Open Access Article
Open Access ArticleA natural solution to photoprotection and isolation of the potent polyene antibiotic, marinomycin A†
Christopher S.
Bailey
a,
Joseph S.
Zarins-Tutt‡
a,
Matthias
Agbo‡§
a,
Hong
Gao‡¶
a,
Alberto
Diego-Taboada
bc,
Maoluo
Gan‡||
 a,
Refaat B.
Hamed‡**
a,
Refaat B.
Hamed‡**
 a,
Emily R.
Abraham
a,
Grahame
Mackenzie
*bc,
P. Andrew
Evans
a,
Emily R.
Abraham
a,
Grahame
Mackenzie
*bc,
P. Andrew
Evans
 *d and
Rebecca J. M.
Goss
*d and
Rebecca J. M.
Goss
 *a
*a
aDepartment of Chemistry, BSRC, University of St Andrews, St Andrews, KY16 9ST, UK. E-mail: rjmg@st-andrews.ac.uk
bDepartment of Chemistry & Biochemistry, University of Hull, HU6 7RX, UK. E-mail: G.Mackenzie@hull.ac.uk
cSporomex Ltd., Medina House, 2 Station Avenue, East Yorkshire, Bridlington, YO16 4LZ, UK
dDepartment of Chemistry, Queen's University, 90 Bader Lane, Kingston, ON K7L 3N6, Canada. E-mail: andrew.evans@chem.queensu.ca
First published on 21st May 2019
Abstract
The photoprotection and isolation of marinomycin A using sporopollenin exine capsules (SpECs) derived from the spores of the plant Lycopodium clavatum is described. The marinomycins have a particularly short half-life in natural light, which severely impacts their potential biological utility given that they display potent antibiotic and anticancer activity. The SpEC encapsulation of the marinomycin A dramatically increases the half-life of the polyene macrodiolide to the direct exposure to UV radiation by several orders of magnitude, thereby making this a potentially useful strategy for other light sensitive bioactive agents. In addition, we report that the SpECs can also be used to selectively extract culture broths that contain the marinomycins, which provides a significantly higher recovery than with conventional XAD resins and provides concomitant photoprotection.
Introduction
Bioactive polyenes are important secondary metabolites that have significant potential for medicinal applications, as exemplified by the antifungal agents, nystatin, natamycin, amphotericin B and candicidin.1a Despite the promising bioactivity of many such agents, the associated photo-instability of the polyene motif adversely impacts their stability,1b and thus hampers their development as drug candidates. The marinomycins A–C (1–3, Scheme 1), which were isolated by Fenical and co-workers from Marinispora CNQ-140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 have attracted considerable synthetic attention3 owing to their potent bioactivity and novel structure. Nevertheless, their demonstrated photo-instability (Scheme 1A) presents a significant challenge, which needs addressing in order to enable the development of these compounds as potential medicinal agents.2 For instance, marinomycin A (1) has an MIC90 of 130 nM against both methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VREF) (both listed “high priority” within the WHO's top 12 bacterial pathogens). Marinomycin A (1) also exhibits anticancer activity against the NCI's 60 cell line panel (LC50 = 0.4–2.7 μM), showing good selectivity against several melanoma cell lines, with particular potency against the SK-MEL-5 melanoma cell line (LC50 of 5.0 nM).3 In contrast to the polyene antibiotics nystatin and amphotericin B,1 the marinomycins show no significant antifungal activity, which implies that they do not act through membrane disruption and that they potentially have a novel mechanism of action.4 The paucity of new classes of antibiotics, coupled to marinomycin A's potency against antibiotic-resistant strains makes it an important lead and thereby provides the impetus to circumvent the problems associated with light sensitivity. Herein, we describe the photoprotection and extraction of marinomycin A (1) using sporopollenin exine capsules (SpECs) derived from the spores of the plant Lycopodium clavatum. (Scheme 1B).
2 have attracted considerable synthetic attention3 owing to their potent bioactivity and novel structure. Nevertheless, their demonstrated photo-instability (Scheme 1A) presents a significant challenge, which needs addressing in order to enable the development of these compounds as potential medicinal agents.2 For instance, marinomycin A (1) has an MIC90 of 130 nM against both methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VREF) (both listed “high priority” within the WHO's top 12 bacterial pathogens). Marinomycin A (1) also exhibits anticancer activity against the NCI's 60 cell line panel (LC50 = 0.4–2.7 μM), showing good selectivity against several melanoma cell lines, with particular potency against the SK-MEL-5 melanoma cell line (LC50 of 5.0 nM).3 In contrast to the polyene antibiotics nystatin and amphotericin B,1 the marinomycins show no significant antifungal activity, which implies that they do not act through membrane disruption and that they potentially have a novel mechanism of action.4 The paucity of new classes of antibiotics, coupled to marinomycin A's potency against antibiotic-resistant strains makes it an important lead and thereby provides the impetus to circumvent the problems associated with light sensitivity. Herein, we describe the photoprotection and extraction of marinomycin A (1) using sporopollenin exine capsules (SpECs) derived from the spores of the plant Lycopodium clavatum. (Scheme 1B).
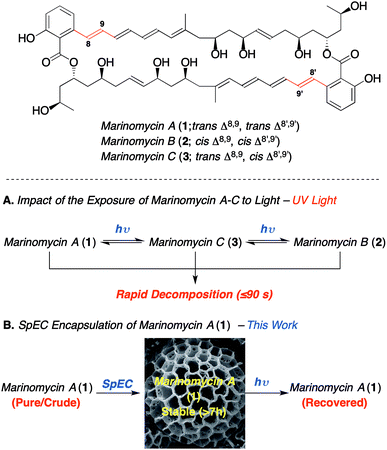 | ||
| Scheme 1 The impact of the photoisomerization and photoprotection on marinomycin A (1).2 | ||
We envisioned that the natural polymer microcapsules, sporopollenin exine capsules (SpECs), could provide a solution to the photoprotection of polyenes and in particular, for marinomycin A (1). Naturally occurring spores consist of an inner layer (intine) and the outer layer (exine) that are made largely of cellulose and a polymer called sporopollenin, respectively (Fig. 1), which have evolved to protect the genetic material of the plant from external damage.4 The genetic content of the spore can be easily removed through the porous exine wall to provide an empty shell, which provides an allergen free non-toxic shell able to uptake a variety of liquids as well as drugs from solution. Since the role of the spore is to protect the plant's genetic material, the exterior layer has a number of important properties that protect it from external factors, such as oxidation, light and physical stress.5 The exine consists of a highly crosslinked polymer of conjugated phenols and polyunsaturated fatty acids, which can block up to 80% UV light.6 Hence, the exines protect the natural content in two ways; namely, as an antioxidant and by minimizing the absorption of ultraviolet light. We envisioned exploring whether the latter property might be harnessed to photo-protect the complex macrodiolide marinomycin A (1), which is known to be extremely sensitive to light.
The spores are porous shells, with multidirectional, nano-diameter-sized, channels that facilitate the transport of water, nutrients and other agents.7 Moreover, the hollow microcapsules are able to encapsulate and release compounds in a controlled manner,8 making them an ideal medium to store and deliver bioactive materials.9 Another critical feature of the shells is their highly uniform size and morphology for a specific plant species, which permits tailoring to encapsulate different molecules. These properties have raised interest in the utilisation of pollen exines (SpECs) for drug delivery, both through inhalation and oral administration.9 For instance, SpECs have been shown to enhance the bioavailability of eicosapentaenoic acid (EPA) via the oral route8a and protect commercial cod liver oil and omega oils from rancidification through oxidation and exposure to UV light.6 In a related study, the SpECs from the plant L. clavatum were used to encapsulated fish oils to mask the taste of the fish oil10 and were utilized in a clinical trial as an oral delivery agent,9a which demonstrated potential for the topical delivery of drug molecules.5c,11 Overall, these microcapsules are generally thought to be beneficial for a variety of applications and in particular provide a safe method for drug-delivery.
Results and discussion
Preliminary studies focused on determining the feasibility of the SpECs extracted from the spores of L. clavatum (Fig. 2; see ESI, Section 4.1.3†) for the photoprotection of the bioactive polyene macrodiolide, marinomycin A (1). To this end, we established a baseline for comparison, by examining the rate of decomposition of the marinomycin A through the direct irradiation of 1 mL of a 10 μM aqueous solution of marinomycin A capped in a 1.5 mL polypropylene microcentrifuge tube, which was directly exposed to a flat UV transilluminator and irradiated with UV light with a wavelength of 312 nm that was generated by 6 × 8 watt UV bulbs placed behind a UV filter. Aliquots (200 μL) were taken from 3 separate microcentrifuge tubes at each time point, diluted with an equal volume of methanol and analysed by UPLC measuring UV absorbance at 360 nm. A predetermined standard curve (see ESI, Section 4.1.1†) was used to determine the concentration of marinomycin A from the UV absorbance. Interestingly, this study indicated that the half-life for marinomycin A photoisomerization was approximately 8 seconds (±1 s) with UV light. Using this as a base line, we set about testing the photoprotection conferred by the SpECs from L. clavatum AT1862. In a dark environment, the SpECs were suspended in a 1 μM aqueous solution of marinomycin A for 30 minutes, centrifuged and the supernatant was discarded and the SpECs were washed with distilled water. The washed and pelleted SpECs were then re-suspended, in their original polypropylene microcentrifuge tubes, with water and capped. The aqueous suspension is needed in this experiment to enable accurate handling of the very small quantities of SpECs that are utilized, and it also maintained consistency with the solvated, unprotected control experiment. The tubes were laid on their side on a flat UV transilluminator, as described above, and irradiated. Sampling was carried out by removing 3 microcentrifuge tubes from UV exposure at each time point. Samples, at the various time points were examined by centrifuging the SpECs to remove them from suspension, removing the aqueous phase, and then eluting marinomycin A from the SpECs by vortexing with DMSO. The DMSO extract was diluted with an equivolume of methanol and analysed by UPLC to evaluate any protective effect of the SpECs on marinomycin A. The half-life for sporopollenin encapsulated marinomycin A (1) in solution was approximately 34 minutes (±7 min) (see ESI Fig. 4.2B†), representing a very dramatic >250-fold improvement over that of the material in solution.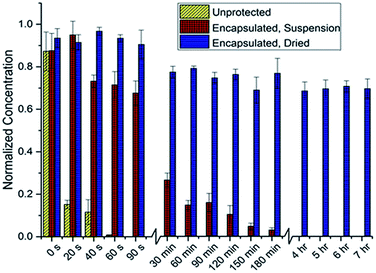 | ||
| Fig. 2 Marinomycin A degradation under direct UV radiation, as determined by the concentration of remaining marinomycin A. | ||
The dramatic increase in the half-life of the marinomycin A conferred by the SpECs, under intense direct UV irradiation, is indeed very promising, albeit we deemed the increase in half-life far too short to impact the utility of this agent for a therapeutic application. To this end, we postulated that the relatively short half-life may be attributed to either the decomposition of the SpECs under these conditions or the diffusion of the natural product from the SpECs in to the aqueous solution. To test this hypothesis, we pre-exposed SpECs to UV light for up to ca. 3 hours prior to loading with marinomycin A (1). The loaded SpECs were than exposed to 5 minutes of UV irradiation, which was intended to decompose any unprotected marinomycin A. The SpECs were then extracted and analysed by UPLC. Analysis of the data showed no change in the quantity of eluted marinomycin A when the SpECs were pre-exposed to UV light. This indicated that the UV protection afforded by the SpECs is related to the interaction between the substrate and the SpECs, and that the gradual photoisomerization observed is likely attributed to the elution of the antibiotic into the water from which the SpECs were initially suspended. To test this notion, the SpECs where loaded with marinomycin A in the same fashion as previously described and lyophilized in the dark. The dried SpECs where then exposed to UV light and the resulting marinomycin A content was analysed in the same manner. Remarkably, approximately 30% of the marinomycin A had degraded after 7 hours. Intriguingly, the degradation occurs within the first 30 minutes, which could potentially be marinomycin A on the surface of the SpECs degrading and thus leaving the remaining agent bound inside the SpECs almost completely photoprotected.
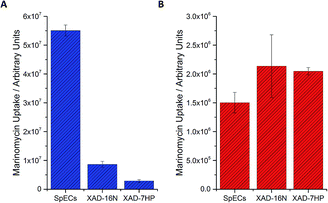
Nevertheless, despite this development the remaining challenges were the low levels of production of marinomycin A (1) and the purification of this natural product, which tend to be common issues with microbial fermentations of natural products. To address this first challenge, namely to increase the amount of isolated marinomycin A for the photoprotection studies, we systematically studied the protocol for improving production by examining the media that was used for the fermentative production of marinomycins (see ESI, Section 3.1†). Since we knew5c that the sporopollenin of SpECs can sequester marinomycin A; we explored whether this phenomenon could be applied to the extraction of it from a fermentation broth. Production cultures of Marinospora CNQ 140 (50 mL), were grown for the standard 6 days and the bacterial cells were removed from the fermentation media by centrifugation. The supernatant was then supplemented with either XAD 16N, XAD7HP or the SpECs. Interestingly, the uptake of marinomycin A by the SpECs from the culture broth was significantly higher than either XAD 16N or XAD 7HP with an improvement of 6 and 19 fold, respectively for the SpECs (Fig. 3A). Hence, the SpECs provide a highly selective method for the extraction for marinomycin A (1) from the culture broth with few other minor metabolic impurities. Elution of marinomycin A from the various resins and the SpECs with DMSO, demonstrates that not only is it extracted, but a high level of purification is conferred by this step. A selectivity ratio (extracted ion chromatogram integration/total ion count integration) of 1.18 × 10−2 was observed, which was 6 and 13 fold more selective than XAD 16N and XAD 7HP, respectively (Fig. 3A). Overall, we envision that given that the SpECs are inexpensive and that pore dimensions can be modified in accordance with plant origin, that this strategy holds significant promise for the isolation and photoprotection of other labile polyene natural products.12
To investigate the selectivity and efficiency of the extraction further, we repeated the experiment by investigating the extraction of a purified aqueous solution of marinomycin A. Under the aforementioned conditions, whilst the SpECs showed excellent and selective uptake of marinomycin A (1) from a crude broth, the resins were very slightly more effective than the SpECs at uptaking marinomycin A from a purified sample (Fig. 3B). Intrigued by this observation we altered the pH of the culture broth to 1.5 pH units either side of the natural pH of the culture broth. This was to determine if we observe any change in affinity for marinomycin A at different pH's. For the SpECs and both of the XAD resins a modest rise in affinity is observed when the pH was increased. However, this increase was not sufficient to explain the slight change in relative binding affinity observed between the SpECs and XAD resins for purified and unpurified marinomycin A (Fig. 3B, see ESI, Section 5.4†). This indicates that pH is not a defining factor in the change in binding affinity. This small change in efficiency is likely to be a result of more selective binding of marinomycin A by the SpECs than the XAD resins. Potentially, XAD's lack of selectivity in binding marinomycins over other components in the fermentation broth, which reduces the number of binding sites available for marinomycin A (1).
Having explored the relative efficiency of the SpECs and XAD resins for extraction, we elected to examine if commercial solid phase extraction resins also provided some form of photoprotection to marinomycin A (1) or if the photoprotection observed was unique to the SpECs. Hence, the XAD 16N and XAD 7HP resins were loaded with marinomycin A in an analogous manner to this agent with the SpECs and then exposed to UV light. In stark contrast to the photoprotection observed for the SpECs no observable photoprotection was evident for either XAD 7HP or XAD 16N. SpECs from the spores of L. clavatum AT1862 showed remarkable photoprotection of the antibiotic and antitumor polyketides, marinomycin A (1, Fig. 2). In addition, it is significantly more effective for extracting the marinomycin A (1) from a culture broth, which has significant potential for natural product isolation.
Conclusion
We have demonstrated the ability to employ naturally occurring, inexpensive and commercially available SpECs to provide a practical solution to the photoprotection and isolation of the light sensitive bioactive polyene antibiotic, marinomycin A (1). The SpEC encapsulation dramatically increases the half-life of the polyene macrodiolide to direct exposure to UV radiation. This unorthodox and ground-breaking finding will likely pave the way and enable future synthetic, biosynthetic and medicinal chemical studies towards the development of these attractive, but challenging light sensitive molecules. There is interest in the utilization of pollen exines (SpECs) for drug delivery, both through inhalation and oral administration.9 These microcapsules have also shown potential for the topical delivery of drug molecules.5c,11 There appears to be a perfect partnership between SpECs from Lycopodium clavatum and marinomycin A (1), in terms of both encapsulation and photoprotection. Varied sized spores from different species may be selected to tailor the encapsulation of different molecules. This work opens interesting avenues to explore the application of this technology as a natural safe and sustainable means of stabilizing other light sensitive bioactive agents, enabling their utilization as important leads in medicinal chemistry.Conflicts of interest
Dr G. Mackenzie is the Technical Director for Sporomex Ltd, which owns patents pertinent to some of the contents in this article.Acknowledgements
This work was supported by an MRC Case Studentship (JZT, RJMG), the European Research Council, funded under the European Union’s Seventh Framework Programme (FP7/2007-2013/ERC consolidator grant GCGXC grant agreement no 614779 to RJMG). We also gratefully acknowledge a CSC Scholarship (MG, RJMG), a BSAC Fellowship (MA, RJMG), and Sporomex Ltd (ADT) for supporting this work. NSERC is thanked for supporting a Tier 1 Canada Research Chair (PAE) and the Royal Society for a Wolfson Research Merit Award (PAE). We also thank W. Fenical (Scripps Institution of Oceanography, San Diego) for the kind provision of Marinispora strain CNQ-140.References
- (a) J. M. T. Hamilton-Miller, Bacteriol. Rev., 1973, 37, 166–196 CAS; (b) For example see: P. Szczeblewski, T. Laskowski, A. Balka, E. Borowski and S. Milewksi, J. Nat. Prod., 2018, 81, 1540–1545 CrossRef CAS PubMed; (c) J. L. Koontz, J. E. Marcy, W. E. Barbeau and S. E. Duncan, J. Agric. Food Chem., 2003, 51, 7111–7114 CrossRef CAS PubMed.
- H. C. Kwon, C. A. Kauffman, P. R. Jensen and W. Fenical, J. Am. Chem. Soc., 2006, 128, 1622–1632 CrossRef CAS PubMed.
- The synthesis of this powerful new antibiotic has attracted much interest, notable total syntheses include: (a) K. C. Nicolaou, A. L. Nold, R. R. Milburn, C. S. Schindler, K. P. Cole and J. Yamaguchi, J. Am. Chem. Soc., 2007, 129, 1760–1768 CrossRef CAS PubMed; (b) P. A. Evans, M.-H. Huang, M. J. Lawler and S. Maroto, Nat. Chem., 2012, 4, 680–684 CrossRef CAS PubMed; (c) T. Nishimaru, M. Kondo, K. Takeshita, K. Takahashi, J. Ishihara and S. Hatakeyama, Angew. Chem., Int. Ed., 2014, 53, 8459–8462 CrossRef CAS PubMed.
- S. Wallace, A. Fleming, C. H. Wellman and D. J. Beerling, AoB Plants, 2011, 1–18 Search PubMed.
- (a) J. Brooks and G. Shaw, Nature, 1968, 219, 532–533 CrossRef CAS PubMed; (b) S. Barrier, A. Diego-Taboada, M. J. Thomasson, L. Madden, J. C. Pointon, J. D. Wadhawan, S. T. Beckett, S. L. Atkin and G. Mackenzie, J. Mater. Chem., 2011, 21, 975–981 RSC; (c) A. Diego-Taboada, P. Cousson, E. Raynaud, Y. Huang, M. Lorch, B. P. Binks, Y. Queneau, A. N. Boa, S. L. Atkin, S. T. Beckett and G. Mackenzie, J. Mater. Chem., 2012, 22, 9767–9773 RSC.
- (a) S. L. Atkin, S. Barrier, Z. Cui, P. D. I. Fletcher, G. Mackenzie, V. Panel, V. Sol and X. Zhang, J. Photochem. Photobiol., B, 2011, 102, 209–217 CrossRef CAS; (b) J. Rozema, R. A. Broekman, P. Blokker, B. B. Meijkamp, N. de Bakker, J. van de Staaij, A. van Beem, F. Ariese and S. M. Kars, J. Photochem. Photobiol., B, 2001, 62, 108–117 CrossRef CAS; (c) P. E. Jardine, F. A. J. Abernethy, B. H. Lomax, W. D. Gosling and W. T. Fraser, Review of Palaeobotany and Palynology, 2017, 238, 1–6 CrossRef.
- L. C. Boavida, J. D. Becker and J. A. Feijó, Int. J. Dev. Biol., 2005, 49, 595–614 CrossRef CAS PubMed.
- (a) I. Sargin, L. Akyuz, M. Kaya, G. Tan, T. Ceter, K. Yildirim, S. Ertosun, G. H. Aydin and M. Topal, Int. J. Biol. Macromol., 2017, 105, 749–756 CrossRef CAS PubMed; (b) N. Sudareva, O. Suvorova, N. Saprykina, A. Vilesov, P. Bel'tiukov, S. Petunov and A. Radilov, J. Mater. Chem. B, 2017, 5, 7711–7720 RSC; (c) M. Mujtaba, I. Sargin, L. Akyuz, T. Ceter and M. Kaya, Mater. Sci. Eng., C, 2017, 77, 263–270 CrossRef CAS PubMed; (d) A. Diego-Taboada, L. Maillet, J. H. Banoub, M. Lorch, A. S. Rigby, A. N. Boa, S. L. Atkin and G. Mackenzie, J. Mater. Chem. B, 2013, 1, 707–713 RSC; (e) T. L. Harris, C. J. Wenthur, A. Diego-Taboada, G. Mackenzie, T. S. Corbitt and K. D. Janda, Chem. Commun., 2016, 52, 4187–4190 RSC; (f) A. Diego-Taboada, S. T. Beckett, S. L. Atkin and G. Mackenzie, Pharmaceutics, 2014, 6, 80–96 CrossRef PubMed; (g) S. L. Atkin, S. T. Beckett and G. Mackenzie, Patent WO2009077749, June 25, 2009.
- (a) A. Wakil, G. Mackenzie, A. Diego-Taboada, J. G. Bell and S. L. Atkin, Lipids, 2010, 45, 645–649 CrossRef CAS PubMed; (b) E. Maltas, I. H. Gubbuk and S. Yildiz, Biochemistry and Biophysics Reports, 2016, 7, 201–205 CrossRef PubMed; (c) M. J. Uddin and H. S. Gill, J. Controlled Release, 2017, 268, 416–426 CrossRef CAS PubMed; (d) S. M. Alshehri, H. A. Al-Lohedan, E. Al-Farraj, N. Alhokbany, A. A. Chaudhary and T. Ahamad, Int. J. Pharm., 2016, 504, 39–47 CrossRef CAS PubMed; (e) S. M. Alshehri, H. A. Al-Lohedan, A. A. Chaudhary, E. Al-Farraj, N. Alhokbany, Z. Issa, S. Alhousine and T. Ahamad, Eur. J. Pharm. Sci., 2016, 88, 158–165 CrossRef CAS PubMed.
- S. Barrier, A. S. Rigby, A. Diego-Taboada, M. J. Thomasson, G. Mackenzie and S. L. Atkin, LWT--Food Sci. Technol., 2010, 43, 73–76 CrossRef CAS.
- S. L. Atkin, S. T. Beckett and G. Mackenzie, Patent WO2007012856, February 1, 2007.
- G. M. Zinkl, B. I. Zwiebel, D. G. Grier and D. Preuss, Development, 1999, 126, 5431–5440 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: NMR characterisation; LC-MS characterisation. See DOI: 10.1039/c9sc01375j |
| ‡ J. S. Zarins Tutt, M. Agbo, H. Gao, M. Gan, R. B. Hamed: work carried out whilst at St Andrews. |
| § Current address: University of Nigeria, Nsukka, Nigeria. |
| ¶ Current address: School of Science, Engineering and Design, Teeside University, UK. |
| || Current address: Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100050, China. |
| ** Current address: Department of Pharmacognosy, Faculty of Pharmacy, Assiut University, 71256, Egypt (on leave). |
| This journal is © The Royal Society of Chemistry 2019 |