaturated aldehydes. In 2004, Glorius and Bode independently developed the fascinating concept of homoenolate equivalent, which was followed up by other researchers as well.20 This idea is illustrated by using a representative example of NHC catalyzed reaction between cinnamaldehyde and 4-chlorobenzaldehyde, as shown in Scheme 8.21 The proposed catalytic cycle involves the initial addition of NHC 31 to α,β-unsaturated aldehyde 32 to form a zwitterionic intermediate 33, which on subsequent proton transfer yields a homoenolate equivalent in the form of the diamino dienol intermediate 34. Species 34 exhibits interesting conjugate umpolung reactivity in its reaction with an aldehyde such as 35 to form a γ-lactone, 38, through an intramolecular cyclization.
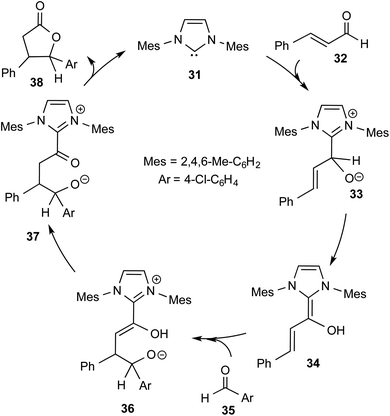 | ||
| Scheme 8 Proposed mechanism for the N-heterocyclic carbene catalyzed reaction between cinnamaldehyde and 4-chlorobenzaldehyde to form a γ-lactone. | ||
The mechanistic proposal was supported by electrospray mass spectrometric measurements on samples drawn from the reaction mixture. The observed values of various m/z peaks revealed the identities of important intermediates. For instance, low intensity peaks pertaining to the protonated form of 33 and the diamino dienol intermediate 34 could be noted. These experiments offer compelling evidence for the participation of the Breslow intermediate under the given reaction conditions. The presence of a high intensity peak at m/z 305 emanating from the protonated catalyst 31 was also a valuable insight into the overall mechanism of NHC catalysis. In another recent study, Douthwaite and co-workers demonstrated the formation of a Breslow type intermediate, 40, derived from unactivated imines through an intramolecular cyclization in the presence of a base (Scheme 9).22 Similar to a Breslow intermediate, the nucleophilic reactivity of an amino analogue such as 40 could be demonstrated through a simple protonation reaction as shown below.
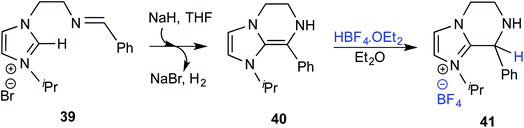 | ||
| Scheme 9 Intramolecular cyclization in the presence of a base to form a Breslow type intermediate, 40. | ||
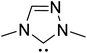
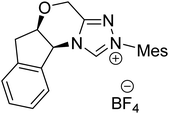
As we begin discussing the isolation and characterization of Breslow intermediates, it is prudent to acknowledge the pioneering contributions from the Berkessel group, by taking one of their examples as a starting point. As the earliest step in this front, back in 2010, they could perform NMR characterization of the keto variant of a Breslow intermediate23 derived from substituted triazolylidene carbenes 42 and an aliphatic aldehyde (Scheme 10(a)). It was noticed that a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
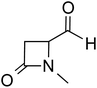
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of the carbene and aldehyde exclusively results in the keto tautomer of the Breslow intermediate (44), while excess aldehyde led to a spiro-dioxolane (43a). The latter could potentially be a resting state in the catalytic cycle, from where dissociation to the monomeric form should take place for its active participation in catalysis. In the presence of an acid, the reversible formation of an isolable intermediate, 43b, was noticed.
1 molar ratio of the carbene and aldehyde exclusively results in the keto tautomer of the Breslow intermediate (44), while excess aldehyde led to a spiro-dioxolane (43a). The latter could potentially be a resting state in the catalytic cycle, from where dissociation to the monomeric form should take place for its active participation in catalysis. In the presence of an acid, the reversible formation of an isolable intermediate, 43b, was noticed.
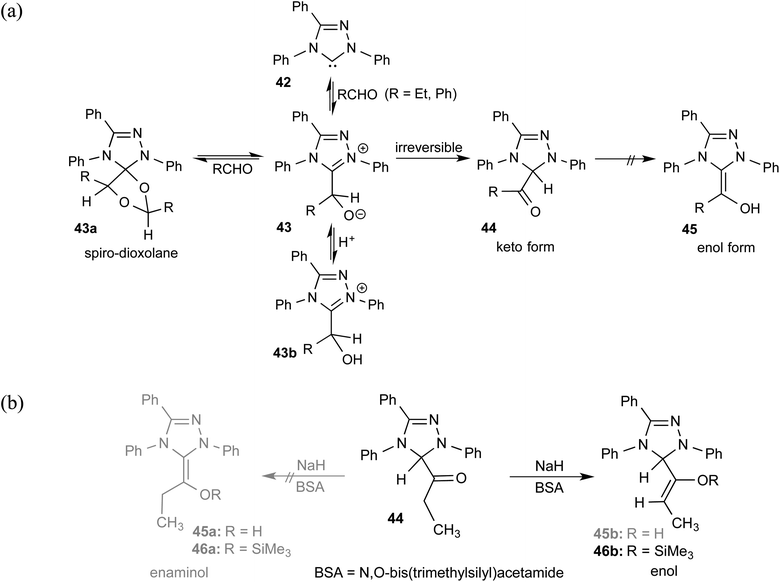 | ||
| Scheme 10 (a) Generation of the keto-tautomer and spiro-dioxolane from a triazolylidene carbene and an aliphatic aldehyde. (b) The formation of the keto-tautomer of the Breslow intermediate. | ||
The B3LYP/6-31G(d,p) computations revealed that the enol intermediate 45 (Scheme 10(a)) is 14 kcal mol−1 higher in energy than the corresponding keto form 44 (when R = Ph). While the keto form was found to be catalytically inactive, attempts at tautomerization to the active enol form by using various acids, bases, and silylating agents also remained unsuccessful (Scheme 10(b)). The tautomerization of 44 to an enaminol (45a) or to an enol (45b) was in vain too, even in the presence of a catalytic amount of acid (TFA, p-Ts-OH). Interestingly, the use of a catalytic amount of NaH base in the presence of a silylating agent, BSA (N,O-bis(trimethylsilyl)acetamide), did provide access to a silylated enol ether, 46b, while the formation of an active enaminol, 46a, could not be established. It is likely that 46a was either not formed or was not amenable to detection under the conditions employed.
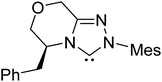
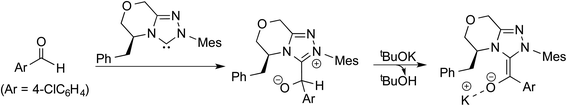
In an interesting study on the triazolinylidene carbene-catalyzed asymmetric intramolecular Stetter reaction (Scheme 11), Rovis and co-workers noted that the proton transfer from the aldehydic carbon to the geminal oxygen is the first irreversible step in the mechanism of formation of the Breslow intermediate.24 Two key pathways for the proton transfer in the initially formed zwitterionic intermediate 47 were envisaged, (a) facilitated by the –OR group of the substrate (via intermediate 48) and (b) by engaging the N-aryl group of the catalyst (involving 50). Carefully designed kinetic experiments using substrates 51 and 53, as shown in Scheme 11(c), could shed more light on the mechanism of 1,2-proton transfer. The faster reaction rate with 51 bearing an ether linkage was considered as evidence for an intramolecular substrate-assisted proton transfer (Scheme 11(a)). To test whether the N-aryl group of the catalyst was involved in the proton transfer, kinetic isotope effect studies were conducted by using deuterated aldehyde 47. The absence of deuterium incorporation at the ortho position of the catalyst N-aryl group (in intermediate 50) confirmed that the proton was not transferred via the catalyst as shown in Scheme 11(b). Interestingly, the formation of a cyclic product was also observed in the case of substrate 53 lacking an ether linkage (Scheme 11(c)), which is an indication that with such substrate an alternative proton transfer mechanism may operate.10a
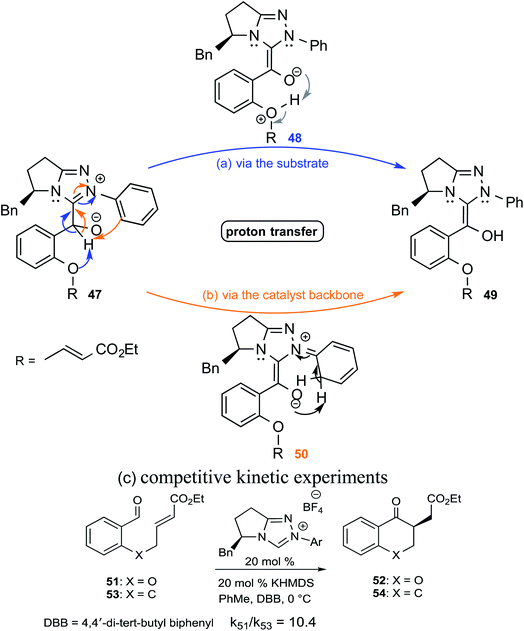 | ||
| Scheme 11 Likely proton transfer pathways as well as competitive experiments between aldehydes 51 and 53. | ||
Similar to the interest in native Breslow intermediates, there have been efforts toward the synthesis of other analogues such as aza-Breslow intermediates. A recent report by the Rovis group is particularly noteworthy where they disclosed the synthesis, detection, and characterization of aza-Breslow intermediates using a combination of UV-Vis, NMR, and X-ray crystallography (Scheme 12) tools.25 This kind of evidence provides a firmer ground for the involvement of Breslow intermediates in NHC catalysis. Besides the detection and characterization of aza-Breslow intermediates (57 and 59), their own catalytic activity was demonstrated in an asymmetric Stetter reaction. Intermediates 57 and 59 are respectively an acyl anion and a homoenolate equivalent, which are typically seen in native NHC catalysis involving Breslow intermediates.
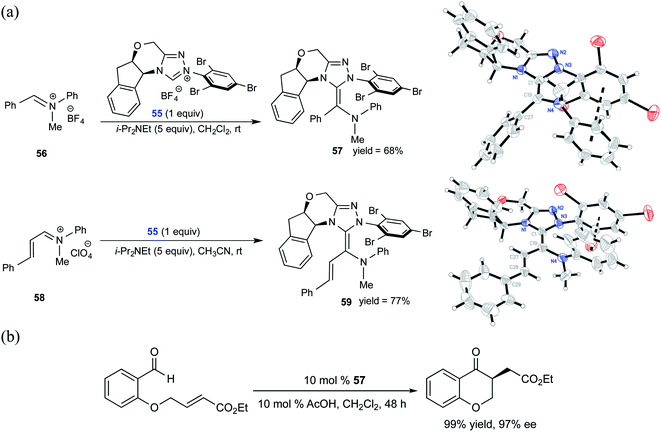 | ||
| Scheme 12 (a) Isolation and characterization of aza-analogues of Breslow intermediate 57 and a homoenolate analogue of Breslow intermediate 59. (b) Stetter reaction catalyzed by an aza-Breslow intermediate. Reprinted with permission from ref. 25. Copyright 2012 American Chemical Society. | ||
In another study, Mayr and co-workers isolated an O-methylated Breslow intermediate by the deprotonation of the corresponding azolium salt in the presence of NaH and catalytic amounts of KOtBu as shown in Scheme 13(a).26 The thiazole derived intermediates 60 and 61 were formed as a mixture of Z and E isomers (Z/E = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) while triazole analogue 64 resulted in Z/E of 1
1) while triazole analogue 64 resulted in Z/E of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (Scheme 13(b)). An interesting structure–activity relationship was established by kinetic experiments using the stabilized benzhydrylium ion 65 as the electrophile (Schemes 13(b) and (c)) and a range of O-methylated Breslow intermediates as the nucleophilic partners. It was found that the thiazole O-methylated Breslow intermediate (61) was less nucleophilic than the corresponding deoxy-Breslow intermediate (66). The relative rates between 62 and 61 indicated that the O-methylated Breslow intermediate derived from thiazole was 2 to 3 orders of magnitude less reactive than the structurally analogous imidazole family.
10 (Scheme 13(b)). An interesting structure–activity relationship was established by kinetic experiments using the stabilized benzhydrylium ion 65 as the electrophile (Schemes 13(b) and (c)) and a range of O-methylated Breslow intermediates as the nucleophilic partners. It was found that the thiazole O-methylated Breslow intermediate (61) was less nucleophilic than the corresponding deoxy-Breslow intermediate (66). The relative rates between 62 and 61 indicated that the O-methylated Breslow intermediate derived from thiazole was 2 to 3 orders of magnitude less reactive than the structurally analogous imidazole family.
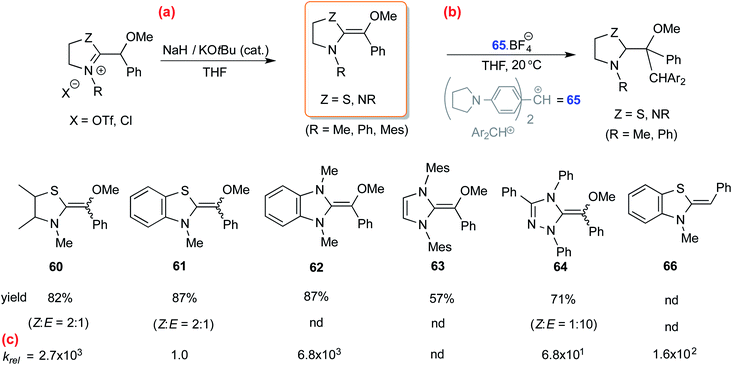 | ||
| Scheme 13 (a) Generation of O-methylated Breslow intermediates from the corresponding azolium salts, (b) their reactivities toward an electrophile, and (c) the relative rates. | ||
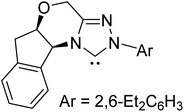
Along similar lines, Berkessel and co-workers reported the synthesis of diamino enols using saturated carbenes. The reaction of dihydro-imidazolidine-2,4,6-trimethylphenyl (SIMes) 31 or dihydro-imidazolidine-2,6-bis(2-propyl)phenyl (SIPr) 68 with benzaldehyde or 2,4-bis(trifluoromethyl)benzaldehyde, respectively, yielded Breslow intermediates 67 and 69 (Scheme 14(a)). A comparison of these adducts of triazolylidene (as shown earlier in Scheme 10) and that formed by dihydro-imidazolylidene, with benzaldehyde as the electrophile, would be of interest here. The former NHC resulted only in the keto form of the Breslow intermediate (44), while the latter offered access to the desired enaminol variants 67 and 69. These Breslow intermediates were further examined using in situ NMR spectroscopy.27 A characteristic peak at δ = 4.40 ppm in the 1H NMR spectrum of 69 (Scheme 14(b)) was confirmed as arising from the diamino enol through rapid H/D exchange upon introducing [D4]MeOH. The use of 13C-labeled 2,4-bis(trifluoromethyl)benzaldehyde (13CHO) resulted in a coupling between 13C (labeled as C6) and the OH group (2JC–OH = 3.23 Hz). The other characteristic 13C peaks observed were at δ = 145.8 ppm (1JC6–C2 = 106.4 Hz, C2) and δ = 142.5 ppm (1JC6–Car = 69.8 Hz, Car). These spectral features confirmed the formation of diamino enol between the saturated carbene SIPr and 2,4-bis(trifluoromethyl)benzaldehyde. These pieces of unequivocal evidence are noteworthy inroads toward a long-standing goal of establishing the true nature of Breslow intermediates.
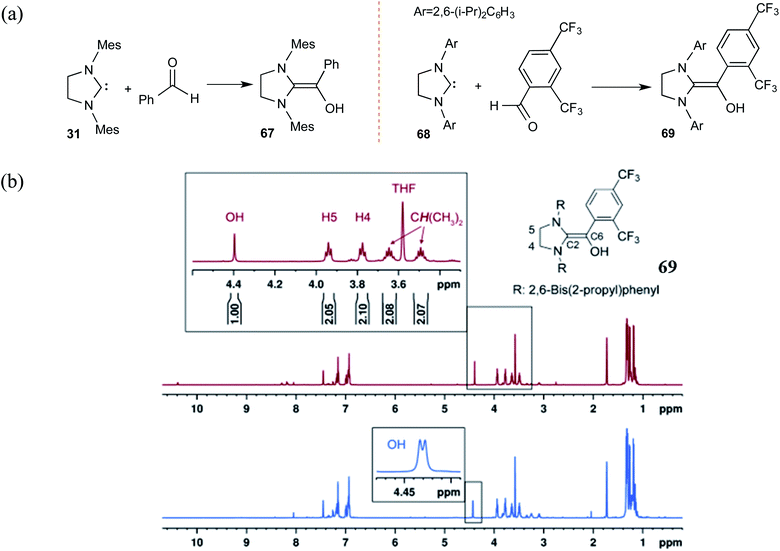 | ||
| Scheme 14 (a) Generation of Breslow intermediates by using saturated carbenes. (b) The 1H NMR spectrum of the diamino enol 69 showing the magnified region of the peaks pertaining to the carbene and the Breslow intermediate protons as marked in the structure. The blue color magnified peaks show the doublet of the diamino enol proton due to the adjacent 13C-labeled carbon. Reprinted with permission from ref. 27. Copyright 2012 John Wiley and Sons. | ||
Interesting crossover experiments were performed to gather additional details regarding the equilibrium of Breslow intermediate formation (Scheme 15). It was noted that enaminol 70 exists in equilibrium with the free carbene 68 and benzaldehyde. The use of a more electrophilic 2,4-bis(trifluoromethyl) benzaldehyde exhibited rapid equilibrium between 70, 68, and 71. The formation of a cross benzoin product (72) as the major product was considered as a tangible indication of a fully reversible equilibrium operating between Breslow intermediates 70 and 71. Experiments of such kind that were able to identify an equilibrium between a free NHC and the corresponding Breslow intermediate do convey a gratifying message that our quest for studying enaminol intermediates has come a long way.
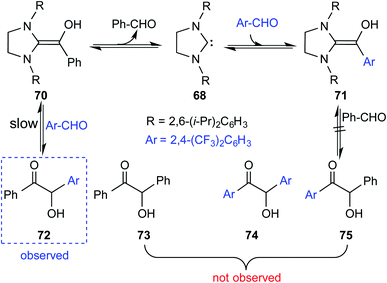 | ||
| Scheme 15 Crossover experiment for deducing the reversibility in the formation of the Breslow intermediate. | ||
In keeping with their continued interest in Breslow intermediates, Berkessel and co-workers characterized a set of key intermediates involved in the NHC catalyzed umpolung reactions of various aldehydes by using X-ray crystallography (Scheme 16).28 Intermediates such as diamino enol 76 (acyl anion equivalent), diamino dienols 77 and 79 (homoenolate equivalent), azolium enolate 78 (enolate equivalent), and azolium enol 80 could be identified. Each of these intermediates hold a prominent place in NHC catalysis as they are known to react with a range of different electrophiles to furnish a good number of valuable products.
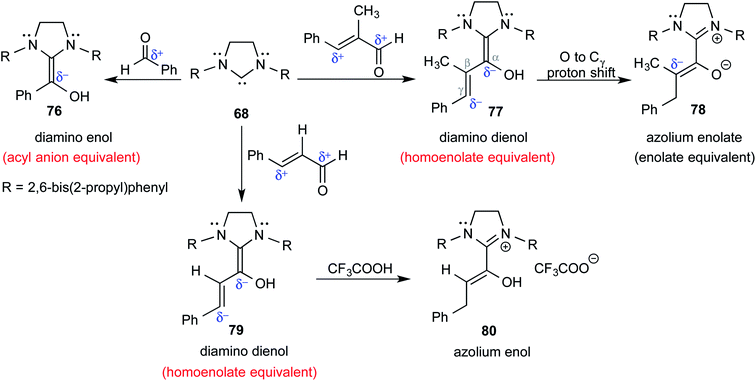 | ||
| Scheme 16 Formation of different likely reactive intermediates in the reaction between NHC and a simple aldehyde or an α,β-unsaturated aldehyde. | ||
Although the isolation and characterization of Breslow intermediates, derived from saturated dihydro-imidazolylidene and aldehydes/enals, were successful, certain puzzling observations pertinent to the formation of Breslow intermediates remained open. For instance, an exclusive formation of the keto form of the Breslow intermediate 82 was noticed in the case of triphenyltriazolylidene carbenes (42), while the saturated imidazolylidene carbenes (68) yielded the enol tautomer (84) in their stoichiometric reaction with benzaldehyde (Scheme 17).29
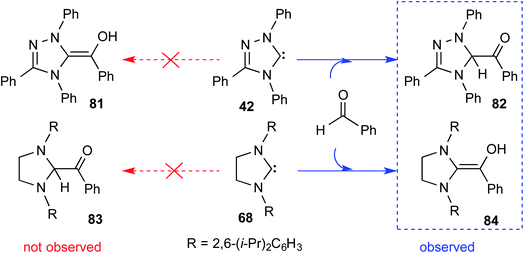 | ||
| Scheme 17 Formation of the keto and enol forms of the Breslow intermediate with triazolylidene and imidazolylidene carbenes with benzaldehyde, respectively. | ||
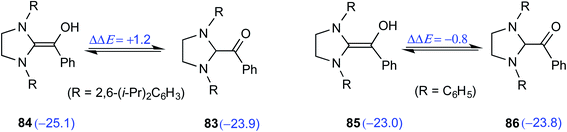
The electronic energy computed at the M06-2X-D3/def2-TZVPP/IEFPCM(THF)//M06-L-D3/6-31+G(d,p) level of theory for the enol was found to be 2 kcal mol−1 lower when 2,6-bis(iso-propyl)phenyl was the N-R substituent as compared to that of a simple phenyl substituted NHC (Scheme 18). The electronic energy for the conversion of the enol to the ketone form for both 84 and 85 was predicted to be similar even as the N-aryl substituents were different. After the success with diamino enols derived from the saturated imidazolidin-2-ylidenes, efforts were expended toward studying aromatic NHCs such as benzimidazolin-2-ylidenes and thiazolin-2-ylidenes. Interestingly in 2019, Berkessel and co-workers could crystallize Breslow intermediates derived from aromatic thiazolin-2-ylidenes and subject them to XRD analysis. Furthermore, solution-phase NMR spectroscopic characterization of another Breslow intermediate obtained from aromatic thiazolin-2-ylidenes and an aliphatic aldehyde (trifluoroacetaldehyde) could also be accomplished.30
After their successful series of reports on the characterization of various Breslow intermediates, both in the solution and in the solid state, Berkessel and co-workers have most recently disclosed a gas phase study of an ammonium charge-tagged Breslow intermediate by using ESI-MS IR ion spectroscopy in conjunction with quantum-chemical calculations.31 The ammonium charge-tag was introduced either on the NHC or aldehyde moiety, as shown in Scheme 19. The Breslow intermediate derived from the imidazolidin-2-ylidene 68 existed as a diamino enol, 87. The presence of IR stretching frequencies at 1538 cm−1 (experimental) and 1524 cm−1 (computed) was attributed to the enol C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(OH) functionality of diamino enol 87. The detection of diamino enol was in concert with the computed energetic preference of 12 kcal mol−1 toward 87 as compared to the corresponding keto form at the M06-2X-D3/def2-TZVPPD//M06-L-D3/6-31+G(d,p) level of theory. On the other hand, attempts to obtain the Breslow intermediate using 1,2,4-triazolin-5-ylidenes 42 and thiazolin-2-ylidenes 89 resulted in keto structures, shown respectively as 88 and 90. Both the experimental and calculated IR spectra showed the presence of a carbonyl C
C(OH) functionality of diamino enol 87. The detection of diamino enol was in concert with the computed energetic preference of 12 kcal mol−1 toward 87 as compared to the corresponding keto form at the M06-2X-D3/def2-TZVPPD//M06-L-D3/6-31+G(d,p) level of theory. On the other hand, attempts to obtain the Breslow intermediate using 1,2,4-triazolin-5-ylidenes 42 and thiazolin-2-ylidenes 89 resulted in keto structures, shown respectively as 88 and 90. Both the experimental and calculated IR spectra showed the presence of a carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band at 1720 cm−1 and at 1670 cm−1 for 88 and 90, respectively.
O stretching band at 1720 cm−1 and at 1670 cm−1 for 88 and 90, respectively.
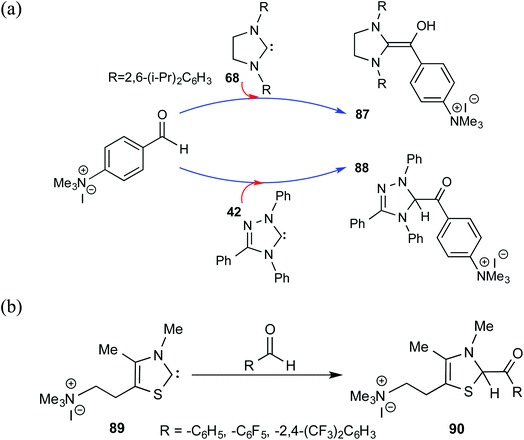 | ||
| Scheme 19 The Breslow intermediate derived from imidazolidin-2-ylidene with ammonium charge-tagged benzaldehyde and the corresponding keto analogue obtained using thiazolin-2-ylidenes. | ||
Summary and outlook
Breslow intermediates occupy a unique position in the contemporary literature owing to the remarkable popularity of N-heterocyclic carbene (NHC) catalysis. In due cognizance of the central role of Breslow intermediates in a myriad of NHC organocatalytic reactions and the lack of a single consolidated resource on their formation, isolation, and characterization, we have compiled the available knowledge in this review and have provided a critical assessment of their developmental strides. As the title implies, this treatise is philosophically organized from ‘then’ to ‘now’, and thus provides chronological details of the evolution of Breslow intermediates.The age-old concept of umpolung, invoked for understanding the homo-dimerization of aldehydes in the presence of cyanide, bears noteworthy semblance to certain catalytic protocols employed in recent times. The suggested role of NHCs in promoting a similar umpolung reactivity became a conspicuous landmark when Ukai and co-workers identified that thiazolium salts could catalyze the benzoin reaction. A few decades later, Breslow proposed the involvement of a nucleophilic amino enol intermediate in the mechanism of a thiazolium catalyzed benzoin reaction. While this putative intermediate remained rather dormant for long, the renaissance in organocatalysis in the last two decades appears to have provided the much-needed impetus. Obviously, recent years witnessed an unprecedented series of applications of such amino enols in NHC catalyzed organocatalytic reactions. In honor of his pioneering work, amino enols have been termed as “Breslow intermediates”.
In the years that followed, efforts toward the generation of structural analogues of Breslow intermediates became an attractive endeavor. The characterization of Breslow intermediates derived from saturated imidazolylidene, unsaturated benzimidazolin-2-ylidenes, and thiazolin-2-ylidenes was realized. The electronic features of the N-substituents on the carbene backbone were identified to influence the formation as well as the subsequent reactivity of nucleophilic Breslow intermediates under different reaction conditions. Experimental endeavors probing the formation of Breslow intermediates were ably complemented by computational studies that helped in gaining key molecular insights. Importantly, computations revealed that after the initial addition of the NHC to a suitable enal/enone or similar electrophilic partner, the ensuing proton/hydride transfer encountered high barriers. A direct proton transfer in the initial zwitterionic intermediate through a strained three-membered transition state, or a 1,2-hydride transfer first to a keto intermediate that tautomerizes via a strained four-membered transition state to the corresponding Breslow intermediate, had a prohibitively high kinetic barrier. Computational studies demonstrated that the vital proton transfer could be assisted by an explicit participation of additives/solvent. Assisted proton transfer promoted by various additives (such as K2CO3) exhibited lower barriers than the corresponding unassisted pathways in the formation of Breslow intermediates, with a general preference for the assisted 1,2-proton transfer than the alternative keto–enol mechanism. Hence, the single step mechanism of formation of Breslow intermediates, with barriers in the range of 7 to 24 kcal mol−1 (depending on the molecular system), was recommended as more favorable over the two-step mechanism (with the overall barriers between 25 and 27 kcal mol−1).
Besides the work on the energetic origin of formation of Breslow intermediates, inexorable efforts led to successful elucidation of their structure by using X-ray crystallography in the solid state, NMR studies in the solution phase, and more recently ESI-MS IR ion spectroscopy in the gas phase. While our ability to perform in situ detection of Breslow intermediates under a plethora of diverse reaction conditions has significantly improved, certain subtle issues related to the energetics of formation that leads to the keto form with imidazolylidene carbenes and enaminol variants with dihydro imidazolylidene seem to demand more careful scrutiny. In summary, we have enunciated the state-of-the-art understanding on the formation and nature of Breslow intermediates in NHC organocatalysis, which is expected to serve as a single source of valuable knowledge on this important topic.
Author contributions
All authors conceptualised the review, M. P. and Y. R. wrote the first draft supervised by R. B. S., who edited the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
M. P. acknowledges CSIR (New Delhi) for a senior research fellowship.Notes and references
- (a) X.-Y. Chen, Z.-H. Gao and S. Ye, Acc. Chem. Res., 2020, 53, 690–702 CrossRef CAS PubMed; (b) Z.-Z. Zhang, Y. Zhang, H.-X. Duan, Z.-F. Deng and Y.-Q. Wang, Chem. Commun., 2020, 56, 1553–1556 RSC; (c) S. Mondal, S. R. Yetra, S. Mukherjee and A. T. Biju, Acc. Chem. Res., 2019, 52, 425–436 CrossRef CAS PubMed; (d) Y. Liu, Q. Chen, C. Mou, L. Pan, X. Duan, X. Chen, H. Chen, Y. Zhao, Y. Lu, Z. Jin and Y. R. Chi, Nat. Commun., 2019, 10, 1675–1682 CrossRef PubMed; (e) K. J. R. Murauski, A. A. Jaworski and K. A. Scheidt, Chem. Soc. Rev., 2018, 47, 1773–1782 RSC; (f) C. Zhang, J. F. Hooper and D. W. Lupton, ACS Catal., 2017, 7, 2583–2596 CrossRef CAS; (g) D. M. Flanigan, F. R. Michailidis, N. A. White and T. Rovis, Chem. Rev., 2015, 115, 9307–9387 CrossRef CAS PubMed; (h) M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485–496 CrossRef CAS PubMed; (i) S. J. Ryan, L. Candish and D. W. Lupton, Chem. Soc. Rev., 2013, 42, 4906–4917 RSC; (j) M. Fevre, J. Pinaud, Y. Gnanou, J. Vignolle and D. Taton, Chem. Soc. Rev., 2013, 42, 2142–2172 RSC; (k) X. Bugaut and F. Glorius, Chem. Soc. Rev., 2012, 41, 3511–3522 RSC; (l) J. Izquierdo, G. E. Hutson, D. T. Cohen and K. A. Scheidt, Angew. Chem., Int. Ed., 2012, 51, 11686–11698 CrossRef CAS PubMed; (m) H. M. Vora and T. Rovis, Aldrichimica Acta, 2011, 44, 3–11 CAS; (n) E. M. Phillips, A. Chan and K. A. Scheidt, Aldrichimica Acta, 2009, 42, 55–83 CAS; (o) V. Nair, S. Vellalath and B. P. Babu, Chem. Soc. Rev., 2008, 37, 2691–2698 RSC; (p) D. Enders, O. Niemeier and A. Henseler, Chem. Rev., 2007, 107, 5606–5655 CrossRef CAS PubMed; (q) R. Song, Z. Jin and Y. R. Chi, Chem. Sci., 2021, 12, 5037–5043 RSC; (r) H. Ohmiya, ACS Catal., 2020, 10, 6862–6869 CrossRef CAS; (s) J. Liu, X.-N. Xing, J.-H. Huang, L.-Q. Lu and W.-J. Xiao, Chem. Sci., 2020, 11, 10605–10613 RSC; (t) R. Song and Y. R. Chi, Angew. Chem., Int. Ed., 2019, 58, 8628–8630 CrossRef CAS PubMed; (u) S. Barik and A. T. Biju, Chem. Commun., 2020, 56, 15484–15495 RSC.
- (a) G. Wittig, P. Davis and G. Koenig, Chem. Ber., 1951, 84, 627–632 CrossRef CAS; (b) D. Seebach, Angew. Chem., Int. Ed., 1979, 18, 239–258 CrossRef.
- F. Wöhler and J. Liebig, Ann. Pharm., 1832, 3, 249–282 CrossRef , (presently part of Eur. J. Org. Chem.).
- A. Lapworth, J. Chem. Soc., Trans., 1903, 83, 995–1005 RSC.
- (a) T. Ukai, R. Tanaka and T. Dokawa, J. Pharm. Soc. Jpn., 1943, 63, 296–300 CrossRef CAS; (b) D. Enders and T. Balensiefer, Acc. Chem. Res., 2004, 37, 534–541 CrossRef CAS PubMed.
- (a) R. Breslow, J. Am. Chem. Soc., 1958, 80, 3719–3726 CrossRef CAS; (b) Involvement of radical transients and discussions on ionic and radical mechanisms of thiazolydene catalyzed benzoin can be found in J. Rehbein, S. M. Ruser and J. Phan, Chem. Sci., 2015, 6, 6013–6018 RSC; (c) T. Ishii, K. Nagao and H. Ohmiya, Chem. Sci., 2020, 11, 5630–5636 RSC; (d) A select set of examples of NHC catalyzed benzoin reactions are S. M. Langdon, M. M. D. Wilde, K. Thai and M. Gravel, J. Am. Chem. Soc., 2014, 136, 7539–7542 CrossRef CAS PubMed; (e) A. Duan, J. S. Fell, P. Yu, C. Y. Lam, M. Gravel and K. N. Houk, J. Org. Chem., 2019, 84, 13565–13571 CrossRef CAS PubMed.
- H.-W. Wanzlick, Angew. Chem., Int. Ed., 1962, 1, 75–80 CrossRef.
- A. J. Arduengo III, R. L. Harlow and M. Kline, J. Am. Chem. Soc., 1991, 113, 361–363 CrossRef.
- (a) D. A. DiRocco, K. M. Oberg, D. M. Dalton and T. Rovis, J. Am. Chem. Soc., 2009, 131, 10872–10874 CrossRef CAS PubMed; (b) J. M. Um, D. A. DiRocco, E. L. Noey, T. Rovis and K. N. Houk, J. Am. Chem. Soc., 2011, 133, 11249–11254 CrossRef CAS PubMed; (c) S. Lu, S. B. Poh and Y. Zhao, Angew. Chem., Int. Ed., 2014, 53, 11041–11045 CrossRef CAS PubMed; (d) R. Maji and S. E. Wheeler, J. Am. Chem. Soc., 2017, 139, 12441–12449 CrossRef CAS PubMed; (e) C. M. Filloux, S. P. Lathrop and T. Rovis, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 20666–20671 CrossRef CAS PubMed; (f) Y. Reddi and R. B. Sunoj, ACS Catal., 2015, 5, 1596–1603 CrossRef CAS; (g) Z. Q. Fu, J. F. Xu, T. S. Zhu, W. W. Y. Leong and Y. R. Chi, Nat. Chem., 2013, 5, 835–839 CrossRef CAS PubMed; (h) Y. Wang, D. Wei, Y. Wang, W. Zhang and M. Tang, ACS Catal., 2016, 6, 279–289 CrossRef CAS; (i) J. Kaeobamrung, J. Mahatthananchai, P. Zheng and J. W. Bode, J. Am. Chem. Soc., 2010, 132, 8810–8812 CrossRef CAS PubMed; (j) E. Lyngvi, J. W. Bode and F. Schoenebeck, Chem. Sci., 2012, 3, 2346–2350 RSC; (k) X. Zhao, D. A. DiRocco and T. Rovis, J. Am. Chem. Soc., 2011, 133, 12466–12469 CrossRef CAS PubMed; (l) M. Pareek and R. B. Sunoj, ACS Catal., 2016, 6, 3118–3126 CrossRef CAS.
- (a) K. J. Hawkes and B. F. Yates, Eur. J. Org. Chem., 2008, 5563–5570 CrossRef CAS; (b) Y. He and Y. Xue, J. Phys. Chem. A, 2011, 115, 1408–1417 CrossRef CAS PubMed; (c) L. R. Domingo, M. J. Aurell and M. Arnó, Tetrahedron, 2009, 65, 3432–3440 CrossRef CAS; (d) P. Verma, P. A. Patni and R. B. Sunoj, J. Org. Chem., 2011, 76, 5606–5613 CrossRef CAS PubMed; (e) R. Kuniyil and R. B. Sunoj, Org. Lett., 2013, 15, 5040–5043 CrossRef CAS PubMed; (f) Y. Wang, L. Zheng, D. Wei and M. Tang, Org. Chem. Front., 2015, 2, 874–884 RSC; (g) Y. Reddi and R. B. Sunoj, ACS Catal., 2017, 7, 530–537 CrossRef CAS; (h) Y. Reddi and R. B. Sunoj, Org. Lett., 2012, 14, 2810–2813 CrossRef CAS PubMed; (i) H. Sun, X. Fnag, Y. R. Chi and G. Li, Chirality, 2013, 25, 521–528 CrossRef CAS PubMed; (j) Z. Li, D. Wei, Y. Wang, Y. Zhu and M. Tang, J. Org. Chem., 2014, 79, 3069–3078 CrossRef CAS PubMed; (k) Y. Wang, B. Wu, L. Zheng, D. Wei and M. Tang, Org. Chem. Front., 2016, 3, 190–203 RSC; (l) L. R. Domingo, R. J. Zaragozá, J. A. Saéz and M. Arnó, Molecule, 2012, 17, 1335–1353 CrossRef CAS PubMed.
- R. B. Sunoj and M. Anand, Phys. Chem. Chem. Phys., 2012, 14, 12715–12736 RSC.
- (a) D. Roy and R. B. Sunoj, Chem.–Eur. J., 2008, 14, 10530–10534 CrossRef CAS PubMed; (b) S. Osuna, A. Dermenci, S. J. Miller and K. N. Houk, Chem.–Eur. J., 2013, 19, 14245–14253 CrossRef CAS PubMed; (c) M. Anand and R. B. Sunoj, Organometallics, 2012, 31, 6466–6481 CrossRef CAS; (d) Y. Reddi and R. B. Sunoj, ACS Catal., 2015, 5, 5794–5802 CrossRef CAS; (e) B. E. Maki, E. V. Patterson, C. J. Cramer and K. A. Scheidt, Org. Lett., 2009, 11, 3942–3945 CrossRef CAS PubMed.
- (a) Y. Li and Z. Zhang, Eur. J. Org. Chem., 2019, 2989–2997 CrossRef CAS; (b) X. Li, Y. Wang, Y. Wang, M. Tang, L.-B. Qu, Z. Li and D. Wei, J. Org. Chem., 2018, 83, 8543–8555 CrossRef CAS PubMed; (c) X. Li, R. Duan, Y. Wang, L.-B. Qu, Z. Li and D. Wei, J. Org. Chem., 2019, 84, 6117–6125 CrossRef CAS PubMed; (d) A. Shyam, A. K. Pradhan and P. Mondal, ChemistrySelect, 2020, 5, 11996–12008 CrossRef CAS; (e) Y. Wang and D. Wei, Mol. Catal., 2020, 489, 110944–110949 CrossRef CAS; (f) X. Li, J. Xu, S.-J. Li, L.-B. Qu, Z. Li, Y. R. Chi, D. Wei and Y. Lan, Chem. Sci., 2020, 11, 7214–7225 RSC; (g) M. Zhang, Y. Wang, S.-J. Li, X. Wang, Q. Shi, X. Li, L.-B. Qu, D. Wei and Y. Lan, ACS Catal., 2021, 11, 3443–3454 CrossRef CAS; (h) Y. Li and Z. Zhang, Int. J. Quantum Chem., 2021, 121, e26557 CAS; (i) X. Wang, S. J. Li, Y. N. Wang, D. Wei and Y. Lan, Org. Lett., 2021, 23, 2421–2425 CrossRef PubMed.
- F. Sun, T. Yin, Y. Wang, A. Feng, L. Yang, W. Wu, C. Yu, T. Li, D. Wei and C. A. Yao, Org. Chem. Front., 2020, 7, 578–583 RSC.
- (a) Y. Xiao, J. Wang, W. Xia, S. Shu, S. Jiao, Y. Zhou and H. Liu, Org. Lett., 2015, 17, 3850–3853 CrossRef CAS PubMed; (b) J.-L. Li, B. Sahoo, C.-G. Daniliuc and F. Glorius, Angew. Chem., Int. Ed., 2014, 53, 10515–10520 CrossRef CAS PubMed; (c) X. Chen, R. Song, Y. Liu, C. Y. Ooi, Z. Jin, T. Zhu, H. Wang, L. Hao and Y. R. Chi, Org. Lett., 2017, 19, 5892–5895 CrossRef CAS PubMed; (d) S. Kuwano, S. Harada, B. Kang, R. Oriez, Y. Yamaoka, K. Takasu and K.-i. Yamada, J. Am. Chem. Soc., 2013, 135, 11485–11488 CrossRef CAS PubMed; (e) Y.-F. Tong, J.-H. Mao, S. Wu, Y. Zhao and Y. Cheng, J. Org. Chem., 2014, 79, 2075–2081 CrossRef CAS PubMed.
- (a) J. Gao and Y. Wang, Org. Biomol. Chem., 2019, 17, 7442–7447 RSC; (b) Y. Wang, L.-B. Qu, Y. Lan and D. Wei, ChemCatChem, 2020, 12, 1068–1074 CrossRef CAS; (c) Y. Li, Z. Li and Z. Zhang, Mol. Catal., 2020, 496, 111183–111191 CrossRef CAS; (d) Y. Li, Z. Li and Z. Zhang, Phys. Chem. Chem. Phys., 2021, 23, 6204–6212 RSC; (e) Y. Xiao, J. Zhao, M. Zhao, R. Chong, X. Li and Y. Qiao, Eur. J. Org. Chem., 2020, 3726–3733 CrossRef CAS; (f) A. A. Rajkiewicz, N. Wojciechowska and M. Kalek, ACS Catal., 2020, 10, 831–841 CrossRef CAS.
- (a) Z. Fu, H. Sun, S. Chen, B. Tiwari, G. Li and Y. R. Chi, Chem. Commun., 2013, 49, 261–263 RSC; (b) A minor point, although not of direct consequence to the formation of the Breslow intermediate, we wish to point out is that the configuration of the NHC used in the computational study was incorrectly drawn in ref. 10j; however, the chiral catalyst with the correct configuration evident from the optimized geometries is the same as the one employed in the original experiments (ref. 17a).
- (a) J. Mahatthananchai and J. W. Bode, Chem. Sci., 2012, 3, 192–197 RSC; (b) C. J. Collett, R. S. Massey, O. R. Maguire, A. S. Batsanov, A. C. O'Donoghue and A. D. Smith, Chem. Sci., 2013, 4, 1514–1522 RSC; (c) R. Kyan, K. Sato, N. Mase, N. Watanabe and T. Narumi, Org. Lett., 2017, 19, 2750–2753 CrossRef CAS PubMed; (d) R. S. Massey, J. Murray, C. J. Collett, J. Zhu, A. D. Smith and A. C. O'Donoghue, Org. Biomol. Chem., 2021, 19, 387–393 RSC.
- F. Jordan, Z. H. Kudzin and C. B. Rios, J. Am. Chem. Soc., 1987, 109, 4415–4416 CrossRef CAS.
- (a) S. S. Sohn, E. L. Rosen and J. W. Bode, J. Am. Chem. Soc., 2004, 126, 14370–14371 CrossRef CAS PubMed; (b) C. Burstein and F. Glorius, Angew. Chem., Int. Ed., 2004, 43, 6205–6208 CrossRef CAS PubMed; (c) C. Burstein, S. Tschan, X. Xie and F. Glorius, Synthesis, 2006, 2418–2439 CAS; (d) M. He and J. W. Bode, Org. Lett., 2005, 7, 3131–3134 CrossRef CAS PubMed; (e) S. S. Sohn and J. W. Bode, Org. Lett., 2005, 7, 3873–3876 CrossRef CAS PubMed.
- W. Schrader, P. P. Handayani, C. Burstein and F. Glorius, Chem. Commun., 2007, 43, 716–718 RSC.
- S. Simonovic, J.-C. Frison, H. Koyuncu, A. C. Whitwood and R. E. Douthwaite, Org. Lett., 2009, 11, 245–247 CrossRef CAS PubMed.
- (a) A. Berkessel, S. Elfert, K. Etzenbach-Effers and J. H. Teles, Angew. Chem., Int. Ed., 2010, 49, 7120–7124 CrossRef CAS PubMed; (b) O. Hollóczki, Z. Kelemen and L. Nyulászi, J. Org. Chem., 2012, 77, 6014–6022 CrossRef PubMed.
- J. L. Moore, A. P. Silvestri, J. Read de Alaniz, D. A. DiRocco and T. Rovis, Org. Lett., 2011, 13, 1742–1745 CrossRef CAS PubMed.
- D. A. DiRocco, K. M. Oberg and T. Rovis, J. Am. Chem. Soc., 2012, 134, 6143–6145 CrossRef CAS PubMed.
- (a) B. Maji and H. Mayr, Angew. Chem., Int. Ed., 2012, 51, 10408–10421 CrossRef CAS PubMed; (b) H. Tan, S.-A. Wang, Z. Yan, J. Liu, J. Wei, S. Song and N. Jiao, Angew. Chem., Int. Ed., 2021, 60, 2140–2144 CrossRef CAS PubMed.
- A. Berkessel, S. Elfert, V. R. Yatham, J.-M. Neudörfl, N. E. Schlörer and J. H. Teles, Angew. Chem., Int. Ed., 2012, 51, 12370–12374 CrossRef CAS PubMed.
- (a) A. Berkessel, V. R. Yatham, S. Elfert and J.-M. Neudörfl, Angew. Chem., Int. Ed., 2013, 52, 11158–11162 CrossRef CAS PubMed; (b) A. Biswas, J.-M. Neudörfl, N. E. Schlörer and A. Berkessel, Angew. Chem., Int. Ed., 2021, 60, 4507–4511 CrossRef CAS PubMed.
- M. Paul, M. Breugst, J.-M. Neudörfl, R. B. Sunoj and A. Berkessel, J. Am. Chem. Soc., 2016, 138, 5044–5051 CrossRef CAS PubMed.
- (a) M. Paul, P. Sudkaow, A. Wessels, N. E. Schlörer, J.-M. Neudörfl and A. Berkessel, Angew. Chem., Int. Ed., 2018, 57, 8310–8315 CrossRef CAS PubMed; (b) M. Paul, J.-M. Neudörfl and A. Berkessel, Angew. Chem., Int. Ed., 2019, 58, 10596–10600 CrossRef CAS PubMed; (c) M. Paul, E. Detmar, M. Schlangen, M. Breugst, J.-M. Neudörfl, H. Schwarz and A. Berkessel, Chem.–Eur. J., 2019, 25, 2511–2518 CrossRef CAS PubMed.
- M. Paul, K. Peckelsen, T. Thomulka, J. Martens, G. Berden, J. Oomens, J.-M. Neudörfl, M. Breugst, A. J. H. M. Meijer, M. Schäfer and A. Berkessel, Chem.–Eur. J., 2021, 27, 2662–2669 CrossRef CAS PubMed.
Footnote |
| † M. P. and Y. R. contributed equally. |
| This journal is © The Royal Society of Chemistry 2021 |