Open Access Article
Open Access ArticleChiral Fe(II) complex catalyzed enantioselective [1,3] O-to-C rearrangement of alkyl vinyl ethers and synthesis of chromanols and beyond†
Lifeng
Wang
 ,
Pengfei
Zhou
,
Pengfei
Zhou
 ,
Qianchi
Lin
,
Qianchi
Lin
 ,
Shunxi
Dong
,
Shunxi
Dong
 ,
Xiaohua
Liu
,
Xiaohua
Liu
 * and
Xiaoming
Feng
* and
Xiaoming
Feng
 *
*
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu 610064, China. E-mail: liuxh@scu.edu.cn; xmfeng@scu.edu.cn
First published on 7th September 2020
Abstract
A highly efficient enantioselective [1,3] O-to-C rearrangement of racemic vinyl ethers that operates under mild conditions was developed. This method with chiral ferrous complex catalyst provided an efficient access to a wide range of chromanols with high yields and excellent enantioselectivities. In addition, an important urological drug (R)-tolterodine and others were easily obtained after simple transformations.
Introduction
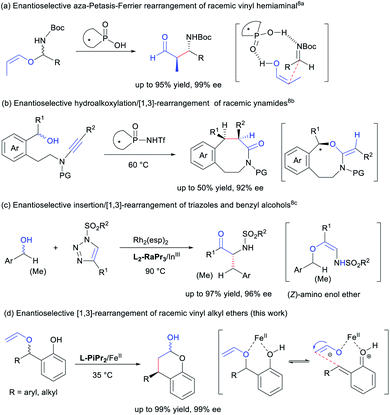
The [1,3] and [3,3] O-to-C rearrangements are powerful methods widely used in the formation of valuable compounds.1 Asymmetric catalytic [3,3] O-to-C rearrangement2 allows stereochemistry to be efficiently relayed through a cyclic transition state, and is more established than its [1,3] counterpart. In comparison, the stereochemistry in [1,3] O-to-C rearrangement is critical, but the development of enantioselective [1,3] O-to-C rearrangement is much slower however.3 Generally, there are three catalytic activation methods for [1,3]-rearrangement of vinyl ether and its variants. Chiral nucleophilic organocatalysts have been generally used for O-carboxyl shift in Steglich-type (Black) rearrangements.4 Transition metal catalysed reactions have been described but none of these are enantioselective thereof.5 Thirdly, chiral Brønsted acids and Lewis acids6 seem to be powerful in asymmetric [1,3] rearrangements of both aryl ethers7 and vinyl ethers.8 For instance, Terada and coworkers investigated asymmetric aza-Petasis–Ferrier rearrangement of hemiaminal vinyl ethers using chiral phosphoric acid catalysts (Scheme 1a).8a Stereoselective hydroalkoxylation/[1,3]-rearrangement of racemic ynamides through kinetic resolution was accomplished by Ye's group (Scheme 1b).8b The stereochemistry of the chiral substrates in the rearrangement could be transferred through zwitterion pairs.9 This result has also been confirmed in the work related to [1,3]-rearrangement of Z-amino enol ethers generated from the insertion of achiral or chiral benzyl alcohols to triazoles (Scheme 1c).8c There is no report that describes enantioselective catalysis in [1,3] O-to-C rearrangement of unsubstituted racemic vinyl ethers. To realize an enantioselective [1,3] O-to-C rearrangement from racemic vinyl alkyl ethers is challenging, and a concerted [1,3] alkyl rearrangement is required to proceed antarafacial which is highly unlikely under thermal conditions.Substituted chromanols or chromanones are important structural motifs in many natural products and pharmaceuticals.10 Despite some asymmetric catalytic examples were reported for the construction of chromanols,11 developing new methods remain highly desirable in order to expand the substrate generality. In recent years, in situ generated o-quinone methide has emerged as a popular synthon in chiral acids promoted reactions.12 We envision that highly enantioselective [1,3] O-to-C arrangement is available from racemic 2-vinyloxymethylphenols in the presence of a chiral Lewis acid catalyst (Scheme 1d). In view of the excellent performance of chiral N,N′-dioxide-metal complex catalysts in asymmetric catalysis,13,14 we utilized chiral Lewis acids for the asymmetric [1,3] O-to-C rearrangement of racemic 2-vinyloxymethylphenols to synthesize chromanols. In this report, we demonstrate that a readily available chiral iron(II) catalyst achieve extremely high enantioselective rearrangement of racemic vinyl alkyl ethers, and establish that an array of chromanols and the corresponding chromanones could be obtained, one of which is transformed into an important urological drug (R)-tolterodine.15
Results and discussion
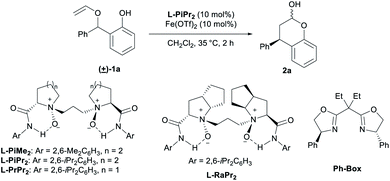
Racemic 2-(phenyl(vinyloxy)methyl)phenol 1a was selected as the model substrate. Upon investigating routine parameters, we found that a Fe(OTf)2/chiral N,N′-dioxide L-PiPr2 catalyst can promote the enantioselective [1,3] O-to-C rearrangement, delivering chromanol 2a in 93% yield and 98% ee (Table 1, entry 1). The use of Sc(OTf)3 as the metal salt resulted in slightly lower yield and enantioselectivity (entry 2). Whereas, an extremely low yield of the product with moderate enantioselectivity was obtained if Zn(OTf)2 was used (entry 3). Interestingly, racemic product was observed in poor yield in the presence of Fe(OTf)3 instead of Fe(OTf)2 (entry 4). According to our current research results,14 most of the chiral N,N′-dioxide-metal complexes, except for lanthanide metal complexes, adopt similar octahedral geometry. We initially proposed that such a sharp contrast in outcomes between Fe(OTf)3 and Fe(OTf)2 might raise from the metal cation radii, and Fe(III) has shorter radii than these other metal salts (entry 4 vs. entries 1–3). X-ray single crystal analysis of Fe(OTf)3, Fe(OTf)2 and Sc(OTf)3 of chiral N,N′-dioxides16 show that the former has a more compact cavity around the metal center, where the distance between the four oxygens of the ligand and metal ion as Fe(III) is shorter than Fe(II) and Sc(III). We probably would have thought that the condensed chiral metal complex might not be beneficial to the coordination of the two oxygen-containing species from the substrate or the intermediates, leading to sharply reduced yield and ee values. However, later we found that when small amount of water was added in connect with the use of Fe(OTf)3 at 10 mol% catalyst loading, the yield and enantioselectivity dramatically recovered (entry 5 vs. entry 4). The influence of water became inapparent when the catalyst loading of Fe(OTf)3/L-PiPr2 was reduced to 1 mol%, and a yield of 63% with 98% ee were still obtained without extra water (entry 6). We rationalized that water might participate in the catalytic cycle, and accelerate the recovery of the metal catalyst. Fe(III) iron has stronger Lewis acidity than Fe(II), and there is closer interaction between Fe(III) and the intermediate species, abating the occurrence of the rearrangement step and its regeneration. The existence of a certain amount of water could overcome the unfavorable issue of Fe(III) catalyst at high catalyst loading. Ferric salts with other counteranions or HOTf as additive was really no good for the outcomes (see ESI† for details). Finally, an excellent result (93% yield with 99% ee) was achieved if 1 mol% of Fe(OTf)2/L-PiPr2 catalyst was employed (entry 7), and an even lower loading of Fe(OTf)2 and chiral ligand L-PiPr2 can be used, without loss in enantioselectivity in the presence of 0.1 mol% of catalyst (entry 8). Other families of N,N′-dioxide ligands are also acceptable under these conditions, regardless the amino acid backbone and the amide substituents (entries 9–11). The Ph-Box ligand could promote the reaction but gave racemic product (entry 12).| Entry | Variation from “standard conditions” | Yield (%) | ee (%) |
|---|---|---|---|
| a “Standard condition”: 1a (0.1 mmol), and L-PiPr2/Fe(OTf)2 (1/1, 10 mol%) in CH2Cl2 (0.1 M) at 35 °C for 2 h. Isolated yield of 2a. Ee was determined by HPLC analysis on a chiral stationary phase. b H2O (5 μL). | |||
| 1 | None | 93 | 98 |
| 2 | Sc(OTf)3 | 86 | 90 |
| 3 | Zn(OTf)2 | 13 | 69 |
| 4 | Fe(OTf)3 | 19 | 0 |
| 5b | Fe(OTf)3 + H2O | 71 | 99 |
| 6 | Fe(OTf)3 in 1 mol% catalyst loading | 63 | 98 |
| 7 | 1 mol% catalyst loading | 93 | 99 |
| 8 | 0.1 mol% catalyst loading | 84 | 98 |
| 9 | L-RaPr2 | 80 | 94 |
| 10 | L-PrPr2 | 80 | 91 |
| 11 | L-PiMe2 | 86 | 96 |
| 12 | Ph-Box | 67 | 0 |
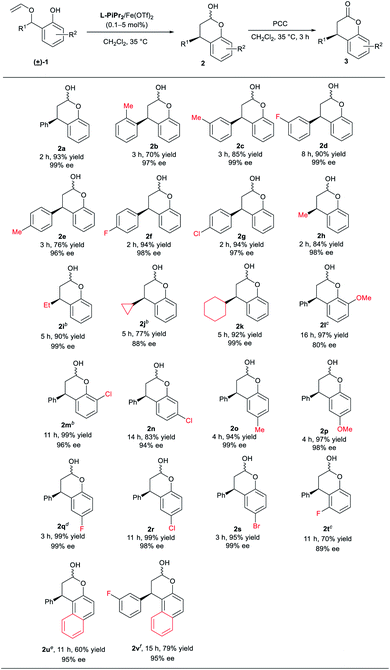
With the optimized conditions in hand, we next explored the substrate scope (Table 2). The [1,3] rearrangement reaction can perform smoothly no matter what electron-donating or electron-withdrawing substituents at meta- or para-position of the aryl group, delivering the corresponding products 2c–2g in good yields (76–94% yield) and excellent ee values (96–99% ee). The ortho-methyl aryl group substituted chromanol 2b was obtained in 70% yield and 97% ee. Moreover, alkyl substituted 2-vinyloxymethylphenols were also tolerated, affording the desired products 2h–2k in 77–92% yields and 88–99% ee. In addition, the effects of the substituent R2 at the phenol unit were investigated. Either electron-withdrawing or electron-donating substituents at para-position of the hydroxyl group of phenol provided the corresponding products 2o–2s in excellent yields (94–99% yield) and enantioselectivities (98–99% ee). It was noteworthy that the catalyst loading in the reaction of the substrate 1q containing a para-fluoro-substituent can decrease to 0.1 mol%. Substituent at meta-position to the hydroxyl group was also tolerable (2n). Nevertheless, the effect of steric hindrance around the hydroxyl group of phenol unit was obvious (2l, 2m, and 2t). In these cases, good yield and enantioselectivity were available at higher catalyst loading (5 mol%) or employing Sc(OTf)3 as the metal precursor. Gratifyingly, naphthyl substituted substrates were proved to be suitable substrates in the reaction, giving the corresponding products 2u and 2v in fair yield and excellent enantioselectivities with 10 mol% of L-RaPr2/Fe(OTf)2 catalyst, albeit in moderate yields.
| a Unless otherwise stated, all reactions were performed with 1 (0.2 mmol) and L-PiPr2/Fe(OTf)2 (1/1, 1 mol%) in CH2Cl2 (0.1 M) at 35 °C. Isolated yield of the product 2. Ee was determined by HPLC analysis based on the related derivative 3. b L-PiPr2/Fe(OTf)2 (5 mol%). c L-PiPr2/Sc(OTf)3 (5 mol%). d L-PiPr2/Fe(OTf)2 (0.1 mol%). e L-RaPr2/Fe(OTf)2 (10 mol%) in CH2ClCH2Cl. f L-RaPr2/Fe(OTf)2 (10 mol%) in CHCl3. |
|---|
|
With respect to the synthetic utility of this new method, an enantioselective synthesis of (R)-tolterodine, an urological drug, has been started in the case of a gram-scale rearrangement of 1o in the presence of 1 mol% of Fe(OTf)2/L-PiPr2, and the carbon–carbon formation proceeded well in 96% yield and 96% ee (Scheme 2a). Subsequently, chroman-2-ol 2o underwent reductive amination in the presence of diisopropylamine and sodium cyanoborohydride to furnish (R)-tolterodine in 72% yield. Its absolute configuration was determined to be R by comparing the optical rotation with the literature value.15e Thus, the stereochemistry of the product 2o and 3o was assigned as (R)-isomers. The configuration of other products was assigned by comparing with the CD spectrum of the compound 3o. Furthermore, the chromanols 2u and 2v were oxidized with PCC to the corresponding chromanones 3u and 3v in excellent yields and enantioselectivities. The chromanone 3u was reported to exhibit activity as an inhibitor of Sir2.17 Subsequently, ring-opened amination reaction of chromanones 3u and 3v using piperidine yields ROR-γ-modulator analogs 4u and 4v with good results (Scheme 2b).18
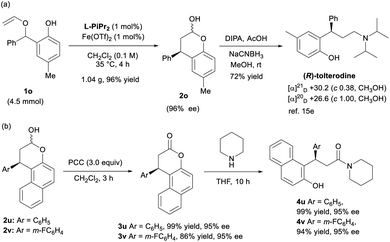 | ||
| Scheme 2 (a) Scale-up synthesis of 2o and concise synthesis of (R)-tolterodine; (b) further transformation of chromanols 2u and 2v. | ||
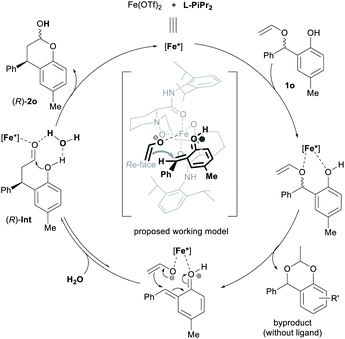
As the second most abundant metal on earth, iron salts are popular Lewis acids and metal complexes catalysts.19 In most cases, ferric salt was used instead of ferrous salt because the former is classified hard acid according to Pearson's HSAB principle. We conducted HRMS spectra to probe into the difference of the two iron species toward water (see ESI† for details). The signals in response to the complexes of iron salt, chiral ligand and water showed that higher peaks was found from the system of Fe(OTf)2 than Fe(OTf)3. It might indicate that the ferrous catalyst is a slightly sensitive to moisture than the corresponding ferric catalyst. Trace amount of water might be brought from the catalysts and other reaction components into the reaction system.
Based on the stereo-outcome of the chiral catalysts and our previous work,16d a possible catalytic model was proposed (Scheme 3). At first, the N,N′-dioxide L-PiPr2 and the two oxygen atoms of 1o coordinated to the Fe(II) center, which undergoes the heterolytic cleavage of alkyl C–O bond to generate enolate anion and o-quinone methide cation, depreciating the formal stereo-arrangement. The activation of protonated o-quinone methide further increases its electrophilicity, so the efficiency of the chiral catalyst is extremely high. Recoordination of the ethenolate and o-quinone methide to the chiral Lewis acid center occurs, followed by bond recombination through 1,4-conjugate addition/cyclization cascade reaction to afford chromanol with the new stereogenic center. As shown in the working mode, the Si-face of o-quinone methide cation was shielded by the bulky 2,6-diisopropylphenyl group of L-PiPr2, thus enolate anion would prefer to attack from the Re-face of o-quinone methide to generate (R)-intermediate. Next, the trace amount of water in the catalytic system assists the formation of acetal, affording the chiral chromanol product 2o and regenerating the chiral catalyst. Additionally, the chiral ligand was found to reduce the generation of 2-methyl-4H-benzo[d][1,3]dioxine byproduct (see ESI† for details).
Conclusions
In summary, we have successfully developed a highly efficient [1,3] O-to-C rearrangement of racemic vinyl alkyl ethers using a chiral N,N′-dioxide/Fe(OTf)2 complex. Various important chiral chromanols were obtained with high yields and excellent enantioselectivities (up to 99% yield and 99% ee). The catalyst loading is as low as 0.1–5.0 mol% in most cases. Moreover, this methodology has been applied in the highly efficient synthesis of an important urological drug (R)-tolterodine. Further investigations on other type of rearrangements are currently underway.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We appreciate the National Natural Science Foundation of China (No. U19A2014 and 21625205) for financial support.Notes and references
- C. M. Rojas, Molecular Rearrangement in Organic Synthesis, Wiley, Hoboken, 2015 Search PubMed.
- (a) H. Ito and T. Taguchi, Chem. Soc. Rev., 1999, 28, 43 RSC; (b) A. M. M. Castro, Chem. Rev., 2004, 104, 2939 CrossRef CAS; (c) E. A. Ilardi, C. E. Stivalaa and A. Zakarian, Chem. Soc. Rev., 2009, 38, 3133 RSC; (d) D. Tejedor, G. M. Abt, L. Cotos and F. G. Tellado, Chem. Soc. Rev., 2013, 42, 458 RSC; (e) J. Rehbein and M. Hiersemann, Synthesis, 2013, 45, 1121 CrossRef CAS; (f) A. C. Jones, J. A. May, R. Sarpong and B. M. Stoltz, Angew. Chem., Int. Ed., 2014, 53, 2556 CrossRef CAS; (g) H. Wu, Q. Wang and J. P. Zhu, Eur. J. Org. Chem., 2019, 1964 CrossRef CAS.
- (a) S. J. Meek and J. P. A. Harrity, Tetrahedron, 2007, 63, 3081 CrossRef CAS; (b) C. G. Nasveschuk and T. Rovis, Org. Biomol. Chem., 2008, 6, 240 RSC; (c) A. Moyano, N. E. Hamdouni and A. Atlamsani, Chem.–Eur. J., 2010, 16, 5260 CrossRef CAS; (d) I. Nakamuraa and M. Terada, Tetrahedron Lett., 2019, 60, 689 CrossRef.
- For selected examples, see: (a) J. C. Ruble and G. C. Fu, J. Am. Chem. Soc., 1998, 120, 11532 CrossRef CAS; (b) I. D. Hills and G. C. Fu, Angew. Chem., Int. Ed., 2003, 42, 3921 CrossRef CAS; (c) S. A. Shaw, P. Aleman and E. Vedejs, J. Am. Chem. Soc., 2003, 125, 13368 CrossRef CAS; (d) J. E. Thomson, K. Rix and A. D. Smith, Org. Lett., 2006, 8, 3785 CrossRef CAS; (e) S. A. Shaw, P. Aleman, J. Christy, J. W. Kampf, P. Va and E. Vedejs, J. Am. Chem. Soc., 2006, 128, 925 CrossRef CAS; (f) C. Joannesse, C. P. Johnston, C. Concellón, C. Simal, D. Philp and A. D. Smith, Angew. Chem., Int. Ed., 2009, 48, 8914 CrossRef CAS; (g) C. K. De, N. Mittal and D. Seidel, J. Am. Chem. Soc., 2011, 133, 16802 CrossRef CAS; (h) E. Gould, D. M. Walden, K. Kasten, R. C. Johnston, J. F. Wu, A. M. Z. Slawin, T. J. L. Mustard, B. Johnston, T. Davies, P. H. Cheong and A. D. Smith, Chem. Sci., 2014, 5, 3651 RSC; (i) M. L. Wang, Z. F. Zhang, S. Liu, F. Xie and W. B. Zhang, Chem. Commun., 2014, 50, 1227 RSC; (j) M. S. Xie, Y. F. Zhang, M. Shan, X. X. Wu, G. R. Qu and H. M. Guo, Angew. Chem., Int. Ed., 2019, 58, 2839 CrossRef CAS.
- (a) J. C. R. Brioche, T. A. Barker, D. J. Whatrup, M. D. Barker and J. P. A. Harrity, Org. Lett., 2010, 12, 4832 CrossRef CAS; (b) T. Toyoshima, Y. Mikano, T. Miura and M. Murakami, Org. Lett., 2010, 12, 4584 CrossRef CAS; (c) D. V. Vidhani, J. W. Cran, M. E. Krafft, M. Manoharan and I. V. Alabugin, J. Org. Chem., 2013, 78, 2059 CrossRef CAS; (d) C. N. Kona and C. V. Ramana, Chem. Commun., 2014, 50, 2152 RSC; (e) J.-O. Zirimwabagaboa and J. P. A. Harrity, Chem. Commun., 2014, 50, 2735 RSC; (f) C. N. Kona, M. N. Patil and C. V. Ramana, Org. Chem. Front., 2016, 3, 453 RSC; (g) J. T. Zhang, Z. H. Liao, L. F. Chen, H. F. Jiang and S. F. Zhu, Chem.–Eur. J., 2018, 24, 6927 CrossRef CAS.
- For selected examples, see: (a) Y. D. Zhang, N. T. Reynolds, K. Manju and T. Rovis, J. Am. Chem. Soc., 2002, 124, 9720 CrossRef CAS; (b) A. Gansäuer, D. Fielenbach, C. Stock and D. G. Gimbel, Adv. Synth. Catal., 2003, 345, 1017 CrossRef; (c) C. G. Nasveschuk and T. Rovis, Angew. Chem., Int. Ed., 2005, 44, 3264 CrossRef CAS; (d) C. G. Nasveschuk and T. Rovis, Org. Lett., 2005, 7, 2173 CrossRef CAS; (e) C. G. Nasveschuk, N. T. Jui and T. Rovis, Chem. Commun., 2006, 3119 RSC; (f) I. Nakamura, M. Owada, T. Jo and M. Terada, Org. Lett., 2017, 19, 2194 CrossRef CAS; (g) J. T. Zhang, Z. H. Liao, L. F. Chen, H. F. Jiang and S. F. Zhu, Chem. Commun., 2019, 55, 7382 RSC.
- (a) A. B. Gade, P. N. Bagle, P. S. Shinde, V. Bhardwaj, S. Banerjee, A. Chande and N. T. Patil, Angew. Chem., Int. Ed., 2018, 57, 5735 CrossRef CAS; (b) L. Yao and K. Ishihara, Chem. Sci., 2019, 10, 2259 RSC.
- (a) M. Terada and Y. Toda, J. Am. Chem. Soc., 2009, 131, 6354 CrossRef CAS; (b) B. Zhou, Y.-Q. Zhang, K. R. Zhang, M.-Y. Yang, Y.-B. Chen, Y. Li, Q. Peng, S.-F. Zhu, Q.-L. Zhou and L.-W. Ye, Nat. Commun., 2019, 10, 3234 CrossRef; (c) Y. S. Chen, Y. Liu, Z. J. Li, S. X. Dong, X. H. Liu and X. M. Feng, Angew. Chem., Int. Ed., 2020, 59, 8052 CrossRef CAS.
- For selected examples, see: (a) Y. D. Zhang and T. Rovis, Tetrahedron, 2003, 59, 8979 CrossRef CAS; (b) S. R. Shenoy and K. A. Woerpel, Org. Lett., 2005, 7, 1157 CrossRef CAS; (c) J. D. Frein and T. Rovis, Tetrahedron, 2006, 62, 4573 CrossRef CAS; (d) B. Peng, D. Geerdink and N. Maulide, J. Am. Chem. Soc., 2013, 135, 14968 CrossRef CAS.
- For selected examples, see: (a) F. Asai, M. Iinuma, T. Tanaka and M. Mizuno, Phytochemistry, 1991, 30, 3091 CrossRef CAS; (b) M. Iinuma, T. Tanaka, M. Takenaka, M. Mizuno and F. Asai, Phytochemistry, 1992, 31, 2487 CrossRef; (c) B. T. Ngadjui, G. W. F. Kapche, H. Tamboue, B. M. Abegaz and J. D. Connolly, Phytochemistry, 1999, 51, 119 CrossRef CAS; (d) X. Sun and A. T. Sneden, Planta Med., 1999, 65, 671 CrossRef CAS; (e) E.-K. Seo, M. C. Wani, M. E. Wall, H. Navarro, R. Mukherjee, N. R. Farnsworth and A. D. Kinghorn, Phytochemistry, 2000, 55, 35 CrossRef CAS; (f) X. M. Li, M. Lin, Y. H. Wang and X. Liu, Planta Med., 2004, 70, 160 CrossRef CAS; (g) B. Wungsintaweekul, K. Umehara, T. Miyase and H. Noguchi, Phytochemistry, 2011, 72, 495 CrossRef CAS; (h) S. Xu, M.-Y. Shang, G.-X. Liu, F. Xu, X. Wang, C.-C. Shou and S.-Q. Cai, Molecules, 2013, 18, 5265 CrossRef CAS.
- For selected examples, see: (a) L. Hong, L. Wang, W. S. Sun, K. Wong and R. Wang, J. Org. Chem., 2009, 74, 6881 CrossRef CAS; (b) X. Jiang, L. Wu, Y. Xing, L. Wang, S. Wang, Z. Chen and R. Wang, Chem. Commun., 2012, 48, 446 RSC; (c) S. K. Alamsetti, M. Spanka and C. Schneider, Angew. Chem., Int. Ed., 2016, 55, 2392 CrossRef CAS; (d) X. Y. Hao, L. L. Lin, F. Tan, S. L. Ge, X. H. Liu and X. M. Feng, Org. Chem. Front., 2017, 4, 1647 RSC; (e) M. Spanka and C. Schneider, Org. Lett., 2018, 20, 4769 CrossRef CAS; (f) F. Göricke and C. Schneider, Angew. Chem., Int. Ed., 2018, 57, 14736 CrossRef.
- For recent selected reviews, see: (a) W.-J. Bai, J. G. David, Z.-G. Feng, M. G. Weaver, K.-L. Wu and T. R. R. Pettus, Acc. Chem. Res., 2014, 47, 3655 CrossRef CAS; (b) Z. Wang and J. Sun, Synthesis, 2015, 47, 3629 CrossRef; (c) L. Caruana, M. Fochi and L. Bernardi, Molecules, 2015, 20, 11733 CrossRef CAS; (d) A. A. Jaworski and K. A. Scheidt, J. Org. Chem., 2016, 81, 10145 CrossRef CAS; (e) C. D. T. Nielsen, H. Abas and A. C. Spivey, Synthesis, 2018, 50, 4008 CrossRef CAS.
- For selected examples, see: (a) Y. B. Liu, H. P. Hu, H. F. Zheng, Y. Xia, X. H. Liu, L. L. Lin and X. M. Feng, Angew. Chem., Int. Ed., 2014, 53, 11579 CrossRef CAS; (b) Y. B. Liu, X. H. Liu, H. P. Hu, J. Guo, Y. Xia, L. L. Lin and X. M. Feng, Angew. Chem., Int. Ed., 2016, 55, 4054 CrossRef CAS; (c) J. Li, L. L. Lin, B. W. Hu, P. F. Zhou, T. Y. Huang, X. H. Liu and X. M. Feng, Angew. Chem., Int. Ed., 2017, 56, 885 CrossRef CAS; (d) H. F. Zheng, Y. Wang, C. R. Xu, X. Xu, L. L. Lin, X. H. Liu and X. M. Feng, Nat. Commun., 2018, 9, 1968 CrossRef; (e) Y. S. Chen, S. X. Dong, X. Xu, X. H. Liu and X. M. Feng, Angew. Chem., Int. Ed., 2018, 57, 16554 CrossRef CAS.
- For reviews of the metal/N,N′-dioxide complex: (a) X. H. Liu, L. L. Lin and X. M. Feng, Acc. Chem. Res., 2011, 44, 574 CrossRef CAS; (b) X. H. Liu, L. L. Lin and X. M. Feng, Org. Chem. Front., 2014, 1, 298 RSC; (c) X. H. Liu, H. F. Zheng, Y. Xia, L. L. Lin and X. M. Feng, Acc. Chem. Res., 2017, 50, 2621 CrossRef CAS; (d) X. H. Liu, S. X. Dong, L. L. Lin and X. M. Feng, Chin. J. Chem., 2018, 36, 791 CrossRef CAS; (e) Z. Wang, X. H. Liu and X. M. Feng, Aldrichimica Acta, 2020, 53, 3 Search PubMed.
- For selected examples, see: (a) P. G. Andersson, H. E. Schink and K. Österlund, J. Org. Chem., 1998, 63, 8067 CrossRef CAS; (b) G. Chen, N. Tokunaga and T. Hayashi, Org. Lett., 2005, 7, 2285 CrossRef CAS; (c) F. Ulgheri, M. Marchetti and O. Piccolo, J. Org. Chem., 2007, 72, 6056 CrossRef CAS; (d) S. Sörgel, N. Tokunaga, K. Sasaki, K. Okamoto and T. Hayashi, Org. Lett., 2008, 10, 589 CrossRef; (e) B. D. Gallagher, B. R. Taft and B. H. Lipshutz, Org. Lett., 2009, 11, 5374 CrossRef CAS; (f) S. Roesner and V. K. Aggarwal, Can. J. Chem., 2012, 90, 965 CrossRef CAS; (g) D. A. Barancelli, A. G. Salles Jr, J. G. Taylor and C. R. D. Correia, Org. Lett., 2012, 14, 6036 CrossRef CAS.
- (a) Y. L. Liu, D. J. Shang, X. Zhou, X. H. Liu and X. M. Feng, Chem.–Eur. J., 2009, 15, 2055 CrossRef CAS; (b) W. Li, X. H. Liu, X. Y. Hao, Y. F. Cai, L. L. Lin and X. M. Feng, Angew. Chem., Int. Ed., 2012, 51, 8644 CrossRef CAS; (c) W. B. Wu, W. D. Cao, L. F. Hu, Z. S. Su, X. H. Liu and X. M. Feng, Chem. Sci., 2019, 10, 7003 RSC; (d) P. F. Zhou, X. H. Liu, W. B. Wu, C. R. Xu and X. M. Feng, Org. Lett., 2019, 21, 1170 CrossRef CAS; (e) F. Wang, L. L. Feng, S. X. Dong, X. H. Liu and X. M. Feng, Chem. Commun., 2020, 56, 3233 RSC.
- (a) J. Posakony, M. Hirao, S. Stevens, J. A. Simon and A. Bedalov, J. Med. Chem., 2004, 47, 2635 CrossRef CAS; (b) R. C. Neugebauer, U. Uchiechowska, R. Meier, H. Hruby, V. Valkov, E. Verdin, W. Sippl and M. Jung, J. Med. Chem., 2008, 51, 1203 CrossRef CAS.
- P. M. Khan, B. E.-D. M. El-Gendy, N. Kumar, R. GarciaOrdonez, L. Lin, C. H. Ruiz, M. D. Cameron, P. R. Griffin and T. M. Kamenecka, Bioorg. Med. Chem. Lett., 2013, 23, 532 CrossRef CAS.
- For selected reviews, see: (a) C. Bolm, J. Legros, J. L. Paih and L. Zani, Chem. Rev., 2004, 104, 6217 CrossRef CAS; (b) A. Correa, O. G. Mancheño and C. Bolm, Chem. Soc. Rev., 2008, 37, 1108 RSC; (c) R. H. Morris, Chem. Soc. Rev., 2009, 38, 2282 RSC; (d) C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Rev., 2011, 111, 1293 CrossRef CAS; (e) K. Gopalaiah, Chem. Rev., 2013, 113, 3248 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H, 13C{1H} and 19F{1H} NMR, HPLC spectra, CD spectra (PDF). See DOI: 10.1039/d0sc04340k |
| This journal is © The Royal Society of Chemistry 2020 |