Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development of tethered dual catalysts: synergy between photo- and transition metal catalysts for enhanced catalysis†
Danfeng
Wang
 a,
Robert
Malmberg
a,
Robert
Malmberg
 a,
Indrek
Pernik‡
a,
Indrek
Pernik‡
 a,
Shyamal K. K.
Prasad
a,
Shyamal K. K.
Prasad
 b,
Max
Roemer‡
b,
Max
Roemer‡
 a,
Koushik
Venkatesan
a,
Koushik
Venkatesan
 a,
Timothy W.
Schmidt
a,
Timothy W.
Schmidt
 b,
Sinead T.
Keaveney
b,
Sinead T.
Keaveney
 *a and
Barbara A.
Messerle‡
*a and
Barbara A.
Messerle‡
 *a
*a
aDepartment of Molecular Sciences, Macquarie University, North Ryde, NSW 2109, Australia. E-mail: barbara.messerle@sydney.edu.au; sinead.keaveney@mq.edu.au
bARC Centre of Excellence in Exciton Science, School of Chemistry, University of New South Wales, Kensington, NSW 2052, Australia
First published on 5th June 2020
Abstract
While dual photocatalysis–transition metal catalysis strategies are extensively reported, the majority of systems feature two separate catalysts, limiting the potential for synergistic interactions between the catalytic centres. In this work we synthesised a series of tethered dual catalysts allowing us to investigate this underexplored area of dual catalysis. In particular, Ir(I) or Ir(III) complexes were tethered to a BODIPY photocatalyst through different tethering modes. Extensive characterisation, including transient absorption spectroscopy, cyclic voltammetry and X-ray absorption spectroscopy, suggest that there are synergistic interactions between the catalysts. The tethered dual catalysts were more effective at promoting photocatalytic oxidation and Ir-catalysed dihydroalkoxylation, relative to the un-tethered species, highlighting that increases in both photocatalysis and Ir catalysis can be achieved. The potential of these catalysts was further demonstrated through novel sequential reactivity, and through switchable reactivity that is controlled by external stimuli (heat or light).
Introduction
Catalysts are essential tools in modern synthetic chemistry, with 90% of industrial chemicals synthesised through a sequence that involves at least one catalytic step.1,2 This extensive use of catalysts arises from their ability to make synthetic processes more efficient, reducing energy consumption and waste generation, as well as their ability to promote otherwise unachievable transformations. Despite their widespread use, there is a continuing need to advance previous methodologies, with targeted catalyst design allowing more efficient and practical chemical transformations to be realised.While the majority of well-established catalytic processes utilise a single catalyst to facilitate the desired chemical transformation, recently there has been a surge in interest in merging different types of catalysis to permit new chemical reactivity.3–6 In particular, there is a growing interest in developing dual-catalytic systems, where cooperation between different catalysts can increase reaction efficiency, or promote reactivity that is not possible using a single catalyst. Significant advances in this emergent field have included the development of a variety of dual-catalytic systems, such as combining: (1) metal catalysis and organocatalysis;5,7,8 (2) photocatalysis and organocatalysis;9–14 and (3) photocatalysis and metal catalysis.15–26 While dual catalysis has emerged as an excellent synthetic platform for discovering new reactivity, most reported dual catalytic systems feature individual catalysts added as independent species to the reaction mixture, with comparatively little focus on single compounds that feature two distinct catalytic sites.27–33 Recent examples of these ‘bifunctional’ catalysts include a photo-palladium catalyst for Sonogashira cross coupling27 and a chiral copper catalyst for enantioselective imine alkylation.30
Chemically tethering different catalysts could permit unique synergy between the catalytic centres, with this approach anticipated to be particularly advantageous for photocatalysis, as ‘heavy atom’ (e.g. halide or metal) incorporation can enhance photocatalytic activity.34–39 In particular, tethering metal complexes to the widely used 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) type dyes can promote intersystem crossing (ISC) from the singlet to the triplet excited state, leading to generation of reactive singlet oxygen which is often key to photocatalysis (Fig. 1).40,41 While tethered BODIPY–metal complex species have been applied as photocatalysts,42 therapeutics,43–46 gas sensing47–51 and as mechanistic probes,48,52–54 their use in dual catalysis is limited.27 As such, if a catalytically active ‘heavy atom’ unit was tethered to BODIPY, this species could have the dual role of enhancing photocatalysis, as well as providing an independent catalytic site to promote alternative reactivity.
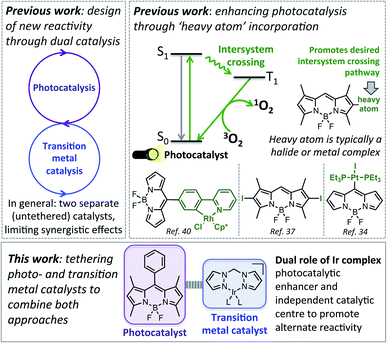 | ||
| Fig. 1 Top: Previous approaches to designing new reactivity using dual catalysis, and more efficient photocatalysts. Bottom: The aim of this work. | ||
In this current work we explored this emergent area of dual catalysis using bifunctional catalysts that feature a photocatalyst tethered to a thermally activated transition metal catalyst. In particular, the BODIPY-type photocatalyst, BDP 1 (Fig. 2) was chosen due to its excellent stability, strong ground-state absorption and ease of modification.35,39,41 The iridium bis(pyrazole)methane based complexes Ir(I) 2 and Ir(III) 3 were chosen due to their high stability, including air tolerance, ease of synthesis and their ability to promote diverse reactivity, including hydroamination,55,56 dihydroalkoxylation57,58 and hydrosilylation.56,59 These properties make Ir(I) 2 and Ir(III) 3 ideal candidates as the ‘heavy atom surrogate’ attached to BDP 1, where it will act as both a photocatalytic enhancer and an independent catalytic site. As there are many different ways to tether the catalysts, three different linking modes were targeted to gain insight into how the tethering mode affects catalytic cooperatively (Fig. 2).
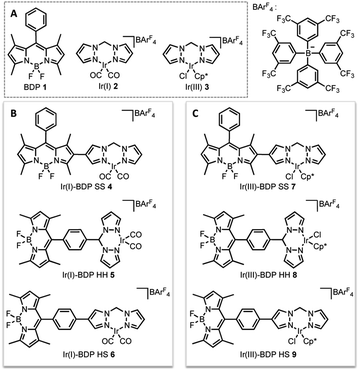 | ||
| Fig. 2 The parent catalysts on which the bifunctional catalysts are based (A); the bifunctional catalysts featuring an Ir(I) (B) or an Ir(III) catalyst (C). | ||
Herein we report a series of tethered dual catalysts, together with a thorough analysis of the structural and physical properties of the new catalysts, providing particular insight into the photophysical properties and catalytic outcomes. This work clearly demonstrates for the first time that chemically tethering heat and light activated catalysts together can allow efficient dual catalytic strategies to be developed, marking a substantial advancement in dual catalyst design.
Results and discussion
Design and synthesis of the bifunctional catalysts
As the target was to assess bifunctional catalysts featuring different tethering modes, a modular synthetic approach was desirable. In addition, an approach that is tolerant to a wide range of functional groups could permit extension to other ligand frameworks and photocatalysts in the future. As such, Suzuki cross-coupling was utilised as the key step in constructing our bifunctional catalysts. This approach is particularly useful as there are numerous synthetic reports of halogenated BDP 1 derivatives.36–38,60 As the BDP motif will feature a halogen, the boronic ester substituted bis(pyrazole)methane 12 was synthesised over two steps from bis(pyrazole)methane. With these building blocks in hand, Suzuki cross-coupling reactions were performed, generating two bifunctional catalyst frameworks in good yield (Scheme 1). These species have different tethering modes between BDP 1 and the ligand that will support iridium, with the connecting modes termed as side–side (SS 13) and head–side (HS 14).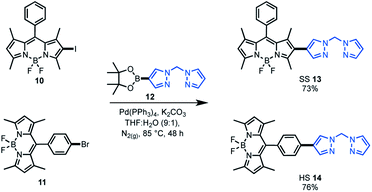 | ||
| Scheme 1 Synthesis of the bifunctional catalyst frameworks SS 13 and HS 14 through Suzuki cross-coupling reactions. | ||
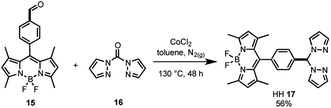
As the synthesis of bis(pyrazole)methane 16 is well-known,61 the bifunctional catalyst framework for the head–head (HH 17) tethering mode was synthesised through a cobalt catalysed condensation reaction between compounds 15 and 16 (Scheme 2). The HH framework 17 was of particular interest due to the sp3 hybridised CH moiety that disrupts conjugation between the BDP and Ir centre, unlike the fully conjugated SS 13 and HS 14 frameworks.
The final step of the synthetic strategy was coordination of the Ir(I) or Ir(III) species to the bis(pyrazole)methane moiety in compounds 13, 14 and 17, and formation of the cationic iridium complex through addition of sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate (NaBArF4). The same methods were used for forming the Ir(I) and Ir(III) derivatives for each framework, with representative reactions with 17 shown in Scheme 3. Overall, these synthetic strategies allowed access to all six of the bifunctional catalysts 4–9.
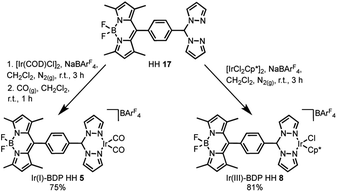 | ||
| Scheme 3 The coordination of Ir(I) or Ir(III) to the bifunctional ligand, with the synthesis of Ir(I)–BDP HH 5 and Ir(III)–BDP HH 8 shown as representative examples. | ||
Structural characterisation
Nuclear Magnetic Resonance (NMR) spectroscopy, high resolution mass spectrometry and elemental analysis confirmed the formation of complexes 4–9. Crystals suitable for X-ray crystallography were obtained for two ligands (14 and 17) and two complexes (5 and a derivative of 9 featuring a BPh4 counterion) (Fig. 3). The structural data, in combination with the NMR spectral data, confirmed the structures and tethering modes of these novel bifunctional catalysts, and indicated that tethering BDP 1 to Ir(I) 2 or Ir(III) 3 led to no significant structural changes in the BDP or iridium units regardless of the level of conjugation between the iridium and boron centre (Tables S1 and S2†).62–64 This is important as it indicates that the photophysical or electrochemical properties of 4–9, or changes in catalytic activity relative to the two catalyst components, are not simply due to structural differences that occur upon tethering.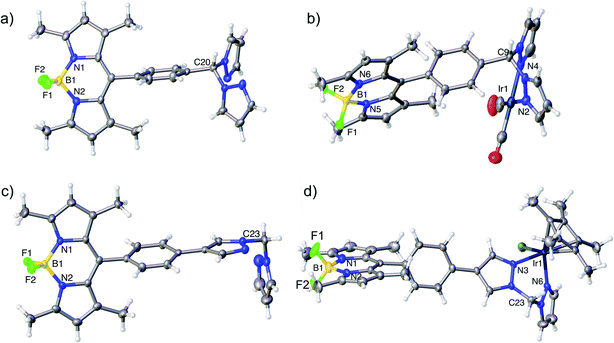 | ||
| Fig. 3 Molecular structures derived from X-ray single crystal diffraction: (a) HH ligand 17 (CCDC: 1955143); (b) Ir(I)–BDP HH 5 (CCDC: 1955144), (c) HS ligand 14 (CCDC: 1955141); (d) an analogue of Ir(III)–BDP HS 9 containing a BPh4 counterion (see ESI† for details, CCDC: 1955142). Thermal ellipsoids are shown at the 50% probability level. BArF4 and BPh4 counterions have been omitted for clarity. | ||
Infrared spectroscopy allowed evaluation of the electronic environment of Ir(I) in the carbonyl ligated species 4–6, relative to the mononuclear complex Ir(I) 2. When moving from 2 to the bifunctional species 4–6 there was a slight decrease in the carbonyl stretching frequencies {2 (2100, 2035)65 > 4 (2098, 2035) > 5 (2093, 2029) > 6 (2091, 2028 cm−1)}. These data are indicative of increased electron donation from the ligand to Ir in the bifunctional catalysts. While this effect was minor, it suggests that tethering Ir(I) 2 and BDP 1 results in increased electron donation to Ir, likely due to electron transfer from the BDP moiety.
Photophysical properties
To understand how the Ir centre influences the properties of BDP 1, detailed photophysical investigations were performed (Table 1). Firstly, analysis of the parent iridium catalysts 2 and 3 showed that these species have very low extinction coefficients (ε) in the visible region (350–650 nm) and do not emit light. This is important as it highlights that any differences in the photophysical behaviour of BDP 1 and the bifunctional catalysts 4–9 are not simply due to the Ir moiety acting as a photocatalyst itself.| Complex | λ abs/nm (ε/×105 M−1 cm−1)a | λ em /nm | τ F /ns | Φ F , /% | k r /108 s−1 | k nr /108 s−1 | τ S /ns | τ T /μs | τ airT /μs | Φ ISC /% | Φ airISC /% | E 1/2 , /V | E red , /V | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ox | red | |||||||||||||
| a Measured in toluene (1 × 10−5 mol L−1) at 298 K. Uncertainty for λabs and λem: ±1 nm. Uncertainty for τF: ±0.3 ns. b Absolute quantum yield measured with an integrated sphere, uncertainty for ΦF: ±5%. c Rates constants of radiative (kr) and non-radiative (knr) decay calculated using the formula kr = ΦF/τF and knr =(1 − ΦF)/τF. d Singlet (τS) and triplet (τT) lifetimes, and intersystem crossing quantum yields (ΦISC) measured using transient absorption spectroscopy in toluene, under an inert atmosphere. Uncertainty for τs: ±0.1 ns, and τT: ±0.1 μs. Uncertainty for ΦISC: ±0.1%. e τ T and ΦISC measurements in air. f Oxidation and reduction potentials determined using cyclic voltammetry in CH2Cl2 (0.1 mol L−1) using TBA-BArF4 as electrolyte, and calibrated using ferrocene. g Half-width potentials, assigned to the BDP moiety. h Irreversible potential of the main cathodic peak reported. i Reported potential is a shoulder on the main BDP-centred reduction. j Ir(I) 2 and Ir(III) 3 have a very weak absorption and no emission, thus limited photophysical data could be obtained. In addition, no clear oxidation wave was observed. k Absorption is outside the wavelength range examined. | ||||||||||||||
| BDP 1 | 504 (1.00) | 513 | 3.26 | 99 | 3.04 | 0.03 | 3.8 | — | — | <2 | <2 | 0.70 | −1.72 | −1.43i |
| Ir(I) 2j | 370 (0.03) | −1.65,−1.28 | ||||||||||||
| BDP 1 + Ir(I) 2 | 503 (0.91) | 514 | 3.29 | 67 | 2.04 | 1.00 | 3.6 | — | — | <2 | <2 | |||
| Ir(I)–BDP SS 4 | 507 (0.76) | 526 | 3.58 | 63 | 1.76 | 1.03 | 3.8 | >500 | 1.6 | 7.0 | 10.4 | 0.88 | −1.69 | −1.55 |
| Ir(I)–BDP HH 5 | 509 (0.76) | 519 | 3.03 | 23 | 0.76 | 2.54 | 3.6 | — | — | — | 0.81 | −1.67 | −1.61i | |
| Ir(I)–BDP HS 6 | 506 (0.21) | 516 | 2.57 | 48 | 1.87 | 2.02 | 2.1 | 160 | 1.0 | 4.3 | 7.3 | 0.75 | −1.72 | −1.58 |
| Ir(III) 3j | −1.55 | |||||||||||||
| BDP 1 + Ir(III) 3 | 503 (0.91) | 514 | 3.26 | 65 | 1.99 | 1.07 | 3.7 | — | — | <2 | <2 | |||
| Ir(III)–BDP SS 7 | 510 (0.83) | 533 | 2.70 | 49 | 1.81 | 1.89 | 2.8 | >500 | 1.1 | 7.2 | 7.3 | 0.69 | −1.69 | −1.15 |
| Ir(III)–BDP HH 8 | 509 (0.81) | 524 | 1.80 | 61 | 3.39 | 2.17 | 1.9 | — | — | — | — | 0.84 | −1.91, −1.62 | |
| Ir(III)–BDP HS 9 | 506 (0.47) | 517 | 2.45 | 47 | 1.92 | 2.16 | 2.8 | — | — | — | — | 0.74 | −1.71 | |
| SS 13 | 519 (0.61) | 565 | 4.93 | 79 | 1.60 | 0.43 | 0.65 | −1.68 | ||||||
| HH 17 | 505 (0.85) | 515 | 2.69 | 64 | 2.38 | 1.34 | 0.75 | −1.66 | ||||||
| HS 14 | 504 (0.83) | 514 | 2.73 | 81 | 2.97 | 0.70 | 0.71 | −1.70 | ||||||
In general, the extinction coefficients of the compounds were found to decrease on moving from BDP 1 to the bifunctional catalysts (Table 1), indicating that catalysts 4–9 are less effective at absorbing light than BDP 1, with the HS tethered catalysts 6 and 9 being the weakest absorbers. The absorption spectra of the HS 14 and HH 17 ligands have similar profiles to BDP 1 (Fig. 4), with a typical absorption near 504 nm, likely due to a ligand centred (LC)  transition.38,39 The HS 14 and HH 17 ligands also had similar absorption profiles to those of the HH and HS tethered bifunctional catalysts 5, 6, 8 and 9, indicating that Ir coordination doesn't affect absorbance maxima (λabs). In contrast, the SS 13 ligand had λabs at 519 nm, with this significant bathochromic shift, relative to BDP 1, suggesting that the pyrazole moiety is involved in the 1LC
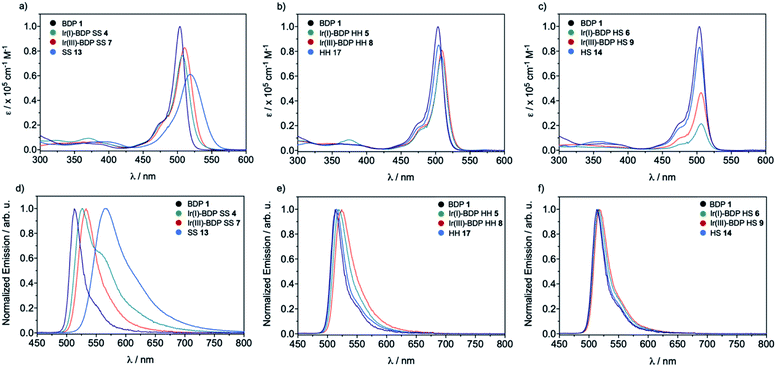
transition.38,39 The HS 14 and HH 17 ligands also had similar absorption profiles to those of the HH and HS tethered bifunctional catalysts 5, 6, 8 and 9, indicating that Ir coordination doesn't affect absorbance maxima (λabs). In contrast, the SS 13 ligand had λabs at 519 nm, with this significant bathochromic shift, relative to BDP 1, suggesting that the pyrazole moiety is involved in the 1LC 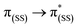 transition for this ligand. In addition, the SS based bifunctional catalysts 4 and 7 had a hypsochromic shift of 9–12 nm, relative to the SS 13 ligand, suggesting that there is a significant electronic interaction between the BDP moiety and the Ir centre, which is consistent with that reported previously for similar species.66
transition for this ligand. In addition, the SS based bifunctional catalysts 4 and 7 had a hypsochromic shift of 9–12 nm, relative to the SS 13 ligand, suggesting that there is a significant electronic interaction between the BDP moiety and the Ir centre, which is consistent with that reported previously for similar species.66
 | ||
| Fig. 4 The UV-Vis absorption spectra ((a–c), 1 × 10−5 mol L−1) and normalised emission spectra (d–f) for BDP 1, catalysts 4–9 and ligands 13, 14 and 17 in toluene. | ||
The emission spectra of BDP 1 and the bifunctional catalysts 4–9 all feature one main band near 500 nm, with a shoulder at lower energy that is most pronounced for catalyst 4 (Fig. 4). In conjunction with the measured lifetimes (τF), this band can be assigned as fluorescence, likely due to a 1LC 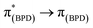 transition. The emission maxima (λem) of the HH and HS based catalysts 5, 6, 8 and 9 were comparable to BDP 1 and the ligands HS 14 and HH 17. However, a significant bathochromic shift (52 nm) was observed for SS 13, relative to BDP 1, suggesting that the pyrazole moiety is involved in the 1LC
transition. The emission maxima (λem) of the HH and HS based catalysts 5, 6, 8 and 9 were comparable to BDP 1 and the ligands HS 14 and HH 17. However, a significant bathochromic shift (52 nm) was observed for SS 13, relative to BDP 1, suggesting that the pyrazole moiety is involved in the 1LC 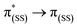 transition for this ligand. In addition, significant hypsochromic shifts of 39 nm and 32 nm for λem were observed for the SS tethered catalysts 4 and 7, relative to SS 13 (Fig. 4 and Table 1). Once again, these data indicate that the excited state of BDP 1 is altered the most when it is tethered to the Ir moiety through the SS tethering mode.
transition for this ligand. In addition, significant hypsochromic shifts of 39 nm and 32 nm for λem were observed for the SS tethered catalysts 4 and 7, relative to SS 13 (Fig. 4 and Table 1). Once again, these data indicate that the excited state of BDP 1 is altered the most when it is tethered to the Ir moiety through the SS tethering mode.
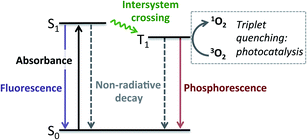
Having established that BDP 1 and the bifunctional catalysts 4–9 are effective at absorbing light, it was important to consider the pathway(s) through which the excited species decay. Following light absorption into the first singlet excited state (S1), the excited photocatalyst can either: (1) decay to the singlet ground state (S0) via radiative (fluorescence) or non-radiative decay; or (2) undergo ISC to the first triplet excited state (T1), followed by radiative (phosphorescence) or non-radiative decay to S0 (Fig. 5). Importantly, for photocatalysis the ISC pathway is desired as this allows singlet oxygen to be generated. As such, the preference for these competing pathways is key for assessing photocatalytic potential.
As discussed above, all catalysts undergo fluorescent decay, which is undesired for photocatalysis, with no phosphorescence observed in our measurements. The fluorescence lifetimes (τF) were all found to be in the lower nanosecond region (2–5 ns), with shorter lifetimes generally observed for the bifunctional catalysts 4–9, relative to BDP 1 (Table 1). Importantly, the fluorescence quantum yield for BDP 1 was very high (ΦF = 99%), indicating that the desired ISC pathway to T1 does not readily occur (<1%), making BDP 1 a poor photocatalyst. Pleasingly, the fluorescence quantum yields were significantly lower for catalysts 4–9 (23–63%), and ligands 13, 14 and 17 (64–81%), indicating that undesired fluorescent decay from S1 is significantly reduced for these compounds. However, these lower ΦF could be due to an increase in the desired ISC to T1,38,48,67 or undesired pathways such as non-radiative decay from S1 to S0 or fluorescence quenching due to intermolecular interactions, as observed for the BDP 1 + Ir(I) 2 and BDP 1 + Ir(III) 3 mixtures.
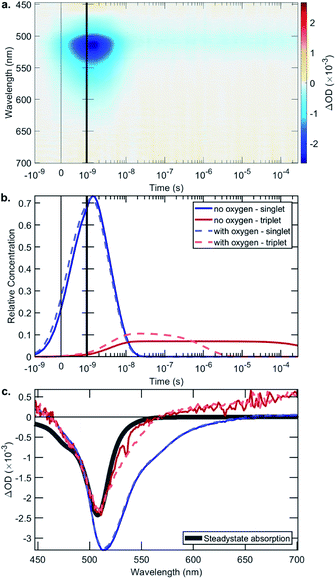
The rate constant data show that in general the bifunctional catalysts 4–9, and ligands 13, 14 and 17, have lower rates of radiative decay (kr), and higher rates of non-radiative decay (knr), relative to BDP 1. This indicates that non-radiative decay pathways contribute significantly to the photophysical behaviour of species 4–9. This is likely due to a large extent of thermal energy loss through rotation of the tethered Ir catalyst about the C–C bond that links BDP 1 and Ir(I) 2/Ir(III) 3.47,68–70 Overall, the increased non-radiative decay from S1 to S0 observed for 4–9, relative to BDP 1, contributes to their lower ΦF. To determine if increased ISC from S1 to T1 is also contributing to these lower ΦF, transient absorption (TA) spectroscopy was used to examine the excited states of BDP 1 and catalysts 4–9 (Table 1, Fig. 6 and S24–S44†).
The TA signal comprises a negative ΔOD (Optical Density) in the 500–550 nm region, which could be caused by ground-state bleaching (GSB) or stimulated emission (SE). A GSB signal is indicative of there being molecules in an excited state, while the presence of SE indicates a singlet state. The surface shown in Fig. 6a is decomposed into relative concentrations (Fig. 6b) and spectra (Fig. 6c) by using a sequential model fit with two exponentials. Fig. 6c shows a comparison of the steady-state absorption, which mirrors the GSB, where it is evident that the first species has an additional lower energy region due to SE, which is not present in the triplet species.
The singlet lifetimes (τS) were comparable to the previously measured τF, as expected (Table 1). A long-lived T1 was not detected for BDP 1, as anticipated based on the reported lifetime of 0.02 μs in acetonitrile.71 Interestingly, triplet excited states were only detected for three of the six bifunctional catalysts, indicating that the mode of tethering the Ir moiety to BDP 1 plays an important role in populating the triplet states (Table 1). The SS based bifunctional catalysts 4 and 7 were found to have the highest extent of ISC from S1 to T1, with ISC quantum yields (ΦISC) of 7.0 and 7.2%, respectively. This is important as it clearly demonstrates that tethering of Ir(I) 2 or Ir(III) 3 to BDP 1 promotes the desired ISC pathway, with the SS tethering mode being most effective. Interestingly, in the presence of air the ΦairISC for the Ir(I) based bifunctional catalysts 4 and 6 increased, whereas ΦairISC for Ir(III)–BDP SS 7 was unchanged.
The triplet state lifetimes (τT) were remarkably high for catalysts 4 and 7 (>500 μs) with Ir(I)–BDP HS 6 also having a long-lived triplet state (160 μs). To the best of our knowledge, the highest reported τT for a BDP-type compound is 539 μs,72 thus catalysts 4 and 7 represent one of the longest reported triplet state lifetimes for BDP-type compounds. These long triplet lifetimes are important for photocatalysis, as they increase the likelihood of productive triplet energy transfer, leading to 1O2 generation. This was confirmed through transient absorption measurements in the presence of air, which resulted in much shorter lifetimes (<2 μs), indicating that quantitative triplet quenching by oxygen occurs (>99%). Overall, these data suggest that Ir(I)–BDP SS 4 and Ir(III)–BDP SS 7 have more desirable photophysical properties than BDP 1 and catalysts 5, 6, 8 and 9, and thus should be more effective photocatalysts.
Cyclic voltammetry
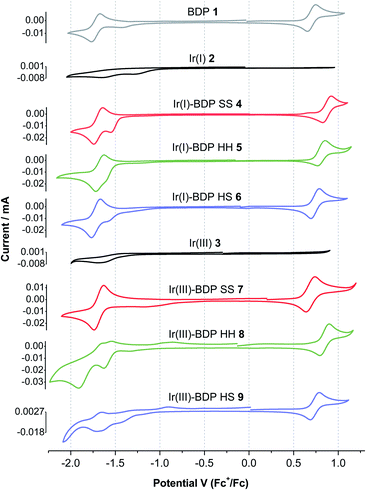
To provide insight into electrochemical behaviour and the potential for redox applications, cyclic voltammetry measurements were performed on catalysts 1–9 and the ligands 13, 14 and 17 (Table 1, Fig. 7 and S45†). Cyclic voltammograms (CVs) were recorded using a three-electrode setup, with a glassy carbon working electrode, in a 0.1 M solution of tetrabutylammonium BArF4 (TBA-BArF4) in dichloromethane. TBA-BArF4 was chosen as the supporting electrolyte as catalysts 2–9 all contain the BArF4 counterion, and as TBA-BArF4 can allow multiple oxidation processes of the analytes to be resolved due to its weakly coordinating nature.73 All potentials are reported vs. the ferrocene/ferrocenium couple.The parent BDP 1 species was found to undergo well-resolved one electron oxidation (0.70 V) and reduction (−1.72 V) events, typical of BDP-type compounds.38,70,74 Good reversibility was observed, with the quotient of the anodic and cathodic peak currents close to unity and an approximately linear relationship between the peak currents and scan rate (Tables S5 and S6†). Similar reversible behaviour was observed for the frameworks 13, 14 and 17, however better reversibility was observed at higher scan rates. In addition, the oxidation and reduction potentials for 13, 14 and 17 were comparable to BDP 1, suggesting that the pyrazole moiety has no significant effect on the redox behaviour of the BDP 1 moiety (Table 1). While BDP 1 and the ligands 13, 14 and 17 exhibited electrochemical reversibility, both the parent Ir(I) 2 and Ir(III) 3 catalysts showed only weakly pronounced irreversible reduction events near −1.5 V, with no distinct oxidation events.
In general, the electrochemical behaviour of catalysts 4–9 was dominated by the BDP 1 fragment, with reversible oxidation and reduction events near 0.7 and −1.7 V, respectively. Poorly defined reduction peaks due to the Ir(I) 2 or Ir(III) 3 moieties were also present in the CVs (Fig. 7). The splitting between the major oxidation and reduction events varies from 2.3 to 2.6 V, which is comparable to reported alkyl,75 phenyl70 and platinum34 substituted BDP derivatives. Comparison of the ligand frameworks 13, 14 and 17 with the Ir(I)-based catalysts 4–6 indicate that coordination of Ir(I) to the ligand has no significant effect on the reduction potential, however the oxidation potential increased, with this increase most pronounced for the SS framework (+0.23 V). This suggests that the Ir(I) species affects the BDP moiety most when it is tethered through the conjugated SS tethering mode, which is consistent with our photophysical measurements.
Coordination of the Ir(III) moiety to the ligand frameworks 13, 14 or 17 generally led to more complex electrochemical behaviour, with multiple reduction events observed for catalysts 8 and 9. The Ir(III)-based catalysts 7–9 had higher oxidation potentials than the corresponding ligand frameworks, as seen for Ir(I), however the increases were less pronounced. In addition, the maximum increase was now seen for the HH catalyst 8 (+0.09 V). This data indicates that coordination of Ir(III)ClCp* to the ligand frameworks leads to different electrochemical behaviour than that seen upon Ir(I)(CO)2 coordination. Overall, catalysts 4–9 exhibit reversible electrochemical behaviour, that is dominated by the BDP 1 fragment, highlighting their potential for use as catalysts for redox processes. There were some interesting trends in potentials observed when changing the Ir species and tethering mode, demonstrating the possibility to tune the redox potential of the catalysts through tethering modes.
X-ray absorption spectroscopy
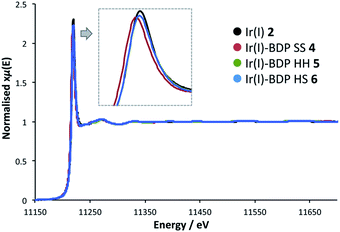
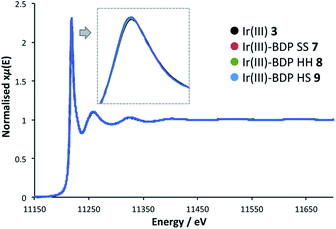
To probe whether BDP is affecting the local electronic structure of Ir in the bifunctional catalysts 4–9, X-ray Absorption Spectroscopy (XAS) measurements were performed at the Ir L3 edge (11–12.5 keV) in transmission mode. The XAS spectra indicate that there is a decrease in the absorption edge energy on moving from Ir(I) 2 to the bifunctional catalysts 4–6 (Fig. 8). This decrease in edge energy is smaller for Ir(I)–BDP HH 5 and Ir(I)–BDP HS 6 (<0.5 eV), with a more significant decrease observed for Ir(I)–BDP SS 4 (1 eV). This decrease in edge energy is characteristic of the metal centre becoming more negative, with a decrease of 1 eV being significant (for example, a difference of 1.6 eV between Ir(IV) and Ir(III) has been reported76). Thus, the trend observed suggests that BDP is transferring electron density to Ir(I), making it less positive, with this effect most pronounced for Ir(I)–BDP SS 4. This is consistent with the IR data, which also suggests electron transfer from BDP to Ir(I) is occurring, and our photophysical and electrochemical studies which suggest that the greatest interaction between Ir and BDP occurs for the SS tethering mode.Interestingly, no shift in edge energy was observed for the Ir(III)-based catalysts 3 and 7–9 (Fig. 9), suggesting that transfer of electron density from BDP to Ir(III) does not occur. Overall, these data indicate that the local electronic structure of Ir(I) 2 becomes less positive when tethered to BDP, while Ir(III) 3 is unaffected.
Catalytic investigations
Photocatalytic applications of BDP-type dyes generally rely on the generation of reactive singlet oxygen,35,37,46,77,78 thus singlet oxygen quantum yields (ΦΔ) are a good indication of photocatalytic potential. Incorporation of heavy atoms into BDP can increase ΦΔ due to enhanced intersystem crossing from S1 to T1.35 This phenomenon was a key part of our bifunctional catalyst design, as we sought to enhance the photocatalytic competency of BDP 1 through incorporation of a ‘heavy atom’, whilst also providing a separate catalytic site that can facilitate complimentary catalytic reactivity. To determine whether our bifunctional catalysts are superior singlet oxygen generators to BDP 1, ΦΔ was determined for each catalyst under green LED irradiation (max wavelength = 510 nm) using 1,3-diphenylisobenzofuran as a singlet oxygen trap (Table 2).36,37,79,80 As alcoholic solvents are often used for photocatalysis, the high boiling tertiary amyl alcohol solvent was used.| Complex | Φ Δ/% |
|---|---|
| BDP 1 | 2.6 ± 0.1 |
| BDP 1 + Ir(I) 2 | 3.2 ± 0.1 |
| Ir(I)–BDP SS 4 | 12.3 ± 0.4 |
| Ir(I)–BDP HH 5 | 3.6 ± 0.1 |
| Ir(I)–BDP HS 6 | 3.0 ± 0.2 |
| BDP 1 + Ir(III) 3 | 3.9 ± 0.2 |
| Ir(III)–BDP SS 7 | 7.5 ± 1.7 |
| Ir(III)–BDP HH 8 | 1.2 ± 0.1 |
| Ir(III)–BDP HS 9 | 2.4 ± 0.2 |
It was found that BDP 1 had a singlet oxygen quantum yield of 2.6% in tertiary amyl alcohol, which is comparable to the reported value of 1% for BDP 1 in dichloromethane.37 Pleasingly, ΦΔ for the side–side tethered bifunctional catalysts 4 and 7 were higher than that for BDP 1, suggesting that tethering Ir(I) 2 or Ir(III) 3 to BDP 1 can promote ISC. The most efficient singlet oxygen generator was Ir(I)–BDP SS 4, with a 5-fold increase in ΦΔ relative to BDP 1. It should be noted that greater increases in ΦΔ would likely be observed if the heavy atom was attached directly to the BDP 1 core,34,35,81 and thus the smaller changes in ΦΔ observed here are likely due to the Ir centre being separated from BDP 1 by the tether. Despite this, the variation in ΦΔ between the bifunctional catalysts indicates that both the tethering mode and the nature of the Ir centre (Ir(I) 2vs. Ir(III) 3) affects singlet oxygen generation. This is important as it clearly highlights the need to consider the tethering mode when developing tethered dual catalysts.
To determine how singlet oxygen quantum yield affects photocatalytic efficiency, the catalytic competency of a representative selection of catalysts was examined using the oxidation of benzylamine 18 as the model reaction. This reaction was chosen as iodo-substituted BDP compounds have previously been shown to effectively promote this oxidation reaction.81 The complexes BDP 1, Ir(I)–BDP SS 4, Ir(I)–BDP HH 5 and Ir(III)–BDP SS 7 were tested as these catalysts cover a range of ΦΔ. It was found that the bifunctional catalysts 4, 5 and 7 were significantly better photocatalysts than BDP 1 (Table 3). Control experiments in the presence of the singlet oxygen scavenger 1,4-diazabicyclo[2.2.2]octane (DABCO) confirm that singlet oxygen is involved in the reaction mechanism for all catalysts (Table S8†). Further control reactions indicate that Ir(I) 2 and Ir(III) 3 are inefficient photocatalysts; this is important as it demonstrates that tethering a transition metal catalyst, that isn't photocatalytically active, to an organic photocatalyst can significantly enhance photocatalytic activity.
| Catalyst | Conversion to product 19/% | ||
|---|---|---|---|
| 4 h | 16 h | 24 h | |
| a Conditions: benzylamine (0.4 mmol), catalyst (0.002 mmol), additive, where appropriate (0.002 mmol) 2,4,6-trimethoxybenzene (internal standard, 0.2 mmol), t-amyl alcohol (0.5 mL) in a vial open to air, with aliquots taken at different time points. Average and error (half the range) of 2 replicate experiments reported. | |||
| BDP 1 | 9 ± 3 | 24 ± 5 | 32 ± 7 |
| Ir(I)–BDP SS 4 | 23 ± 4 | 59 ± 7 | 79 ± 1 |
| Ir(I)–BDP HH 5 | 18 ± 1 | 50 ± 3 | 73 ± 5 |
| Ir(III)–BDP SS 7 | 18 ± 4 | 46 ± 5 | 72 ± 1 |
| Ir(I) 2 | 0 | 6 | 10 |
| Ir(III) 3 | 0 | 0 | 1 |
| BDP 1 + Ir(I) 2 | 12 ± 1 | 42 ± 1 | 59 ± 2 |
| BDP 1 + Ir(III) 3 | 12 ± 1 | 41 ± 3 | 57 ± 3 |
| BDP 1 + NaBArF4 | 8 ± 4 | 24 ± 2 | 42 ± 3 |
| BDP 1 + NaCl | 6 ± 3 | 21 ± 2 | 35 ± 2 |
Comparison of the extent of conversion to the product 19 with ΦΔ (Fig. S53†) gives a moderate correlation (R2 = 0.58), indicating that singlet oxygen generation is not rate-determining for this reaction, as seen for other processes involving singlet oxygen.37 This is supported by the catalytic enhancements observed when using untethered mixtures of ‘BDP 1 + Ir(I) 2’ or ‘BDP 1 + Ir(III) 3’, relative to BDP 1 (Table 3); this was unexpected as these mixtures have ΦΔ similar to BDP 1 (Table 2). These data suggest that factors, other than simply ΦΔ, contribute to the synergistic effects observed when using dual BDP 1–Ir(I) 2/Ir(III) 3 systems in photocatalysis. Control reactions using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of BDP 1 and NaBArF4 suggest that the BArF4 anion isn't contributing to the enhancements seen, while reactions using a 1
1 mixture of BDP 1 and NaBArF4 suggest that the BArF4 anion isn't contributing to the enhancements seen, while reactions using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of BDP 1 and NaCl confirm that it is not simply a salt effect (Table 3). Therefore, we postulate that the Ir centre is interacting with specie(s) along the reaction coordinate, contributing to the catalytic enhancements observed when using either tethered or untethered BDP 1−Ir(I) 2/Ir(III) 3 systems (Table 3). While mechanistic investigations into this phenomenon are ongoing, it is likely that the effect of the Ir centre in the tethered dual catalysts is two-fold; it increases ΦΔ and is also directly involved in the benzylamine 18 oxidation reaction mechanism.
1 mixture of BDP 1 and NaCl confirm that it is not simply a salt effect (Table 3). Therefore, we postulate that the Ir centre is interacting with specie(s) along the reaction coordinate, contributing to the catalytic enhancements observed when using either tethered or untethered BDP 1−Ir(I) 2/Ir(III) 3 systems (Table 3). While mechanistic investigations into this phenomenon are ongoing, it is likely that the effect of the Ir centre in the tethered dual catalysts is two-fold; it increases ΦΔ and is also directly involved in the benzylamine 18 oxidation reaction mechanism.
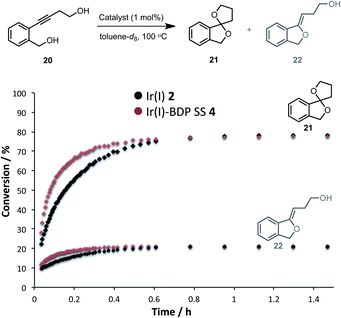
Having established that the bifunctional species are superior photocatalysts to BDP 1, attention will turn to the Ir(I) and Ir(III) moieties. In this section the parent catalysts 2 and 3 were compared with the SS based complexes 4 and 7, as the SS framework was most favourable for photocatalysis. The catalytic reactivity of the Ir(I) moiety was assessed for promoting the dihydroalkoxylation of 4-(2-(hydroxymethyl)-phenyl)but-3-yn-1-ol 20 to produce products 21 and 22. Kinetic analyses using in situ1H NMR spectroscopy indicate that both Ir(I) 2 and Ir(I)–BDP SS 4 are effective at facilitating this reaction, and comparable product ratios were observed (Fig. 10). However, Ir(I)–BDP SS 4 was more efficient than Ir(I) 2, indicating that tethering BDP to Ir(I) enhances the catalytic reactivity of the Ir(I) moiety, possibly due to electron transfer from BDP to Ir(I) as suggested by the XAS data. This is important as it indicates that there are two types of synergy between the catalytic centres in Ir(I)–BDP SS 4: Ir(I) 2 enhances the photocatalytic ability of BDP 1 (Table 3) and BDP 1 enhances the catalytic reactivity of Ir(I) 2 (Fig. 10).
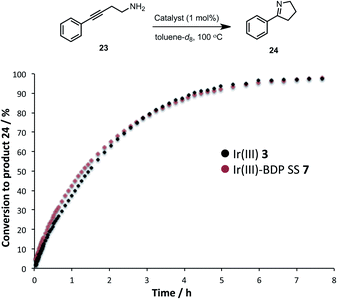
The activity of the Ir(III) based compounds 3 and 7 were assessed for promoting the intramolecular hydroamination of 4-phenylbut-3-yn-1-amine 23 (Fig. 11). Kinetic analyses using in situ1H NMR spectroscopy indicate that Ir(III)–BDP SS 7 can effectively promote hydroamination, with identical reaction profiles obtained for Ir(III) 3 and Ir(III)–BDP SS 7. This indicates that the Ir(III) 3 moiety remains catalytically active in Ir(III)–BDP SS 7. Overall, these data indicate that the reactivity of the Ir(I) 2 and Ir(III) 3 moieties are not inhibited when tethered to BDP 1, with comparable, or better, catalytic results obtained for the reactions considered. This is important, as it demonstrates that the Ir centre is still able to act as a competent catalyst when incorporated into the bifunctional catalyst, highlighting the dual role of Ir as both a photocatalytic enhancer and a unique reaction centre available to promote alternate reactivity.
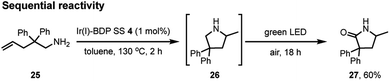
To further validate the bifunctional character of the novel tethered catalysts 4–9, their ability to promote both sequential and stimuli-responsive chemical reactivity was demonstrated using Ir(I)–BDP SS 4 as a representative catalyst. This bifunctional catalyst could promote a novel tandem reaction, where the amine 25 first undergoes Ir(I) catalysed intramolecular hydroamination to produce the intermediate 26, followed by BDP promoted photocatalytic oxidation to generate product 27 in 60% isolated yield over two steps (Scheme 4). This is important as it provides an alternative synthetic approach to the medicinally important lactam framework 27. In addition, there was a significant advantage to chemically tethering the catalysts together, as demonstrated by the much lower yield of the product 27 (17%) obtained when using a mixture of BDP 1 and Ir(I) 2.
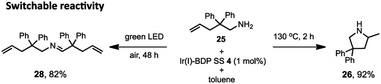
Lastly, switchable chemical reactivity was demonstrated using the amine 25 under different external stimuli, where use of heat activated the Ir(I) moiety in Ir(I)–BDP SS 4, resulting in hydroamination to compound 26. Conversely, light irradiation activated the BDP moiety in catalyst 4, leading to photocatalytic oxidation of 25 to the product 28 (Scheme 5). This stimuli-responsive behaviour of the tethered dual catalyst is significant, as controlling reactivity through external stimuli is an emergent field as it is central to the development of programmable and adaptive materials, and controllable sequential reactions.82,83 Overall, these are the first reported examples of sequential and switchable reactivity using BDP-based tethered dual catalysts.
Conclusions
In summary, we have synthesised a series of novel tethered dual catalysts that feature a BDP photocatalyst and a thermally activated iridium catalyst, with synergistic interactions between the catalysts examined using a range of techniques. Absorption and emission spectroscopy revealed that the excited state is centred on the BDP 1 moiety of the bifunctional catalysts, with the excited state altered most when the SS tethering mode is used. Interaction between BDP and Ir(I) was also observed in the XAS data for catalysts 4–6, with the greatest extent of electron transfer from BDP to Ir(I) seen for the SS-tethered catalyst 4. Interestingly, the XAS data suggest that electron transfer from BDP to Ir(III) does not occur in catalysts 7–9.Transient absorption spectroscopy indicated that tethering Ir(I) 2 or Ir(III) 3 to BDP 1 can increase intersystem crossing from the singlet to the triplet excited state, with long lived triplet states located for Ir(I)–BDP SS 4, Ir(I)–BDP HS 6 and Ir(III)–BDP SS 7. The highest extent of ISC and the longest triplet lifetimes (>500 ns) were observed for the SS-tethered catalysts 4 and 7, suggesting that the SS tethering mode will be most effective for photocatalysis. The superior photocatalytic ability of catalysts 4 and 7 was confirmed through singlet oxygen quantum yield measurements and photocatalytic investigations. In addition, cyclic voltammetry indicated that catalysts 4–9 exhibit reversible electrochemical behaviour that is dominated by the BDP moiety. The oxidation and reduction potentials varied depending on the nature of the Ir species and tethering mode, highlighting the tunability of the bifunctional catalysts' redox potentials.
Importantly, Ir(I) and Ir(III) were shown to remain catalytically active in the bifunctional catalysts 4 and 7 for representative hydroamination and dihydroalkoxylation reactions. This allowed the first demonstration of tethered photo–transition metal dual catalysts to promote both sequential and stimuli-responsive chemical reactivity. The key fundamental insight into catalytic cooperatively presented in this manuscript lays the groundwork for rationally designing tethered photo–transition metal dual catalysts in the future, and utilising these species to develop novel chemical reactivity.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the Chemical Analysis Facility at Macquarie University, in particular Dr Nicole Cordina and Dr Remi Rouquette. We also thank Dr Mohan Bhadbhade (UNSW Australia) for the X-ray diffraction measurements. TWS and SKKP are supported by the ARC Centre of Excellence in Exciton Science (CE170100026). The XAS measurements were performed on the XAS beamline at the Australian Synchrotron, part of ANSTO (Project 14297), and we would like to thank Dr Peter Kappen and Dr Jeremy Wykes for their assistance. DW and RM acknowledge the support of Macquarie University through the iMQRES scholarship program. STK acknowledges the support of Macquarie University through the receipt of a Macquarie University Research Fellowship (MQRF).References
- Q.-L. Zhou, Angew. Chem., Int. Ed., 2016, 55, 5352–5353 CrossRef CAS PubMed.
- I. Fechete, Y. Wang and J. C. Védrine, Catal. Today, 2012, 189, 2–27 CrossRef CAS.
- J. Twilton, C. Le, P. Zhang, M. H. Shaw, R. W. Evans and D. W. C. MacMillan, Nat. Rev. Chem., 2017, 1, 0052 CrossRef CAS.
- K. L. Skubi, T. R. Blum and T. P. Yoon, Chem. Rev., 2016, 116, 10035–10074 CrossRef CAS PubMed.
- S. Afewerki and A. Córdova, Chem. Rev., 2016, 116, 13512–13570 CrossRef CAS PubMed.
- A. E. Allen and D. W. C. MacMillan, Chem. Sci., 2012, 3, 633–658 RSC.
- Z. Du and Z. Shao, Chem. Soc. Rev., 2013, 42, 1337–1378 RSC.
- Z. Shao and H. Zhang, Chem. Soc. Rev., 2009, 38, 2745–2755 RSC.
- M. N. Hopkinson, B. Sahoo, J.-L. Li and F. Glorius, Chem.–Eur. J., 2014, 20, 3874–3886 CrossRef CAS PubMed.
- V. Dimakos, H. Y. Su, G. E. Garrett and M. S. Taylor, J. Am. Chem. Soc., 2019, 141, 5149–5153 CrossRef CAS PubMed.
- J. Zelenka, E. Svobodová, J. Tarábek, I. Hoskovcová, V. Boguschová, S. Bailly, M. Sikorski, J. Roithová and R. Cibulka, Org. Lett., 2019, 21, 114–119 CrossRef CAS PubMed.
- H. Hou, S. Zhu, I. Atodiresei and M. Rueping, Eur. J. Org. Chem., 2018, 2018, 1277–1280 CrossRef CAS.
- D. A. DiRocco and T. Rovis, J. Am. Chem. Soc., 2012, 134, 8094–8097 CrossRef CAS PubMed.
- D. A. Nicewicz and D. W. C. MacMillan, Science, 2008, 322, 77–80 CrossRef CAS PubMed.
- Y.-M. Tian, X.-N. Guo, M. W. Kuntze-Fechner, I. Krummenacher, H. Braunschweig, U. Radius, A. Steffen and T. B. Marder, J. Am. Chem. Soc., 2018, 140, 17612–17623 CrossRef CAS PubMed.
- H.-H. Zhang, J.-J. Zhao and S. Yu, J. Am. Chem. Soc., 2018, 140, 16914–16919 CrossRef CAS PubMed.
- Y. Shen, Y. Gu and R. Martin, J. Am. Chem. Soc., 2018, 140, 12200–12209 CrossRef CAS PubMed.
- H. Yue, C. Zhu and M. Rueping, Angew. Chem., Int. Ed., 2018, 57, 1371–1375 CrossRef CAS PubMed.
- D. Wang, N. Zhu, P. Chen, Z. Lin and G. Liu, J. Am. Chem. Soc., 2017, 139, 15632–15635 CrossRef CAS PubMed.
- M. El Khatib, R. A. M. Serafim and G. A. Molander, Angew. Chem., Int. Ed., 2016, 55, 254–258 CrossRef CAS PubMed.
- K. Nakajima, S. Nojima and Y. Nishibayashi, Angew. Chem., Int. Ed., 2016, 55, 14106–14110 CrossRef CAS PubMed.
- B. J. Shields and A. G. Doyle, J. Am. Chem. Soc., 2016, 138, 12719–12722 CrossRef CAS PubMed.
- S. Z. Tasker and T. F. Jamison, J. Am. Chem. Soc., 2015, 137, 9531–9534 CrossRef CAS PubMed.
- J. A. Terrett, J. D. Cuthbertson, V. W. Shurtleff and D. W. C. MacMillan, Nature, 2015, 524, 330 CrossRef CAS PubMed.
- J. C. Tellis, D. N. Primer and G. A. Molander, Science, 2014, 345, 433 CrossRef CAS PubMed.
- Y. Ye and M. S. Sanford, J. Am. Chem. Soc., 2012, 134, 9034–9037 CrossRef CAS PubMed.
- K. C. Dissanayake, P. O. Ebukuyo, Y. J. Dhahir, K. Wheeler and H. He, Chem. Commun., 2019, 55, 4973–4976 RSC.
- T. Rigotti, A. Casado-Sánchez, S. Cabrera, R. Pérez-Ruiz, M. Liras, V. A. de la Peña O'Shea and J. Alemán, ACS Catal., 2018, 8, 5928–5940 CrossRef CAS.
- X. Shen, Y. Li, Z. Wen, S. Cao, X. Hou and L. Gong, Chem. Sci., 2018, 9, 4562–4568 RSC.
- Y. Li, K. Zhou, Z. Wen, S. Cao, X. Shen, M. Lei and L. Gong, J. Am. Chem. Soc., 2018, 140, 15850–15858 CrossRef CAS PubMed.
- W. Ding, L.-Q. Lu, Q.-Q. Zhou, Y. Wei, J.-R. Chen and W.-J. Xiao, J. Am. Chem. Soc., 2017, 139, 63–66 CrossRef CAS PubMed.
- X. Lang, J. Zhao and X. Chen, Chem. Soc. Rev., 2016, 45, 3026–3038 RSC.
- Q. M. Kainz, C. D. Matier, A. Bartoszewicz, S. L. Zultanski, J. C. Peters and G. C. Fu, Science, 2016, 351, 681–684 CrossRef CAS.
- P. Irmler and R. F. Winter, Organometallics, 2018, 37, 235–253 CrossRef CAS.
- J. Zhao, K. Xu, W. Yang, Z. Wang and F. Zhong, Chem. Soc. Rev., 2015, 44, 8904–8939 RSC.
- X.-F. Zhang and X. Yang, J. Phys. Chem. B, 2013, 117, 5533–5539 CrossRef CAS PubMed.
- W. Li, L. Li, H. Xiao, R. Qi, Y. Huang, Z. Xie, X. Jing and H. Zhang, RSC Adv., 2013, 3, 13417–13421 RSC.
- V. Lakshmi and M. Ravikanth, Dalton Trans., 2012, 41, 5903–5911 RSC.
- A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891–4932 CrossRef CAS PubMed.
- J. Zhao, W. Wu, J. Sun and S. Guo, Chem. Soc. Rev., 2013, 42, 5323–5351 RSC.
- A. Kamkaew, S. H. Lim, H. B. Lee, L. V. Kiew, L. Y. Chung and K. Burgess, Chem. Soc. Rev., 2013, 42, 77–88 RSC.
- G. M. Chu, I. Fernández, A. Guerrero-Martínez, C. Ramírez de Arellano and M. A. Sierra, Eur. J. Inorg. Chem., 2016, 2016, 844–852 CrossRef CAS.
- N. E. Aksakal, E. Tanrıverdi Eçik, H. H. Kazan, G. Yenilmez Çiftçi and F. Yuksel, Photochem. Photobiol. Sci., 2019, 18, 2012–2022 RSC.
- B. Bertrand, K. Passador, C. Goze, F. Denat, E. Bodio and M. Salmain, Coord. Chem. Rev., 2018, 358, 108–124 CrossRef CAS.
- J. M. Zimbron, K. Passador, B. Gatin-Fraudet, C.-M. Bachelet, D. Plażuk, L.-M. Chamoreau, C. Botuha, S. Thorimbert and M. Salmain, Organometallics, 2017, 36, 3435–3442 CrossRef CAS.
- M. Üçüncü, E. Karakuş, E. Kurulgan Demirci, M. Sayar, S. Dartar and M. Emrullahoğlu, Org. Lett., 2017, 19, 2522–2525 CrossRef PubMed.
- K. S. Choung, K. Marroquin and T. S. Teets, Chem. Sci., 2019, 10, 5124–5132 RSC.
- O. Halter, J. Spielmann, Y. Kanai and H. Plenio, Organometallics, 2019, 38, 2138–2149 CrossRef CAS.
- O. Halter, I. Fernández and H. Plenio, Chem.–Eur. J., 2017, 23, 711–719 CrossRef CAS PubMed.
- F. Geist, A. Jackel and R. F. Winter, Inorg. Chem., 2015, 54, 10946–10957 CrossRef CAS PubMed.
- B. W. Michel, A. R. Lippert and C. J. Chang, J. Am. Chem. Soc., 2012, 134, 15668–15671 CrossRef CAS PubMed.
- M. Navarro, S. Wang, H. Müller-Bunz, G. Redmond, P. Farràs and M. Albrecht, Organometallics, 2017, 36, 1469–1478 CrossRef CAS.
- J. D. Ng, S. P. Upadhyay, A. N. Marquard, K. M. Lupo, D. A. Hinton, N. A. Padilla, D. M. Bates and R. H. Goldsmith, J. Am. Chem. Soc., 2016, 138, 3876–3883 CrossRef CAS.
- P. Kos and H. Plenio, Chem.–Eur. J., 2015, 21, 1088–1095 CrossRef CAS.
- T. O. Nguyen, B. Y.-W. Man, R. Hodgson and B. A. Messerle, Aust. J. Chem., 2011, 64, 741–746 CrossRef CAS.
- L. D. Field, B. A. Messerle and S. L. Wren, Organometallics, 2003, 22, 4393–4395 CrossRef CAS.
- M. R. D. Gatus, R. T. McBurney, M. Bhadbhade and B. A. Messerle, Dalton Trans., 2017, 46, 7457–7466 RSC.
- J. H. H. Ho, R. Hodgson, J. Wagler and B. A. Messerle, Dalton Trans., 2010, 39, 4062–4069 RSC.
- L. D. Field, B. A. Messerle, M. Rehr, L. P. Soler and T. W. Hambley, Organometallics, 2003, 22, 2387–2395 CrossRef CAS.
- S. Guo, L. Ma, J. Zhao, B. Küçüköz, A. Karatay, M. Hayvali, H. G. Yaglioglu and A. Elmali, Chem. Sci., 2014, 5, 489–500 RSC.
- D. L. Reger, K. J. Brown, J. R. Gardinier and M. D. Smith, Organometallics, 2003, 22, 4973–4983 CrossRef CAS.
- C. M. Wong, K. Q. Vuong, M. R. D. Gatus, C. Hua, M. Bhadbhade and B. A. Messerle, Organometallics, 2012, 31, 7500–7510 CrossRef CAS.
- J. H. H. Ho, J. Wagler, A. C. Willis and B. A. Messerle, Dalton Trans., 2011, 40, 11031–11042 RSC.
- Z.-H. Pan, G.-G. Luo, J.-W. Zhou, J.-X. Xia, K. Fang and R.-B. Wu, Dalton Trans., 2014, 43, 8499–8507 RSC.
- S. L. Dabb, J. H. H. Ho, R. Hodgson, B. A. Messerle and J. Wagler, Dalton Trans., 2009, 634–642, 10.1039/B814168A.
- J. Sun, F. Zhong, X. Yi and J. Zhao, Inorg. Chem., 2013, 52, 6299–6310 CrossRef CAS.
- T. Slanina, P. Shrestha, E. Palao, D. Kand, J. A. Peterson, A. S. Dutton, N. Rubinstein, R. Weinstain, A. H. Winter and P. Klán, J. Am. Chem. Soc., 2017, 139, 15168–15175 CrossRef CAS.
- V. Leen, T. Leemans, N. Boens and W. Dehaen, Eur. J. Org. Chem., 2011, 2011, 4386–4396 CrossRef CAS.
- J. Chen, A. Burghart, A. Derecskei-Kovacs and K. Burgess, J. Org. Chem., 2000, 65, 2900–2906 CrossRef CAS PubMed.
- A. Burghart, H. Kim, M. B. Welch, L. H. Thoresen, J. Reibenspies, K. Burgess, F. Bergström and L. B. Å. Johansson, J. Org. Chem., 1999, 64, 7813–7819 CrossRef CAS.
- W. Wu, H. Guo, W. Wu, S. Ji and J. Zhao, J. Org. Chem., 2011, 76, 7056–7064 CrossRef CAS PubMed.
- Y. Dong, A. A. Sukhanov, J. Zhao, A. Elmali, X. Li, B. Dick, A. Karatay and V. K. Voronkova, J. Phys. Chem. C, 2019, 123, 22793–22811 CrossRef CAS.
- F. Barrière and W. E. Geiger, J. Am. Chem. Soc., 2006, 128, 3980–3989 CrossRef PubMed.
- M. Kollmannsberger, T. Gareis, S. Heinl, J. Daub and J. Breu, Angew. Chem., Int. Ed., 1997, 36, 1333–1335 CrossRef CAS.
- R. Y. Lai and A. J. Bard, J. Phys. Chem. B, 2003, 107, 5036–5042 CrossRef CAS.
- A. Minguzzi, O. Lugaresi, E. Achilli, C. Locatelli, A. Vertova, P. Ghigna and S. Rondinini, Chem. Sci., 2014, 5, 3591–3597 RSC.
- M. Liras, M. Iglesias and F. Sánchez, Macromolecules, 2016, 49, 1666–1673 CrossRef CAS.
- S. G. Awuah, J. Polreis, V. Biradar and Y. You, Org. Lett., 2011, 13, 3884–3887 CrossRef CAS PubMed.
- W. Hu, Y. Lin, X.-F. Zhang, M. Feng, S. Zhao and J. Zhang, Dyes Pigm., 2019, 164, 139–147 CrossRef CAS.
- T. Yogo, Y. Urano, Y. Ishitsuka, F. Maniwa and T. Nagano, J. Am. Chem. Soc., 2005, 127, 12162–12163 CrossRef CAS PubMed.
- L. Huang, J. Zhao, S. Guo, C. Zhang and J. Ma, J. Org. Chem., 2013, 78, 5627–5637 CrossRef CAS PubMed.
- H. A. Houck, F. E. Du Prez and C. Barner-Kowollik, Nat. Commun., 2017, 8, 1869 CrossRef PubMed.
- V. Blanco, D. A. Leigh and V. Marcos, Chem. Soc. Rev., 2015, 44, 5341–5370 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedures; characterisation data; crystallographic data; NMR spectra; details of the photophysical, singlet oxygen quantum yield, cyclic voltammetry and XAS measurements; catalytic procedures and control experiments. CCDC 1955141–1955144. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc02703k |
| ‡ Current address: School of Chemistry, The University of Sydney, Sydney, NSW, 2006, Australia. |
| This journal is © The Royal Society of Chemistry 2020 |