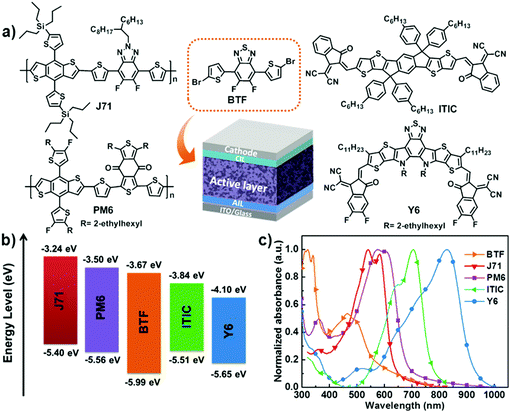
A minimal benzo[c][1,2,5]thiadiazole-based electron acceptor as a third component material for ternary polymer solar cells with efficiencies exceeding 16.0%†
Yunlong
Ma
a,
Xiaobo
Zhou
b,
Dongdong
Cai
a,
Qisheng
Tu
ac,
Wei
Ma
 b and
Qingdong
Zheng
b and
Qingdong
Zheng
 *a
*a
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, 155 Yangqiao West Road, Fuzhou, Fujian 350002, China. E-mail: qingdongzheng@fjirsm.ac.cn
bState Key Laboratory for Mechanical Behavior of Materials, Xi’an Jiaotong University, Xi’an, Shaanxi 710049, China
cUniversity of Chinese Academy of Sciences, 19 Yuquan Road, Beijing 100049, China
First published on 29th July 2019
Abstract
Ternary polymer solar cells (PSCs) with three active layer components (two donors with one acceptor, or two acceptors with one donor) have been demonstrated as an emerging strategy to improve the power conversion efficiencies (PCEs). So far, most of the third components utilized in the ternary PSCs are relatively complex molecules or polymers which are expensive due to complicated multi-step syntheses. Here, a simple small molecule of 4,7-bis(5-bromothiophen-2-yl)-5,6-difluorobenzo[c][1,2,5]thiadiazole (BTF), is first used as a second acceptor material to fabricate efficient ternary PSCs. The incorporation of BTF into a binary active layer based on a wide-bandgap polymer of J71 and a low-bandgap acceptor of ITIC, results in ternary PSCs with the best PCE of 12.35%, which is higher than the control J71:ITIC-based binary device with the best PCE of 10.79%. This significantly enhanced PCE results from the simultaneously improved short-circuit current density, open-circuit voltage, and fill factor in the ternary PSCs, and they are in part attributed to the increased light-harvesting with the BTF incorporation and in part attributed to the more balanced charge transportation induced by the improved polymer crystallinity. To determine the generality of BTF as a second acceptor in ternary PSCs, another benchmark binary active layer of PM6:Y6 has been incorporated with 10 wt% BTF, leading to a best-performance device with an outstanding PCE of 16.53% which is the highest among all ternary PSCs, to the best of our knowledge. Our work provides a simple strategy to efficiently boost the performance of ternary PSCs by using an easily available, low-cost acceptor of BTF.
New conceptsTernary polymer solar cells (PSCs) with three light-absorbing materials (components) are regarded as an emerging strategy to improve the power conversion efficiencies (PCEs). Up to now, the third components in the ternary PSCs are limited to some relatively complex molecules or copolymers which are expensive with complicated multi-step syntheses. Here, we show a minimal low-cost small molecule of 4,7-bis(5-bromothiophen-2-yl)-5,6-difluorobenzo[c][1,2,5]thiadiazole (BTF) which is introduced as an efficient acceptor in the binary blends of J71:ITIC and PM6:Y6 to achieve ternary PSCs with improved PCEs of 12.35% and 16.53%, respectively. With a strong absorption band of BTF in the range from 350 to 500 nm, incorporation of BTF can enhance light harvesting of the active layer and thus increase the short-circuit current. Furthermore, the introduction of highly crystalline BTF can help to improve the crystallinity, charge transport and exciton dissociation of the active layer thereby leading to enhancements of both the open-circuit voltage and fill factor. Our work not only offers an affordable strategy to efficiently boost the performance of ternary PSCs, but also provides a new concept that small molecules as simple as only four aromatic rings in the chemical structure can be an efficient third component material. |
Introduction
With the salient features of low manufacturing cost, light-weight, good flexibility and distinct colors, polymer solar cells (PSCs) have emerged as a promising technology to directly convert sunlight into electricity.1–6 In the past two decades, photovoltaic performances of PSCs have been greatly improved, thanks to the synergistic developments in active materials (both donor and acceptor materials), interfacial materials, and various morphology and device engineering techniques.7–17So far, the commonly investigated PSCs are based on binary bulk-heterojunction (BHJ) active layers which are formed by blending a donor material with an acceptor material.1 It has been known that the donor and acceptor materials in the BHJ active layer should have complementary absorption bands and matched energy levels in order to achieve efficient PSCs. However, the absorption bands of the active layers in most binary devices only roughly match the solar spectrum thereby leading to a limited photocurrent.18 Thus, ternary PSCs based on multiple materials with more complementary absorption profiles have been proposed to enhance the light-harvesting ability of the active layers.19–23 For example, when a small amount of fullerene acceptor (PC71BM) was added into a nonfullerene binary blend (PTB7-Th:COi8DFIC), the resulting ternary PSC showed significantly increased short-circuit current density (Jsc) from 23.84 mA cm−2 (binary) to 28.20 mA cm−2 (ternary).23 Besides the enhancement in photocurrent, open-circuit voltages (Vocs) of ternary PSCs can also be tuned by using third components with suitable energy levels.24,25 Furthermore, the third components may help to improve the charge carrier transport and reduce the recombination rates thereby enhancing the fill factors (FFs).26 In other words, the ternary-blend strategy can be used to increase the power conversion efficiencies (PCEs) of PSCs by simultaneously improving their Jsc, Voc, and FF values. To date, PCEs exceeding 14% have been reported by a number of research groups through the judicious selection of three components and the optimization of the blending ratio.27–30 For the studies at the early stage, the active layers of ternary PSCs are mainly obtained by blending two donors with one fullerene acceptor.20 Due to the availability of various high-performance non-fullerene acceptors in recent years, considerable efforts have been made on the development of non-fullerene-based ternary PSCs. So far, many non-fullerene acceptors have been used as third components to fabricate ternary PSCs, most of which showed better photovoltaic performance and stability as compared to the corresponding binary devices.31–37 For ternary PSCs, both small molecules and polymers can be used as a third component. For example, Wei and coworkers fabricated a high-performance ternary PSC with a PCE of 10.5% by incorporating a highly crystalline small molecule of p-DTS(FBTTH2)2 into the binary PTB7-Th:PC71BM blend. They reported that the addition of p-DTS(FBTTH2)2 not only enhanced the crystallinity of the blend film but also facilitated a face-on preferential orientation of PTB7-Th, all of which are beneficial for the improved device performance.20 By incorporating a strongly aggregating polymer of P1 into the binary blend of PBDB-T:IT-M, Nian et al. fabricated efficient ternary PSCs with PCEs as large as 13.52%.31 More recently, when a nonfullerene acceptor of IT-4F was used as the third component in ternary PSCs, a record high PCE of 16.27% was achieved by Zhang and co-workers.30 Nonetheless, most of the small molecules or polymers for the ternary PSCs have relatively complex molecular structures and they are relatively expensive with time-consuming multi-step syntheses. Therefore, from the viewpoint of commercial application, simple molecules with low cost would be better third component candidates for ternary PSCs.
Here, to our surprise, when a common low-cost small molecule of 4,7-bis(5-bromothiophen-2-yl)-5,6-difluorobenzo[c][1,2,5]thiadiazole (BTF) is incorporated as the third component in the traditional binary blend of J71:ITIC, the resulting ternary PSCs showed enhanced photovoltaic performance. The introduction of BTF (10 wt%) into the J71:ITIC blend not only enhanced the EQEs in the short-wavelength region but also balanced the hole and electron transportation thereby leading to enhanced FF and Jsc values. Furthermore, the Voc of the ternary device was also improved due to the higher-lying lowest unoccupied molecular orbital (LUMO) of BTF in comparison with that of ITIC. In going from binary to ternary devices, the PCE increased from 10.79% to 12.35%, Jsc increased from 17.22 to 18.48 mA cm−2, FF increased from 66.53% to 70.03%, and Voc increased from 0.941 to 0.952 V. In order to test the generality of BTF as the third component material, we fabricated other ternary PSCs by incorporating 10 wt% BTF into another benchmark binary active layer of PM6:Y6.11 In comparison with the binary devices showing a best PCE of 15.65%, the ternary PSCs based on PM6:Y6:BTF exhibited an increased PCE of 16.53% which is record-high among all ternary PSCs reported to date, to the best of our knowledge. It should be noted that BTF is a common monomer intermediate with only four aromatic rings in its chemical structure, and it is among the minimal electron acceptors for ternary PSCs so far.
Results and discussion
Fig. 1a shows the molecular structures of the materials utilized for the ternary PSCs in this work. The thermal behavior of BTF was investigated using differential scanning calorimetry (DSC) and the result is presented in Fig. S1 (ESI†). BTF features a distinct endothermic peak at 215.4 °C and an exothermic peak at 109.9 °C, indicating its good crystallinity. For this reason, BTF has good potential to be used as a morphology regulator to optimize the morphology of the binary active layer, which is helpful for enhancing the Jsc and FF values of the resulting devices. The highest occupied molecular orbital (HOMO) and LUMO levels are −5.40/−3.24 eV, −5.51/−3.84 eV, and −5.99/−3.67 eV for J71, ITIC, and BTF, respectively (Fig. 1b). Clearly, the charge transfer is energetically favorable in both binary systems of J71:ITIC and J71:BTF. The HOMO energy level of ITIC lies in between J71 and BTF, implying that cascade charge transfer cannot be formed in their ternary blend. In addition, due to the relatively higher-lying LUMO level of BTF than that of ITIC, a higher Voc is expected when BTF is used as the second acceptor material for the ternary devices.Normalized UV-vis absorption spectra for J71, ITIC, PM6, Y6 and BTF neat films are shown in Fig. 1c. The main absorption band of BTF is located in the wavelength range from 320 to 550 nm which is complementary with the absorption profile of binary J71:ITIC. The absorption spectra of J71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC
ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BTF blend films with various BTF weight ratios are shown in Fig. S2 (ESI†). All ternary blend films display broad absorption in the wavelength range from 300 to 800 nm. When the content of BTF in the J71
BTF blend films with various BTF weight ratios are shown in Fig. S2 (ESI†). All ternary blend films display broad absorption in the wavelength range from 300 to 800 nm. When the content of BTF in the J71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC (1
ITIC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) blend increases, the absorption intensities in the wavelength range of 300–500 nm are gradually enhanced. This implies that adding BTF as the third component could help to harvest more photons in the short wavelength region which would, therefore, enhance Jsc of the resulting devices.
1, w/w) blend increases, the absorption intensities in the wavelength range of 300–500 nm are gradually enhanced. This implies that adding BTF as the third component could help to harvest more photons in the short wavelength region which would, therefore, enhance Jsc of the resulting devices.
As shown in Fig. S3 (ESI†), the emission spectrum of the BTF film overlaps with the absorption spectra of both J71 and ITIC films indicating the possibility of Förster resonance energy transfer (FRET) from BTF to both J71 and ITIC.38,39 Both steady-state photoluminescence (PL) and time-resolved photoluminescence (TR-PL) studies were carried out to experimentally verify the existence of the FRET processes mentioned above. The PL spectra for individual components and binary blends in the thin-film state with 10 wt% BTF:J71 or 10 wt% BTF:ITIC are shown in Fig. S4 (ESI†). When excited at 450 nm, the 10 wt% BTF:J71 blend film showed a PL peak at a position similar to J71, but the PL intensity of the 10 wt% BTF:J71 blend was greater than that of the reference J71 film. Similarly, the 10 wt% BTF:ITIC blend film showed a higher PL intensity compared to the pure ITIC film, while the PL of BTF was completely quenched in the blend. These results reveal the dual FRET processes involved in the ternary blend system.
It has been shown that FRET provides an additional non-radiative decay route for the energy donor in the donor and acceptor FRET pair which will lead to a decrease in the excited-state lifetime of the energy donor.39 Fig. S4b (ESI†) shows TR-PL spectra of the pristine BTF, 10 wt% BTF:J71 and 10 wt% BTF:ITIC in a thin film. All the samples were pumped at 450 nm and probed at 600 nm, and their PL decay curves were fitted using a bi-exponential model. The average fluorescence lifetime of pristine BTF film was estimated to be 3.00 ns. However, the average fluorescence lifetime reduces to 1.13 ns in the binary blend of 10 wt% BTF:J71, and to 1.25 ns in the binary blend of 10 wt% BTF:ITIC. The TR-PL studies further confirm the hypothesis of dual FRET processes between J71 and BTF as well as between ITIC and BTF. The efficiency of FRET (E) can be calculated using the equation of E = 1 − (τblend/τD),38 where τblend and τD are the fluorescence lifetimes of the binary blend and pure donor films, respectively. Based on the fluorescence lifetime data shown in Fig. S4b (ESI†), the energy transfer efficiencies from BTF to J71 and ITIC were determined to be 62.33% and 58.33%, respectively.
To study the influence of BTF incorporation in the binary J71:ITIC system, both binary and ternary solar cells were fabricated using an inverted device structure of indium tin oxide (ITO)/ZnO/active layer/MoO3/Ag. The weight ratio of J71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC was fixed at 1
ITIC was fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, with BTF weight ratios varied from 0 wt% to 15 wt%. The active layers with a thickness of around 130 nm were obtained by spin-coating the chloroform solution blends, followed by thermal annealing at 110 °C for 3 min. It should be noted that other annealing temperatures were also tried during the device optimizations, and the related device parameters are summarized in Table S3 (ESI†). The current density–voltage (J–V) characteristics of the best-performance devices are shown in Fig. 2a and the detailed device parameters are summarized in Table 1.
1, with BTF weight ratios varied from 0 wt% to 15 wt%. The active layers with a thickness of around 130 nm were obtained by spin-coating the chloroform solution blends, followed by thermal annealing at 110 °C for 3 min. It should be noted that other annealing temperatures were also tried during the device optimizations, and the related device parameters are summarized in Table S3 (ESI†). The current density–voltage (J–V) characteristics of the best-performance devices are shown in Fig. 2a and the detailed device parameters are summarized in Table 1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC
ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BTF-based devices with different weight ratios of BTF
BTF-based devices with different weight ratios of BTF
| BTF (wt%) | V oc (V) | J sc (mA cm−2) | FF (%) | PCEb (%) |
|---|---|---|---|---|
| a J sc values in parentheses calculated from EQE curves. b In parentheses are average PCEs from 16 devices. | ||||
| 0 | 0.941 | 17.22 (16.65) | 66.53 | 10.79 (10.61 ± 0.23) |
| 5 | 0.946 | 17.91 (17.29) | 68.41 | 11.59 (11.36 ± 0.26) |
| 10 | 0.952 | 18.48 (17.86) | 70.03 | 12.35 (12.14 ± 0.22) |
| 15 | 0.960 | 18.25 (17.63) | 68.38 | 11.97 (11.48 ± 0.25) |
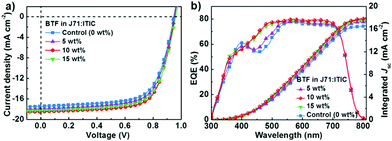
Similar to the literature reported results,40 the best-performance binary J71:ITIC-based device exhibited a PCE of 10.79% with a Voc of 0.941 V, a Jsc of 17.22 mA cm−2 and an FF of 66.53%. Incorporating 5 wt% BTF into the J71:ITIC blend boosted the PCE to 11.59% with a Voc of 0.946 V, a Jsc of 17.91 mA cm−2 and an FF of 68.41%. When the BTF content increased to 10 wt%, the best-performance ternary solar cells exhibited an average PCE of 12.14% and a maximum PCE of 12.35%, with a Voc of 0.952 V, a Jsc of 18.48 mA cm−2 and an FF of 70.03%. The simultaneous enhancements in Voc, Jsc and FF resulted in a 14% improvement in PCE compared to the best-performance J71:ITIC-based solar cell. As the BTF content in the ternary blends increased to 15%, the ternary devices showed a slightly reduced PCE of 11.97%, which is still higher than that of the control binary device. The decreased PCE for the 15% BTF-based ternary device could be related to a deteriorated charge transportation with too much BTF additive. It is interesting to observe that the Voc of the ternary devices gradually increased with increasing BTF content in the ternary blends. This behavior is related to the relatively higher-lying LUMO level of BTF. The improved Jsc values were verified by external quantum efficiency (EQE) measurements. As shown in Fig. 2b, the BTF-containing ternary devices exhibited an excellent photocurrent response over the whole visible absorption spectral range from 300 to 800 nm. When BTF was added in the binary system, EQE values in the range of 400-500 nm were clearly increased. According to the optical absorption spectra of the three materials, the enhancement of EQE in the short wavelength region is related to the enhanced optical absorption by BTF. The Jsc values calculated by integrating the EQE spectra were 16.65, 17.29, 17.86, and 17.63 mA cm−2 for the devices with 0 wt%, 5 wt%, 10 wt% and 15 wt% BTF, respectively. Obviously, the Jsc values integrated from the EQE curves were in good agreement with the Jsc values obtained from the J–V measurements, exhibiting small mismatches of less than 3.5%.
To determine the possibility of intermolecular charge transfer between BTF and J71 as well as between BTF and ITIC in ternary blends, PSCs using J71, ITIC, J71:BTF or ITIC:BTF as the active layers were fabricated. It should be noted that the devices based on the pristine BTF film could not work due to its poor film-forming ability. As shown in Fig. S5 (ESI†), Jsc values of solar cells based on J71 and ITIC neat films were 0.44 and 0.15 mA cm−2, respectively. The device based on the J71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BTF (1
BTF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) blend showed a Jsc of 0.66 mA cm−2, which is higher than that of the J71-based device, suggesting that charge transfer could occur between J71 and BTF. However, Jsc (0.07 mA cm−2) of the ITIC
1, w/w) blend showed a Jsc of 0.66 mA cm−2, which is higher than that of the J71-based device, suggesting that charge transfer could occur between J71 and BTF. However, Jsc (0.07 mA cm−2) of the ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BTF-based (1
BTF-based (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) device is lower than that of the ITIC-based device, revealing negligible charge transfer between ITIC and BTF. The results confirm that in the ternary blend of J71:ITIC:BTF, BTF plays the role of an electron acceptor instead of an electron donor.
1, w/w) device is lower than that of the ITIC-based device, revealing negligible charge transfer between ITIC and BTF. The results confirm that in the ternary blend of J71:ITIC:BTF, BTF plays the role of an electron acceptor instead of an electron donor.
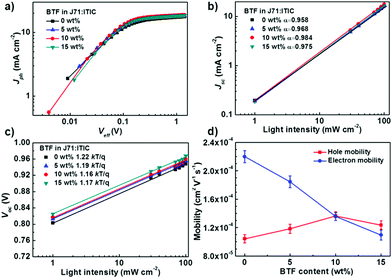
To investigate the effect of BTF content on the exciton dissociation probability and charge collection efficiency in the active layers, we plotted photocurrent density (Jph) versus the effective voltage (Veff) of the devices. The Jph and Veff can be defined by equations as the following:41Jph = JL − JD, Veff = Vo − Va, in which JL and JD represent the current densities under light illumination and in the dark, respectively. Va represents the applied bias and Vo represents the voltage at which JL = JD. As shown in Fig. 3a, Jph showed a strong dependence on the voltage at low Veff and reached saturation (Jsat) when Veff is larger than 1 V. The exciton dissociation and charge collection efficiencies of the devices can be assessed by the Jph/Jsat ratio under short-circuit conditions. The efficiencies were 96.4%, 97.2%, 98.6%, and 97.8% for the ternary blends with 0 wt%, 5 wt%, 10 wt%, and 15 wt% BTF, respectively. These results indicate that the ternary PSCs with an optimal BTF content (10 wt%) facilitated a higher exciton dissociation rate, thus giving rise to larger Jsc and FF values.
After the dissociation of a photogenerated exciton, the separated free carriers suffer from recombination if they cannot be swept-out immediately by the internal electric field.42 The recombination losses can be estimated by measuring the dependence of Jsc and Voc on the light intensity.43,44 Generally, Jsc shows a power-law connection with light intensity (I), which can be written as Jsc ∝ Iα, in which α is the power-law exponent referring to the degree of bimolecular recombination. If bimolecular recombination is completely suppressed in a device, the Jsc would linearly depend on the light intensity with an α value equal to 1. As shown in Fig. 3b, the α values increased gradually from 0.958 to 0.984 and then decreased to 0.975 with the increasing BTF content, indicating the reduced bimolecular recombination enabled by the ternary strategy. The ternary device with the highest α value suggested that the bimolecular recombination was effectively suppressed after adding 10 wt% BTF, which was consistent with its higher Jsc and FF values.
To further identify whether trap-assisted or bimolecular recombination is the dominant mechanism, we measured the Voc of devices as a function of light intensity (I).45 The recombination mechanism can be evaluated according to the equation of Voc ∝ (nkT/q)ln(I), where q, T, k, and n represent the elementary charge, the absolute temperature, Boltzmann constant, and the recombination factor, respectively. When the n value is close to 1, the bimolecular recombination is the primary mechanism, while the n value should be close to 2 when the trap-induced recombination dominated in the active layers.46 The fitting results for the binary and ternary devices are shown in Fig. 3c. The slopes of Vocversus the natural logarithm of light intensity were 1.22, 1.19, 1.16, and 1.17 kT/q for the devices with 0 wt%, 5 wt%, 10 wt%, and 15 wt% BTF, respectively, suggesting that bimolecular recombination should be the dominant mechanism for all the devices at the open-circuit condition. In addition, smaller slopes were observed for the devices with 10 wt% BTF compared to the control device, indicating that the trap density and trap-assisted recombination could be restrained after the addition of BTF into the ternary blends.
To investigate the effect of the third component BTF on the charge transfer properties, hole (μh) and electron (μe) mobilities of the binary and ternary blends were measured by using the space charge limited current (SCLC) model. The electron- and hole-only diodes were fabricated with the structures of ITO/ZnO (30 nm)/active layer (130 nm)/Ca (10 nm)/Al (100 nm) and ITO/PEDOT:PSS (40 nm)/active layer (130 nm)/Au (50 nm), respectively. The J–V plots of the devices with different BTF contents under dark condition are shown in Fig. S6 (ESI†), and the corresponding data are summarized in Table S1 (ESI†). The addition of BTF is detrimental to the electron transportation, and the mobilities dropped from 2.20 × 10−4 to 1.18 × 10−4 cm2 V−1 s−1 as the BTF content increased from 0 to 15 wt% in the ternary blends. This might be due to the fact that the favorable backbone orientation of ITIC for vertical electron transport is disrupted by the addition of BTF, which can be confirmed by the GIWAXS results in the coming paragraph. On the other hand, the hole-mobilities increased from 1.10 × 10−4 to 1.41 × 10−4 cm2 V−1 s−1 and then decreased to 1.26 × 10−4 cm2 V−1 s−1 with increasing BTF ratio. The unbalanced charge mobilities of the binary blend device resulted from its relatively poor morphology leads to a low FF value. The ternary blend with 10 wt% BTF has the most balanced hole (1.41 × 10−4 cm2 V−1 s−1) and electron-mobilities (1.39 × 10−4 cm2 V−1 s−1) with a μh/μe ratio of 1.01, which well correlates with the best FF value of 70.03% for the corresponding solar cell. The efficient exciton dissociation, reduced recombination, and balanced charge transport synergistically contribute to the higher Jsc and FF values for the ternary devices compared to those for the binary counterparts.
Considering the correlation between the morphology of the active layer and the device performance, morphological characteristics of the binary and ternary blends were studied in detail by tapping-mode atomic force microscopy (AFM), transmission electron microscopy (TEM) and grazing incidence wide-angle X-ray scattering (GIWAXS).47 All the blend films were prepared in an identical fashion to those for the best-performance devices. Fig. S7a–d (ESI†) shows the AFM topography images for ternary blend films with 0 wt%, 5 wt%, 10 wt%, and 15 wt% of BTF. All the blend films exhibited a smooth surface with relatively small root-mean-square roughness (RMS) values ranging from 0.60 to 0.75 nm, implying excellent miscibility between the BTF and J71:ITIC host system. Interestingly, by introducing BTF into the J71:ITIC binary blend, slightly increased RMS values of 0.66, 0.70, and 0.75 nm were found for 5 wt%, 10 wt%, and 15 wt% BTF-containing ternary blends, suggesting the improved crystallinity induced by BTF incorporation. As shown in the AFM phase images (Fig. S7e–h, ESI†), the incorporation of BTF enlarged the domain sizes and induced more obvious phase separation in the ternary blends as compared to the binary blend without BTF. In particular, the phase separation feature is the most clear and well-distributed for the ternary blend with 10 wt% BTF. Such phase separation behaviors are further confirmed by TEM images. As shown in Fig. S7i–l (ESI†), the ternary blends with BTF, especially for that with 10 wt% BTF, revealed more distinct and denser interpenetrating nanofibrillar networks compared to the control binary blend. The morphological results are generally consistent with the trend of device performance variation as summarized in Table 1. Therefore, BTF can be used as a morphology regulator to optimize the morphology of the binary active layers.
In order to study the BTF effect on the molecular orientation and packing behavior, GIWAXS results of the blend films with various BTF contents are shown in Fig. 4 and Table S2 (ESI†). Ternary blend films with different BTF contents exhibited similar diffraction characters. As shown in Fig. 4a–d, in all cases, an obvious (010) diffraction corresponding to the π–π stacking appeared in the out-of-plane direction and a (100) diffraction corresponding to the lamellar stacking appeared in the in-plane direction, indicating that all blend films prefer a face-on molecular orientation with respect to the substrate. According to the previous reports, the (010) diffraction peaks located at qxy ≈ 1.5 and 1.7 Å in the binary film correspond to the π–π stacking of ITIC and J71, respectively.40,48 As shown in Fig. 4e, the π–π stacking diffraction feature of ITIC in the blend gradually disappeared upon adding incremental amounts of BTF, which suggests that BTF could suppress the π–π molecular packing of ITIC. In contrast, as the BTF content increased from 0 wt% to 15 wt% in the ternary blends, the π–π stacking peak of J71 steadily enhanced, and the corresponding coherence lengths increased from 20.58 to 21.80 Å, indicating the increased crystallinity. On the other hand, we found that the π–π stacking distance decreased first from 3.68 to 3.62 Å with 10 wt% BTF loading, and then increased slightly to 3.63 Å with 15 wt% BTF loading. Note that in all cases, the π–π stacking distances in the blend films with BTF were smaller than that in the control binary film. The proper crystallinity together with tight π–π stacking in the ternary blend with 10 wt% BTF could effectively improve charge transport and prevent the charge carrier recombination, eventually enhancing the photovoltaic performance.
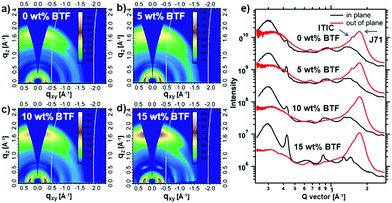 | ||
| Fig. 4 (a–d) 2D GIWAXS patterns and (e) the in-plane and out-of-plane line-cut curves of binary and ternary blend films. | ||
The greatly enhanced performance for the J71:ITIC:BTF-based ternary device in comparison with the control binary device, motivates us to learn the generality of BTF as the third component for other ternary PSCs. Consequently, the binary active layer of PM6:Y6 was used as another example considering the impressive PCE of 15.70% for the binary device as disclosed by Zou et al.11 The molecular structures and absorption spectra of PM6 and Y6 have been depicted in Fig. 1. Similar to the case of J71:ITIC, the incorporation of BTF can also enhance the light-harvesting ability of the blend films in the spectral range from 300 to 500 nm (Fig. S2, ESI†). PM6:Y6:BTF-based ternary devices were fabricated using a conventional device structure of ITO/poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS)/active layer/PDIN/Ag. PDIN is 2,9-bis(3-(dimethylamino)propyl)anthrax[2,1,9-def:6,5,10-d′e′f′]diisoquinoline-1,3,8,10(2H,9H)tetraone which was used as the cathode interlayer to facilitate the electron extraction.49 The J–V curves of the best-performance binary and ternary devices are shown in Fig. 5a and the detailed photovoltaic parameters are summarized in Table 2. The photovoltaic parameters of ternary devices based on PM6:Y6:BTF with different annealing temperatures are listed in Table S4 (ESI†). In this work, the best-performance PM6:Y6-based binary device (annealed at 85 °C) exhibited a Voc of 0.845 V, a Jsc of 25.80 mA cm−2 and an FF of 71.77%, and a final PCE of 15.65% which is comparable to the previously reported PCE of 15.70%.11 In contrast, the best-performance ternary PSC based on PM6:Y6:BTF showed an increased PCE of 16.53% with the simultaneously increased Voc of 0.853 V, Jsc of 26.11 mA cm−2 and FF of 74.22%. The results are consistent with those observed in the J71:ITIC system suggesting the generality of BTF as the third component for ternary PSCs. Fig. 5b depicts the EQE spectra of the best-performance binary and ternary devices based on PM6:Y6 and PM6:Y6:BTF, respectively. The best-performance BTF-containing ternary device exhibited slightly higher EQEs compared to its binary counterpart, especially in the spectral region where BTF has strong absorption. The integrated Jsc values from the EQE spectra are 25.19 and 25.49 mA cm−2, respectively, within 2.4% mismatches with those obtained from the J–V measurements. The results indicate that BTF may also be used as a third component material to enhance the light harvesting and to optimize the film morphology of other binary active layers thereby leading to a great number of ternary PSCs with improved PCEs.
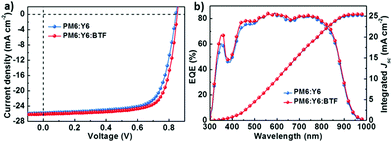 | ||
| Fig. 5 (a) J–V curves for the binary and ternary devices; (b) EQE curves and integrated Jsc for the binary and ternary devices. | ||
Conclusions
In summary, we have shown that a simple benzo[c][1,2,5]thiadiazole-based small molecule of BTF can be incorporated into the binary J71:ITIC blend as an electron acceptor to achieve ternary PSCs with improved performance. The incorporation of 10 wt% BTF in the J71:ITIC blend can increase the light harvesting of the active layer in the wavelength region from 300 to 500 nm which results in enhanced EQEs for the ternary PSCs in the corresponding wavelengths. Moreover, owing to the higher-lying LUMO energy level of BTF than that of ITIC, the Vocs of ternary devices based on J71:ITIC:BTF are enhanced compared to their binary counterpart. Detailed morphological studies illustrate that BTF can also function as a processing additive to enhance the crystallinity of J71 and reduce the π–π stacking distance. As a result, a higher hole-mobility together with a more balanced charge transport was observed for the ternary blend with 10 wt% BTF. The above beneficial effects induced by the introduction of 10 wt% BTF into the binary J71:ITIC synergistically yielded ternary devices with a champion PCE of 12.35% which is higher than that for the J71:ITIC-based binary devices (10.79%). The generality of BTF as a third component for ternary devices with improved performance has been successfully demonstrated by using another benchmark active layer system of PM6:Y6. The best-performance ternary PSCs based on PM6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y6
Y6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BTF (1
BTF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) displayed an outstanding PCE of 16.53%, representing the best value for all ternary PSCs reported in the literature. With the simple fabrication process of ternary PSCs, and the low cost and multiple beneficial functions of the BTF acceptor, this strategy can be easily applied for future industrial production of PSCs.
0.1) displayed an outstanding PCE of 16.53%, representing the best value for all ternary PSCs reported in the literature. With the simple fabrication process of ternary PSCs, and the low cost and multiple beneficial functions of the BTF acceptor, this strategy can be easily applied for future industrial production of PSCs.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. U1605241, 51703226, 21704082, 51561165011, 21875182), the Strategic Priority Research Program of CAS (No. XDB20030300), the Key Research Program of Frontier Sciences, Chinese Academy of Sciences (No. QYZDB-SSW-SLH032), and the Natural Science Foundation of Fujian Province, China (No. 2019J01124). We thank Prof. He Yan from the Hong Kong University of Science and Technology for the helpful discussions.Notes and references
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789–1791 CrossRef CAS.
- Y. J. Cheng, S. H. Yang and C. S. Hsu, Chem. Rev., 2009, 109, 5868–5923 CrossRef CAS PubMed.
- G. Li, R. Zhu and Y. Yang, Nat. Photonics, 2012, 6, 153–161 CrossRef CAS.
- L. Lu, T. Zheng, Q. Wu, A. M. Schneider, D. Zhao and L. Yu, Chem. Rev., 2015, 115, 12666–12731 CrossRef CAS.
- J. Tong, S. Xiong, Y. Zhou, L. Mao, X. Min, Z. Li, F. Jiang, W. Meng, F. Qin, T. Liu, R. Ge, C. Hernandez, B. Kippelen and Y. Zhou, Mater. Horiz., 2016, 3, 452–459 RSC.
- H. Fu, Z. Wang and Y. Sun, Angew. Chem., Int. Ed., 2019, 58, 4442–4453 CrossRef CAS.
- S. Zhang, Y. Qin, J. Zhu and J. Hou, Adv. Mater., 2018, 30, 1800868 CrossRef.
- L. Meng, Y. Zhang, X. Wan, C. Li, X. Zhang, Y. Wang, X. Ke, Z. Xiao, L. Ding, R. Xia, H.-L. Yip, Y. Cao and Y. Chen, Science, 2018, 361, 1094–1098 CrossRef CAS.
- F. Zhao, S. Dai, Y. Wu, Q. Zhang, J. Wang, L. Jiang, Q. Ling, Z. Wei, W. Ma, W. You, C. Wang and X. Zhan, Adv. Mater., 2017, 29, 1700144 CrossRef PubMed.
- B. Fan, X. Du, F. Liu, W. Zhong, L. Ying, R. Xie, X. Tang, K. An, J. Xin, N. Li, W. Ma, C. J. Brabec, F. Huang and Y. Cao, Nat. Energy, 2018, 3, 1051–1058 CrossRef CAS.
- J. Yuan, Y. Zhang, L. Zhou, G. Zhang, H.-L. Yip, T.-K. Lau, X. Lu, C. Zhu, H. Peng, P. A. Johnson, M. Leclerc, Y. Cao, J. Ulanski, Y. Li and Y. Zou, Joule, 2019, 3, 1140–1151 CrossRef CAS.
- C. Sun, F. Pan, H. Bin, J. Zhang, L. Xue, B. Qiu, Z. Wei, Z.-G. Zhang and Y. Li, Nat. Commun., 2018, 9, 743 CrossRef PubMed.
- J. Zhao, Y. Li, G. Yang, K. Jiang, H. Lin, H. Ade, W. Ma and H. Yan, Nat. Energy, 2016, 1, 15027 CrossRef CAS.
- Y. Liu, Z. Zhang, S. Feng, M. Li, L. Wu, R. Hou, X. Xu, X. Chen and Z. Bo, J. Am. Chem. Soc., 2017, 139, 3356–3359 CrossRef CAS.
- Y. Cui, H. Yao, J. Zhang, T. Zhang, Y. Wang, L. Hong, K. Xian, B. Xu, S. Zhang, J. Peng, Z. Wei, F. Gao and J. Hou, Nat. Commun., 2019, 10, 2515 CrossRef.
- X. Xu, K. Feng, Z. Bi, W. Ma, G. Zhang and Q. Peng, Adv. Mater., 2019, 31, 1901872 CrossRef.
- B. Fan, D. Zhang, M. Li, W. Zhong, Z. Zeng, L. Ying, F. Huang and Y. Cao, Sci. China: Chem., 2019, 62, 746 CrossRef CAS.
- Y. Yang, W. Chen, L. Dou, W. H. Chang, H. S. Duan, B. Bob and G. Li, Nat. Photonics, 2015, 9, 190–198 CrossRef CAS.
- P. Cheng and X. Zhan, Mater. Horiz., 2015, 2, 462–485 RSC.
- J. Zhang, Y. Zhang, J. Fang, K. Lu, Z. Wang, W. Ma and Z. Wei, J. Am. Chem. Soc., 2015, 137, 8176–8183 CrossRef CAS.
- W. Xu and F. Gao, Mater. Horiz., 2018, 5, 206–221 RSC.
- L. Zhan, S. Li, H. Zhang, F. Gao, T.-K. Lau, X. Lu, D. Sun, P. Wang, M. Shi, C.-Z. Li and H. Chen, Adv. Sci., 2018, 5, 1800755 CrossRef.
- Z. Xiao, X. Jia and L. Ding, Sci. Bull., 2017, 62, 1562–1564 CrossRef CAS.
- R. A. Street, D. Davies, P. P. Khlyabich, B. Burkhart and B. C. Thompson, J. Am. Chem. Soc., 2013, 135, 986–989 CrossRef CAS PubMed.
- S. A. Mollinger, K. Vandewal and A. Salleo, Adv. Energy Mater., 2015, 5, 1501335 CrossRef.
- N. Gasparini, X. Jiao, T. Heumueller, D. Baran, G. J. Matt, S. Fladischer, E. Spiecker, H. Ade, C. J. Brabec and T. Ameri, Nat. Energy, 2016, 1, 16118 CrossRef CAS.
- Q. An, J. Wang and F. Zhang, Nano Energy, 2019, 60, 768–774 CrossRef CAS.
- R. Geng, X. Song, H. Feng, J. Yu, M. Zhang, N. Gasparini, Z. Zhang, F. Liu, D. Baran and W. Tang, ACS Energy Lett., 2019, 4, 763–770 CrossRef CAS.
- X. Ma, M. Luo, W. Gao, J. Yuan, Q. An, M. Zhang, Z. Hu, J. Gao, J. Wang, Y. Zou, C. Yang and F. Zhang, J. Mater. Chem. A, 2019, 7, 7843–7851 RSC.
- Q. An, X. Ma, J. Gao and F. Zhang, Sci. Bull., 2019, 64, 504 CrossRef CAS.
- L. Nian, Y. Kan, H. Wang, K. Gao, B. Xu, Q. Rong, R. Wang, J. Wang, F. Liu, J. Chen, G. Zhou, T. P. Russell and A. K. Y. Jen, Energy Environ. Sci., 2018, 11, 3392–3399 RSC.
- L. Xiao, B. He, Q. Hu, L. Maserati, Y. Zhao, B. Yang, M. A. Kolaczkowski, C. L. Anderson, N. J. Borys, L. M. Klivansky, T. L. Chen, A. M. Schwartzberg, T. P. Russell, Y. Cao, X. Peng and Y. Liu, Joule, 2018, 2, 2154–2166 CrossRef CAS.
- T. Kumari, S. M. Lee, S.-H. Kang, S. Chen and C. Yang, Energy Environ. Sci., 2017, 10, 258–265 RSC.
- X. Ma, W. Gao, J. Yu, Q. An, M. Zhang, Z. Hu, J. Wang, W. Tang, C. Yang and F. Zhang, Energy Environ. Sci., 2018, 11, 2134–2141 RSC.
- Z. Zhou, S. Xu, J. Song, Y. Jin, Q. Yue, Y. Qian, F. Liu, F. Zhang and X. Zhu, Nat. Energy, 2018, 3, 952–959 CrossRef CAS.
- W. Jiang, R. Yu, Z. Liu, R. Peng, D. Mi, L. Hong, Q. Wei, J. Hou, Y. Kuang and Z. Ge, Adv. Mater., 2018, 30, 1703005 CrossRef.
- D. Baran, R. S. Ashraf, D. A. Hanifi, M. Abdelsamie, N. Gasparini, J. A. Röhr, S. Holliday, A. Wadsworth, S. Lockett, M. Neophytou, C. J. M. Emmott, J. Nelson, C. J. Brabec, A. Amassian, A. Salleo, T. Kirchartz, J. R. Durrant and I. McCulloch, Nat. Mater., 2016, 16, 363–369 CrossRef PubMed.
- J.-S. Huang, T. Goh, X. Li, M. Y. Sfeir, E. A. Bielinski, S. Tomasulo, M. L. Lee, N. Hazari and A. D. Taylor, Nat. Photonics, 2013, 7, 479–485 CrossRef CAS.
- V. Gupta, V. Bharti, M. Kumar, S. Chand and A. J. Heeger, Adv. Mater., 2015, 27, 4398–4404 CrossRef CAS PubMed.
- H. Bin, L. Gao, Z.-G. Zhang, Y. Yang, Y. Zhang, C. Zhang, S. Chen, L. Xue, C. Yang, M. Xiao and Y. Li, Nat. Commun., 2016, 7, 13651 CrossRef CAS.
- V. D. Mihailetchi, L. J. A. Koster, J. C. Hummelen and P. W. M. Blom, Phys. Rev. Lett., 2004, 93, 216601 CrossRef CAS PubMed.
- L. G. Kaake, J. J. Jasieniak, R. C. Bakus, G. C. Welch, D. Moses, G. C. Bazan and A. J. Heeger, J. Am. Chem. Soc., 2012, 134, 19828–19838 CrossRef CAS.
- Z. He, C. Zhong, X. Huang, W.-Y. Wong, H. Wu, L. Chen, S. Su and Y. Cao, Adv. Mater., 2011, 23, 4636–4643 CrossRef CAS PubMed.
- A. K. K. Kyaw, D. H. Wang, V. Gupta, W. L. Leong, L. Ke, G. C. Bazan and A. J. Heeger, ACS Nano, 2013, 7, 4569–4577 CrossRef CAS.
- L. J. A. Koster, V. D. Mihailetchi, R. Ramaker and P. W. M. Blom, Appl. Phys. Lett., 2005, 86, 123509 CrossRef.
- R. A. Street, S. Cowan and A. J. Heeger, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 121301 CrossRef.
- A. Hexemer, W. Bras, J. Glossinger, E. Schaible, E. Gann, R. Kirian, A. MacDowell, M. Church, B. Rude and H. Padmore, J. Phys.: Conf. Ser., 2010, 247, 012007 CrossRef.
- G. Ding, J. Yuan, F. Jin, Y. Zhang, L. Han, X. Ling, H. Zhao and W. Ma, Nano Energy, 2017, 36, 356–365 CrossRef CAS.
- Y. Ma, S.-C. Chen, Z. Wang, W. Ma, J. Wang, Z. Yin, C. Tang, D. Cai and Q. Zheng, Nano Energy, 2017, 33, 313–324 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, DSC thermogram, cyclic voltammogram, absorption and photoluminescence spectra, SCLC and GIWAXS results, and AFM and TEM images. See DOI: 10.1039/c9mh00993k |
| This journal is © The Royal Society of Chemistry 2020 |