Graphdiyne: a superior carbon additive to boost the activity of water oxidation catalysts†
Panyong
Kuang
a,
Bicheng
Zhu
a,
Yuliang
Li
 c,
Huibiao
Liu
c,
Huibiao
Liu
 c,
Jiaguo
Yu
c,
Jiaguo
Yu
 *ab and
Ke
Fan
*ab and
Ke
Fan
 *a
*a
aState Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan 430070, P. R. China. E-mail: jiaguoyu@yahoo.com; kefan@whut.edu.cn
bDepartment of Physics, Faculty of Science, King Abdulaziz University, Jeddah 21589, Saudi Arabia
cBeijing National Laboratory for Molecular Sciences (BNLMS), CAS Key Laboratory of Organic Solids, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China
First published on 15th March 2018
Abstract
A new carbon allotrope, graphdiyne (GDY), with a highly π-conjugated structure of sp- and sp2-hybridized carbon networks, has recently emerged and been used as a superior carbon additive to boost the activity of water oxidation catalysts. In this work, a hybrid GDY/NiFe-LDH electrocatalyst was prepared by a facile hydrothermal treatment, which shows an outstanding catalytic activity with a low overpotential of 260 mV at 10 mA cm−2 catalytic current density and good durability towards oxygen evolution in 1.0 M KOH solution. Density functional theory (DFT) calculations prove that the sp- and sp2-hybridized GDY shows both superior electron capture and excellent electron transfer ability compared to that of the conventional sp2-hybridized carbon materials.
Conceptual insightsFull-carbon materials are promising additives to boost water oxidation activity, however, the improvement of oxygen evolution derived from the commonly used carbon additives of sp2-hybridized graphene and carbon nanotubes is limited. Graphdiyne (GDY), a new carbon allotrope with a highly π-conjugated structure of sp- and sp2-hybridized carbon networks, has recently been emerging. Herein, GDY serves as an additive to boost the electrocatalytic activity of the prototype of water oxidation catalysts, nickel-iron layered double hydroxide (NiFe-LDH). The as-prepared GDY/NiFe-LDH shows an outstanding catalytic activity with a low overpotential of 260 mV at 10 mA cm−2 catalytic current density and good durability towards the oxygen evolution reaction. Remarkably, compared with the other carbon additives (reduced graphene oxide (RGO) and carbon nanotubes (CNT)) cooperating with NiFe-LDH, GDY/NiFe-LDH shows superior OER activity, attributed to the larger π-conjugated structure of GDY and the strong chemical and electronic interactions between the diacetylenic linkages of GDY and the transition metals. Density functional theory (DFT) calculations prove that the sp- and sp2-hybridized GDY shows both superior electron capture and excellent electron transfer compared to RGO and CNT, and thus greatly enhances the OER performance. Our research verifies that GDY can serve as a superior carbon additive to boost the activity of water oxidation catalysts. |
Introduction
Electrochemical water splitting represents a promising approach to enable the storage and utilization of a considerable amount of energy.1–5 Nevertheless, the oxygen evolution reaction (OER) half reaction is kinetically sluggish due to its multistep proton-coupled electron transfer process (4OH− → 2H2O + O2 + 4e−), and thus a relatively higher overpotential is required to drive the overall water splitting (H2O → 1/2O2 + H2).6,7 At present, the state-of-the-art electrocatalysts for the OER are mostly based on noble metals such as RuO2 and IrO2.8,9 However, the scarcity and high cost of Ru and Ir make them impractical on a large scale.10–12 Thus, searching for an efficient, earth-abundant and nonprecious metal-based OER electrocatalyst represents an urgent target in the regard of science and application.As one of the most successful low-cost electrocatalysts, two-dimensional (2D) layered double hydroxide (LDH), NiFe-LDH, has been widely employed for the OER, and exhibits outstanding electrocatalytic activity and durability in alkaline electrolytes due to its layered and relatively open structure.13–16 However, the low electrical conductivity of NiFe-LDH seriously impedes the electron transfer, and becomes the main obstacle to its electrocatalytic activity. To address this issue, various highly conductive carbon materials, such as reduced graphene oxide (RGO),17–19 carbon nanotubes (CNT)20,21 and carbon quantum dots (CQDs),22 have been popularly attempted to hybridize with NiFe-LDH to strengthen the electron transfer and OER performance. However, the improvement of OER by these carbon additives is limited. Moreover, harsh conditions (high temperature and inert atmosphere) are always demanded to prepare these carbon materials,23–27 which are fatal drawbacks and inevitably lower the economic profits. Therefore, a new highly efficient carbon additive prepared under relatively mild conditions is highly required, but still remains a great challenge.
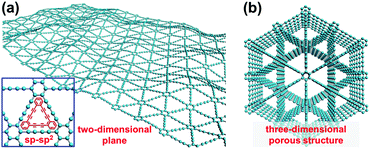
Compared with the natural carbon materials (sp2-hybridized graphite and sp3-hybridized diamond), a new carbon allotrope, graphdiyne (GDY), has attracted increasing focus recently due to its unique structure assembled by periodic motifs within networks of sp- and sp2-hybridized carbon atoms.28–30 This 2D and lamellar carbon material comprises neighbored benzene rings and diacetylenic linkages (–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C
C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–), and was first prepared by Li et al. in 2010 under mild conditions of low temperature and ordinary pressure, achieving the breakthrough from theoretical predictions to practical construction.31,32 These flat sp- and sp2-hybridized carbon networks (2D plane with high degree of π-conjunction, Fig. 1a) endow GDY with richer carbon chemical bonds, higher electrical conductivity and better chemical stability than bare sp2-hybridized carbon frameworks. In particular, the honeycomb lattice structure and three-dimensional (3D) porous structure of GDY provide more homodisperse pores for accessible active sites, electrolyte and electron transfer (Fig. 1b). These outstanding merits make GDY widely used in various research fields including catalysis,33–35 Li-ion batteries,36–38 photoelectric devices,39,40 and solar cells.41–43 Density functional theory (DFT) calculations indicated that the strong chemical interaction between transition metal (TM) atoms (e.g., Ni, Fe and Co) and the surrounding acetylenic bonds of GDY leads to fast electron transfer from the TMs to the GDY, thus enriching the electron density of GDY and improving the OER activity.35,44 More interestingly, TMs adsorbed on GDY are more stable than those adsorbed on graphene and CNT, which is quite beneficial for the fabrication of catalysts with high activity and long-term durability.44
C–), and was first prepared by Li et al. in 2010 under mild conditions of low temperature and ordinary pressure, achieving the breakthrough from theoretical predictions to practical construction.31,32 These flat sp- and sp2-hybridized carbon networks (2D plane with high degree of π-conjunction, Fig. 1a) endow GDY with richer carbon chemical bonds, higher electrical conductivity and better chemical stability than bare sp2-hybridized carbon frameworks. In particular, the honeycomb lattice structure and three-dimensional (3D) porous structure of GDY provide more homodisperse pores for accessible active sites, electrolyte and electron transfer (Fig. 1b). These outstanding merits make GDY widely used in various research fields including catalysis,33–35 Li-ion batteries,36–38 photoelectric devices,39,40 and solar cells.41–43 Density functional theory (DFT) calculations indicated that the strong chemical interaction between transition metal (TM) atoms (e.g., Ni, Fe and Co) and the surrounding acetylenic bonds of GDY leads to fast electron transfer from the TMs to the GDY, thus enriching the electron density of GDY and improving the OER activity.35,44 More interestingly, TMs adsorbed on GDY are more stable than those adsorbed on graphene and CNT, which is quite beneficial for the fabrication of catalysts with high activity and long-term durability.44
On the basis of these theoretical and experimental results, GDY is supposed as a promising scaffold for the OER electrocatalyst NiFe-LDH to further improve its catalytic activity. Despite the tremendous interest in GDY, to the best of our knowledge, no previous reports have appeared regarding the use of GDY in NiFe-LDH electrocatalysts for the OER under strong alkaline conditions.
Herein, in this work, by using NiFe-LDH as the prototype of low-cost OER catalysts, we report a simple and in situ method for fabricating a GDY/NiFe-LDH composite catalyst for the OER. GDY/NiFe-LDH exhibits an extraordinary catalytic activity with a low overpotential of 260 mV at 10 mA cm−2 catalytic current density and a considerable stability on a glassy carbon electrode in 1.0 M KOH solution, which is ascribed to the larger π-conjugated structure of GDY, and the strong chemical and electronic interaction between –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C
C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C– and TMs that contribute to the fast electron transfer. In addition, DFT calculations prove that GDY is a superior electron transfer candidate as compared with its sisters RGO and CNT, and serves as a promising additive to boost the water oxidation activity of NiFe-LDH. We conclude that the GDY is a superior carbon additive compared to the commonly used RGO and CNT in OER catalysts, and the GDY/NiFe-LDH composite is one of the best of the state-of-the-art OER electrocatalysts.
C– and TMs that contribute to the fast electron transfer. In addition, DFT calculations prove that GDY is a superior electron transfer candidate as compared with its sisters RGO and CNT, and serves as a promising additive to boost the water oxidation activity of NiFe-LDH. We conclude that the GDY is a superior carbon additive compared to the commonly used RGO and CNT in OER catalysts, and the GDY/NiFe-LDH composite is one of the best of the state-of-the-art OER electrocatalysts.
Results and discussion
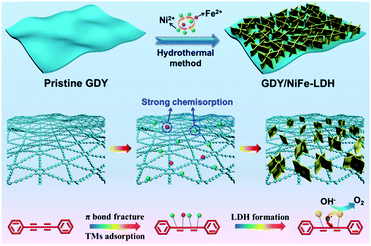
Fig. 2 shows the typical fabrication process of GDY/NiFe-LDH in our case (see the Experimental section for details). Generally, the π bond of acetylenic bond is very active, and subsequently Ni/Fe could be adsorbed on the GDY due to the strong chemisorption and then forms the GDY/NiFe-LDH composite. The transmission electron microscopy (TEM) images (Fig. 3a and b) indicate that the GDY is composed of a few dispersed thin layers, which is conducive to the adsorption of abundant TMs and fast charge transfer. Fig. 3c presents the high-resolution transmission electron microscopy (HRTEM) image of GDY, and the interlayer spacing of 0.35 nm is very close to the theoretical value (inset, Fig. 3c).45 Moreover, the thin film of GDY is also confirmed by atomic force microscopy (AFM), showing the average thickness of ∼1.8 nm (Fig. 3d). Note that some graininess and porous structure of GDY can also be observed, which are beneficial for the diffusion of the electrolyte into GDY (Fig. S1, ESI†). Moreover, the energy-dispersive spectroscopy (EDS) mapping images (Fig. S2, ESI†) reveal the homogeneous distribution of C and O elements. The oxygen-containing functional groups could also contribute to the formation of strong chemical bonds between Ni/Fe and surrounding acetylenic bonds, which can lead to fast charge transfer from NiFe-LDH to GDY, and subsequently results in promoted catalytic activity (see the following sections).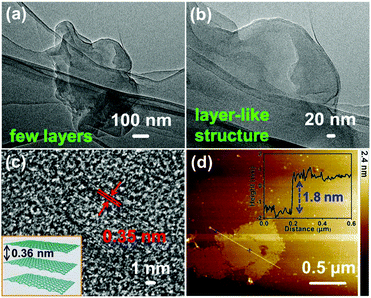 | ||
| Fig. 3 (a and b) TEM images, (c) HRTEM image, and (d) AFM image of the GDY. The inset in (c) is the theoretical interlayer spacing of GDY. | ||
GDY/NiFe-LDH samples with varied GDY amounts (1%, 5%, 10%, 20% and 33%, weight ratio) were firstly prepared to optimize the composition of the catalyst. As shown in Fig. S3 (ESI†), the overpotential to achieve 10 mA cm−2 catalytic current density (η10) reaches the lowest value of 260 mV for 5% GDY, which is confirmed as the optimized GDY content, and further experiments were carried out with 5% GDY/NiFe-LDH catalysts (for convenience, it is still denoted as GDY/NiFe-LDH in the following sections unless noted otherwise). Fig. 4a presents the representative scanning electron microscopy (SEM) image of GDY/NiFe-LDH. The dark wrinkled strips in the spherical aberration corrected transmission electron microscopy (ACTEM) image and TEM images (Fig. 4b and Fig. S4a, b, ESI†) indicate the thin flake feature of NiFe-LDH. The average thickness of LDH is ∼2.9 nm according to the AFM test (Fig. S4c and d, ESI†). A discernible lattice fringe of 0.25 nm, which corresponds to the (012) crystal plane of NiFe-LDH, is observed in the HRTEM image of GDY/NiFe-LDH (Fig. 4c). Fig. 4d shows an enlarged view of the GDY component in the GDY/NiFe-LDH composite, which confirms the existence of GDY in the composite. In addition, the high-angle annular dark field (HAADF) and EDS mapping images indicate the homogeneous distribution of Ni, Fe, C and O in GDY/NiFe-LDH (Fig. 4e and i). These investigations strongly suggest the successful preparation of the hybrid material of GDY/NiFe-LDH.
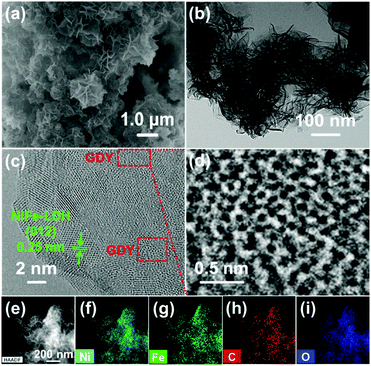 | ||
| Fig. 4 (a) SEM image, (b) ACTEM image, (c and d) HRTEM images, and (e–i) HAADF and EDS mapping images of GDY/NiFe-LDH. | ||
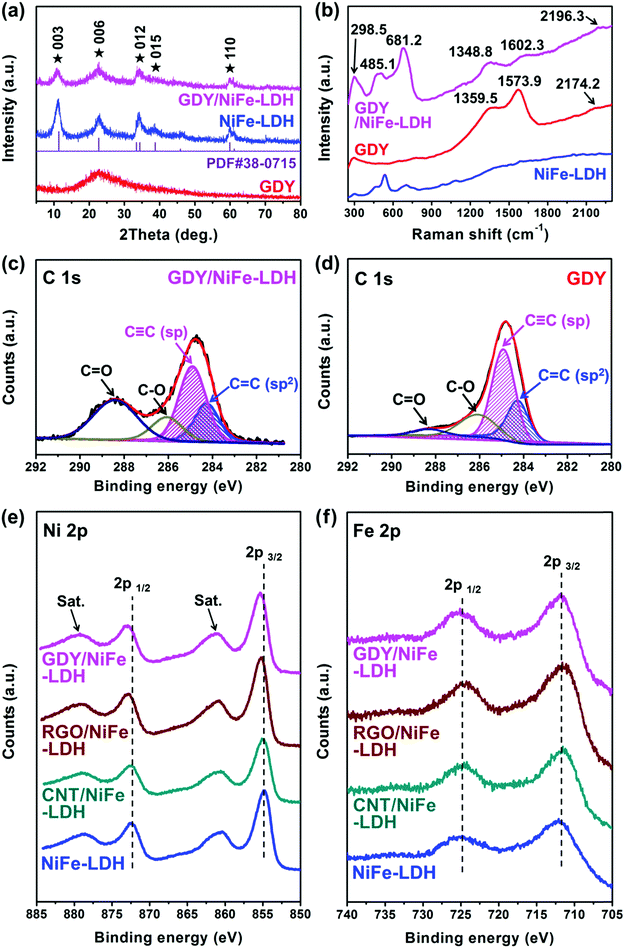
Fig. 5a shows the powder X-ray diffraction (XRD) patterns of the bare GDY, NiFe-LDH and GDY/NiFe-LDH. GDY shows a broad diffraction peak located at around 23°, corresponding to the (002) plane of graphite-type carbon with a d-spacing of 0.37 nm, which is very close to the theoretical interlayer spacing of 0.36 nm of GDY in Fig. 3c. The broad feature reveals a distortion from the ordered arrangement of GDY along its stacking direction. Both the NiFe-LDH and GDY/NiFe-LDH exhibit the typical hydrotalcite-like characteristic of the rhombohedral phase of α-Ni(OH)2 (JCPDS no. 38-0715). The characteristic peak for GDY at around 23° is hard to distinguish in GDY/NiFe-LDH, due to the strong (006) peak of NiFe-LDH and small amount of GDY (5%). Therefore, thermogravimetric (TG) analysis and differential thermal analysis (DTA) curves were measured to further confirm the existence of GDY in GDY/NiFe-LDH. Compared with the TG and DTA curves of bare NiFe-LDH (Fig. S5a, ESI†), the emerging exotherm peak in TDA of GDY/NiFe-LDH at around 400 °C is attributed to the combustion of GDY (carbon material) under air atmosphere (Fig. S5b, ESI†).46 The relatively strong background noise of DTA for GDY/NiFe-LDH can also be attributed to GDY, which reduces the diffraction peak intensity of NiFe-LDH nanoplates. Thus, the GDY composition in GDY/NiFe-LDH can be verified. In addition, Fourier transform infrared spectroscopy (FT-IR) spectra (Fig. S6, ESI†) of the samples also confirm the existence of GDY composition in GDY/NiFe-LDH. Fig. 5b shows the Raman spectra of GDY/NiFe-LDH, NiFe-LDH and GDY. For the bare GDY, the Raman peaks located at 1359.5 and 1573.9 cm−1 correspond to the D band (disordered carbon) and G band (ordered graphitic carbon), respectively, and the peak at 2174.2 cm−1 represents the vibrations of the conjugated diyne links (–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C
C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–).47 This is a clear characteristic of GDY that differentiates it from the sp2-hybridized carbon materials (Fig. S7, ESI†).48,49 The Raman spectrum of GDY/NiFe-LDH features peaks at 1348.8, 1602.3 and 2196.3 cm−1 assigned to the D band, G band and vibrations of the conjugated diyne links, respectively. These observations suggest that the GDY framework is undamaged after the hydrothermal treatment. Furthermore, the peaks located at around 298.5, 485.1 and 681.2 cm−1 correspond to the typical characteristics of NiFe-LDH.50 Notably, compared with the Raman spectrum of bare GDY, blue shift of the G band peak and signals for the vibrations of conjugated diyne links in GDY/NiFe-LDH can be clearly observed, indicating the chemical interaction and electron transfer between GDY and NiFe-LDH.47,51,52 Moreover, the greatly enhanced ID (intensity of D band)/IG (intensity of G band) ratio of GDY/NiFe-LDH (0.96 vs. 0.86 for bare GDY) reveals the large increase in structural defect sites with more exposed active sites, which are considered to be beneficial for electrocatalysis improvement.47 According to the above analyses, it is validated that the GDY/NiFe-LDH composite catalyst has been successfully prepared.
C–).47 This is a clear characteristic of GDY that differentiates it from the sp2-hybridized carbon materials (Fig. S7, ESI†).48,49 The Raman spectrum of GDY/NiFe-LDH features peaks at 1348.8, 1602.3 and 2196.3 cm−1 assigned to the D band, G band and vibrations of the conjugated diyne links, respectively. These observations suggest that the GDY framework is undamaged after the hydrothermal treatment. Furthermore, the peaks located at around 298.5, 485.1 and 681.2 cm−1 correspond to the typical characteristics of NiFe-LDH.50 Notably, compared with the Raman spectrum of bare GDY, blue shift of the G band peak and signals for the vibrations of conjugated diyne links in GDY/NiFe-LDH can be clearly observed, indicating the chemical interaction and electron transfer between GDY and NiFe-LDH.47,51,52 Moreover, the greatly enhanced ID (intensity of D band)/IG (intensity of G band) ratio of GDY/NiFe-LDH (0.96 vs. 0.86 for bare GDY) reveals the large increase in structural defect sites with more exposed active sites, which are considered to be beneficial for electrocatalysis improvement.47 According to the above analyses, it is validated that the GDY/NiFe-LDH composite catalyst has been successfully prepared.
X-ray photoelectron spectroscopy (XPS) measurements were further performed to provide more information of insightful chemical interaction and electron transfer of GDY/NiFe-LDH. Meanwhile, NiFe-LDHs with other commonly used carbon additives (5% RGO and 5% CNT, weight ratio) were investigated as well; for convenience, they are denoted as RGO/NiFe-LDH and CNT/NiFe-LDH, respectively. The XPS survey spectra in Fig. S8 (ESI†) confirm the Ni, Fe, O and C elements in all the carbon-containing samples. Particularly, the C 1s XPS spectrum of GDY/NiFe-LDH is fitted into four peaks located at 284.3, 284.9, 286.1 and 288.4 eV, which are ascribed to the C 1s orbitals of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (sp2), C
C (sp2), C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C (sp), C–O and C
C (sp), C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively (Fig. 5c).53 Notably, similar to the bare GDY (Fig. 5d), the area ratio of the sp and sp2 hybridized carbon atom in GDY/NiFe-LDH is also ∼2 (GDY is theoretically composed of 66.7% sp and 33.3% sp2 carbons),45 suggesting the intact structure of GDY even after being hybridized with NiFe-LDH. In comparison with the C
O, respectively (Fig. 5c).53 Notably, similar to the bare GDY (Fig. 5d), the area ratio of the sp and sp2 hybridized carbon atom in GDY/NiFe-LDH is also ∼2 (GDY is theoretically composed of 66.7% sp and 33.3% sp2 carbons),45 suggesting the intact structure of GDY even after being hybridized with NiFe-LDH. In comparison with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak of the bare GDY, the significantly increased intensities of the C
O peak of the bare GDY, the significantly increased intensities of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak in GDY/NiFe-LDH, RGO/NiFe-LDH, CNT/NiFe-LDH and NiFe-LDH (Fig. 5c and Fig. S9, ESI†) are derived from the carbonate species in NiFe-LDH.54 Besides, the high-resolution Ni 2p XPS spectra of GDY/NiFe-LDH, RGO/NiFe-LDH and CNT/NiFe-LDH exhibit peak shift to higher binding energy compared with the pure NiFe-LDH (Fig. 5e and Table S1, ESI†),55–57 indicating the modulated local electronic structure of Ni cations by the carbon additive. Notably, GDY/NiFe-LDH exhibits the largest shift of the Ni 2p peaks to higher binding energy (ca. 0.5 eV) compared with RGO/NiFe-LDH (ca. 0.3 eV) and CNT/NiFe-LDH (ca. 0.2 eV), indicating that GDY can form the strongest chemical interaction and electronic coupling with NiFe-LDH among all the used carbon additives in this research. In addition, the Fe 2p XPS spectra of all the samples (Fig. 5f) remain almost the same, which demonstrates the oxidation state of Fe3+.58,59 It is reported that Fe is the possible active site in NiFe-LDH,60–62 while the Ni (oxy)hydroxide serves as an electron transfer-favourable layered host with high surface area.63 The greatest extent of electron transfer from Ni to GDY revealed by XPS suggests the electron-defect environment for the Ni electronic structure, which can promote the electric conductivity of NiFe-LDH, and consequently contribute to the improved catalytic activity (see the following sections).
O peak in GDY/NiFe-LDH, RGO/NiFe-LDH, CNT/NiFe-LDH and NiFe-LDH (Fig. 5c and Fig. S9, ESI†) are derived from the carbonate species in NiFe-LDH.54 Besides, the high-resolution Ni 2p XPS spectra of GDY/NiFe-LDH, RGO/NiFe-LDH and CNT/NiFe-LDH exhibit peak shift to higher binding energy compared with the pure NiFe-LDH (Fig. 5e and Table S1, ESI†),55–57 indicating the modulated local electronic structure of Ni cations by the carbon additive. Notably, GDY/NiFe-LDH exhibits the largest shift of the Ni 2p peaks to higher binding energy (ca. 0.5 eV) compared with RGO/NiFe-LDH (ca. 0.3 eV) and CNT/NiFe-LDH (ca. 0.2 eV), indicating that GDY can form the strongest chemical interaction and electronic coupling with NiFe-LDH among all the used carbon additives in this research. In addition, the Fe 2p XPS spectra of all the samples (Fig. 5f) remain almost the same, which demonstrates the oxidation state of Fe3+.58,59 It is reported that Fe is the possible active site in NiFe-LDH,60–62 while the Ni (oxy)hydroxide serves as an electron transfer-favourable layered host with high surface area.63 The greatest extent of electron transfer from Ni to GDY revealed by XPS suggests the electron-defect environment for the Ni electronic structure, which can promote the electric conductivity of NiFe-LDH, and consequently contribute to the improved catalytic activity (see the following sections).
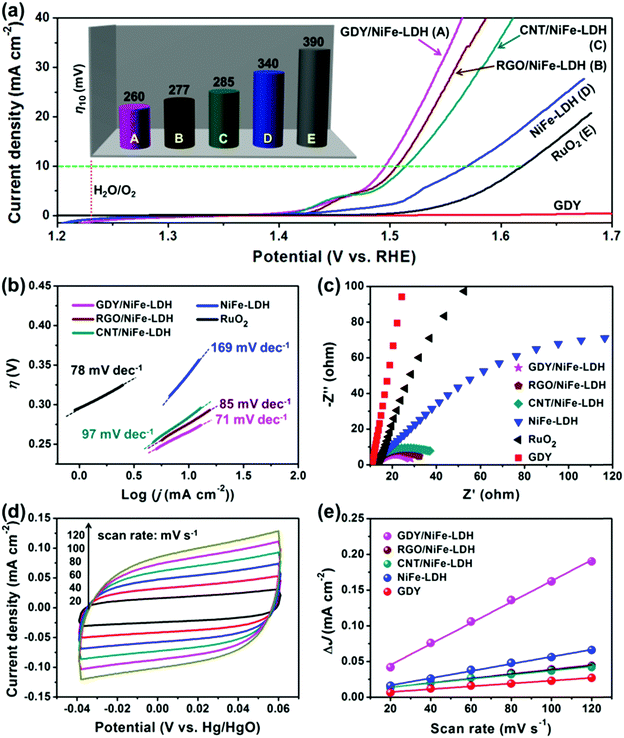
The OER activity of GDY/NiFe-LDH was evaluated using a standard three-electrode system in 1.0 M KOH. Bare NiFe-LDH, commercial RuO2, RGO/NiFe-LDH and CNT/NiFe-LDH were used for comparison. Their SEM images, XRD patterns and EDS analyses are presented in Fig. S10 and S11 (ESI†). Fig. 6a shows the polarization curves of all the samples. Dramatically, GDY/NiFe-LDH exhibits a remarkable OER activity, whose current density increases rapidly along the increased applied potential. The required overpotential at 10 mA cm−2 catalytic current density (η10) of GDY/NiFe-LDH is 260 mV, much lower than that of RGO/NiFe-LDH (η10 = 277 mV), CNT/NiFe-LDH (η10 = 285 mV), NiFe-LDH (η10 = 340 mV) and commercial RuO2 (η10 = 390 mV), as exhibited in the inset of Fig. 6a and Table S2 (ESI†). Such an excellent catalytic activity was further confirmed by its lowest onset overpotential among all the samples (Fig. S12, ESI†). This striking improvement in OER activity of GDY/NiFe-LDH could be ascribed to two plausible aspects: (1) fast and efficient electron transfer from NiFe-LDH to GDY due to the strong chemical interaction between TMs and acetylenic bonds. (2) Abundant active sites and sufficient space for the evolution and release of gases provided by a three-dimensional (3D) geometry structure, leading to high oxygen yield. As further compared with the recently reported state-of-the-art and carbon material-included OER catalysts (Table S3, ESI†), our GDY/NiFe-LDH performs as one of the best candidates.
For obtaining further insight into the OER activity of GDY/NiFe-LDH, a comparison of the Tafel slopes was performed. In Fig. 6b, the Tafel slope for GDY/NiFe-LDH is 71 mV dec−1, which is lower than that of RGO/NiFe-LDH (85 mV dec−1), CNT/NiFe-LDH (97 mV dec−1), NiFe-LDH (169 mV dec−1) and RuO2 (78 mV dec−1), suggesting favorable catalytic kinetics of GDY/NiFe-LDH during the OER. According to the results of inductively coupled plasma atomic emission spectroscopy (ICP-AES), the molar ratio of Ni/Fe in GDY/NiFe-LDH is ∼3, and the turnover frequency (TOF) of GDY/NiFe-LDH is calculated to be 0.025 s−1 at an overpotential (η) of 300 mV (Fig. S13, ESI†), which is 1.32, 1.67 and 4.17 times higher than that of the RGO/NiFe-LDH (0.019 s−1), CNT/NiFe-LDH (0.015 s−1) and NiFe-LDH (0.006 s−1) at the same overpotential, respectively. TOF generally reveals the OER intrinsic activity of the catalysts, and the results suggested that the GDY/NiFe-LDH can serve as an intrinsically active catalyst for outstanding OER performance. Electrochemical impedance spectroscopy (EIS) measurements in Fig. 6c reveal that GDY/NiFe-LDH exhibits greatly reduced charge transfer resistance compared to pure GDY and NiFe-LDH, indicating that the electrical conductivity of NiFe-LDH was significantly improved by adding GDY. Moreover, compared to the bigger semicircle of RGO/NiFe-LDH and CNT/NiFe-LDH, the smaller semicircle of GDY/NiFe-LDH suggests the enhanced electron transfer between GDY and NiFe-LDH, which should be attributed to the strong chemical interaction between –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C
C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C– and TMs. To further confirm the advantages of chemical interaction between GDY and NiFe-LDH, the polarization curve and Nyquist plots of the physically mixed GDY and NiFe-LDH counterpart (GDY + NiFe-LDH, 5% weight ratio of GDY) were performed. As shown in Fig. S14 (ESI†), GDY + NiFe-LDH exhibits inferior OER activity compared to GDY/NiFe-LDH. Thus, it is inferred that the superior performance of GDY/NiFe-LDH is from the strong chemical interaction between –C
C– and TMs. To further confirm the advantages of chemical interaction between GDY and NiFe-LDH, the polarization curve and Nyquist plots of the physically mixed GDY and NiFe-LDH counterpart (GDY + NiFe-LDH, 5% weight ratio of GDY) were performed. As shown in Fig. S14 (ESI†), GDY + NiFe-LDH exhibits inferior OER activity compared to GDY/NiFe-LDH. Thus, it is inferred that the superior performance of GDY/NiFe-LDH is from the strong chemical interaction between –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C
C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C– and TMs, which greatly promotes the electron transfer ability. The lowest Tafel slope and smallest charge transfer resistance of GDY/NiFe-LDH indicate the facile kinetics for OER, consistent with the largest electrochemical active surface area (ECSA) (Fig. 6d, e and Fig. S15, Table S2, ESI†), which demonstrates the most exposed active sites. Generally, the electrocatalytic activity of LDH is limited by its MO6 center with fully occupied bonding t2g orbitals, which results in poor electronic conductivity. This disadvantage is reflected by the sluggish reaction between OH− and adsorbed oxygen atoms on the active sites to form the adsorbed –OOH group. Moreover, the insufficient contact between the active sites and electrolytes also decreases the OER activity. From these perspectives, the highly π-conjugated and lamellar structure of GDY has great potential to address the above problems, since it can act as an excellent electronic transmission channel to improve the electron transfer kinetics, and simultaneously serve as the backbone to support the NiFe-LDH.
C– and TMs, which greatly promotes the electron transfer ability. The lowest Tafel slope and smallest charge transfer resistance of GDY/NiFe-LDH indicate the facile kinetics for OER, consistent with the largest electrochemical active surface area (ECSA) (Fig. 6d, e and Fig. S15, Table S2, ESI†), which demonstrates the most exposed active sites. Generally, the electrocatalytic activity of LDH is limited by its MO6 center with fully occupied bonding t2g orbitals, which results in poor electronic conductivity. This disadvantage is reflected by the sluggish reaction between OH− and adsorbed oxygen atoms on the active sites to form the adsorbed –OOH group. Moreover, the insufficient contact between the active sites and electrolytes also decreases the OER activity. From these perspectives, the highly π-conjugated and lamellar structure of GDY has great potential to address the above problems, since it can act as an excellent electronic transmission channel to improve the electron transfer kinetics, and simultaneously serve as the backbone to support the NiFe-LDH.
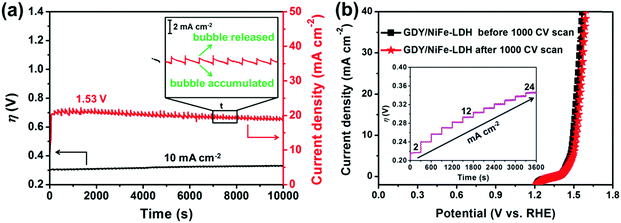
The long-term OER of GDY/NiFe-LDH was tested by chronopotentiometry at 10 mA cm−2 and chronoamperometry under 300 mV overpotential in 1.0 M KOH. As displayed in Fig. 7a, GDY/NiFe-LDH exhibits satisfactory stable OER catalytic performance. As observed from the chronopotentiometry test, the GDY/NiFe-LDH showed excellent catalytic stability with an inessential voltage loss of about 2.8% at 104 seconds continuous operation. During the OER process, O2 gas bubbles were successively detected on the electrode surface. As for the chronoamperometry measurement, the small fluctuation in current density in the chronoamperometry curve was caused by the accumulation of the produced O2 bubbles on the surface of the electrode and their subsequent release. The inset of Fig. 7a shows that the fluctuation range of the current density of GDY/NiFe-LDH is smaller than 2 mA cm−2. The small fluctuations denote that the unique structure of GDY/NiFe-LDH is favourable to the evolution of O2 bubbles from the material. Moreover, a stability test of the GDY/NiFe-LDH electrode was also performed at different current densities and higher overpotential (Fig. 7b, inset and Fig. S16, ESI†), and it almost retains a constant overpotential for each applied current density and stable current density under high overpotential. The structure and morphology of GDY/NiFe-LDH are fully preserved after the long-term stability test (Fig. S17, ESI†), demonstrating the satisfactory durability of GDY/NiFe-LDH in strong alkaline solution. In addition to these long-term stability tests, GDY/NiFe-LDH exhibits only a slight loss in current density after continuous cyclic voltammetry scanning for 1000 cycles (Fig. 7b), further suggesting the excellent durability of GDY/NiFe-LDH towards OER application.
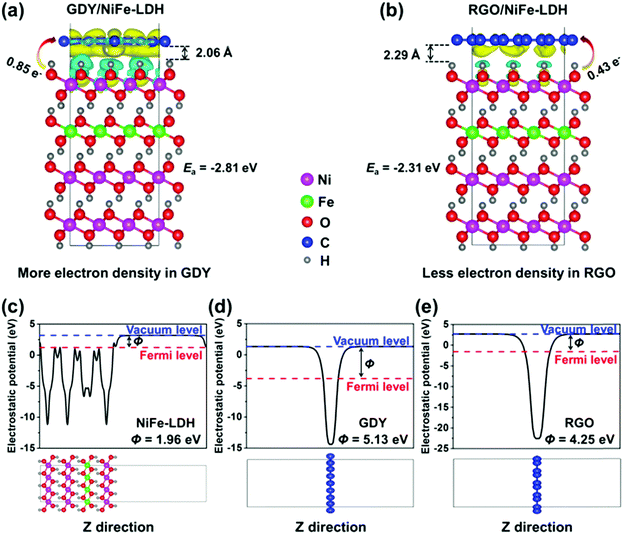
DFT calculations were performed to further understand the superior electron transfer ability of GDY compared to the commonly used RGO and CNT. Owing to the same structural unit of RGO and CNT, only 2D structural RGO was chosen as the reference. To obtain the optimal Fe position, several feasible positions of Fe (position 1 to 4) in NiFe-LDH were chosen, as shown in Fig. S18 (ESI†). Position 2 is found to be the most stable position for Fe in NiFe-LDH and used for the following calculations. Fig. S19 (ESI†) shows the top and side views of the optimized structures of GDY/NiFe-LDH and RGO/NiFe-LDH, respectively. As shown in Fig. 8a and b, the distance between GDY and NiFe-LDH (2.06 Å) is smaller than that between RGO and NiFe-LDH (2.29 Å), indicating the stronger chemical interaction between GDY and TMs. Moreover, the adhesive energies (Ea) of GDY/NiFe-LDH and RGO/NiFe-LDH are calculated to be −2.81 and −2.31 eV, respectively. The more negative Ea of the former suggests that the GDY/NiFe-LDH system is structurally more stable than RGO/NiFe-LDH. Besides, the differential electron densities of the GDY/NiFe-LDH and RGO/NiFe-LDH systems were also calculated. The yellow and cyan iso-surfaces depict the region of net electron accumulation or deficit on intercalation. The Bader charge analysis shows that 0.85 and 0.43 e− are transferred from NiFe-LDH to the GDY and RGO, respectively, which distinctly suggests the stronger electron-withdrawing effect on GDY. This effect is also verified by the distribution of yellow and cyan regions, in which the GDY/NiFe-LDH has a much wider region than RGO/NiFe-LDH in the same iso-surface value (0.0007 e Å−3). The work function (Φ) of the material is a crucial parameter to investigate the electron transfer of the interface.64 As demonstrated in Fig. 8c–e, the Φ values of NiFe-LDH, GDY and RGO are calculated to be 1.96, 5.13 and 4.25 eV,65 respectively. The electrons are inclined to flow from NiFe-LDH to GDY and RGO as they are combined. In particular, more electrons would be transferred from NiFe-LDH to GDY as GDY has a larger Φ than RGO. This result, together with the shorter distance and more electron transfer between GDY and LDH, sufficiently suggests stronger chemical and electronic interactions in GDY/NiFe-LDH than those in RGO/NiFe-LDH and CNT/NiFe-LDH. Hence, the DFT calculations are in accordance with the above XPS and experimental results.
Based on the experimental and theoretical calculation results presented above, we proposed a mechanism to interpret the excellent OER activity of the GDY/NiFe-LDH catalyst (Fig. 9). To sum up, the extraordinary electrocatalytic properties of GDY/NiFe-LDH are derived from several advantageous features: (1) the excellent electronic structure and conductivity of GDY lead to efficient electron capture and transfer abilities in catalysts. Moreover, the strong interaction between the GDY layers provides good mechanical adhesion and electrical connection between them, making them highly conductive supporting matrices. (2) The layered GDY provides a large number of surface oxygen-containing species on GDY (like C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) which facilitate the formation of GDY/NiFe-LDH. (3) Last but not the least, due to the strong chemical and electronic interactions between –C
O) which facilitate the formation of GDY/NiFe-LDH. (3) Last but not the least, due to the strong chemical and electronic interactions between –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C
C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C– and TMs, and the synergistic effect between NiFe-LDH and GDY afforded by direct integration of LDH with surface functional groups, much more exposed active sites are provided for highly efficient mass and electron transfer, which contribute to the superior OER activity of GDY/NiFe-LDH catalysts. These advantages significantly distinguish GDY from the commonly used carbon additives of RGO and CNT, and endow it with great potential to boost the activity of electrocatalysts for the OER.
C– and TMs, and the synergistic effect between NiFe-LDH and GDY afforded by direct integration of LDH with surface functional groups, much more exposed active sites are provided for highly efficient mass and electron transfer, which contribute to the superior OER activity of GDY/NiFe-LDH catalysts. These advantages significantly distinguish GDY from the commonly used carbon additives of RGO and CNT, and endow it with great potential to boost the activity of electrocatalysts for the OER.
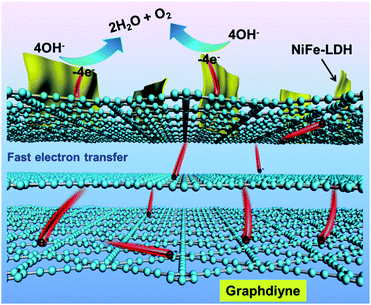 | ||
| Fig. 9 Schematic diagram of the role of GDY in the high OER activity of the GDY/NiFe-LDH electrocatalyst. | ||
Conclusions
In summary, we have prepared a GDY/NiFe-LDH catalyst and applied it for efficient water oxidation reaction. The as-prepared GDY/NiFe-LDH shows outstanding catalytic activity (with a low overpotential of 260 mV at 10 mA cm−2 catalytic current density) and durability towards oxygen evolution in 1.0 M KOH. In particular, it shows superior OER activity compared to its sister composites RGO/NiFe-LDH and CNT/NiFe-LDH, which is ascribed to the larger π-conjugated structure of GDY. On the other hand, strong interaction between –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C
C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C– and TMs contributes to the fast electron transfer and good durability. In addition, DFT calculations confirmed that GDY could serve as a highly efficient electron transfer phase for the OER process due to its sp- and sp2-hybridized and lamellar structure, exhibiting superior electron capture and excellent electron transfer. Our research indicates that GDY can serve as a superior carbon additive to boost the activity of water oxidation catalysts, and possesses great potential for other energy conversion-related fields.
C– and TMs contributes to the fast electron transfer and good durability. In addition, DFT calculations confirmed that GDY could serve as a highly efficient electron transfer phase for the OER process due to its sp- and sp2-hybridized and lamellar structure, exhibiting superior electron capture and excellent electron transfer. Our research indicates that GDY can serve as a superior carbon additive to boost the activity of water oxidation catalysts, and possesses great potential for other energy conversion-related fields.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was partially supported by NSFC (51320105001, 21433007, 51772234 and U1705251), the Natural Science Foundation of Hubei Province of China (2015CFA001) and Innovative Research Funds of SKLWUT (2017-ZD-4).Notes and references
- Y. P. Zhu, C. Guo, Y. Zheng and S. Z. Qiao, Acc. Chem. Res., 2017, 50, 915–923 CrossRef CAS PubMed.
- Y. Hou, M. R. Lohe, J. Zhang, S. Liu, X. Zhuang and X. Feng, Energy Environ. Sci., 2016, 9, 478–483 CAS.
- N. T. Suen, S. F. Hung, Q. Quan, N. Zhang, Y. J. Xu and H. M. Chen, Chem. Soc. Rev., 2017, 46, 337–365 RSC.
- M. Gong and H. Dai, Nano Res., 2014, 8, 23–39 CrossRef.
- Y. Zhang, B. Ouyang, J. Xu, G. Jia, S. Chen, R. S. Rawat and H. J. Fan, Angew. Chem., Int. Ed., 2016, 55, 8670–8674 CrossRef CAS PubMed.
- K. Fan, H. Chen, Y. Ji, H. Huang, P. M. Claesson, Q. Daniel, B. Philippe, H. Rensmo, F. Li, Y. Luo and L. Sun, Nat. Commun., 2016, 7, 11981 CrossRef CAS PubMed.
- A. Vasileff, S. Chen and S. Z. Qiao, Nanoscale Horiz., 2016, 1, 41–44 RSC.
- L. Zhao, B. Dong, S. Li, L. Zhou, L. Lai, Z. Wang, S. Zhao, M. Han, K. Gao, M. Lu, X. Xie, B. Chen, Z. Liu, X. Wang, H. Zhang, H. Li, J. Liu, H. Zhang, X. Huang and W. Huang, ACS Nano, 2017, 11, 5800–5807 CrossRef CAS PubMed.
- G.-F. Chen, Y. Luo, L.-X. Ding and H. Wang, ACS Catal., 2018, 8, 526–530 CrossRef CAS.
- Z. Q. Liu, H. Cheng, N. Li, T. Y. Ma and Y. Z. Su, Adv. Mater., 2016, 28, 3777–3784 CrossRef CAS PubMed.
- H. Cheng, M.-L. Li, C.-Y. Su, N. Li and Z.-Q. Liu, Adv. Funct. Mater., 2017, 1701833 CrossRef.
- Z. Wang, M. Li, L. Fan, J. Han and Y. Xiong, Appl. Surf. Sci., 2017, 401, 89–99 CrossRef CAS.
- X. Zhu, C. Tang, H.-F. Wang, Q. Zhang, C. Yang and F. Wei, J. Mater. Chem. A, 2015, 3, 24540–24546 CAS.
- B. M. Hunter, H. B. Gray and A. M. Müller, Chem. Rev., 2016, 116, 14120–14136 CrossRef CAS PubMed.
- R. Chen, G. Sun, C. Yang, L. Zhang, J. Miao, H. Tao, H. Yang, J. Chen, P. Chen and B. Liu, Nanoscale Horiz., 2016, 1, 156–160 RSC.
- J. Luo, J. H. Im, M. T. Mayer, M. Schreier, M. K. Nazeeruddin, N. G. Park, S. D. Tilley, H. J. Fan and M. Gratzel, Science, 2014, 345, 1593–1596 CrossRef CAS PubMed.
- X. Long, J. Li, S. Xiao, K. Yan, Z. Wang, H. Chen and S. Yang, Angew. Chem., 2014, 53, 7584–7588 CrossRef CAS PubMed.
- C. Tang, H. S. Wang, H. F. Wang, Q. Zhang, G. L. Tian, J. Q. Nie and F. Wei, Adv. Mater., 2015, 27, 4516 CrossRef CAS PubMed.
- H.-F. Wang, C. Tang and Q. Zhang, J. Mater. Chem. A, 2015, 3, 16183–16189 CAS.
- M. Gong, Y. Li, H. Wang, Y. Liang, J. Z. Wu, J. Zhou, J. Wang, T. Regier, F. Wei and H. Dai, J. Am. Chem. Soc., 2013, 135, 8452–8455 CrossRef CAS PubMed.
- J. Ruan, W. Zhao, L. Wu, X. Li, X. Zheng, Q. Ye, X. Xu and F. Wang, Appl. Surf. Sci., 2017, 396, 1146–1154 CrossRef CAS.
- D. Tang, J. Liu, X. Wu, R. Liu, X. Han, Y. Han, H. Huang, Y. Liu and Z. Kang, ACS Appl. Mater. Interfaces, 2014, 6, 7918–7925 CAS.
- A. M. El-Sawy, I. M. Mosa, D. Su, C. J. Guild, S. Khalid, R. Joesten, J. F. Rusling and S. L. Suib, Adv. Energy Mater., 2016, 6, 1501966 CrossRef.
- Z. Lin, G. H. Waller, Y. Liu, M. Liu and C. Wong, Nano Energy, 2013, 2, 241–248 CrossRef CAS.
- L. Shi, K. Chen, R. Du, A. Bachmatiuk, M. H. Rümmeli, K. Xie, Y. Huang, Y. Zhang and Z. Liu, J. Am. Chem. Soc., 2016, 138, 6360–6363 CrossRef CAS PubMed.
- N. Daems, X. Sheng, I. F. J. Vankelecom and P. P. Pescarmona, J. Mater. Chem. A, 2014, 2, 4085–4110 CAS.
- M. Miao, Carbon, 2011, 49, 3755–3761 CrossRef CAS.
- Z. Jia, Y. Li, Z. Zuo, H. Liu, C. Huang and Y. Li, Acc. Chem. Res., 2017, 50, 2470–2478 CrossRef CAS PubMed.
- Y. Li, L. Xu, H. Liu and Y. Li, Chem. Soc. Rev., 2014, 43, 2572–2586 RSC.
- Q. Peng, A. K. Dearden, J. Crean, L. Han, S. Liu, X. Wen and S. De, Nanotechnol., Sci. Appl., 2014, 7, 1–29 CAS.
- G. Li, Y. Li, H. Liu, Y. Guo, Y. Li and D. Zhu, Chem. Commun., 2010, 46, 3256–3258 RSC.
- J. Zhou, X. Gao, R. Liu, Z. Xie, J. Yang, S. Zhang, G. Zhang, H. Liu, Y. Li, J. Zhang and Z. Liu, J. Am. Chem. Soc., 2015, 137, 7596–7599 CrossRef CAS PubMed.
- N. Yang, Y. Liu, H. Wen, Z. Tang, H. Zhao, Y. Li and D. Wang, ACS Nano, 2013, 7, 1504–1512 CrossRef CAS PubMed.
- H. Qi, P. Yu, Y. Wang, G. Han, H. Liu, Y. Yi, Y. Li and L. Mao, J. Am. Chem. Soc., 2015, 137, 5260–5263 CrossRef CAS PubMed.
- Y. Xue, Y. Guo, Y. Yi, Y. Li, H. Liu, D. Li, W. Yang and Y. Li, Nano Energy, 2016, 30, 858–866 CrossRef CAS.
- S. Zhang, H. Liu, C. Huang, G. Cui and Y. Li, Chem. Commun., 2015, 51, 1834–1837 RSC.
- S. Zhang, H. Du, J. He, C. Huang, H. Liu, G. Cui and Y. Li, ACS Appl. Mater. Interfaces, 2016, 8, 8467–8473 CAS.
- J. He, N. Wang, Z. Cui, H. Du, L. Fu, C. Huang, Z. Yang, X. Shen, Y. Yi, Z. Tu and Y. Li, Nat. Commun., 2017, 8, 1172 CrossRef PubMed.
- J. Li, X. Gao, B. Liu, Q. Feng, X. B. Li, M. Y. Huang, Z. Liu, J. Zhang, C. H. Tung and L. Z. Wu, J. Am. Chem. Soc., 2016, 138, 3954–3957 CrossRef CAS PubMed.
- Z. Jin, Q. Zhou, Y. Chen, P. Mao, H. Li, H. Liu, J. Wang and Y. Li, Adv. Mater., 2016, 28, 3697–3702 CrossRef CAS PubMed.
- C. Kuang, G. Tang, T. Jiu, H. Yang, H. Liu, B. Li, W. Luo, X. Li, W. Zhang, F. Lu, J. Fang and Y. Li, Nano Lett., 2015, 15, 2756–2762 CrossRef CAS PubMed.
- J. Xiao, J. Shi, H. Liu, Y. Xu, S. Lv, Y. Luo, D. Li, Q. Meng and Y. Li, Adv. Energy Mater., 2015, 5, 1401943 CrossRef.
- H. Ren, H. Shao, L. Zhang, D. Guo, Q. Jin, R. Yu, L. Wang, Y. Li, Y. Wang, H. Zhao and D. Wang, Adv. Energy Mater., 2015, 5, 1500296 CrossRef.
- J. He, S. Y. Ma, P. Zhou, C. X. Zhang, C. He and L. Z. Sun, J. Phys. Chem. C, 2012, 116, 26313–26321 CAS.
- Z. Zou, H. Shang, Y. Chen, J. Li, H. Liu, Y. Li and Y. Li, Chem. Commun., 2017, 53, 8074–8077 RSC.
- T. Xiong, X. Yuan, X. Chen, Z. Wu, H. Wang, L. Leng, H. Wang, L. Jiang and G. Zeng, Appl. Surf. Sci., 2018, 427, 1107–1117 CrossRef CAS.
- Y. Xue, J. Li, Z. Xue, Y. Li, H. Liu, D. Li, W. Yang and Y. Li, ACS Appl. Mater. Interfaces, 2016, 8, 31083–31091 CAS.
- F. Razmjooei, K. P. Singh, D.-S. Yang, W. Cui, Y. H. Jang and J.-S. Yu, ACS Catal., 2017, 7, 2381–2391 CrossRef CAS.
- Z. Kou, B. Guo, D. He, J. Zhang and S. Mu, ACS Energy Lett., 2017, 3, 184–190 CrossRef.
- M. A. Oliver-Tolentino, J. Vázquez-Samperio, A. Manzo-Robledo, R. G. González-Huerta, J. L. Flores-Moreno, D. Ramírez-Rosales and A. Guzmán-Vargas, J. Phys. Chem. C, 2014, 118, 22432–22438 CAS.
- J. Li, X. Gao, X. Jiang, X.-B. Li, Z. Liu, J. Zhang, C.-H. Tung and L.-Z. Wu, ACS Catal., 2017, 7, 5209–5213 CrossRef CAS.
- X. Yan, T. Itoh, Y. Kitahama, T. Suzuki, H. Sato, T. Miyake and Y. Ozaki, J. Phys. Chem. C, 2012, 116, 17897–17903 CAS.
- Y. Xue, Z. Zuo, Y. Li, H. Liu and Y. Li, Small, 2017, 1700936 CrossRef PubMed.
- C. Lei, X. Zhu, B. Zhu, C. Jiang, Y. Le and J. Yu, J. Hazard. Mater., 2017, 321, 801–811 CrossRef CAS PubMed.
- G.-F. Chen, T. Y. Ma, Z.-Q. Liu, N. Li, Y.-Z. Su, K. Davey and S.-Z. Qiao, Adv. Funct. Mater., 2016, 26, 3314–3323 CrossRef CAS.
- K. Xiao, L. Xia, G. Liu, S. Wang, L.-X. Ding and H. Wang, J. Mater. Chem. A, 2015, 3, 6128–6135 CAS.
- P. Kuang, T. Tong, K. Fan and J. Yu, ACS Catal., 2017, 7, 6179–6187 CrossRef CAS.
- L. Qian, Z. Lu, T. Xu, X. Wu, Y. Tian, Y. Li, Z. Huo, X. Sun and X. Duan, Adv. Energy Mater., 2015, 5, 1500245 CrossRef.
- K. Fan, Y. Ji, H. Zou, J. Zhang, B. Zhu, H. Chen, Q. Daniel, Y. Luo, J. Yu and L. Sun, Angew. Chem., Int. Ed., 2017, 56, 3289–3293 CrossRef CAS PubMed.
- L. Trotochaud, S. L. Young, J. K. Ranney and S. W. Boettcher, J. Am. Chem. Soc., 2014, 136, 6744–6753 CrossRef CAS PubMed.
- M. W. Louie and A. T. Bell, J. Am. Chem. Soc., 2013, 135, 12329–12337 CrossRef CAS PubMed.
- D. Friebel, M. W. Louie, M. Bajdich, K. E. Sanwald, Y. Cai, A. M. Wise, M. J. Cheng, D. Sokaras, T. C. Weng, R. Alonso-Mori, R. C. Davis, J. R. Bargar, J. K. Norskov, A. Nilsson and A. T. Bell, J. Am. Chem. Soc., 2015, 137, 1305–1313 CrossRef CAS PubMed.
- M. S. Burke, M. G. Kast, L. Trotochaud, A. M. Smith and S. W. Boettcher, J. Am. Chem. Soc., 2015, 137, 3638–3648 CrossRef CAS PubMed.
- J. Liu, B. Cheng and J. Yu, Phys. Chem. Chem. Phys., 2016, 18, 31175–31183 RSC.
- Y. Pan, Y. Wang, L. Wang, H. Zhong, R. Quhe, Z. Ni, M. Ye, W. N. Mei, J. Shi, W. Guo, J. Yang and J. Lu, Nanoscale, 2015, 7, 2116–2127 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8nh00027a |
| This journal is © The Royal Society of Chemistry 2018 |