Open Access Article
Open Access ArticleTwo-dimensional transition metal dichalcogenide-based counter electrodes for dye-sensitized solar cells
Eric Singh abc,
Ki Seok Kim
abc,
Ki Seok Kim b,
Geun Young Yeom
b,
Geun Young Yeom *bc and
Hari Singh Nalwa
*bc and
Hari Singh Nalwa *d
*d
aDepartment of Computer Science, Stanford University, Stanford, California 94305, USA
bSchool of Advanced Materials Science and Engineering, Sungkyunkwan University, 2066 Seobu-ro, Jangan-gu, Suwon-si, Gyeonggi-do 16419, South Korea. E-mail: gyyeom@skku.edu
cSKKU Advanced Institute of Nano Technology, Sungkyunkwan University, 2066 Seobu-ro, Jangan-gu, Suwon-si, Gyeonggi-do 16419, South Korea
dAdvanced Technology Research, 26650 The Old Road, Valencia, California 91381, USA. E-mail: nalwa@mindspring.com
First published on 30th May 2017
Abstract
Dye-sensitized solar cells (DSSCs) are gaining considerable interest as alternatives to semiconductor-based thin film solar cells. The noble metal platinum (Pt) is conventionally used as a counter electrode (CE) material for fabricating DSSCs, since Pt is expensive and scarce, therefore, new materials have been explored to develop cost-effective Pt-free counter electrodes. Two-dimensional (2D) graphene-based counter electrodes have achieved the highest power conversion efficiency (PCE, η) of 13%, which has stimulated research activities in 2D layered transition metal dichalcogenides (TMDs) for developing Pt-free DSSCs. In this review, progress made on alternative counter electrodes for fabricating low-cost Pt-free DSSCs, based on earth-abundant 2D TMDs including MoS2, WS2, TiS2, FeS2, CoS2, NiS2, SnS2, MoSe2, NbSe2, TaSe2, NiSe2, FeSe2, CoSe2, Bi2Se3 and their based composites, are discussed and summarized. Also, the considerable progress made on thin films of MoS2 and MoS2 based carbon, graphene, carbon nanotubes (CNTs), carbon nanofibers (CNFs), and poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) composites as efficient counter electrodes (CEs) for DSSCs are discussed, in terms of their electrochemical and photovoltaic properties. At present, PCE values higher than that of standard Pt CE have been recorded for a number of TMD-based CEs, which include MoS2 and MoSe2/thin films deposited on Mo foil, MoS2/CNTs, MoS2/graphene, MoS2/carbon, MoSe2/PEDOT:PSS, NbSe2, FeS2, FeSe2 nanosheets, TiS2/graphene, and NiS2/graphene hybrid systems in DSSCs, for the reduction of triiodide (I3−) to iodide (I−). The highest PCE (η = 10.46%) versus Pt CE (η = 8.25%) at 1 Sun (100 mW cm−2, AM 1.5G) was measured for DSSCs having a low cost and flexible CoSe2/carbon-nanoclimbing wall counter electrode deposited on a nickel foam. Though TMD-based materials show great potential for solar cell devices, their long-term stability is equally important. The DSSCs with a TiS2/graphene hybrid, and TiS2/PEDOT:PSS composite CEs, showed stability up to 20 to 30 days, respectively, without any measurable degradation in the photovoltaic performance. The long-term stability of TMDs-based DSSCs under different environmental conditions is also described in view of their commercial applications.
1. Introduction: dye-sensitized solar cells (DSSCs)
Silicon has been traditionally used in fabricating solar cells. O'Regan and Grätzel1 in 1991 demonstrated dye-sensitized solar cells (DSSC) as a low-cost approach and as an alternative to silicon solar cells. The concept of a DSSC system was hypothesized based on photosynthesis in nature. The first DSSC was fabricated using nanocrystalline porous TiO2 as optically transparent thin-film photoelectrodes and a ruthenium(II)–bipyridyl complex as a photosensitizer (dye), which generated a power conversion efficiency of 7.9% for the triiodide/iodide (I3−/I−) redox couple. This inspired a new field of research based on DSSCs where a broad range of novel materials are now being explored for electrodes (photoanode and counter electrode (CE; cathode)), photosensitizers (dyes), and reduction–oxidation (redox) electrolytes for fabricating DSSC devices.The commonly used components in dye-sensitized solar cells are a photoanode, a CE, a photosensitizing dye, and an electrolyte. These include tris(2,2′-bipyridyl)cobalt(II/III) [Co(bpy)3] (Co2+/Co3+), triiodide/iodide (I3−/I−) and 5,5′-dithiobis(1-methyltetrazole)/1-methyltetrazole-5-thiolate (T2/T−) as redox couples, poly(3,4-ethylenedioxythiophene) (PEDOT), poly(styrenesulfonate) (PSS), poly(3-hexylthiophene) (P3HT), 2,2′,7,7′-tetrakis(N,N-p-dimethoxy-phenylamino)-9,9′-spirobifluorene (spiro-OMETAD) as solid-state hole-transport materials (HTM); cis-bis(isothiocyanato)bis(2,2′-bipyridyl-4,4′-dicarboxylato)ruthenium(II), (N3 dye), ditetrabutylammonium cis-bis(isothiocyanato)bis(2,2′-bipyridyl-4,4′-dicarboxylato)ruthenium(II), where tetrabutyl-ammonium cation: [(C4H9)4N+] (N719 dye), and (tris(cyanato)-2,2′,2′′-terpyridyl-4,4′,4′′-tricarboxylate)ruthenium(II) (N749 or black dye) as photosensitizing dyes, are regularly used. The dye-sensitized mesoporous TiO2 is used as a photoanode, while the Pt-coated fluorine-doped tin oxide (FTO) on a glass substrate is used as a CE, which facilitates the catalysis process. The counter electrode in a DSSC device acts as a catalyst. Pt counter electrodes yield the maximum electrocatalytic activity for the triiodide/iodide (I3−/I−) redox couple, but are poorly effective for iodine-free redox couple such as Co2+/Co3+ and T2/T−. Transparent conducting oxides such as FTO or indium–tin oxide (ITO) on glass is the common substrate used in assembling DSSCs with different CE materials. The power conversion efficiency of DSSCs is governed by many factors including light absorbing capacity of photosensitizing dyes and catalytic materials.
The classical photosensitizing dyes were developed by Grätzel's research team,2,3 including N3 dye that absorbs up to 800 nm, and N749 dye (also known as black dye) which absorbs sunlight in the longer wavelength region up to 920 nm. In 2014, Mathew et al.4 used graphene nanoplatelets as a CE, a new push–pull porphyrin with a donor–π–bridge acceptor (D–π–A) chemical structure as a sensitizing dye, and Co(II)/Co(III) redox mediator for developing a DSSC, which exhibited a Jsc of 18.1 mA cm−2, Voc of 0.91 V, FF of 0.78 and the highest power conversion efficiency (PCE) of 13% under 100 mW cm−2 (AM 1.5) illumination. The high PCE of DSSCs originated from a molecularly engineered porphyrin dye, 4-(7-{2-[(2Z,7Z,11E,16Z)-7,17-bis[2,6-bis(octyloxy)phenyl]-12-[bis({4-[2,4-bis(hexyloxy)phenyl]phenyl})amino]-21,23,24,25-tetraaza-22-zincahexacyclo[9.9.3.13,6.113,16.08,23.018,21]pentacosa 1(20),2,4,6(25),7,9,11,13(24),14,16,18-undecane-2-yl]ethynyl}-2,1,3-benzothiadiazole-4-yl)benzoic acid, coded as SM315 dye. The sensitizing dyes as a light harvester play a very significant role in achieving high PCE for DSSCs, therefore, dyes such as N3, N719, N749, Y123, Z907, JK-303 those absorb as much sunlight as possible is of a great interest, i.e., Y123 dye = 3-[6-[4-[bis(2′,4′-dihexyloxybiphenyl-4-yl)amino-]phenyl[-4,4-dihexylcyclopenta-[2,1-b:3,4-b]dithiphene-2-yl]-2-cyanoacrylic acid; Z907 = cis-bis(isothiocyanato) (2,2′-bipyridyl-4,4′-dicarboxylato) (4,4′-di-nonyl-2′-bipyridyl)ruthenium(II); and JK-306 = (E)-3-{5′-{4-[bis(2′,4′-dihexyloxybiphenyl-4-yl)amino]phenyl}-2,2′-bithiophene-5-yl}-2-cyanoacrylic acid. The many types of dye-sensitizers such as ruthenium dyes, metal-free organic dyes, porphyrin dyes, quantum dots, and perovskites have been explored for DSSCs. Over the past 30 years, significant progress has been made in exploring diverse aspects of DSSC components, including dye-sensitizers, CEs, electrolytes and photoanodes, and many outstanding reviews are available in the literature on this exciting research topic.5–17 Chemical structures of photosensitizing dyes generally used in evaluating the photovoltaic performance of CEs in DSSCs are also discussed elsewhere. Many different types of Pt-free CE materials have been studied for DSSCs including carbon-based materials such as carbon black, mesoporous carbon, carbon nanotubes (CNTs), carbon fibers, fullerenes, graphene-based materials, metals, transition metal oxides, sulfides, carbides, nitrides, selenides, tellurides, chalcogenides, layered double hydroxides, phosphides and their alloys, conducting polymers such as PEDOT:PSS and other composites materials.18–22
Platinum (Pt) and ITO are the traditional materials used in fabricating different types of solar cell devices. In DSSCs, the Pt deposited on a FTO transparent conductive glass substrate is used as a CE for the reduction of triiodide (I3−) ions to iodide (I−) ions. In this case, Pt acts as a catalyst for the regeneration of redox couples while FTO acts as an electron collector. Pt is a highly expensive metal which has been identified as one of the most critically important metals for the U.S. economic growth.23,24 Among electrocatalysts for DSSCs, Pt shows the best photovoltaic performance due to its high conductivity, chemical stability, and electrochemical activity but it is scarce. The current idea is to explore new alternative catalytic materials to replace the conventional Pt CE in DSSCs using easily available, inexpensive, highly electrically conductive, and high electrocatalytic activity materials. Earth-abundant 2D materials that offer high optical transparency and high electrocatalytic activity have been explored as potential candidate materials for CEs (cathode) and for replacing Pt in DSSC devices. During the last decade, graphene-based materials in the form of their thin films, nanosheets, fibers, multilayers, nanoplatelets, quantum dots, nanofoams and their nanocomposites with metals, CNTs, organic polymers, upconversion nanoparticles, titanium dioxide (TiO2), ionic liquids, and halide perovskites, have been extensively investigated as Pt-free CEs for DSSCs25 and heterojunction solar cells.26,27 The merit of graphene-based CEs in DSSC devices includes their high optical transparency, high electrical conductivity, large effective specific surface area, and the flexibility to fabricate them on different types of substrates.
Layered 2D TMDs are graphene analogs, therefore, research activities have been strongly diverted towards this new class of low-cost 2D materials. Among 2D TMDs, transition-metal disulfides and diselenides such as MoS2, MoSe2, WS2, TiS2, NbSe2, TaSe2, NiSe2, FeSe2, CoSe2, SnS2, Bi2Se3 and other TMD thin films have been investigated as CEs to fabricate Pt-free DSSCs. The CE acts as an electrocatalyst, and is one of the main components of a DSSC device which facilitates the reduction of triiodide (I3−) ions to iodide (I−) ions in a redox electrolyte for dye generation. In this review, electrochemical and photovoltaic properties of low-cost catalytic CEs developed from earth-abundant TMDs and their composites with carbon, graphene, CNTs, carbon nanofibers, PEDOT:PSS and other materials are discussed. The impact of materials processing and morphology associated with PCEs of DSSCs has also been analyzed. Finally, this review discusses the electrochemical and environmental stability of TMDs-based CEs for DSSCs.
2. Two-dimensional transition metal dichalcogenides (2D TMDs)
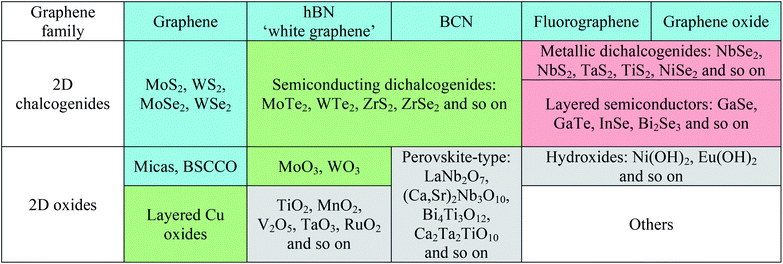
Two-dimensional (2D) transition metal dichalcogenides (TMDs), i.e. MX2 where M is a transition metal (Mo, W, Ti, Zr, Hf, V, Nb, Ta, Tc, Re, Pd, Pt) and X is a chalcogen (S, Se, Te), such as MoS2, WS2, MoSe2, WSe2, form 2D layered structures and are abundantly available in nature. In a MX2 monolayer structure (X–M–X), an atomic layer of a transition metal (M) is sandwiched between two chalcogen (X) atomic layers, where transition metal atoms (M) such as Mo and W are covalently bonded with chalcogen atoms (X) such as S, Se, Te. Each layer is 6–7 Å thick in the TMD layered structures. Weak van der Waals interactions occur between two adjacent MX2 layers of TMDs, whereas the intra-layer M–X bonds are covalent.28–31 From an electrical point of view, MoS2, MoSe2, WS2 and WSe2 are semiconductors, WTe2 and TiSe2 are semimetals, VSe2 and NbS2 are metals, while NbSe2 and TaS2 are superconductors in their bulk crystalline forms.26,30 Geim and Grigorieva32 created a library of 2D materials (Table 1) where the 2D materials were classified into three different classes. Their graphene family includes graphene-based materials hBN, and BCN; the 2D chalcogenides include a large family of TMDs having insulator to semiconducting to metallic behavior; and the 2D oxides include layered oxides, perovskite-based materials, hydroxides, and others. The 2D TMDs include MoS2, WS2, VS2, NbS2, ZrS2, TiS2, NbSe2, WSe2, MoSe2, ZrSe2, WTe2, and MoTe2, and layered semiconductor chalcogenides such as GaSe, GaTe, InSe, Bi2Se3, and Bi2Te3. 2D materials represented by different colors denote their stability under ambient conditions. 2D materials among the graphene family have been extensively studied so far. 2D TMDs such as MoS2, WS2, WSe2 and MoSe2 are structurally similar. Among 2D TMDs, W based materials have stronger spin–orbit coupling while Se materials exhibit lower stability.Research activities into 2D TMDs were intensified after the monolayers of MoS2 showed high carrier mobility of 100 cm2 V−1 s−1 and on/off current ratios of >108 because of the interesting bandgap of MoS2.33 The monolayers of MoS2 are direct semiconductors whereas MoS2 bilayers, trilayers, few-layers are indirect semiconductors. Thickness dependent bandgap energies of 2D TMDs-based semiconductors, including MoS2, MoSe2, MoTe2, WS2, and WSe2 (2H–MX2), were calculated by Yun et al.34 using the first-principles calculations as shown in Fig. 1.34 As the number of layers in the TMDs is reduced to a single layer, the band curvatures lead to significant changes of effective masses. When a single layer of the TMDs is strained, the direct bandgap turns to an indirect bandgap. It was found that bandgap energy and effective masses are reduced by tensile strain, contrary to the compressive strain that increases both parameters. Also the applied larger tensile stress gives rise to a metallic character. This study emphasized the electronic structures of 2D TMDs.
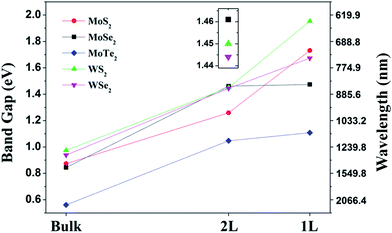 | ||
| Fig. 1 Colored lines representing thickness dependence of bandgap energies of 2D TMDs including MoS2, MoSe2, MoTe2, WS2, and WSe2 attain (2H–MX2 semiconductors). Reprinted with permission from ref. 34, W. S. Yun, S. W. Han, S. C. Hong, I. G. Kim and J. D. Lee, thickness and strain effects on electronic structures of transition metal dichalcogenides: 2H–MX2 semiconductors (M = Mo, W; X = S, Se, Te). Phys. Rev. B, 2012, 85, 033305. Copyright© American Physical Society. | ||
The photoluminescence and absorption spectra of monolayers of MoS2, MoSe2, WS2, and WSe2 have been measured.35 TMDs as bulk crystals are indirect-bandgap semiconductors, however they become direct-bandgap semiconductors as their thickness is reduced to monolayers. The bandgap increases as the number of layers decreases. Photoluminescence peaks at 1.84 eV for MoS2, 1.65 eV for WSe2 and 1.56 eV for MoSe2 have been observed.36 The indirect bandgap at 1.2 eV for bulk crystals of WSe2 was reported,37 whereas the monolayer showed a photoluminescence peak at 1.65 eV (752 nm). In the case of bulk crystals of MoSe2, the indirect bandgap was measured as 1.1 eV (1.13 μm) while the monolayer exhibited a photoluminescence peak at 1.57 eV (792 nm). The photoluminescence emission peak for monolayer WS2 appeared at 1.97 eV.38
Mechanical exfoliation is the most common approach for peeling off monolayers or a few-layers of TMD materials from their bulk crystals,39–41 which is a top-down method. A second widely used approach is chemical vapor deposition (CVD), which is a bottom-up method to consecutively deposit desired thickness layers of TMDs.42–45 Other approaches for preparing layers of TMDs include chemical,46 lithium intercalation,47–49 and ultrasonic-assisted liquid exfoliation in organic solvents,50–54 and salt-assisted liquid-phase exfoliation,55 all of which can prepare mono-layers to multi-layers of TMDs, such as MoS2, MoSe2, WS2, and WSe2.
Raman scattering methods for single-layer, multi-layer and bulk 2D TMDs, including MoS2, MoSe2, WS2, and WSe2, in terms of phonons with respect to the number of layers, has been reviewed and analyzed.56,57 Tonndorf et al.37 studied photoluminescence and Raman characteristics of the monolayers of MoS2, MoSe2, and WSe2. Fig. 2a shows a schematic representation of the four Raman active modes and two Raman inactive modes of TMDs MX2 (M = Mo, W and X = Se, Se) as predicted for the D6h point group.58 The Raman active modes include three in-plane modes referred as E1g, E12g, and E22g, and one out-of-plane mode referred as A1g. However, experimentally only the two active Raman modes E12g and A1g were observed. The active Raman E22g, mode appears at very low frequencies, whereas the E1g mode is forbidden.59 A systematic study of the optical absorption of single-layer and few-layers of MoS2 from the 385 nm (3.22 eV) to 1000 nm (1.24 eV) spectral range was conducted by Castellanos-Gomez et al.60 using a hyperspectral imaging technique. Poly(dimethylsiloxane) (PDMS) was used as a transparent substrate for depositing mechanically exfoliated MoS2 thin films. The optical absorbance spectra of MoS2 flakes consisting of single-layer to six-layer were recorded as a function of different excitation wavelengths. The bandgap of a monolayer MoS2 was measured as 1.85 eV, which decreased with increasing number of MoS2 layers, reaching a bandgap value of 1.35 eV for the bulk MoS2. Fig. 2b and c shows Raman spectra for the E12g and A1g modes of the exfoliated MoS2 flakes ranging from single-layer to six-layer, and the variation of peak frequency difference (Δ) between the E12g and A1g modes as a function of the number of MoS2 layers. The Δ value increases with the increasing number of layers from 19.8 cm−1 for a single-layer to 25 cm−1 for bulk MoS2.
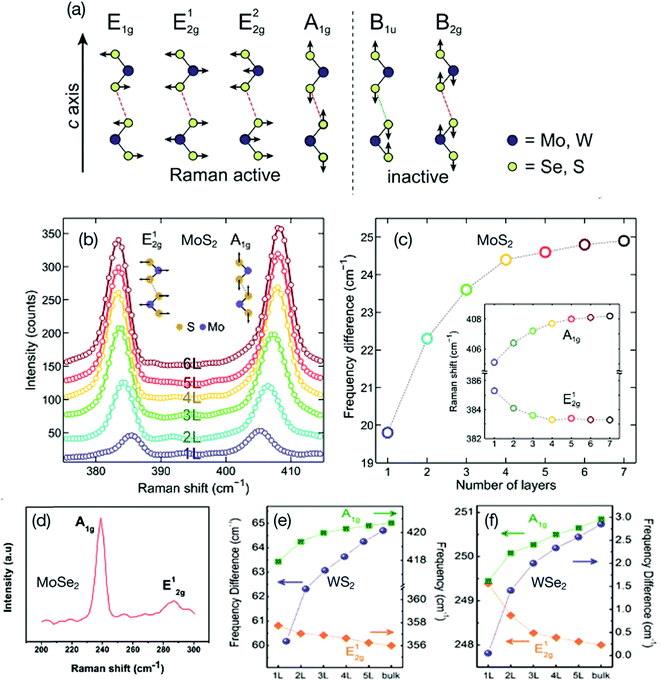 | ||
| Fig. 2 (a) Schematic representation of the four Raman active modes and two Raman inactive modes of TMDs MX2 (M = Mo, W and X = Se, Se). Reprinted with permission from ref. 37, P. Tonndorf, R. Schmidt, P. Böttger, X. Zhang, J. Borner, A. Liebig, M. Albrecht, C. Kloc, O. Gorgan, D. R. T. Zahn, S. M. de Vasconcellos and R. Bratschitsch, Photoluminescence Emission and Raman Response of Monolayer MoS2, MoSe2, and WSe2. Opt. Express, 2013, 21, 4908–4916. Copyright© Optical Society of America. (b) Raman spectra of mechanically exfoliated MoS2 flakes deposited onto a transparent poly(dimethylsiloxane) (PDMS) substrate with different number of layers, single-layer to six-layers of MoS2. (c) Peak frequency difference (Δ) between Raman modes as a function of the number of MoS2 layers. Reprinted with permission from ref. 60, A. Castellanos-Gomez, J. Quereda, H. P. van der Meulen, N. Agraït and G. Rubio-Bollinger, spatially resolved optical absorption spectroscopy of single-and few-layer MoS2 by hyperspectral imaging. Nanotechnol., 2016, 27, 115705. Copyright© Institute of Physics (IOP). (d) Raman spectrum of few-layer MoSe2 nanosheets showing two distinct Raman active modes; A1g and E12g. Reprinted with permission from ref. 61, S. K. Balasingam, J. S. Lee and Y. Jun, few-layered MoSe2 nanosheets as an advanced electrode material for supercapacitors, Dalton Trans., 2015, 44, 15491–15498. Copyright© Royal Society of Chemistry. (e, f) Thickness dependence of Raman A1g and E12g modes of 1 to 5 layers and bulk WS2 and WSe2. Reprinted with permission from ref. 62, W. Zhao, Z. Ghorannevis, K. K. Amara, J. R. Pang, M. Toh, X. Zhang, C. Kloc, P. H. Tan and G. Eda, lattice dynamics in mono-and few-layer sheets of WS2 and WSe2. Nanoscale, 2013, 5, 9677–9683. Copyright© Royal Society of Chemistry. | ||
Raman spectroscopy for few-layer MoSe2 nanosheets was recorded by Balasingam et al.61 which showed Raman peaks at 239 cm−1 and 287.11 cm−1 (Fig. 2d) corresponding to the A1g mode (out-of-plane) and E12g mode (in-plane) of MoSe2, respectively. For bulk crystal MoSe2, the A1g and E12g modes appear at 242 cm−1 and 286 cm−1, respectively. The red shift in the A1g mode and a blue shift in the E12g mode indicate the formation of a few-layered MoSe2 nanosheet. Raman spectra of monolayers, few-layers and bulk WS2 and WSe2 were evaluated by Zhao et al.62 Thickness dependent Raman A1g and E12g modes of 1 to 5 layers, as well as bulk WS2 and WSe2, are shown in (Fig. 2e and f). As discussed above, the frequency difference (Δ) can be used to distinguish the number of layers. McCreary et al.38 reported the E12g mode at 357.5 cm−1 (in-plane) and A1g mode at 419 cm−1 (out-of-plane) with a frequency difference (Δ) of 61.5 cm−1 for WS2 monolayer. Photoluminescence emission and Raman scattering of 2D TMDs have been extensively studied and analyzed by several research groups around the world.63–71
3. TMD-based counter electrodes for DSSCs
The new materials used as CEs in DSSC devices are generally characterized by their molecular structure and surface morphology using a wide variety of spectroscopic techniques, including X-ray diffraction (XRD), Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), UV-vis (Ultraviolet-visible) spectrometry, atomic force microscopy (AFM), field-emission scanning electron microscopy (FESEM), high-resolution transmission electron microscopy (HRTEM) and energy dispersive X-ray spectroscopy (EDX). The electrochemical catalytic activity and photovoltaic performance of CEs in DSSCs are evaluated using different methods. Electrocatalytic activity can be evaluated by cyclic voltammetry (CV), rotating disk electrode (RDE) for determining rate constant and effective catalytic surface area, interfacial charge transfer parameters (series resistance and charge-transfer resistance) measurements by electrochemical impedance spectroscopy (EIS) and Tafel polarization plots for electrocatalytic ability, incident photon-to-current conversion efficiency (IPCE) spectra measurements; these measurements of a DSSC device and their electro-catalytic activities are compared with a standard Pt CE. Photovoltaic tests of a DSSC device are performed by measuring photocurrent density–voltage (J–V) characteristic curves of CEs under a simulated solar illumination at 100 mW cm−2 (AM 1.5G) corresponding to 1 Sun intensity unless specified for other sunlight intensity. The photovoltaic and electrochemical parameters of the DSSC system include short-circuit photocurrent density (Jsc), open-circuit voltage (Voc), fill factor (FF), power conversion efficiency (PCE) which is the solar-to-electricity conversion efficiency (η), series resistance (Rs), charge-transfer resistance (RCT) at the CE/electrolyte interface, capacitance, and Nernst diffusion impededance (ZN). When using a new material as a CE catalyst, the electrochemical and photovoltaic properties of a DSSC device are compared with a conventional Pt CE due to its optimal electrocatalytic activity and chemical stability in the electrolyte. The dye-adsorbed TiO2 mesoporous film deposited on a FTO glass substrate is used as a photoanode (working electrode) of the DSSC device. The ruthenium N719 dye has been commonly used for the reduction of triiodide (I3−) to iodide (I−). DSSCs are assembled with different CEs to be studied, and filled with the same redox electrolyte solution such as triiodide/iodide (I3−/I−) redox couple. The CE reduces the triiodide (I3−) ions to iodide ions (I−). For example, a typical iodide electrolyte solution contains 0.1 M of LiI, 0.05 M of iodine (I2), 0.6 M of 1,2-dimethyl-3-n-propylimidazolium iodide (DMPII), and 0.5 M of 4-tert-butylpyridine (TBP) in acetonitrile. All these spectroscopic techniques and electrochemical measurements were performed, which have been discussed throughout this review as needed in order to evaluate the potential of 2D TMDs (such as MoS2, WS2, TiS2, FeS2, CoS2, NiS2, SnS2, MoSe2, NbSe2, TaSe2, NiSe2, FeSe2, CoSe2, Bi2Se3 and their based hybrids/composites) as CEs for fabricating low-cost Pt-free DSSCs.3.1 MoS2 counter electrodes
TMDs are one of the 2D graphene analogs which show great potential for both bulk-heterojunction (BHJ) and dye-sensitized solar cells. A review article on atomically thin MoS2 layers used as the electron-transport layer (ETL), hole-transport layer (HTL), interfacial layer, and protective layer in fabricating bulk-heterojunction (BHJ) solar cells has been recently published by Singh et al.118 Here, the applications of MoS2 thin films as CEs for fabricating Pt-free dye-DSSCs are discussed.
Polytypism in MoS2 has been studied by using Raman spectroscopy.121 Layered MoS2 exists in 2H, 3R and 1T phases where monolayers are stacked in a different sequence.122–124 The semiconducting 2H–MoS2 phase of the bulk crystal contains two-layer per unit cell stacked in a hexagonal symmetry, where each Mo atom is coordinated with six sulfur (S) atoms. The 3R–MoS2 phase contains three-layer per unit cell stacked in a rhombohedral symmetry. The metallic 1T phase contains one MoS2 layer per unit cell in tetragonal symmetry with octahedral coordination. A structural phase transition of 2H–MoS2 to 1T–MoS2 (2H → 1T) has been reported due to a lithium ion intercalation process.122–125 The metallic 1T phase of MoS2 exhibits very interesting electronic properties.126–129 Therefore, Wei et al.130 used metastable 1T metallic phase MoS2 in fabricating DSSCs. In that work, MoS2 films were deposited on FTO glass substrate by a hydrothermal method, performing reactions at 180 or 200 °C for 24 hours. The MoS2 films prepared at 200 °C showed lumps, while those grown at 180 °C had flower-like structures. High-angle annular dark-field scanning transmission electron microscopy (HAAD-FSTEM) images, Raman spectroscopy, and XPS also showed the formation of 2H and 1T metallic phases of MoS2. Fig. 3 represents the J–V curves of 2H–MoS2 and flower-structured 1T metallic phase MoS2. The flower-structured 1T metallic MoS2 film was grown onto an FTO substrate as a CE of DSSC, which exhibited PCE (η) of 7.08%, that is a three times higher PCE compared with 2H phase MoS2 (η = 1.72%) and comparable to a Pt CE (η = 7.25%). Such a large difference in PCE values of 1T and 2H phases occurs because the electrical conductivity of 1T–MoS2 is 107 times larger compared with 2H–MoS2, which gives rise to a higher electrocatalytic activity for the 1T phase than that of the 2H phase. The 1T–MoS2 CE also demonstrated lower charge-transfer resistance (RCT). The IPCE curves and CV measurements also showed a better electrocatalytic activity of 1T–MoS2 CE in DSSC than that of 2H–MoS2.
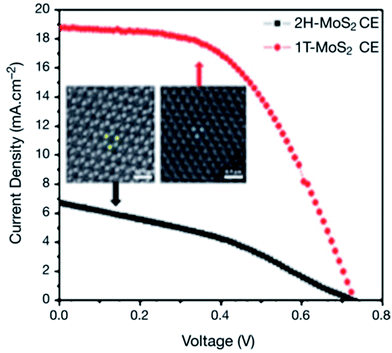 | ||
| Fig. 3 Photocurrent density–voltage (J–V) curves of 2H-type MoS2 and flower-structured 1T metallic phase MoS2. Insert shows high-angle annular dark-field scanning transmission electron microscopy (HAAD-FSTEM) confirming 2H and 1T metallic phase of MoS2. Reprinted with permission from ref. 130, W. Wei, K. Sun and Y. H. Hu, an efficient counter electrode material for dye-sensitized solar cells—flower-structured 1T metallic phase MoS2. J. Mater. Chem. A, 2016, 4, 12398–12401. Copyright© Royal Society of Chemistry. | ||
A very interesting study was conducted by Infant et al.131 who developed CE materials for DSSCs by vertically oriented MoS2 on an FTO substrate, in order to increase the reflectivity of MoS2 CE. Fig. 4 shows high resolution SEM images of CVD-deposited MoS2 thin films, the reflectivity of MoS2 CE measured by UV-vis spectrophotometer, and CV measurements using standard hydrogen electrode (SHE) as a reference electrode. The high quality thin films of MoS2 were obtained at the optimum conditions of 600 °C with a 15 minute reaction time at a flow rate of 50 sccm. The FTO substrate is damaged if temperature is increased above 600 °C and the reaction time is over 15 minutes, leading to excessive deposition of sulfur on the surface. The layered MoS2 thin films are polycrystalline and have 0.18–0.27 nm spacing as evidenced by the TEM image and SEAD pattern. The CVD-prepared MoS2 thin films are vertically oriented on the FTO substrate, which yields more active sites and eventually enhance the reflectivity so that more photons are absorbed, and also created active edge sites facilitating the generation of the I−/I3− redox couple. The reflectance of vertically inclined MoS2 films on the FTO substrate was measured by a UV-vis spectrophotometer between 350–800 nm wavelength for reaction temperatures varying from 400 to 700 °C, a reaction time ranging from 5 to 30 minutes, and different flow rates of Ar gas (Fig. 4). The maximum reflectance of 38% was observed for a vertically inclined MoS2 thin film under optimized conditions, due to high crystallinity, and the inclined angle of 26° was estimated that supports the reflectivity of photons to dye molecules. The reflectivity of 38% was observed for MoS2 thin films prepared at 50 sccm due to its crystallinity and higher inclination angle, which contributes to more absorption photons, and hence leads to a higher PCE of 7.50% compared to PCE of 7.38% for 150 sccm with 25% reflectivity, due to smaller angle of MoS2 inclination. This approach yielded a PCE of 7.50% for the MoS2 CE, exceeding the PCE of Pt based CE (η = 7.28%). The MoS2 CE shows a higher Jsc value of 15.2 mA cm−2, and is higher than Pt CE (Jsc = 14.6 mA cm−2) due to high reflectivity. The RCT of MoS2 CE was lower than Pt CE, indicating the higher electro-catalytic activity of vertically inclined MoS2 film on the FTO substrate was due to more active edge sites, which gives rise to enhanced electrocatalytic activity of the MoS2 CE. Two redox peaks are observed in the CV curves of Pt and MoS2 CEs, one corresponding to the reduction of I3−, which is a cathodic peak (Vpc), and the other corresponding to the oxidation of I−, which is an anodic peak (Vpa). The value of anodic peak to cathodic peak separation (Vpp) was found to be less for the MoS2 CE (0.456 V) than that of Pt (0.484 V), which also indicates a better electrocatalytic activity of MoS2 CE due to the presence of active edge sites, as evidenced in the SEM images.
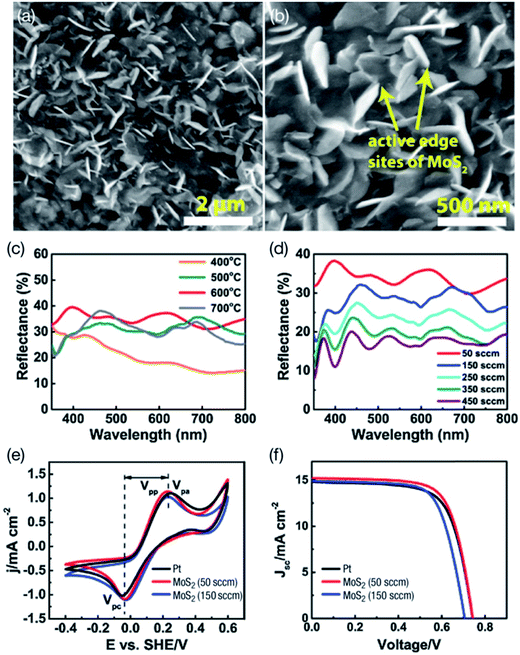 | ||
| Fig. 4 SEM (a) and high resolution SEM (b) of CVD-deposited MoS2 thin films. The highest quality thin films of MoS2 were prepared at 600 °C with 15 minute reaction time at the Ar flow rate of 50 sccm. (c) Optical properties of MoS2 thin films between 350–800 nm range prepared at different temperature. (d) Flow rates of Ar gas between 50 to 450 sccm for preparing MoS2 thin films by CVD technique. (e) Cyclic voltammetry (CV) plots using standard hydrogen electrode (SHE) as a reference electrode and (f) photocurrent density–voltage (J–V) curves of MoS2 and Pt based CEs prepared at 50 and 150 sccm flow rates of Ar gas. Reprinted with permission from ref. 131, R. S. Infant, X. Xu, W. Yang, F. Yang, L. Hou and Y. Li, highly active and reflective MoS2 counter electrode for enhancement of photovoltaic efficiency of dye sensitized solar cells. Electrochim. Acta, 2016, 212, 614–620. Copyright© Elsevier. | ||
Vertical MoS2 nanosheets on different substrates using CVD and CS2 as a sulfur precursor have been developed.132 The DSSC CEs with vertical MoS2 nanosheets showed a comparable electro-catalytic activity to Pt CE for the triiodide (I3−) reduction, resulting from large specific surface areas and more active edges. Li et al.133 prepared molybdenum disulfide-based CEs for DSSCs with different morphologies (multilayers, few-layers and nanoparticles) using the thermal decomposition method. The X-ray diffraction and transmission electron microscopy showed edge area to basal-plane ratio in the following order: MoS2 nanoparticles > multilayered MoS2 > few-layered MoS2. A similar order was observed for the PCE values with corresponding CEs-based DSSCs. The MoS2 nanoparticles-based CE had the minimum RCT, while the few-layered MoS2 based CE had the maximum, as measured by EIS. The active sites of MoS2 responsible for the reduction of triiodide lie on the edges of layered materials, instead of their basal planes. MoS2 nanoparticle CE showed the highest PCE value of 5.41%, compared with 6.58% of Pt CE. A novel approach for improving PCE of MoS2 CE based DSSCs has been developed,134 where electrocatalytic activity was enhanced by artificially generating active edge sites on MoS2 atomic layers by hole patterning. The PCE of the DSSC increased from 2% to 5.8% after applying the hole patterning approach. Al-Mamun et al.135 deposited MoS2 nanoscale thin films onto FTO substrates using a low temperature one-pot hydrazine assisted hydrothermal method. Both the hydrothermal reaction temperature as well as the different molar ratio of reaction precursors was found to impact the structure and performance of MoS2 films used as CEs for DSSCs. The MoS2 thin films having surface exposed layered nanosheets were obtained by the hydrothermal process with a molar ratio of reaction precursors as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 of (NH4)6Mo7O24·4H2O and NH2CSNH2 (thiourea) at 150 °C for 24 hours, referred to as MS-150-28. The molar ratio of (NH4)6Mo7O24·4H2O and NH2CSNH2 was fixed at 1
28 of (NH4)6Mo7O24·4H2O and NH2CSNH2 (thiourea) at 150 °C for 24 hours, referred to as MS-150-28. The molar ratio of (NH4)6Mo7O24·4H2O and NH2CSNH2 was fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 1
7, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14, 1
14, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 and 1
28 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 and the hydrothermal temperature was maintained at either 120, 150, 180 or 210 °C. The DSSCs having MoS2 CEs fabricated using different molar ratios of reaction precursors at a temperature of 150 °C (referred to as MS-150-7, MS-150-14, MS-150-28 and MS-150-42) exhibited PCEs of 3.70, 4.97, 7.41 and 4.96%, respectively. The DSSCs with MS-120-28, MS-180-28, and MS-210-28 CEs showed PCEs of 5.52, 7.15 and 5.47%, respectively. The MoS2 film based CEs showed a PCE of 7.41%, higher than the Pt electrode based DSSCs (η = 7.13%) using TiO2 photoanodes sensitized by N719 dye.
42 and the hydrothermal temperature was maintained at either 120, 150, 180 or 210 °C. The DSSCs having MoS2 CEs fabricated using different molar ratios of reaction precursors at a temperature of 150 °C (referred to as MS-150-7, MS-150-14, MS-150-28 and MS-150-42) exhibited PCEs of 3.70, 4.97, 7.41 and 4.96%, respectively. The DSSCs with MS-120-28, MS-180-28, and MS-210-28 CEs showed PCEs of 5.52, 7.15 and 5.47%, respectively. The MoS2 film based CEs showed a PCE of 7.41%, higher than the Pt electrode based DSSCs (η = 7.13%) using TiO2 photoanodes sensitized by N719 dye.
Pulse electrochemical deposited thin films of molybdenum sulfide (MoSx) on indium tin oxide/poly(ethylene naphthalate) (ITO/PEN) substrates have been studied as flexible CEs for DSSC, and these showed a PCE of 4.39% for the triiodide (I3−) reduction.136 The nanostructured MoS2 thin films developed by a low-temperature thermally reduced technique on a FTO substrate have also been used for DSSCs.137 MoS2 thin film annealed at 300 °C were also used as CEs for DSSCs, which showed a PCE of 6.351%, slightly lower than the Pt reference CE (η = 6.929%). The performance of DSSCs was impacted by the molar ratio of reaction precursors and the temperature of thermal reaction. Thermally reduced (TR) MoS2 thin film annealed at 250 °C showed PCE of 1.917%, while those annealed at 350 °C showed PCE of 3.479%. The 300 °C annealed TR–MoS2 CE also has larger exchange current density than those of 250 °C and 350 °C annealed TR–MoS2 CEs and comparable with thermally deposited (TD) Pt CE. The RCT values that correspond to the charge-transfer resistance at the electrolyte–electrode interface were 14.98 Ω cm2 for TD–Pt CE and 30.98, 141.41 Ω cm2 for TR–MoS2 CEs annealed at 300 °C and 350 °C, respectively. TR–MoS2 CEs annealed at 250 °C has no RCT value being too large. The annealing temperature of 300 °C generates much larger active area, providing the highest electrocatalytic activity for the reduction of I3−, while 350 °C annealing decreases the active sites of the edge-planes in MoS2. A PCE of 7.01% was achieved for DSSCs using pristine MoS2 as a CE, chemically synthesized by low-temperature wet-chemical process, which has a comparable PCE of 7.31% for DSSCs with Pt CEs.138 The Rs and RCT values of 23.51 and 18.50 Ω cm2, respectively, for the MoS2 CE were lower than those of Pt CE (26.73 and 22.88 Ω cm2), suggesting better electro-catalytic activity of MoS2 for the reduction of triiodide (I3−).
A correlation between the electrical conductivity of the CE, PCE, and the crystallinity of MoS2 was also demonstrated.139 The XRD, XPS, EIS and Hall measurements established a link between the PCE, carrier concentration, mobility, and Jsc value. The DSSCs having pristine (non-annealed), vacuum-annealed and N2-annealed MoS2 CEs showed PCE values of 1.0, 1.7 and 0.8%, respectively. Thermal annealing in vacuum was found to reduce the over-potential that leads to an increased Jsc value of 7.95 mA cm−2 due to high MoS2 crystallinity, whereas the N2-annealing of MoS2 CEs increases over-potential, which gives rise to lower Jsc value of 4.35 mA cm−2 due to the poor crystallinity of MoS2. Interestingly, the electrical conductivity of MoS2 CEs follows the order: N2-annealed > vacuum-annealed > non-annealed MoS2. This indicates that the PCE of the DSSCs is influenced by the over-potential that involves an electron transferring from the MoS2 CE to the electrolyte, instead of the electrical conductivity of the CE. Antonelou et al.140 reported the growth of monolayer and few-layer MoS2 films by the sulfurization of molybdenum (Mo) foils at 800 °C. The few-layer thick MoS2 films were used as CEs for DSSCs for the reduction of I3− to I−. The electrocatalytic activity of MoS2 CE on flexible Mo substrates depends upon the number of monolayers in the DSSC. DSSCs having the MoS2/Mo CE yield a PCE of 8.4%, close to Pt/FTO-based DSSCs (PCE of 8.7%). Stability of a three-monolayer thick MoS2 CE was studied for 100 consecutive cycles, where no degradation of the peak current density was noticed for 100 repeated cycles, confirming long term electrochemical stability in an electrolyte solution. MoS2 layers with 1–2 nm thickness showed long term chemical stability of the DSSC device for the electrolyte solution comparable to Pt CE. The increased number of active sites due to a grainy surface texture of Mo foil leads to the higher electro-catalytic activity of MoS2 films.
A very interesting comparison was made by Wu et al.141 for chemically synthesized MoS2 and WS2 as CEs in the reduction of I3− to I− and disulfide/thiolate (T2/T−) based DSSCs. The RCT values of 0.5 Ω cm2 for MoS2 and 0.3 Ω cm2 for WS2, respectively, compared with RCT of 3.0 Ω cm2 for Pt CE, indicates that both MoS2 and WS2 are as effective as CEs as standard Pt for triiodide (I3−) reduction in DSSCs. The high FFs of 73% for MoS2 CEs and 70% for WS2 CEs also confirm high electro-catalytic activities for the reduction of triiodide (I3−) to iodide (I−). Therefore, high PCE values of 7.59% for MoS2 and 7.73% for WS2 were observed, which are comparable to the PCE of 7.64% for Pt CEs in DSSCs under simulated AM 1.5 illumination. The ZN of >100 Ω for triiodide (I3−) reduction on the sulfide electrodes was found to be higher compared with a ZN of 9.5 Ω on the Pt CE. The photocurrent density voltage (J–V) curves of the DSSCs having MoS2, WS2, and Pt based CEs for the triiodide/iodide (I3−/I−) redox couple and disulfide/thiolate (T2/T−) redox couple are shown in (Fig. 5). The TiO2 film photoanode was obtained after pre-heating at 80 °C and immersing in a 5 × 10−4 M solution of N719 dye in acetonitrile/tert-butyl alcohol for 20 hours. The triiodide/iodide (I3−/I−) electrolyte is made of 0.06 M of lithium iodide (LiI), 0.03 M of I2, 0.5 M of 4-tert-butyl pyridine, 0.6 M of 1-butyl-3-methylimidazolium iodide, and 0.1 M of guanidinium thiocyanate in acetonitrile. The 5-mercapto-1-methyltetrazole di-5-(1-methyltetrazole) disulfide/N-tetramethylammonium salt (+NMe4T−) (T2/T−) electrolyte consists of 0.4 M of +NMe4T−, 0.05 M of LiClO4, 0.4 M of di-5-(1-methyltetrazole) disulfide (T2), and 0.5 M 4-tert-butylpyridine in acetonitrile and ethylene carbonate solution. For both DSSCs, a spray-coating technique was used for fabricating MoS2 and WS2 CEs. The J–V curves of the DSSCs having sulfide CEs and T2/T− electrolyte show PCE values of 4.97% for MoS2, 5.24% for WS2, and 3.70% for Pt CE. The PCE values were increased 36% for MoS2 and 41% for WS2 compared to the Pt CE, showing that DSSCs having MoS2 and WS2 are better than that of the Pt CE for the T2/T− redox couple.
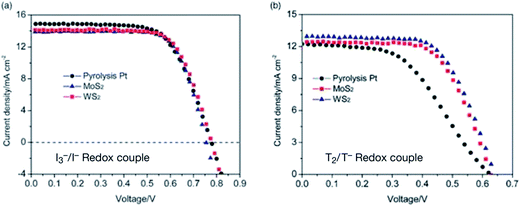 | ||
| Fig. 5 (a) Photocurrent density–voltage (J–V) curves of the DSSCs having MoS2, WS2, and Pt counter electrodes for triiodide to iodide (I3−/I−) reduction. (b) J–V curves of the DSSCs using MoS2, WS2 and Pt CEs for redox couple of disulfide/thiolate (T2/T−). Reprinted with permission from ref. 141, M. Wu, Y. Wang, X. Lin, N. Yu, L. Wang, L. Wang, A. Hagfeldt and T. Ma, economical and effective sulfide catalysts for dye-sensitized solar cells as counter electrodes. Phys. Chem. Chem. Phys., 2011, 13, 19298–19301. Copyright© Royal Society of Chemistry/Owner Societies. | ||
The interfaced exfoliated MoS2 thin films with different porphyrin molecules, where the MoS2 was functionalized with zinc(II) porphyrin (ZnPP), showed a 10-fold increase in photocurrent compared to MoS2 films.142 Exfoliated ultrathin porous MoS2 sheets prepared by a tetraethylorthosilicate (TEOS)-assisted hydrothermal method were used as CEs in DSSCs.143 The cyclic voltammograms and electrochemical impedance spectroscopy showed low RCT and high electro-catalytic activity for porous MoS2 sheets CEs in DSSCs, with a PCE of 6.35%, slightly better than that of Pt CEs (η = 6.19%) under similar experimental conditions. A CE created by spin-coating of MoS2 nanosheets followed by thermal treatment was also prepared.144 DSSCs having MoS2 nanosheets thermally treated at 100 °C showed a PCE value of 7.35%, comparable to conventional a Pt CE (7.53%). When MoS2 nanosheets were thermally treated at 300 °C, the PCE value decreased significantly due to the transformation of MoS2 to MoO3. MoS2 films deposited on FTO glass using an RF sputtering method were also used as CEs for TiO2-based DSSCs.145 CV, EIS, and Tafel polarization curve measurements conducted on the MoS2 CE showed high electrocatalytic activity, low charge-transfer resistance as well as the fast reaction kinetics for triiodide (I3−) reduction. The MoS2 CE prepared after 5 minutes of sputtering showed a PCE of 6.0%, comparable to Pt CEs (η = 6.6%) in DSCCs. The PCE of DSSCs having MoS2 CEs sputtered for 1, 3, 5 and 7 minutes were 5.7, 5.8, 6.0, and 5.2%, respectively. MoS2 CEs were also developed by synthesizing MoS2 films at 70 °C using molybdenum(V) chloride and thioacetamide, followed by near-infrared laser-sintering for 1 minute to enhance crystallinity and interconnectivity between the MoS2 particles.146 The DSSC with laser-sintered MoS2 CE exhibited a PCE of 7.19%, much higher than heat-sintered MoS2 CE (5.96%) and comparable with a Pt CE (η = 7.42%). The laser-sintered MoS2 CE offers superior electrocatalytic activity for the triiodide (I3−) to iodide (I−) redox couple. Also, a solution-phase process was used to grow MoS2 nanofilms on FTO glass as a CE for a DSSC, which showed a PCE of 8.3%.147 Finally, exfoliated and annealed MoS2xSe2(1 − x) as well as exfoliated-MoS2 films were used as CEs.148 The thickness and size of exfoliated MoS2 nanosheets were 0.9 by 1.2 nm and 0.2 by 2 μm, corresponding to a single-layer. The MoS2 based CE showed a PCE of 6%, compared to 5.1% for the annealed MoS2 films.
To fabricate a Pt-free DSCC, Lin et al.151 used a MoS2/graphene nanosheet composite as a CE and nanocrystalline TiO2 as a photoanode. The redox electrolyte solution in the DSSC was made of 1 M 1,3-dimethylimidazolium iodide, 0.15 M iodine, 0.5 M 4-tert-butylpyridine and 0.1 M guanidine thiocyanate in 3-methoxypropionitrile. The MoS2/graphene nanosheet based CE showed a PCE of 5.81%, in comparison to a PCE of 6.24% for the conventional sputtered Pt CE. Yue et al.152 used a MoS2/graphene flake composite film as the CEs for DSSCs with N719 dye for the reduction of triiodide (I3−) to iodide (I−). The MoS2 powder (ARCOS, 99%) and 8 nm flakes of multi-layer graphene nanopowder (Uni-Onward Corp., 99.5%) were mixed in specific ratios to prepare the composites. The electrocatalytic activity increased after adding graphene flakes to the MoS2 film. Charge-transfer resistances (RCT) of 3.98 Ω cm2 for graphene, 2.71 Ω cm2 for MoS2, 2.09 Ω cm2 for MoS2/graphene, and 2.01 Ω cm2 for Pt CEs were measured. The current density of the MoS2/graphene composite CE was recorded to be higher than that of the MoS2, graphene and Pt CEs. The J–V characteristics of the DSSCs having MoS2/graphene as CEs and ranging in thickness from single-layer to six-layer were investigated and compared with conventional Pt CEs. The thickness of the MoS2/graphene layers affected the PCE of the DSSCs, and Jsc and Voc increased with increasing number of plaster layers from single to 3-layer, and, thereafter, a decrease was noticed for 4-layers and 5-layers. The effect of graphene content was also studied, where, the RCT was found to decrease for the MoS2/graphene CE, from 0.5 wt% to 1.5 wt% of graphene content. The PCE of the MoS2/graphene composite CE having 1.5 wt% graphene was 5.98%, compared to the PCE of 6.23% for Pt CE. Yu et al.153 used MoS2 nanosheets and graphene composites for fabricating CEs for the triiodide (I3−) reduction. Graphene thin films were prepared by the chemical vapor deposition (CVD) technique in combination with a hydrothermal process. MoS2 nanosheets with 210 nm thickness were in situ grown on FTO glass substrate, and uniformly dispersed on the surface of a graphene film. Fig. 6 shows the morphology of the synthesized graphene–MoS2 composites, using field-emission scanning electron microscopy (FESEM) and high-resolution transmission electron microscopy (HRTEM) with different magnifications. The thickness of the graphene film was found to be 2.48 nm by atomic force microscopy, which corresponds to seven layers of graphene. The top-view FESEM images of graphene–MoS2 hybrids showed fully covered graphene film with 5 to 20 nm thick MoS2 nanosheets. The nucleation and growth of MoS2 nanosheets depends upon the graphene film. Graphene films play an active role for higher electrical conductivity by speeding up the charge transfer process and generating active sites for dispersion and integration of MoS2 nanosheets. The presence of MoS2 nanosheets increases the electrode–electrolyte contact area, and therefore helps in improving the electrocatalytic activity. The low RCT of 1.5 Ω cm2 for MoS2/graphene CE, 1.7 Ω cm2 for MoS2, 1.70 Ω cm2 for graphene, and a high RCT of 2.1 Ω cm2 for a Pt CE, indicates better interaction and contact formation of MoS2 nanosheets and graphene film with fluorine doped tin oxide (FTO) substrates and a fast charge transfer. The FF of graphene is 24% compared to a FF of 65% for MoS2, however when both materials are combined into a composite system, the FF raises to 68%. Therefore, MoS2 nanosheet/graphene composite CEs resulted in a higher PCE of 7.1% because of the synergetic interactions between graphene and MoS2, compared to the low PCE values of 2.8% for graphene and 5.6% for MoS2, and a comparable PCE of 7.4% for Pt reference CEs. MoS2/graphene based CEs in a DSSC offer higher electrocatalytic activity for triiodide (I3−) reduction induced by the synergetic interactions. Fig. 7 represents the J–V characteristics of DSSCs having graphene–MoS2 as CEs with increasing number of layers152 and graphene–MoS2 nanosheet based CEs for the triiodide (I3−) reduction.153
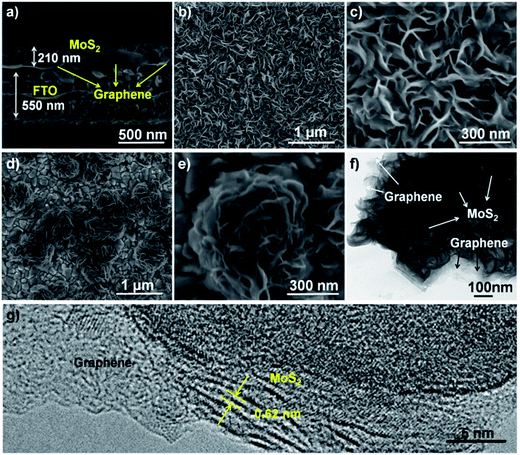 | ||
| Fig. 6 Field-emission scanning electron microscopy (FESEM) images of graphene–MoS2 composites with different magnifications; (a) side-view, (b) and (c) top-view. (d) and (e) top-view FESEM images of flower-like MoS2 clusters without graphene film. (f) and (g) high-resolution transmission electron microscopy (HRTEM) images of as-prepared graphene–MoS2 hybrids. Reprinted with permission from ref. 153, C. Yu, X. Meng, X. Song, S. Liang, Q. Dong, G. Wang, C. Hao, X. Yang, T. Ma, P. M. Ajayan and J. Qiu, graphene-mediated highly-dispersed MoS2 nanosheets with enhanced triiodide reduction activity for dye-sensitized solar cells. Carbon, 2016, 100, 474–483. Copyright© Elsevier. | ||
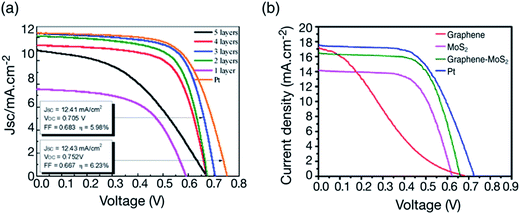 | ||
| Fig. 7 (a) J–V characteristics of DSSCs having MoS2/graphene as counter electrodes with different thickness ranging from single-layer to six-layer and a comparison with sputtered Pt counter electrode (PEC of 6.23%). Reprinted with permission from ref. 152, G. Yue, J. Y. Lin, S. Y. Tai, Y. Xiao and J. Wu, A catalytic composite film of MoS2/graphene flake as a counter electrode for Pt-free dye-sensitized solar cells. Electrochim. Acta, 2012, 85, 162–168. Copyright© Elsevier. (b) J–V curves of DSSCs based on Pt, graphene/MoS2, MoS2, and graphene CEs. Reprinted with permission from ref. 153, C. Yu, X. Meng, X. Song, S. Liang, Q. Dong, G. Wang, C. Hao, X. Yang, T. Ma, P. M. Ajayan and J. Qiu, graphene-mediated highly-dispersed MoS2 nanosheets with enhanced triiodide reduction activity for dye-sensitized solar cells. Carbon, 2016, 100, 474–483. Copyright© Elsevier. | ||
MoS2 nanosheets/graphene electrodes were also studied by Lynch et al.154 the PCE of 95% of the Pt electrode was achieved after adding 10 wt% MoS2 nanosheets to a graphene film CE. This again confirms that the MoS2 nanosheet/graphene composite ECs have higher catalytic activity than graphene CEs, though the graphene nanosheets contribute to higher electrical conductivity in the composite. An electrochemical strategy155 was used for preparing MoS2/graphene composite as CEs of DSSCs, which included electro-deposition and electro-reduction of graphene oxide, and thereafter electro-deposition of MoS2 on reduced graphene oxide (GO) layers. The MoS2/graphene composites were characterized by SEM, TEM and Raman spectroscopy. The MoS2/graphene CEs based DSCCs exhibited a PCE of 8.01%, comparable to a PCE of 8.21% for the Pt CE. In another study, nanocomposites of MoS2 and nitrogen-doped graphene oxide (N–GO) were used as a CE for DSSCs.156 The MoS2/N–GO nanocomposites were characterized by HRTEM, XPS, and Raman spectroscopy, and their electrochemical properties were evaluated by EIS, CV, and Tafel measurements. The MoS2/N–GO nanocomposite formation offered high specific surface area of N–GO and many edge sites of MoS2. The MoS2/N–GO nanocomposite based CE exhibited a PCE of 5.95%, lower than the standard Pt CE (PCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) of
of![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.43%).
6.43%).
Composite films of MoS2 with nitrogen-doped graphene (N–graphene) were prepared using a drop-coating method and used as a CE of DSSCs.157 The N–graphene supported an increase in electrical conductivity, whereas MoS2 increased the electrocatalytic activity in the composite thin film. The N–graphene/MoS2 composite film showed a PCE of 7.82%, lower than the Pt CE (η = 8.25%) based DSSC. The electrocatalytic capability of N–graphene/MoS2 composite films for the triiodide (I3−) reduction was much higher compared with pristine N–graphene and MoS2 thin films, as studied using CV, RDE, the Tafel polarization curve, and EIS. The graphene flakes (GF) into a nanosheet-like MoS2 matrix were dispersed using an in situ hydrothermal method, and used a MoS2/GF hybrid as a CE to fabricate Pt-free DSSCs.158 The incorporation of GFs into the MoS2 matrix was confirmed using scanning electron microscopy (SEM), transmission electron microscopy (TEM), XRD, and Raman spectroscopy. The electrochemical measurements showed improvement in the electrocatalytic activity after the GFs were integrated into the MoS2 matrix, where the hybrid containing 1.5 wt% of graphene flakes exhibited the highest electrocatalytic activity. The DSSC with the MoS2/GF hybrid CE showed a PCE of 6.07%, which was 95% of the Pt CE (η = 6.41%).
The MoS2 nanosheets anchored onto the CNT surfaces were used in Pt-free CEs for DSSCs.161 The MoS2 nanosheets offer edge-plane electrocatalytically active sites for the reduction of I3−. The large surface area of CNTs supports the loading of MoS2 nanosheets in order to increase the electrochemical activity. The CNTs deposited onto the FTO substrate promoted charge transport, leading to a higher exchange current density and also to the lower charge-transfer resistance. The MoS2/CNT hybrid based DSSCs achieved a PCE of 7.83%, which is 9.5% higher than that of the Pt CE (η = 7.15%) based DSSC.
Another research group162 used flower-like MoS2 and a multi-walled carbon nanotubes (MoS2/MWCNTs) hybrid as a CE for dye-sensitized solar cells. The flower-like MoS2/MWCNTs hybrid contains a large specific surface area and lamellar structure, as evidenced by field emission scanning electron microscopy (FESEM). The optimized MoS2/MWCNTs has a RCT of 2.05 Ω cm2 and series resistance (Rs) of 1.13 Ω cm2 as measured by electrochemical impedance spectroscopy. Cyclic voltammogram measurements showed larger current density for MoS2/MWCNTs based CEs than those of MoS2, MWCNTs, and Pt CEs. MoS2/MWCNTs CE based DSSCs exhibited a PCE of 7.50%, comparable with the DSSC based on the Pt CE (η = 7.49%). A carbon nanotubes–MoS2–carbon (CNTs–MoS2–carbon) hybrid was prepared using wet impregnation and calcination with polyethylene glycol as a CE for DSSCs.163 Spectroscopic characterization by Raman spectra, XRD, TEM, XPS, BET and thermal methods indicated a homogenous coating of CNTs with thin layers of MoS2, as a result of wetting and emulsification of polyethylene glycol 400. The CNTs–MoS2–carbon heterostructure was used as CEs for DSSCs, and showed high stability and electrocatalytic activity in the reduction of I3− to I− because of low RCT. Interestingly, a PCE of 7.23% achieved for the CNTs–MoS2–carbon CEs based DSSC was higher than Pt CEs (η = 6.19%).
In another study, nanocomposites of MoS2 and CNTs using a glucose aided (G–A) hydrothermal method were prepared by Yue et al.164 The (G–A)MoS2/CNTs nanocomposites obtained by adding 0.5, 1.0, 1.5, 2.0 and 2.5 wt% of acid-treated CNTs were deposited onto a FTO substrate and used as CEs in DSSCs for the reduction of triiodide (I3−) to iodide (I−). The dye-sensitized TiO2 anode was prepared by dipping the TiO2 anode in 0.3 mM of dye N719 ethanol for 24 hours. Fig. 8 shows the SEM images of MoS2 nanopower and MoS2–MWCNTs composites, J–V characteristics of the DSSCs fabricated with MoS2, MoS2/C, (G–A)MoS2/CNTs and Pt CEs, and the effect of CNT contents on the PCE of the DSSCs using (G–A)MoS2/CNTs. Scanning and transmission electron microscopy showed tentacle-like structures of the MoS2/CNTs composites, having large active surface area and interconnected networks for fast transport for the electrolyte. The CNTs functionalized with a –COOH functional group were used and MoS2 was anchored onto the functionalized CNTs. The (G–A)MoS2/CNTs had specific surface area of 411.7 m2 g−1 and exhibited a small overpotential and better conductivity. The Nernst diffusion impedance (Zw) values of 1.85 Ω cm2 for (G–A)MoS2/CNTs CEs and 2.25 Ω cm2 for Pt CEs were measured, which indicates that the (G–A)MoS2/CNTs catalyst accelerated the reduction of triiodide ions (I3−) to iodide ions (I−). The (G–A)MoS2/CNTs based CEs were found to exhibit enhanced electrocatalytic activity as evidenced by the CV, EIS, and Tafel polarization measurements. The photovoltaic performance of the DSSCs having MoS2, MoS2/C, (G–A)MoS2/CNTs, and Pt CEs were compared. The photovoltaic performance of (G–A)MoS2/CNTs CEs were also studied as a function of the CNT contents. The PCE of the DSSCs increased as the contents of the CNTs increased from 0 to 1.5 wt%, however, further increase in CNT content leads to a decreased PCE. Likely, the maximum photovoltaic performance of the (G–A)MoS2/CNTs CE in the DSSCs was achieved for a film thickness of 12 μm. The (G–A)MoS2/CNTs composite CE has a lower RCT of 1.77 Ω cm2 at the electrolyte/electrode interface than those of MoS2, MoS2/carbon and conventional sputtered Pt CEs, and a PCE of 7.92% higher than the PCE of 7.11% for the Pt electrode in DSSCs for the triiodide/iodide (I3−/I−) system. Lin et al.165 fabricated nanocomposites of MoS2/reduced graphene oxide (RGO) with CNTs using electrophoretic deposition. The MoS2/RGO–CNTs hybrid was then used as a CE in DSSCs. In this hybrid, CNTs offer conductive networks for electron transport to increase the rate of charge-transfer at the CE/electrolyte interface. The MoS2/RGO–CNTs hybrid CEs show improved electrocatalytic activity in comparison with the MoS2/RGO alone. The DSSC having a MoS2/RGO–CNTs hybrid CE achieved a PCE of 7.46%, exceeding PCE values of DSSCs containing MoS2/RGO CE (η = 6.82%) and Pt CE (η = 7.23%). Thin films of MoS2/carbon nanotube composites have also been applied as electrodes for lithium ion batteries.166
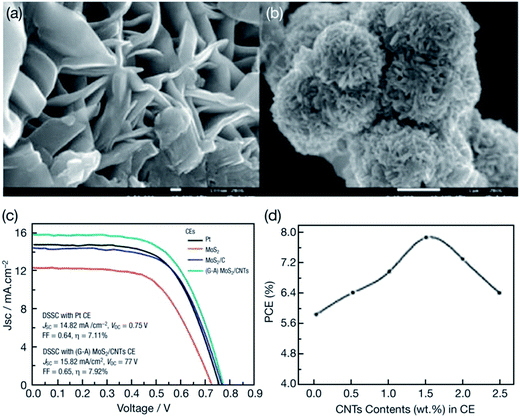 | ||
| Fig. 8 Scanning electron microscopic image of MoS2 nanopower and MoS2–MWCNTs composites prepared by glucose aided (G–A) hydrothermal method. Photocurrent–voltage (J–V) characteristics of the DSSCs fabricated with MoS2, MoS2/C, (G–A)MoS2/CNTs and Pt CEs. Relationship between the contents of CNTs in (G–A)MoS2/CNTs CE and the PCE of DSSCs. Reprinted with permission from ref. 164, G. Yue, W. Zhang, J. Wu and Q. Jiang, glucose aided synthesis of molybdenum sulfide/carbon nanotubes composites as counter electrode for high performance dye-sensitized solar cells. Electrochim. Acta, 2013, 112, 655–662. Copyright© Elsevier. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Thermal annealing of the MoS2 and MoS2/TiO2 CEs was performed at 400 °C in vacuum for 2 hours. Fig. 9 shows the J–V curves for MoS2, MoS2/TiO2, and Pt CEs in the DSSCs. The addition of TiO2 nanoparticles into the MoS2 CE significantly increased the Jsc value from 7.24 mA cm−2 for the MoS2 CE to 13.76 mA cm−2 for the MoS2/TiO2 CE. The MoS2/TiO2 CE based DSSC (η = 5.08%) shows two times better photovoltaic performance compared with the MoS2 CE (2.54%) and comparable to the Pt CE (5.27%), because of an increased active surface area from the incorporated TiO2 nanoparticles and the low overpotential loss. Though the MoS2/TiO2 CE exhibited high PCE, its electrical conductivity was found to be lower compared to the MoS2 CE, and, therefore, the high electrocatalytic activity was not related to its electrical conductivity.
1. Thermal annealing of the MoS2 and MoS2/TiO2 CEs was performed at 400 °C in vacuum for 2 hours. Fig. 9 shows the J–V curves for MoS2, MoS2/TiO2, and Pt CEs in the DSSCs. The addition of TiO2 nanoparticles into the MoS2 CE significantly increased the Jsc value from 7.24 mA cm−2 for the MoS2 CE to 13.76 mA cm−2 for the MoS2/TiO2 CE. The MoS2/TiO2 CE based DSSC (η = 5.08%) shows two times better photovoltaic performance compared with the MoS2 CE (2.54%) and comparable to the Pt CE (5.27%), because of an increased active surface area from the incorporated TiO2 nanoparticles and the low overpotential loss. Though the MoS2/TiO2 CE exhibited high PCE, its electrical conductivity was found to be lower compared to the MoS2 CE, and, therefore, the high electrocatalytic activity was not related to its electrical conductivity.
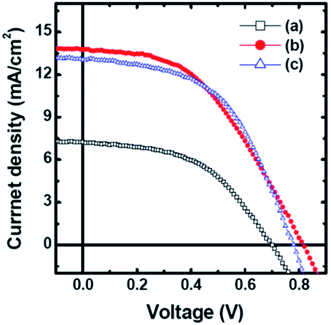 | ||
| Fig. 9 Photocurrent density–voltage (J–V) characteristics curves of DSSCs with (a) MoS2, (b) MoS2/TiO2 and (c) Pt counter electrodes measured under the solar light illumination of 100 mW cm−2 (AM 1.5G). Reprinted with permission from ref. 168, W. H. Jhang and Y. J. Lin, interface modification of MoS2 counter electrode/electrolyte in dye-sensitized solar cells by incorporating TiO2 nanoparticles. Curr. Appl. Phys., 2015, 15, 906–909. Copyright© Elsevier. | ||
The MoS2/TiO2 based CE doped with different Co contents for the DSSCs were found to influence the PCE by improving electrocatalytic activity.169 The Co content-optimized MoS2/TiO2 CE had enhanced catalytic activity at the electrolyte interfaces. The Co 3d orbit plays a role in increasing in the reduction of I3− to I−. The photoanodes were developed using MoS2 and TiO2 nanoparticles.170 The DSSCs with MoS2@TiO2 photoanode showed a PCE of 6.02%, 1.5 times higher than that of the TiO2 film photoanode (η = 4.43%).
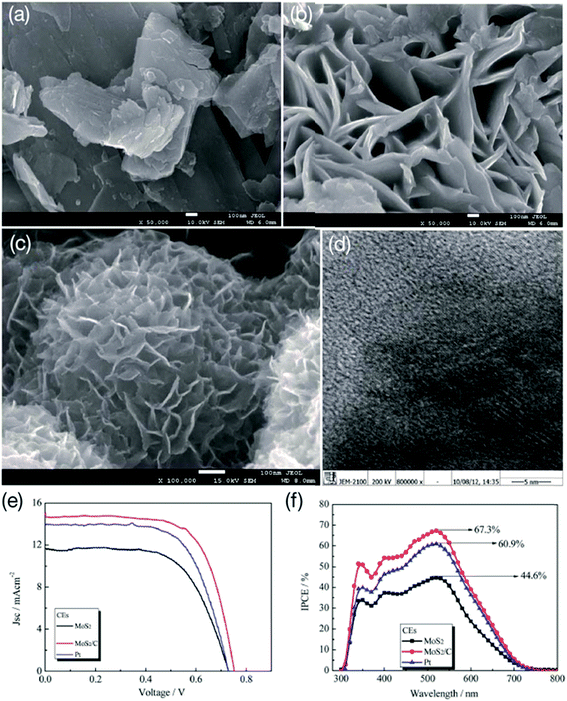 | ||
| Fig. 10 Scanning electron microscopy (SEM) images of (a) commercial MoS2, (b) hydrothermal route synthesized MoS2, (c) porous MoS2–carbon hybrid prepared by a hydrothermal route, (d) high-resolution TEM image of MoS2–carbon hybrid, (e) photocurrent–voltage (J–V) curves of the DSSCs with MoS2, MoS2–C and Pt CEs and (f) incident photon-to-current efficiency (IPCE) spectra of the DSSCs with MoS2, MoS2–C and Pt CEs. Reprinted with permission from ref. 171, G. Yue, J. Wu, Y. Xiao, M. Huang, J. Lin and J. Y. Lin, high performance platinum-free counter electrode of molybdenum sulfide–carbon used in dye-sensitized solar cells. J. Mater. Chem. A, 2013, 1, 1495–1501. Copyright© Royal Society of Chemistry. | ||
In yet another study, MoS2/carbon fibers were used as CEs for DSSCs.172 Both electrocatalytic activity and the PCE (3.26%) of the MoS2/carbon fiber based CE was found be better than that of Pt/carbon fiber CE (η = 2.93%). In another study, composites of flower-like MoS2 microspheres and carbon materials such as vulcan carbon, acetylene black, MWCNTs, carbon nanofibers (CNFs), and rice husk ash were studied as cost-effective Pt-free CEs for DSSCs.173 The electrolyte used in the DSSC was a phthaloylchitosan-based polymer. The carbon materials/MoS2 CEs showed low RCT at the CE/electrolyte interface and high electro-catalytic activity for I3− reduction. The DSSC with MoS2/CNF CE showed a PCE of 3.17%, compared to a PCE of 1.04% for the pure MoS2 CE.
Another study used MoS2 and PEDOT:PSS composites as CEs for DSSCs.174 The MoS2/PEDOT:PSS composite CE exhibits a PCE of 5.7% and FF of 58%, comparable to the Pt CE. The high PCE of the MoS2/PEDOT:PSS CE originated from high electrocatalytic activity of the MoS2 active sites for triiodide (I3−) reduction and high conductivity of PEDOT:PSS. The inorganic/organic MoS2/PEDOT:PSS composite may be useful as a low-cost Pt-free CEs for DSSCs. Another study used Bi5FeTi3O15 (BFTO) nanofibers of 40–100 nm diameter developed by a sol–gel aided electrospinning method.175 The MoS2/BFTO nanocomposite-based CE for DSSCs was prepared by uniformly dispersing MoS2 nanoparticles into the BFTO matrix. The optical bandgap of the MoS2/BFTO nanocomposites was found to decrease with increasing MoS2 contents. The DSSC with a MoS2/BFTO nanocomposite-based CE showed a PCE of 5.20%, 24 times higher than that of the pure BFTO nanofiber based CE. Table 2 summarizes the electrochemical and photovoltaic properties of all types of MoS2 based CEs discussed in this section above, and a comparison is made with conventional Pt CEs for DSSCs.
| Counter electrodes | Redox couple | Dye | Jsc (mA cm−2) | Voc (V) | FF (%) | PCE (η, %) | Rs (Ω cm2) | RCT (Ω cm2) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| a Disulfide/thiolate (T2/T−) redox couple. In the case of Rs and RCT: some of the authors used Ω instead of Ω cm2 for the resistances without mentioning the size of the electrode. | |||||||||
| 2H–MoS2 (hydrothermal, 200 °C) | I−/I3− | N719 | 6.78 | 0.73 | 35 | 1.72 | 16 | 49 | 130 |
| 1T–MoS2 (hydrothermal, 180 °C) | I−/I3− | N719 | 8.76 | 0.73 | 52 | 7.08 | 16 | 19 | 130 |
| Pt reference | I−/I3− | N719 | 17.75 | 0.702 | 58 | 7.25 | — | — | 130 |
| MoS2 (CVD) vertically inclined | I−/I3− | N719 | 15.2 | 0.707 | 69.7 | 7.50 | 9.5 | 3.10 | 131 |
| Pt reference | I−/I3− | N719 | 14.6 | 0.712 | 70.0 | 7.28 | 6.7 | 5.36 | 131 |
| MoS2/graphite | I−/I3− | N719 | 15.64 | 0.685 | 67 | 7.18 | — | 8.05 | 132 |
| Graphite | I−/I3− | N719 | 11.62 | 0.445 | 63 | 3.26 | — | 15.70 | 132 |
| Pt reference | I−/I3− | N719 | 15.84 | 0.735 | 65 | 7.57 | — | 6.35 | 132 |
| MoS2 (multi-layer) | I−/I3− | N719 | 15.81 | 0.745 | 25 | 2.92 | 27.3 | 186.2 | 133 |
| MoS2 (few-layer) | I−/I3− | N719 | 14.90 | 0.744 | 16 | 1.74 | 35.8 | 281.2 | 133 |
| MoS2 (nanoparticle) | I−/I3− | N719 | 14.72 | 0.745 | 49 | 5.41 | 26.9 | 93.0 | 133 |
| Pt reference | I−/I3− | N719 | 13.41 | 0.754 | 65 | 6.58 | 34.2 | 3.9 | 133 |
| MoS2 (hydrothermal method) | I−/I3− | N719 | 18.37 | 0.698 | 57.8 | 7.41 | — | 0.619 | 135 |
| Pt reference | I−/I3− | N719 | 16.78 | 0.722 | 58.8 | 7.13 | — | 3.78 | 135 |
| MoS2 (300 °C annealed) | I−/I3− | N719 | 16.905 | 0.727 | 51.7 | 6.351 | 23.89 | 30.98 | 137 |
| Pt reference | I−/I3− | N719 | 17.056 | 0.724 | 55.7 | 6.929 | 27.17 | 14.98 | 137 |
| MoS2 (chemical deposition) | I−/I3− | N719 | 18.46 | 0.68 | 58 | 7.01 | 23.51 | 18.50 | 138 |
| Pt reference | I−/I3− | N719 | 16.80 | 0.71 | 60 | 7.31 | 26.73 | 22.88 | 138 |
| MoS2 (non-annealed) | I−/I3− | N719 | 5.24 | 0.74 | 27 | 1.0 | 28 | — | 139 |
| MoS2 (vacuum-annealed) | I−/I3− | N719 | 7.95 | 0.74 | 29 | 1.7 | 22 | — | 139 |
| MoS2 (N2-annealed) | I−/I3− | N719 | 4.35 | 0.67 | 27 | 0.8 | 44 | — | 139 |
| MoS2/Mo (in situ sulfurization) | I−/I3− | N719 | 22.6 | 0.74 | 50 | 8.4 | — | — | 140 |
| Pt reference | I−/I3− | N719 | 21.9 | 0.735 | 53.4 | 8.7 | — | — | 140 |
| MoS2 (hydrothermal method) | I−/I3− | N719 | 13.84 | 0.76 | 73 | 7.59 | 20.8 | 0.5 | 141 |
| WS2 (hydrothermal method) | I−/I3− | N719 | 14.13 | 0.78 | 70 | 7.73 | 19.4 | 0.3 | 141 |
| Pt reference | I−/I3− | N719 | 14.89 | 0.78 | 66 | 7.64 | 12.7 | 3.0 | 141 |
| MoS2 (hydrothermal method) | T2/T− | N719 | 12.52 | 0.63 | 63 | 4.97 | — | — | 141 |
| WS2 (hydrothermal method) | T2/T− | N719 | 12.99 | 0.64 | 64 | 5.24 | — | — | 141 |
| Pt reference | T2/T− | N719 | 12.23 | 0.63 | 48 | 3.70 | — | — | 141 |
| MoS2 (porous sheets) | I−/I3− | N719 | 15.40 | 0.763 | 53 | 6.35 | 7.95 | 1.73 | 143 |
| MoS2 (flower-shaped) | I−/I3− | N719 | 13.73 | 0.700 | 52 | 5.23 | 7.89 | 2.67 | 143 |
| Pt reference | I−/I3− | N719 | 16.34 | 0.745 | 51 | 6.19 | 8.06 | 1.82 | 143 |
| MoS2 (sputtering, 5 min) | I−/I3− | N719 | 13.17 | 0.71 | 64 | 6.6 | 30.1 | 2.2 | 145 |
| Pt reference | I−/I3− | N719 | 14.70 | 0.71 | 66 | 6.0 | 3.1 | 1.5 | 145 |
| MoS2 (as-prepared) | I−/I3− | N719 | 11.92 | 0.656 | 35 | 2.74 | — | 1.01 × 104 | 146 |
| MoS2 (heat-sintered) | I−/I3− | N719 | 13.01 | 0.705 | 65 | 5.96 | — | 18.50 | 146 |
| MoS2 (laser-sintered) | I−/I3− | N719 | 14.94 | 0.718 | 67 | 7.19 | — | 15.29 | 146 |
| Pt reference | I−/I3− | N719 | 14.30 | 0.741 | 70 | 7.42 | — | 3.99 | 146 |
| MoS2 (growth time, 5 h) | I−/I3− | N719 | 15.15 | 0.76 | 52 | 5.96 | 50.9 | 118.8 | 147 |
| MoS2 (growth time, 10 h) | I−/I3− | N719 | 15.94 | 0.71 | 63 | 7.14 | 50.4 | 21.2 | 147 |
| MoS2 (growth time, 15 h) | I−/I3− | N719 | 16.96 | 0.74 | 66 | 8.28 | 38.8 | 12.9 | 147 |
| Pt reference | I−/I3− | N719 | 13.77 | 0.74 | 74 | 7.53 | 36.3 | 13.1 | 147 |
| MoS2 (exfoliated) | I−/I3− | N719 | 11.54 | 0.80 | 65 | 6.0 | 29.60 | 19.60 | 148 |
| MoS2 (annealed) | I−/I3− | N719 | 10.92 | 0.80 | 58 | 5.1 | 28.10 | 121.10 | 148 |
| Pt reference | I−/I3− | N719 | — | 148 | |||||
| MoS2 | I−/I3− | N719 | 12.92 | 0.701 | 46 | 4.15 | 11.69 | 3.65 | 151 |
| Graphene nanosheet | I−/I3− | N719 | 11.99 | 0.754 | 30 | 2.68 | 9.31 | 6.24 | 151 |
| MoS2–graphene nanosheet | I−/I3− | N719 | 12.79 | 0.773 | 59 | 5.81 | 9.52 | 2.34 | 151 |
| Pt reference | I−/I3− | N719 | 13.12 | 0.763 | 62 | 6.24 | 9.11 | 1.79 | 151 |
| MoS2/graphene | I−/I3− | N719 | 12.41 | 0.71 | 68 | 5.98 | 24.42 | 4.94 | 152 |
| Pt reference | I−/I3− | N719 | 12.43 | 0.73 | 67 | 6.23 | 24.72 | 4.74 | 152 |
| MoS2 (CVD) | I−/I3− | N719 | 14.0 | 0.62 | 65 | 5.6 | 1.5 | 2.3 | 153 |
| MoS2/graphene (CVD) | I−/I3− | N719 | 16.1 | 0.66 | 67 | 7.1 | 1.7 | 1.6 | 153 |
| Graphene | I−/I3− | N719 | 16.9 | 0.68 | 24 | 2.8 | 1.7 | 2.8 | 153 |
| Pt reference | I−/I3− | N719 | 17.2 | 0.72 | 60 | 7.4 | 2.1 | 1.8 | 153 |
| MoS2 | I−/I3− | N719 | 9.14 | 0.589 | 47 | 2.53 | — | — | 154 |
| Graphene | I−/I3− | N719 | 10.7 | 0.652 | 51.9 | 3.62 | — | — | 154 |
MoS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) graphene (10 graphene (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) 90) |
I−/I3− | N719 | 11.91 | 0.646 | 56.5 | 4.35 | — | — | 154 |
| Pt reference | I−/I3− | N719 | 13.39 | 0.657 | 50 | 4.40 | — | — | 154 |
| MoS2/graphene oxide (N2 doped) | I−/I3− | N719 | 15.98 | 0.70 | 53 | 5.95 | 25.7 | 5.4 | 156 |
| Graphene oxide (N2 doped) | I−/I3− | N719 | 14.66 | 0.71 | 38 | 3.95 | 25.7 | 21.3 | 156 |
| MoS2 | I−/I3− | N719 | 15.39 | 0.69 | 39 | 4.09 | 26.0 | 10.1 | 156 |
| Pt reference | I−/I3− | N719 | 16.14 | 0.70 | 57 | 6.43 | 26.3 | 4.3 | 156 |
| MoS2 (drop-coating) | I−/I3− | N719 | 13.46 | 0.79 | 58 | 6.20 | 21.14 | 24.93 | 157 |
| Nitrogen-doped graphene (NGr) | I−/I3− | N719 | 12.72 | 0.68 | 64 | 5.50 | 16.31 | 30.17 | 157 |
| MoS2/NGr (8 wt%) | I−/I3− | N719 | 15.36 | 0.77 | 66 | 7.82 | 15.60 | 16.73 | 157 |
| Pt reference | I−/I3− | N719 | 15.71 | 0.77 | 68 | 8.25 | 15.23 | 10.15 | 157 |
| MoS2 | I−/I3− | N719 | 10.56 | 0.67 | 58 | 4.10 | — | — | 158 |
| Graphene flake (GF) | I−/I3− | N719 | 10.96 | 0.69 | 48 | 3.63 | — | — | 158 |
| MoS2 (GF is 0.5 wt%) | I−/I3− | N719 | 12.09 | 0.69 | 59 | 4.85 | — | — | 158 |
| MoS2 (GF is 1 wt%) | I−/I3− | N719 | 12.68 | 0.74 | 59 | 5.35 | — | — | 158 |
| MoS2 (GF is 1.5 wt%) | I−/I3− | N719 | 13.27 | 0.75 | 61 | 6.07 | — | — | 158 |
| MoS2 (GF is 2 wt%) | I−/I3− | N719 | 12.95 | 0.74 | 58 | 5.56 | — | — | 158 |
| MoS2 (GF is 2.5 wt%) | I−/I3− | N719 | 12.63 | 0.70 | 58 | 5.04 | — | — | 158 |
| Pt reference | I−/I3− | N719 | 12.95 | 0.75 | 66 | 6.41 | — | — | 158 |
| MoS2 | I−/I3− | N719 | 11.25 | 0.72 | 61 | 4.99 | 11.37 | 2.43 | 160 |
| Multi-walled CNT (MWCNT) | I−/I3− | N719 | 9.11 | 0.65 | 58 | 3.53 | 9.91 | 8.59 | 160 |
| MoS2/MWCNT | I−/I3− | N719 | 13.69 | 0.73 | 65 | 6.45 | 10.22 | 1.69 | 160 |
| Pt reference | I−/I3− | N719 | 13.24 | 0.74 | 66 | 6.41 | 9.06 | 1.91 | 160 |
| MoS2 | I−/I3− | N719 | 14.44 | 0.74 | 64 | 6.81 | — | — | 161 |
| Carbon nanotubes | I−/I3− | N719 | 13.33 | 0.75 | 62 | 6.15 | — | — | 161 |
| MoS2/carbon nanotubes | I−/I3− | N719 | 16.65 | 0.74 | 66 | 7.83 | — | — | 161 |
| Pt reference | I−/I3− | N719 | 14.83 | 0.74 | 65 | 7.15 | — | — | 161 |
| MoS2/CNTs | I−/I3− | N719 | 14.93 | 0.65 | 47 | 4.51 | 8.42 | 4.35 | 163 |
| CNTs/MoS2/carbon | I−/I3− | N719 | 16.44 | 0.79 | 57 | 7.23 | 8.14 | 1.73 | 163 |
| Pt reference | I−/I3− | N719 | 15.40 | 0.75 | 55 | 6.19 | 8.31 | 1.95 | 163 |
| MoS2/CNTs (G–A) | I−/I3− | N719 | 15.82 | 0.77 | 65 | 7.92 | 5.20 | 1.77 | 164 |
| MoS2/carbon | I−/I3− | N719 | 14.52 | 0.76 | 64 | 7.06 | 5.24 | 2.35 | 164 |
| MoS2 | I−/I3− | N719 | 12.33 | 0.72 | 61 | 5.42 | 5.33 | 4.16 | 164 |
| Pt reference | I−/I3− | N719 | 14.82 | 0.75 | 64 | 7.11 | 5.06 | 2.22 | 164 |
| MoS2/reduced graphene oxide (RGO) | I−/I3− | N719 | 14.31 | 0.76 | 63 | 6.82 | 20.58 | 4.42 | 165 |
| MoS2/RGO–CNTs | I−/I3− | N719 | 14.59 | 0.76 | 67 | 7.46 | 20.37 | 3.31 | 165 |
| Pt reference | I−/I3− | N719 | 14.53 | 0.77 | 65 | 7.23 | 20.13 | 4.06 | 165 |
| MoS2 (spin-coating) | I−/I3− | N719 | 7.24 | 0.70 | 49 | 2.54 | 59.5 | — | 168 |
MoS2/TiO2 (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt ratio) 1 wt ratio) |
I−/I3− | N719 | 13.76 | 0.82 | 45 | 5.08 | 56.5 | — | 168 |
| Pt reference | I−/I3− | N719 | 13.06 | 0.78 | 52 | 5.27 | — | — | 168 |
| MoS2/TiO2 | I−/I3− | N719 | 4.67 | 0.68 | 44 | 1.4 | — | — | 169 |
| MoS2/TiO2/Co | I−/I3− | N719 | 9.21 | 0.70 | 50 | 3.2 | — | — | 169 |
| MoS2/carbon (C is 2.23 wt%) | I−/I3− | N719 | 13.98 | 0.74 | 68 | 7.03 | 5.87 | 2.67 | 171 |
| MoS2/carbon (C is 3.30 wt%) | I−/I3− | N719 | 15.07 | 0.75 | 68 | 7.69 | 5.77 | 2.07 | 171 |
| MoS2/carbon (C is 4.35 wt%) | I−/I3− | N719 | 14.37 | 0.75 | 68 | 7.33 | 5.83 | 2.40 | 171 |
| MoS2 | I−/I3− | N719 | 11.66 | 0.73 | 63 | 5.36 | 5.87 | 4.13 | 171 |
| Pt reference | I−/I3− | N719 | 13.98 | 0.73 | 66 | 6.74 | 5.79 | 2.29 | 171 |
| MoS2/PEDOT–PSS | I−/I3− | N719 | 14.55 | 0.68 | 58 | 5.7 | — | — | 174 |
| PEDOT–PSS | I−/I3− | N719 | 14.6 | 0.68 | 26 | 2.5 | — | — | 174 |
| Pt reference | I−/I3− | N719 | 15.26 | 0.73 | 59 | 6.6 | — | — | 174 |
3.2 WS2 counter electrodes
Tungsten disulfide (WS2), traditionally used as a lubricant, is a semiconductor having van der Waals bonding which forms 2D layered-structures similar to other TMDs. WS2 can form atomically thin nanosheets,176,177 nanorods,178 and nanotubes,179,180 which have been actively studied for potential applications.Carbon-coated WS2 CEs have been fabricated for DSSCs at low temperature and characterized using FESEM, XRD, and Raman spectroscopy.181 The electrocatalytic activity of the WS2 CEs was studied using CV and EIS. The DSSCs with carbon-coated WS2 CEs show a PCE of 5.5%, comparable to that of Pt CE based DSSCs (η = 5.6%). A DSSC having plastic WS2 CEs exhibited a PCE of 5.0%. Carbon-coated WS2 seems promising to develop low cost Pt-free CEs for DSSCs. The WS2 films were deposited by radio frequency (RF) sputtering and a sulfurization process as CE for DSSCs.182 The WS2 films were characterized using XRD, FESEM, Raman spectroscopy, and XPS techniques. The transparent WS2 CEs demonstrated high electrocatalytic activity and fast reduction of triiodide, (I3−) as characterized using CV, EIS, and Tafel polarization curve. WS2 CE sputtered for 10 minutes showed a PCE of 6.3%, slightly lower than the Pt-based CE (η = 6.64%) used in the DSSC. The J–V characteristics as a function of sputtering time used to prepare WS2 films as a CE were also studied. WS2 film CEs prepared at sputtering time of 5, 10 and 15 minutes showed PCEs of 5.4%, 6.3% and 5.8%, respectively.
Another research team183 used edge-oriented WS2 based CEs for DSSCs. Edge-oriented WS2 was obtained from mesoporous interconnected WO3 structures using a high temperature sulfurization process. The DSSCs with edge-oriented WS2 CEs show a PCE of 8.85%, higher compared to the Pt CE (η = 7.20%). The large number of active edge sites in edge-oriented WS2 is responsible for high electrocatalytic activity for the reduction of triiodide (I3−) in the DSSCs. The WS2 films were fabricated by the doctor-blade method (or tape casting method; a method removing excessive liquid material using a moving blade for uniform coating) to use as CEs for DSSCs.184 The TiO2 (P25) and carbon nanoparticles were introduced into WS2 films to increase electrical conductivity and bonding strength. The electrochemical catalytic activity of WS2/P25/C CEs was compared with Pt for the triiodide (I3−) to iodide (I−) electrolyte system using CV and EIS measurements. The DSSC developed with WS2/P25/C CE was shown to yield a PCE of 4.56%.
Yue et al.185 prepared WS2 decorated multi-walled carbon nanotubes (MWCNTs) by applying a hydrothermal method, for use as a low-cost Pt-free CE for DSSCs. The contents of MWCNTs in MWCNTs–WS2 CEs varied from 1 to 10 wt%. PCE values of 5.20, 5.45, 6.41, 5.53 and 5.22% were measured in the DSSCs for CEs having 1, 3, 5, 7 and 10 wt% contents of MWCNTs, respectively. CV and EIS showed high electrocatalytic activity for the MWCNTs–WS2 CE for the triiodide (I3−) reduction. The RCT of MWCNTs–WS2 CEs having 1, 3, 7, and 10 wt% contents of MWCNTs were 4.54, 3.47, 3.24, and 4.59 Ω cm2, respectively. The RCT of the MWCNTs–WS2 CE with 5 wt% contents of MWCNTs shows 2.53 Ω cm2, comparable to the RCT of 2.74 Ω cm2 for a Pt CE. The DSSCs based on WS2/MWCNTs CEs showed a PCE of 6.41% for 5 wt% MWCNTs, comparable to the PCE of 6.56% for Pt CE under a simulated AM 1.5 solar illumination (100 mW cm−2). The low RCT of WS2/MWCNTs CEs at the electrolyte/electrode interface contributed to the higher PCEs.
The WS2 based CEs prepared by a hydrothermal method was also used for DSSC by Wu et al.,141 which exhibited a PCE of 7.73%. The same research team186 also synthesized WS2/MWCNTs hybrids by a glucose-aided (G–A) hydrothermal route, which is discussed here in detail. Fig. 11 presents the SEM images of WS2, MWCNTs, and (G–A)WS2/MWCNTs composites, and J–V curves of the DSSCs with WS2, MWCNTs, WS2/MWCNTs* (prepared without the aid of glucose), WS2/MWCNTs, and Pt CEs, under a simulated solar illumination of 100 mW cm−2. The WS2 exhibits graphene-like lamellar structure, whereas the MWCNTs have a fiber-like structure, indicating both materials have a large specific surface area. The specific surface area of the (G–A)WS2/MWCNTs composite was estimated to be 230 m2 g−1 by the Brunauer–Emmett–Teller (BET) technique, indicating high electrochemical activity as well as photovoltaic efficiency for CEs. The cathodic peak potentials of the WS2, WS2/MWCNTs, and WS2/MWCNTs* CEs showed cathodic peak potentials of −0.14, −0.13 and −01.7 V, respectively, which implies that the MWCNTs help in improving the electrocatalytic activity, and a lower cathodic peak potential observed for (G–A)WS2/MWCNTs as opposed to WS2/MWCNTs* results from the large specific surface area generated by glucose aided preparation. The EIS measurements yielded an RCT of 2.49 Ω cm2 and Rs of 2.54 Ω cm2 for the WS2/MWCNTs hybrid CE, which is smaller compared with WS2 CE, and indicates the synergistic effect between WS2 and MWCNTs that enhanced the electrical conductivity of the hybrid. The (G–A)WS2/MWCNT CE based DSSC resulted in a PCE of 7.36%, comparable to WS2 CE (5.32%), MWCNTs CE (4.34%), and the Pt CE (7.54%). The Jsc and PCE values increased with increasing content of MWCNTs, up to 5 wt% in the WS2/MWCNT hybrid CEs, and thereafter started decreasing with further increases in MWCNTs content. The (G–A)WS2/MWCNT (5 wt%) film also exhibits a smaller transmission between 320 to 800 nm than Pt film, therefore the WS2/MWCNT film absorbs more incident light, which also further improves photovoltaic performance. The glucose aided (G–A)WS2/MWCNTs (5 wt%) CE in the DSSC had low RCT and high electrocatalytic activity for the reduction of triiodide (I3−), due to the synergistic effects induced by glucose.
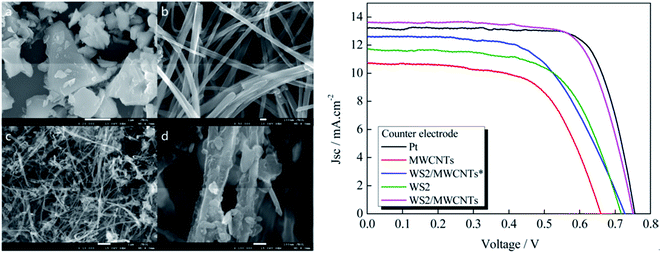 | ||
| Fig. 11 (Left) SEM images of (a) WS2, (b) MWCNTs, and (c and d) WS2/MWCNTs composites. (Right) photocurrent–voltage (J–V) curves of the DSSCs with Pt, WS2, MWCNTs, WS2/MWCNTs* (prepared without glucose aid), and (G–A)WS2/MWCNTs counter electrodes under a simulated solar illumination of 100 mW cm−2. Reprinted with permission from ref. 186, J. Wu, G. Yue, Y. Xiao, M. Huang, J. Lin, L. Fan, Z., Lan and J. Y. Lin, glucose aided preparation of tungsten sulfide/multi-wall carbon nanotube hybrid and use as counter electrode in dye-sensitized solar cells. ACS Appl. Mater. Interfaces, 2012, 4, 6530–6536. Copyright© American Chemical Society. | ||
3.3 TiS2 counter electrodes
Titanium disulfide (TiS2) is a metallic thermoelectric material which attains unique morphologies such as nanotubes, nanoclusters and nanodisks, and exhibits interesting physical properties.187–198 Meng et al.199 has prepared 2D TiS2 nanosheets decorated on graphene using a ball milling method and followed by high-temperature annealing. The electroactive surface areas of 1.70 cm2 for TiS2–graphene and 0.232 cm2 for Pt electrodes, measured by CV, indicate more active sites for the hybrid. Fig. 12 compares J–V curves of DSSCs having TiS2–graphene and Pt/FTO CEs. The CEs based on TiS2–graphene hybrids exhibited higher electrocatalytic activity for the reduction of triiodide (I3−) to iodide (I−) in electrolyte with a PCE of 8.80%, which is higher than the Pt CE (η = 8.00%). The Rs of TiS2–graphene CEs (2.32 Ω cm2) was found to be smaller compared to the Pt CEs (6.90 Ω cm2), showing better contact between the hybrid CE and FTO glass. Furthermore, the RCT of TiS2–graphene CE (0.63 Ω cm2) was also lower than the Pt CE (1.32 Ω cm2), which again indicates a higher electrocatalytic activity for the reduction of triiodide (I3−). Also, the ZN of TiS2–graphene CE (10.52 Ω cm2) was found to be higher than the Pt CE (6.89 Ω cm2). The high electrocatalytic activity of the TiS2–graphene hybrids is attributed to the highly electroactivity of TiS2 and enhanced transport facilitated by the graphene conductive network.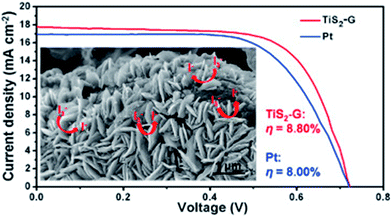 | ||
| Fig. 12 A comparison of photocurrent density–voltage (J–V) curves of DSSCs having TiS2–graphene hybrid and Pt counter electrodes. Reprinted with permission from ref. 199, X. Meng, C. Yu, B. Lu, J. Yang and J. Qiu, dual integration system endowing two-dimensional titanium disulfide with enhanced triiodide reduction performance in dye-sensitized solar cells. Nano Energy, 2016, 22, 59–69. Copyright© Elsevier. | ||
Li et al.200 deposited composite films of TiS2/PEDOT:PSS on ITO substrates by drop coating, to study CEs of DSSCs. The wt% of TiS2 particles in TiS2/PEDOT:PSS composite films varied from 5 to 15 wt%. TiS2 particles were dispersed in a PEDOT:PSS matrix to be used as an electrocatalyst for the I−/I3− redox reaction. In the composite, conducing polymer PEDOT:PSS plays the role of a binder for the TiS2 nanoparticles, as well as a linking agent between TiS2 particles and the ITO substrate, and also facilitates electron transfer. The TiO2 photoanode for a DSSC was prepared by immersing it in N719 dye solution for 24 hours at room temperature. Fig. 13 shows the photocurrent density–voltage curves and IPCE curves of the DSSCs with Pt, bare TiS2, bare PEDOT:PSS, and 10 wt% TiS2/PEDOT:PSS composite CEs. The TiS2/PEDOT:PSS composite CE offered a large surface area, yielding a high PCE of 7.04%. The CEs of bare TiS2, bare PEDOT:PSS, the TiS2/PEDOT:PSS composite, and Pt were characterized by AFM, SEM, and EDX. AFM images indicated a roughness of 45 nm for PEDOT:PSS and of 378 nm for TiS2/PEDOT:PSS composite film; therefore, the higher roughness could lead to a larger active surface area and consequently to the higher electrocatalytic activity for the TiS2/PEDOT:PSS composite thin. The electrocatalytic properties of the DSSCs using the CEs of bare TiS2, bare PEDOT:PSS, the TiS2/PEDOT:PSS composite, and Pt were evaluated by CV, RDE, EIS, and Tafel polarization measurements. The high PCE of the TiS2/PEDOT:PSS composite CE based DSSC was also measured by IPCE curves. The maximum of the IPCE spectra at 520 nm increased from 52% to 63% as the content of TiS2 particles increased from 0 to 10 wt%, respectively, and similar characteristics were observed for the Jsc values from the J–V curves of the DSSCs. The 10 wt% TiS2/PEDOT:PSS composite film based CE shows a higher redox current density compared with bare TiS2 and PEDOT:PSS CEs, therefore, it possesses high electrocatalytic activity for triiodide (I3−) reduction, and it also exhibits high electrochemical stability after 100 consecutive cycles in the I−/I3− redox electrolyte.
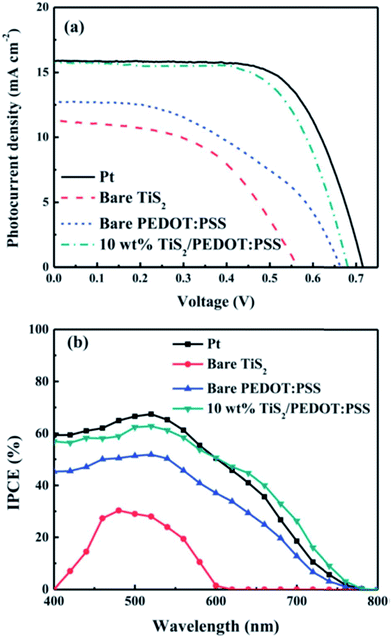 | ||
| Fig. 13 (a) Photocurrent density–voltage curves of DSSCs with Pt, bare TiS2, bare PEDOT:PSS, and 10 wt% TiS2/PEDOT:PSS composite based CEs recorded under light illumination of 100 mW cm−2 (AM 1.5). (b) Incident photon-to-current conversion efficiency (IPCE) curves of the DSSCs with similar CEs. Reprinted with permission from ref. 200, C. T. Li, C. P. Lee, Y. Y. Li, M. H. Yeh and K. C. Ho, a composite film of TiS2/PEDOT:PSS as the electrocatalyst for the counter electrode in dye-sensitized solar cells. J. Mater. Chem. A, 2013, 1, 14888–14896. Copyright© Royal Society of Chemistry. | ||
3.4 NiS2 counter electrodes
Nickel disulfide (NiS2) is a semiconducting material with pyrite structure which acquires unique morphologies such as hollow prisms, nano/microspheres, nanocubes, nanosheets, nanoparticles, and also exhibits interesting electrical, optical, magnetic, and catalytic properties.201–205 Depending upon the synthesis procedures, the stoichiometric composition of nickel sulfide varies to a great extent (NiS, NiS2, Ni3S2, Ni3S4, Ni6S5, Ni7S6, Ni9S8, etc.).In one study, hierarchical NiS2 hollow microspheres on a FTO substrate were prepared by a hydrothermal method to use as a CE for a DSSC.206 The NiS2 hollow microspheres were partially broken, offering more active sites for electrocatalysis and electrolyte adsorptions. The IPCE values of 81.3% for the NiS2 microspheres CE and 76.6% for the Pt CEs at 500 nm were observed. The peak current density of the NiS2 microspheres CE was found to be higher than the Pt CE, whereas the peak-to-peak separation (Epp) value was lower by 10 mV compared to Pt CE, which suggests a high electrocatalytic activity for the NiS2 microspheres CE in the reduction of triiodide (I3−) in the electrolyte. The NiS2 hollow microspheres CE based DSSC showed a PCE of 7.84%, equal to the Pt CE (7.89%), indicating their potential as low-cost CEs for DSSCs. The NiS/NiS2 composite hollow spheres prepared by a solvothermal method exhibited a low RCT of 0.34 Ω cm−2 at the CE/electrolyte interface, and a PCE of 7.66%, outperforming the Pt CE (7.01%), and showed high electrocatalytic activity for I3− reduction, and also better electrochemical stability.207 The NiS and NiS2 hollow spheres were synthesized through a solvothermal process.208 The Ni/S molar ratio controlled the different stoichiometric ratios of nickel sulfides. The NiS2 CE based DSSC showed a higher electrocatalytic activity than that of the NiS CE for I3− reduction. The DSSC with NiS2 CE yielded a PCE value of 7.13% in comparison to 6.49% for NiS CE.
NiS2 polyhedrons were studied as CEs for DSSCs by Zheng et al.209 Fig. 14 shows SEM, TEM, and selected area electron diffraction (SAED) images of NiS2 octahedrons and NiS2 cubes, and electrochemical characteristics of DSSCs having NiS2 octahedrons, NiS2 cubes and Pt CEs, under simulated AM1.5G solar light. The average size of NiS2 octahedrons and cubes were about 250 nm. The electrochemical performance of NiS2 octahedron and cube based CEs were evaluated using CV, J–V characteristics, EIS, and the Tafel polarization method. The NiS2 octahedron CEs showed peak current density of 1.40 mA cm−2, compared to 1.22 mA cm−2 for the NiS2 cubes, indicating better electrocatalytic activity. The NiS2 octahedron CEs also exhibited a higher Jsc of 13.55 mA cm−2 and FF of 62%, higher than that of the NiS2 cube CE (Jsc of 12.62 mA cm−2 and FF of 60%), giving rise to a higher PCE. Octahedral NiS2 nanocrystals based CEs incorporated into DSSCs exhibited a PCE of 5.98%, slightly higher than that of the NiS2 cube nanocrystals (η = 5.43%). The NiS2 octahedron CE had a PCE of up to 91% of the conventional Pt CE in DSSCs (η = 6.55%). The Rs of the NiS2 octahedron based CE was 13.14 Ω cm2, somewhat lower than that of the NiS2 cube CE (Rs of 14.98 Ω cm2), indicating higher electrical conductivity of the NiS2 octahedron based CE. The RCT of the NiS2 octahedron CE was measured as 9.86 Ω cm2, also lower than that of the NiS2 cube CE (RCT of 13.17 Ω cm2), which demonstrates that the NiS2 octahedrons with {111} facets possesses better electrocatalytic activity than the NiS2 cubes with {100} facets.
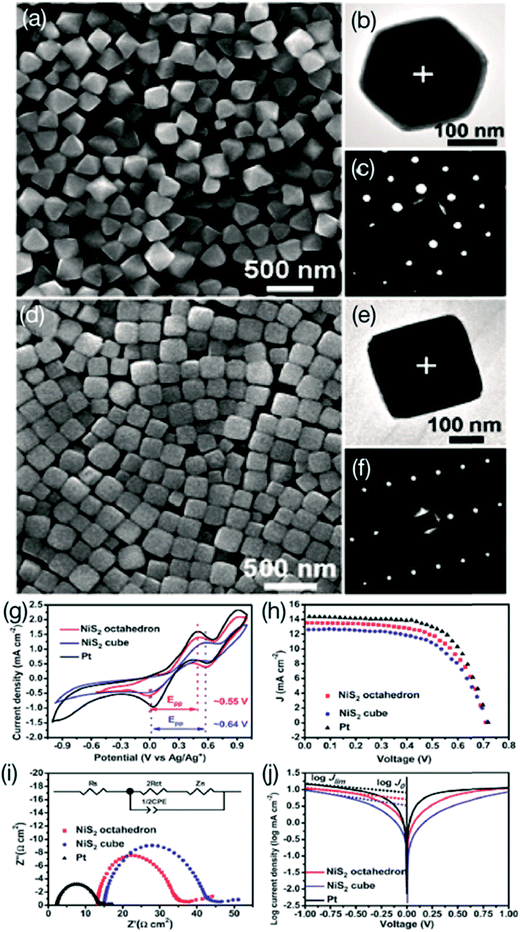 | ||
| Fig. 14 SEM, TEM, and SAED images of NiS2 octahedrons (a–c) and NiS2 cubes (d–f). SAED patterns of NiS2 octahedrons (c) and NiS2 cubes (f) were recorded from the corresponding particles depicted (b) and (e), respectively. (g) C–V curves of DSSCs having counter electrodes of NiS2 octahedrons, NiS2 cubes and Pt for the reduction of tri-iodide (h) J–V curves of DSSCs with NiS2 octahedrons, NiS2 cubes and Pt CEs under simulated AM1.5G solar light. Nyquist plots (i) and Tafel polarization curves (j) of DSSCs having NiS2 octahedrons, NiS2 cubes and Pt CEs. Reprinted with permission from ref. 209, J. Zheng, W. Zhou, Y. Ma, W. Cao, C. Wang and L. Guo, facet-dependent NiS2 polyhedrons on counter electrodes for dye-sensitized solar cells. Chem. Commun., 2015, 51, 12863–12866. Copyright© Royal Society of Chemistry. | ||
The electrocatalytic performance of NiS2 nanoparticles and their nanocomposites with RGO were compared by Li et al.210 In a hydrothermal process, graphene oxide was transformed to RGO, and then NiS2@RGO nanocomposites were formed by depositing NiS2 nanoparticles on the surface of RGO. CEs for DSSCs were fabricated by drop-casting solutions of NiS2, NiS2@RGO, and RGO nanocomposites on FTO-coated glass substrate. The surface areas measured by the BET method were 11.4, 9.4, and 8.6, and 5.8 m2 g−1 for NiS2@RGO, NiS2, and RGO, respectively. Fig. 15 shows J–V curves of DSSCs with bare NiS2, NiS2@RGO nanocomposites, bare RGO, and Pt CEs. The NiS2@RGO nanocomposites based CE showed a PCE of 8.55% (Jsc = 16.55 mA cm−2, Voc = 0.749 V, and FF = 0.69), much higher than that of the NiS2 CE (η = 7.02%), RGO CE (η = 3.14%), or standard Pt CE (η = 8.15%) for the DSSCs under the same experimental conditions. The larger RCT values of 100.2 Ω cm2 for RGO and 8.8 Ω cm2 for NiS2 also suggest the low electrocatalytic activity. On the other hand, the smaller RCT value of 2.9 Ω cm2 for the NiS2@RGO nanocomposite indicates much higher electrocatalytic activity for the reduction of triiodide (I3−) in electrolyte due the cooperative synergetic effect and the increased conductivity from the RGO nanosheets. This study demonstrates that NiS2@RGO nanocomposites are a promising alternate CE to conventional Pt CE for DSSC devices.
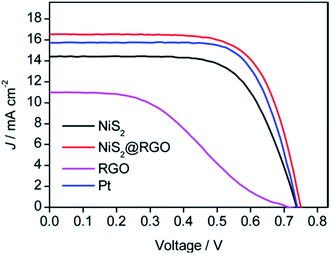 | ||
| Fig. 15 (a) Photocurrent density–voltage (J–V) curves of DSSCs with bare NiS2, NiS2@RGO nanocomposites, bare RGO, and Pt CEs measured under light illumination of 100 mW cm−2 (AM 1.5). Reprinted with permission from ref. 210, Z. Li, F. Gong, G. Zhou and Z. S. Wang, NiS2/reduced graphene oxide nanocomposites for efficient dye-sensitized solar cells. J. Phys. Chem. C, 2013, 117, 6561–6566. Copyright© American Chemical Society. | ||
3.5 FeS2 based counter electrodes
Pyrite iron disulfide (NiS2) acquires unique morphological structures including nanocrystals, nanowires, nanosheets, nanocubes, and exhibits interesting electrical, photovoltaic, and catalytic properties.211–220 An interesting comparative study was done by Shukla et al.221 on pyrite iron disulfide (FeS2) as a CE material in comparison with Pt and PEDOT CEs in DSSCs. The FeS2 film CEs were fabricated on a FTO glass substrate by a spray pyrolysis method and used in I3−/I− and Co(III)/Co(II) electrolyte-mediated DSSCs. N719 dye was used for the DSSC with I3−/I− redox electrolyte and C128 dye for the [Co(bpy)3]2+/3+ redox electrolyte. The I3−/I− redox electrolyte contained 1.0 mM of 1,3-dimethylimidazolium iodide, 50 mM of LiI, 30 mM of I2, 0.5 mM of tert-butylpyridine, and 0.1 mM of guanidinium thiocyanate in a acetonitrile and valeronitrile (v/v, 85/15) mixed solution. The cobalt electrolyte was made up of 0.22 M of Co(bpy)3(TFSI)2, (bpy = 2,2′-bipyridine and TFSI = [bis(trifluoromethane)-sulfonimide]), 0.05 M of Co(bpy)3(TFSI)3, 0.1 M of lithium bis(trifluoromethanesulfonyl)imide (LiTFSI), and 0.2 M of tert-butylpyridine (tBP) in acetonitrile. Fig. 16 shows the J–V curves and IPCE of the DSSCs with FeS2 and Pt CEs in I3−/I− electrolyte, and DSSCs with FeS2 and PEDOT CEs in the Co(III)/Co(II) electrolyte. The catalytic activity of the FeS2 film CEs was found to be comparable to the Pt and PEDOT CEs, which were both in I3−/I− and Co2+/Co3+ electrolytes, respectively. With the I3−/I− electrolyte, a PCE of 7.97% was observed for the FeS2 film CE and a PCE of 7.54% for the Pt CE in the I3−/I− electrolyte, whereas the PCEs were almost the same (6.3%) for the FeS2 film and PEDOT CEs in the [Co(bpy)3]2+/3+ redox-mediated DSSCs. The performance of the DSSCs with FeS2 and Pt CEs was studied by varying solar light illumination intensities between 1 Sun and 0.1 Sun. The current density was observed to be higher for the FeS2 CE (15.20 mA cm−2) than for the Pt CE (14.77 mA cm−2) at 1 Sun light intensity. When the solar light intensity was reduced to 0.5 and 0.1 Sun, the difference in current density between the FeS2 and Pt CEs was not noticeable, with the Jsc values being 8.97 and 8.91 mA cm−2 at 0.5 Sun, and 1.75 and 1.74 mA cm−2 at 0.1 Sun for the Fe and Pt CEs, respectively. The PCE values for the FeS2 and Pt CEs were 9.27% and 8.92% at 0.5 Sun, and 8.68% and 8.32% at 0.1 Sun, respectively. The excellent performance of FeS2 film in both electrolyte systems makes it very interesting for applications in DSSCs.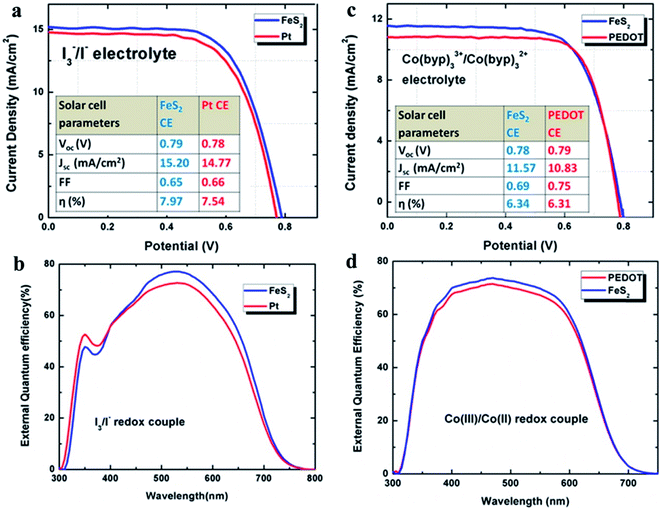 | ||
| Fig. 16 (a) Photocurrent density–voltage (J–V) curves and (b) IPCE of the DSSCs with FeS2 and Pt counter electrodes using N719 dye in I3−/I− electrolyte. (c) J–V curve and (d) IPCE of the DSSCs with FeS2 and PEDOT counter electrodes suing C128 dye in Co3+/Co2+ electrolyte. Reprinted with permission from ref. 221, S. Shukla, N. H. Loc, P. P. Boix, T. M. Koh, R. R. Prabhakar, H. K. Mulmudi, J. Zhang, S. Chen, C. F. Ng, C. H. A. Huan and N. Mathews, iron pyrite thin film counter electrodes for dye-sensitized solar cells: high efficiency for iodine and cobalt redox electrolyte cells. ACS Nano, 2014, 8, 10597–10605. Copyright© American Chemical Society. | ||
In an interesting study, FeS2 nanorod arrays were fabricated on a FTO substrate after sulfurizing FeO(OH) nanorods, and used as a CE for DSSCs.222 The FeS2 nanorods exhibited better electrocatalytic activity than FeS2 films and Pt-based CEs due to more active sites, which resulted in high Jsc value of the DSSCs. The FeS2 nanorods-based CEs showed lower interface resistance compared with FeS2 thin films, which leads to a higher FF and hence a higher PCE comparable to Pt CE based DSSCs. The electrochemical stability of the FeS2 nanorod arrays-based CE measured in I3−/I− electrolyte showed a slight change in CV plots up to 10 consecutive days of aging time. In another study, FeS2 powder prepared through a hydrothermal method was used as a CE for fabricating DSSCs.223 The effect of NaOH addition on FeS2 crystal size and electrocatalytic activities was then studied. It was observed that the size of FeS2 nanoparticles decreased after adding NaOH, and the resulting photovoltaic performance and electrocatalytic activity of DSSC with FeS2 powder significantly increased, achieving a PCE of 5.78% under simulated sunlight irradiation of 1 Sun.
A chemically prepared FeS2 nanocrystal ink was used to fabricate a CE for a DSSC, which showed a PCE of 7.31% after ethanedithiol (EDT) treatment.224 FeS2 nanocrystal ink casted on a flexible ITO/PET substrate exhibited a Jsc of 14.93 mA cm−2, Voc of 0.71 V, FF of 0.60, and a PCE of 6.36%. The semi-transparent FeS2 nanocrystal/ITO glass CE has an optical transmittance of 50–70% between 300 to 800 nm in comparison to 15% transmittance for the reference Pt/ITO glass CE. When the DSSC with the FeS2 nanocrystal CE was illuminated from the rear side, it showed a PCE of 4.17%, which was 57% of the front illumination value, whereas opaque Pt CE had a PCE of 1.06% from the rear side. Semi-transparent FeS2 nanocrystal CEs offer bifacial DSSCs utilizing incident light from both front and rear sides, and thus could be cost-effective for energy production. The FeS2 nanocrystal ink also demonstrated high electrocatalytic activity and electrochemical stability. Additionally, MWCNT/TiO2 hybrid and pure TiO2 mesoporous photoanodes with FeS2 thin films as CEs were studied for DSSCs by Kilic et al.225 In the MWCNT/TiO2 hybrid photoanode, CNT played an important role of increasing the optical absorption and shifting it toward a longer wavelength region, where the bandgap of 3.15 eV for mesoporous TiO2 shifted to 2.5 eV for the MWCNT/TiO2 hybrid. The DSSC with the MWCNT/TiO2 hybrid photoanode and the Pt CE showed values of Jsc of 15.96 mA cm−2, Voc of 0.77 V, FF of 0.57 and a PCE of 7.0%. The DSSC with the pure mesoporous TiO2 photoanode and the Pt CE both resulted in PCEs of 6.51%. The enhancement in PCE value of the hybrid photoanode is associated with MWCNTs, which offer an electrical conduction pathway for speedy electron transport. The MWCNT/TiO2 hybrid photoanode also showed an increase in IPCE in the 350–600 nm wavelength range compared to the mesoporous TiO2 photoanode. When FeS2 thin films were used as a CE with a MWCNT/TiO2 hybrid photoanode, the PCE of the DSSC increased to 7.27% under 1 Sun. The DSSC with a FeS2 CE and a pure TiO2 photoanode both yielded a PCE of 6.65%. The FeS2 thin films showed an optical bandgap of 1.27 eV and large effective surface area, which contribute to more light absorption and increased electrocatalytic activity for the reduction of triiodide (I3−).
3.6 CoS2 counter electrodes
Cobalt disulfide (CoS2) is a semiconducting material that exhibits interesting magnetic, electrical, and catalytic properties for energy storage applications.226–231 The properties of CoS2 nanocrystalline thin films prepared by a hydrothermal method were reported by Jin et al.232 CoS2 powder was dispersed in ethanol in order to prepare a nanoink (40 mg m L−1) for fabricating a CE. The CoS2 nanoink (10 μL) was drop-cast on an FTO glass or flexible ITO/PET substrate, followed by ethanol evaporation. The self-assembled CoS2 film CE has a thickness of 2.5 μm which can be controlled by the nanoink content. DSSCs were assembled by using a N719 dye-sensitized TiO2 photoanode as the working electrode, a CoS2 nanocrystal film or a Pt as the CE, and an electrolyte solution containing LiI (0.1 M), I2 (0.05 M) I2, 1,2-dimethyl-3-n-propylimidazolium iodide (DMPII, 0.6 M), and 4-tert-butylpyridine (TBP, 0.5 M) in acetonitrile. The morphology and structure of the CoS2 self-assemblies were studied by SEM and TEM techniques. Fig. 17 shows an SEM image of a self-assembled CoS2 nanocrystal film, a photographic image of CoS2 nanoink, a self-assembled CoS2 CE, and J–V curves of the DSSCs, fabricated with the CoS2 nanocrystal thin film and a Pt CE on the FTO and flexible ITO/polyethylene terephthalate (PET) substrate. The CoS2 nanoparticles were stabilized with poly(vinylpyrrolidone) (PVP) to form an oriented structure. The DSSC with self-assembled CoS2 CE shows a Jsc of 14.62 mA cm−2, Voc of 0.71 V, and a FF of 0.64, resulting in a PCE of 6.78%, which is comparable to a Pt CE with a value of 7.38%. The PCE was found to decrease when the thickness of the self-assembled CoS2 film was either higher or lower than 2.5 μm. The CoS2 shows a Rs value of 34.20 Ω cm2, slightly higher compared to Pt (27.13 Ω cm2), indicating comparable electrical conductivity. The RCT value of 7.21 Ω cm2 for the CoS2 CE indicates higher electrocatalytic activity for the reduction of triiodide (I3−). The CoS2 CE has a high chemical capacitance (Cμ) of 27.57 μF, compared to 2.76 μF for the Pt CE, which also is evidence of a high surface area for CoS2 CE which is beneficial to electrocatalysis. The DSSCs with CoS2 deposited on a flexible ITO/PET substrate showed a Jsc of 13.17 mA cm−2, Voc of 0.70 V, FF of 0.68 and a PCE of 6.40%. The fabrication of a CoS2 CE is low-cost and solution processable at room temperature, which could make it an alternative to Pt CEs for flexible DSSCs.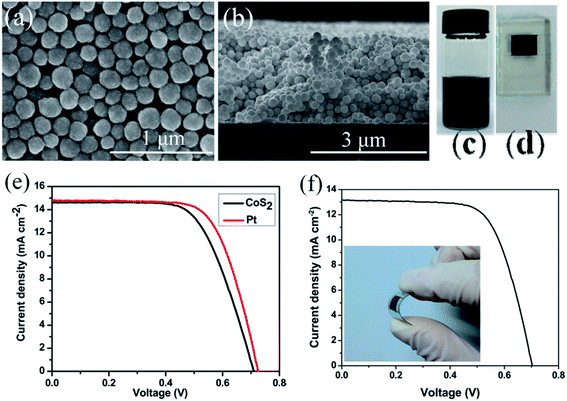 | ||
| Fig. 17 (a) SEM image of a self-assembled CoS2 nanocrystal film on FTO glass substrate, (b) cross-sectional SEM image of the CoS2 counter electrode (CE), (c) photographic image of CoS2 nanoink, and (d) self-assembled CoS2 CE. (e) Photocurrent density–voltage (J–V) curves of the DSSCs with CoS2 nanocrystal thin film and Pt CEs. (f) J–V curves of the DSSCs with CoS2 deposited on a flexible ITO/PET substrate. Reprinted with permission from ref. 232, J. Jin, X. Zhang and T. He, self-assembled CoS2 nanocrystal film as an efficient counter electrode for dye-sensitized solar cells. J. Phys. Chem. C, 2014, 118, 24877–24883. Copyright© American Chemical Society. | ||
Another study used mesoporous CoS2 nanotube arrays deposited on an FTO glass substrate and used as a CE for a DSSC.233 The CoS2 nanotube arrays were characterized by SEM, TEM, and XRD techniques for their morphology and crystal structures. The electrocatalytic properties of the CoS2 nanotube arrays were measured using CV and Tafel polarization curve measurements. The DSSCs having CoS2 CEs achieved a PCE of 6.13%, comparable to that of sputtered Pt CE (6.04%). The RCT of the mesoporous CoS2 nanotube array CE was found to be 3.51 Ω cm2 and comparable to the Pt CE (5.78 Ω cm2). The CoS2 nanotube array based CEs have large active surface area due to the mesoporous nanotube structure. The CoS2 nanotube array CE also exhibits electrocatalytic activities comparable to Pt CE. Tsai et al.234 also prepared CoS2 nanoflake arrays from Co(OH)2 nanoflake arrays through an ion exchange reaction to develop CEs for DSSCs. The CoS2 nanoflakes were found to be composed of CoS2 single crystals as well as their aggregates. The DSSC with CoS2 nanoflake arrays as a CE showed a PCE of 5.20%, comparable to a sputtered Pt CE (5.34%).
Different types of cobalt sulfide (CoS) have been used as CEs for DSSCs. The CoS nanoparticles deposited onto FTO glass substrates showed good transparency and high electrocatalytic activity for the I−/I−3 redox couple for a DSSC.235 CoS nanoparticle CEs showed a low RCT value of 1.3 Ω cm2, less than that of Pt on FTO glass (RCT of 2.3 Ω cm2) and achieved a PCE of 6.6%. An optimized CoS nanoparticle CE was also studied for a ferrocene-based liquid electrolyte. The rose-petal like CoS2 was deposited on an FTO as CE using a chemical bath deposition method.236 The DSSC assembled with a CoS2 CE achieved a PCE of 5.32%, higher than that of a Pt CE (5.02%). The PCEs of CoS2 CEs depend on the deposition parameters including the concentrations of urea and thioacetamide, and the deposition time of the CEs. Also, CoS2 embedded carbon nanocages were fabricated as CEs for DSSCs through a zeolitic imidazolate framework-67, Co(2-methylimidolate)2 template.237 The performance of the CoS2 CE in a DSSC was optimized via a sulfurization process, where CoS2 nanoparticles with embedded carbon nanocages were sulfurized for a period of 4 hours, and showed the highest PCE of 8.20%, even higher than Pt-based CE (7.88%). The synergic effect of CoS2 nanoparticles and the carbon matrix resulted in the CE having high electrical conductivity and catalytic activity. Kim et al.238 deposited CoS2, nickel sulfide (NiS), and Ni-doped CoS2 nanoparticles on a FTO substrate as CEs for DSSCs via a chemical bath deposition method. The surface morphology of the thin films was analyzed by SEM. Electrochemical properties of Ni-doped CoS2 thin films evaluated by EIS, CV, and Tafel polarization curves indicated increased electrocatalytic activity for the reduction of I3− in the DSSCs compared to Pt CEs. The Ni-doped CoS2 CE (15% Ni) showed a PCE of 5.50% under 1 Sun illumination, exceeding the PCE of the Pt CE (η = 5.21%). PCE and RCT values of the DSSCs were found to depend on the amount of Ni-doping of the CoS2 nanoparticles. These yielded PCEs of 4.81, 5.17, 5.50, and 4.12%, for RCT values of 279.7, 36.63, 8.53 and 82.72 Ω cm2, at 5, 10, 15 and 20% Ni contents in the CoS2 CE, respectively. Comparatively, DSSCs with bare CoS2 and NiS CEs showed poor electrocatalytic activity of I3− reduction.
In another study, CoS2/graphene composites were prepared via a hydrothermal method using Co ions with thiourea in the presence of graphene oxide (GO).239 The distribution and size of the CoS2 nanoparticles deposited onto a flexible graphene sheet was controlled in order to optimize the electrocatalytic activity for I3− reduction. A CoS2 nanoparticles/graphene sheet (CoS2/G50) CE was prepared by incorporating 50 mg graphene oxide, and exhibited the lowest electrolyte diffusion resistance and the highest electrocatalytic activity. The DSSC with CoS2/G50 CE achieved a PCE of 6.55%, higher than bare CoS2 or graphene CEs or a conventional Pt CE (η = 6.20%). Also, CoS2/RGO composite films for a CE of a DSSC were prepared using the layer-by-layer (LbL) assembly method, followed by thermal annealing.240 The photovoltaic parameters of the CoS2/RGO CE based DSSCs were found to depend upon the deposition times of graphene oxide. PCE values of 2.6, 4.1, 5.4, 2.9 and 1.4% were measured for 2, 4, 6, 8 and 10 deposition times of graphene oxide, respectively. It appeared that the lowest RCT of 4.8 Ω cm2 was observed for a CoS2/RGO CE prepared with 6 deposition times.
3.7 SnS2 counter electrodes
Tin disulfide (SnS2) attains morphological structures which include nanocrystals, nanosheets, nanowires, nanobelts, and these show potential for field-effect transistors, gas sensors, photocatalysts, and solar cells.241–248 In one study, semitransparent SnS2 nanosheets were prepared as a CE to develop a Pt-free DSSC for the reduction of I3−.249 The SnS2-based CE with 300 nm thickness showed high electrocatalytic activity, with a PCE of 7.64% compared to a PCE of 7.71% for a Pt CE based DSSC. When SnS2 nanosheets were functionalized with carbon nanoparticles, the CE exhibited a PCE of 8.06%, a better electrocatalytic performance than a Pt CE. SnS2 nanosheets could therefore be used as a low cost electrocatalytic electrode material for DSSCs. Yang et al.250 prepared SnS2 nanoparticles and a RGO nanocomposite as a Pt-free CE for a DSSC. The SnS2 nanoparticles dispersed onto RGO sheets exhibited improved electrocatalytic activity for reducing I3−, and also increased conductivity. Fig. 18 illustrates the preparation of SnS2@RGO nanocomposites and compares J–V curves for DSSCs having Pt, RGO, SnS2, and SnS2@RGO composite CEs. The DSSC with SnS2@RGO nanocomposite CE showed a PCE of 7.12%, much higher than that of the RGO sheet alone (3.73%) and SnS2 nanoparticles (5.58%), and a comparable PCE value to Pt CE (6.79%). The ZN values were 0.64, 4.36, 5.01 and 0.95 Ω for the SnS2@RGO nanocomposite, SnS2, RGO and Pt CEs, respectively. The RGO and SnS2 CEs have larger RCT values, hence lower electrocatalytic ability among these CEs, whereas the SnS2@RGO composite has a lower RCT value, leading to a higher electrocatalytic activity. The role of the RGO sheets is to facilitate the conduction pathway. The Epp of the SnS2@RGO nanocomposite CE is 593 mV, similar to a Pt CE (Epp of 605 mV). This indicates that the electrocatalytic activity of the SnS2@RGO nanocomposite CE is similar to that of a conventional Pt CE. The electrochemical stability of the SnS2@RGO nanocomposite CE was analyzed up to 25 cycles of CV curves, where no drastic change was noticed in peak current density, confirming that SnS2@RGO nanocomposite CEs are stable for electrocatalysis of I3− in the electrolyte. The SnS2@RGO nanocomposite CEs are as promising as Pt CEs in DSSCs. Table 3 lists a summary of the photovoltaic parameters of WS2, NiS2, TiS2, FeS2, CoS2, and SnS2 based CEs for DSSCs and their comparison with a standard Pt CE.| Counter electrodes | Redox couples | Dye | Jsc (mA cm−2) | Voc (V) | FF (%) | PCE (η, %) | Rs (Ω cm2) | RCT (Ω cm2) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| a WS2/MWCNTs* prepared without glucose aid. In the case of Rs and RCT: some of the authors used Ω instead of Ω cm2 for the resistances without mentioning the size of the electrode. | |||||||||
| WS2 | I−/I3− | N719 | 12.1 | 0.662 | 55 | 4.4 | — | — | 181 |
| WS2 (glucose solution, 0.3 M) | I−/I3− | N719 | 12.8 | 0.658 | 63 | 5.3 | — | — | 181 |
| WS2 (glucose solution, 0.6 M) | I−/I3− | N719 | 13.1 | 0.670 | 62 | 5.5 | — | — | 181 |
| WS2 (glucose solution, 1.2 M) | I−/I3− | N719 | 12.4 | 0.675 | 63 | 5.3 | — | — | 181 |
| Pt reference | I−/I3− | N719 | 13.2 | 0.668 | 63 | 5.6 | — | — | 181 |
| WS2 (sputtering time, 10 min) | I−/I3− | N719 | 13.43 | 0.71 | 66 | 6.3 | — | — | 182 |
| WS2 (sputtering time, 15 min) | I−/I3− | N719 | 15.01 | 0.69 | 55 | 5.8 | — | — | 182 |
| Pt reference | I−/I3− | N719 | 16.50 | 0.66 | 61 | 6.8 | — | — | 182 |
| WS2 (hydrothermal method) | I−/I3− | N719 | 11.28 | 0.72 | 59 | 4.79 | 4.86 | 5.13 | 185 |
| WS2/MWCNTs (3 wt%) | I−/I3− | N719 | 12.65 | 0.73 | 59 | 5.45 | 3.75 | 3.47 | 185 |
| WS2/MWCNTs (5 wt%) | I−/I3− | N719 | 13.51 | 0.73 | 65 | 6.41 | 3.01 | 2.53 | 185 |
| WS2/MWCNTs (10 wt%) | I−/I3− | N719 | 12.09 | 0.72 | 60 | 5.22 | 4.17 | 4.59 | 185 |
| MWCNTs | I−/I3− | N719 | 10.77 | 0.66 | 61 | 4.34 | 6.52 | 6.60 | 185 |
| Pt reference | I−/I3− | N719 | 13.23 | 0.74 | 67 | 6.56 | 2.26 | 2.74 | 185 |
| WS2 (hydrothermal method) | I−/I3− | N719 | 11.72 | 0.72 | 63 | 5.32 | 2.86 | 4.60 | 186 |
| WS2/MWCNTs (1 wt%) | I−/I3− | N719 | 11.95 | 0.73 | 63 | 5.50 | 2.80 | 3.86 | 186 |
| WS2/MWCNTs (5 wt%) | I−/I3− | N719 | 13.63 | 0.75 | 72 | 7.36 | 2.54 | 2.49 | 186 |
| WS2/MWCNTs (7 wt%) | I−/I3− | N719 | 12.75 | 0.75 | 68 | 6.50 | 2.67 | 2.94 | 186 |
| WS2/MWCNTs (10 wt%) | I−/I3− | N719 | 12.47 | 0.74 | 68 | 6.27 | 2.78 | 3.46 | 186 |
| WS2/MWCNTs* | I−/I3− | N719 | 12.65 | 0.73 | 59 | 5.45 | 2.85 | 3.47 | 186 |
| MWCNTs | I−/I3− | N719 | 10.77 | 0.66 | 61 | 4.34 | 2.95 | 6.60 | 186 |
| Pt reference | I−/I3− | N719 | 13.23 | 0.76 | 75 | 7.54 | 2.27 | 2.74 | 186 |
| TiS2 nanosheets | I−/I3− | N719 | 17.48 | 0.73 | 60.3 | 7.66 | — | — | 199 |
| TiS2/graphene hybrid | I−/I3− | N719 | 17.76 | 0.72 | 68.5 | 8.80 | 2.32 | 0.63 | 199 |
| Graphene | I−/I3− | N719 | 15.41 | 0.71 | 48.4 | 5.33 | — | — | 199 |
| Pt reference | I−/I3− | N719 | 16.93 | 0.72 | 65.6 | 8.00 | 6.90 | 1.32 | 199 |
| TiS2 (drop coating method) | I−/I3− | N719 | 11.27 | 0.565 | 51 | 3.24 | 16.12 | — | 200 |
| TiS2/PEDOT:PSS (5 wt%) | I−/I3− | N719 | 13.81 | 0.686 | 62 | 5.91 | — | — | 200 |
| TiS2/PEDOT:PSS (10 wt%) | I−/I3− | N719 | 15.78 | 0.681 | 66 | 7.04 | 15.78 | 4.78 | 200 |
| PEDOT:PSS | I−/I3− | N719 | 12.74 | 0.664 | 46 | 3.91 | 14.92 | 7.27 | 200 |
| Pt reference | I−/I3− | N719 | 15.83 | 0.716 | 68 | 7.65 | 14.29 | 3.02 | 200 |
| NiS2–hollow microspheres | I−/I3− | N719 | 17.48 | 0.712 | 63 | 7.84 | 10.56 | 9.68 | 206 |
| Pt reference | I−/I3− | N719 | 17.04 | 0.747 | 62 | 7.89 | 12.78 | 8.76 | 206 |
| NiS2–octahedron | I−/I3− | N719 | 13.55 | 0.712 | 62 | 5.98 | 13.14 | 9.86 | 209 |
| NiS2–cube | I−/I3− | N719 | 12.62 | 0.715 | 60 | 5.43 | 14.98 | 13.17 | 209 |
| Pt reference | I−/I3− | N719 | 14.37 | 0.718 | 63 | 6.55 | 2.24 | 6.25 | 209 |
| NiS2 (hydrothermal method) | I−/I3− | N719 | 14.42 | 0.738 | 66 | 7.02 | 5.1 | 8.8 | 210 |
| NiS2/RGO | I−/I3− | N719 | 16.55 | 0.749 | 69 | 8.55 | 6.4 | 2.9 | 210 |
| Reduced graphene oxide (RGO) | I−/I3− | N719 | 10.98 | 0.716 | 40 | 3.14 | 14.2 | 100.2 | 210 |
| Pt reference | I−/I3− | N719 | 15.75 | 0.739 | 70 | 8.15 | 2.2 | 0.5 | 210 |
| FeS2 (spray pyrolysis) | I−/I3− | N719 | 15.20 | 0.79 | 65 | 7.97 | — | — | 221 |
| Pt reference | I−/I3− | N719 | 14.77 | 0.78 | 66 | 7.54 | — | — | 221 |
| FeS2 | Co2+/Co3+ | C128 | 11.57 | 0.78 | 69 | 6.34 | 4.9 | 7.2 | 221 |
| PEDOT | Co2+/Co3+ | C128 | 10.83 | 0.79 | 75 | 6.31 | 6.0 | 3.9 | 221 |
| FeS2 films | I−/I3− | N719 | 12.56 | 0.658 | 57.8 | 4.78 | 9.60 | 213.1 | 222 |
| FeS2 nanorods | I−/I3− | N719 | 13.68 | 0.653 | 65.7 | 5.88 | 9.61 | 11.0 | 222 |
| Pt reference | I−/I3− | N719 | 13.36 | 0.685 | 68.2 | 6.23 | 5.62 | 9.2 | 222 |
| FeS2 (without NaOH) | I−/I3− | N719 | 10.20 | 0.70 | 66 | 4.76 | 3.73 | 13.6 | 223 |
| FeS2 (with NaOH) | I−/I3− | N719 | 12.08 | 0.74 | 64 | 5.78 | 2.91 | 5.99 | 223 |
| Pt reference | I−/I3− | N719 | 11.58 | 0.74 | 69 | 5.93 | 3.09 | 1.16 | 223 |
| FeS2 (with ethanedithiol) | I−/I3− | N719 | 15.14 | 0.71 | 68 | 7.31 | — | 1.60 | 224 |
| FeS2 (without ethanedithiol) | I−/I3− | N719 | 12.63 | 0.71 | 64 | 5.74 | — | 4.45 | 224 |
| Pt reference | I−/I3− | N719 | 15.37 | 0.71 | 69 | 7.52 | — | 1.47 | 224 |
| FeS2 (MWCNT/TiO2 photoanode) | I−/I3− | N719 | 16.86 | 0.77 | 56 | 7.27 | — | — | 225 |
| FeS2 (TiO2 photoanode) | I−/I3− | N719 | 15.16 | 0.77 | 57 | 6.65 | — | — | 225 |
| Pt (MWCNT/TiO2 photoanode) | I−/I3− | N719 | 15.96 | 0.77 | 57 | 7.00 | — | — | 225 |
| Pt (TiO2 photoanode) | I−/I3− | N719 | 15.68 | 0.77 | 54 | 6.51 | — | — | 225 |
| CoS2 nanocrystals | I−/I3− | N719 | 14.62 | 0.71 | 64 | 6.78 | 34.20 | 7.21 | 232 |
| Pt reference | I−/I3− | N719 | 14.78 | 0.72 | 68 | 7.38 | 27.13 | 4.57 | 232 |
| CoS2 nanotube (NT1) | I−/I3− | N719 | 5.26 | 0.765 | 52.5 | 2.13 | — | — | 233 |
| CoS2 nanotube (NT2) | I−/I3− | N719 | 10.68 | 0.794 | 64.6 | 5.48 | — | — | 233 |
| CoS2 nanotube (NT3) | I−/I3− | N719 | 11.70 | 0.797 | 65.5 | 6.11 | — | — | 233 |
| CoS2 nanotube (NT4) | I−/I3− | N719 | 11.58 | 0.804 | 65.8 | 6.13 | — | — | 233 |
| Pt reference | I−/I3− | N719 | 12.28 | 0.770 | 63.9 | 6.04 | — | — | 233 |
| CoS2 nanoflakes | I−/I3− | N719 | 10.13 | 0.747 | 68.8 | 5.20 | — | — | 234 |
| Pt reference | I−/I3− | N719 | 10.04 | 0.767 | 69.4 | 5.34 | — | — | 234 |
| CoS(50) (chloroform, 50 mM) | I−/I3− | N3 | 8.48 | 0.703 | 53.1 | 3.4 | — | — | 235 |
| CoS(25) (chloroform, 25 mM) | I−/I3− | N3 | 9.23 | 0.700 | 51.3 | 3.5 | — | — | 235 |
| CoS(25)A (annealed for 240 min) | I−/I3− | N3 | 3.86 | 0.560 | 23.2 | 0.5 | — | — | 235 |
| CoS(12.5) (chloroform, 12.5 mM) | I−/I3− | N3 | 6.08 | 0.701 | 54.1 | 2.3 | — | — | 235 |
| CoS(2.5) (chloroform, 2.5 mM) | I−/I3− | N3 | 4.32 | 0.700 | 47.9 | 1.4 | — | — | 235 |
| Pt reference | I−/I3− | N3 | 9.05 | 0.702 | 56.4 | 3.6 | — | — | 235 |
| CoS(25) (chloroform, 25 mM) | I−/I3− | N719 | 14.15 | 0.703 | 66.7 | 6.6 | — | — | 235 |
| Pt reference | I−/I3− | N719 | 13.90 | 0.692 | 62.4 | 6.0 | — | — | 235 |
| CoS2 rose–petal structure | I−/I3− | N719 | 12.14 | 0.64 | 68 | 5.32 | 9.88 | 3.52 | 236 |
| Pt reference | I−/I3− | N719 | 13.35 | 0.61 | 61 | 5.02 | 9.09 | 3.65 | 236 |
| CoS2 (chemical bath deposition) | I−/I3− | N719 | 8.41 | 0.628 | 73.6 | 4.01 | 12.02 | 326.6 | 238 |
| CoS2 (Ni-doped, 15%) | I−/I3− | N719 | 12.12 | 0.649 | 69.8 | 5.50 | 9.21 | 8.53 | 238 |
| NiS nanoparticles | I−/I3− | N719 | 9.54 | 0.592 | 67.8 | 3.83 | — | — | 238 |
| Pt reference | I−/I3− | N719 | 12.33 | 0.649 | 65.0 | 5.21 | 10.4 | 14.18 | 238 |
| CoS2 | I−/I3− | N719 | 6.25 | 0.58 | 51 | 1.86 | 9.7 | 3.4 | 239 |
| CoS2–G20 (GO powder, 20 mg) | I−/I3− | N719 | 14.38 | 0.71 | 57 | 5.86 | 7.7 | 2.3 | 239 |
| CoS2–G50 (GO powder, 50 mg) | I−/I3− | N719 | 15.12 | 0.73 | 60 | 6.55 | 7.6 | 1.3 | 239 |
| CoS2–G80 (GO powder, 80 mg) | I−/I3− | N719 | 13.11 | 0.71 | 52 | 4.83 | 7.9 | 2.7 | 239 |
| Graphene | I−/I3− | N719 | 3.68 | 0.65 | 58 | 1.37 | 7.6 | 4.0 | 239 |
| Pt reference | I−/I3− | N719 | 14.69 | 0.73 | 58 | 6.20 | 7.2 | 1.9 | 239 |
| CoS2/reduced graphene oxide | I−/I3− | Z907 | 12.87 | 0.67 | 63 | 5.4 | 22.5 | 4.8 | 240 |
| SnS2 (350 °C, 30 min) | I−/I3− | N719 | 15.63 | 0.725 | 55.3 | 6.27 | 21.5 | 8.3 | 249 |
| SnS2 (400 °C, 30 min) | I−/I3− | N719 | 16.96 | 0.743 | 60.7 | 7.64 | 18.6 | 5.6 | 249 |
| C/SnS2 (400 °C, 30 min) | I−/I3− | N719 | 17.47 | 0.745 | 61.9 | 8.06 | 17.4 | 5.2 | 249 |
| SnS2 (450 °C, 30 min) | I−/I3− | N719 | 13.37 | 0.734 | 62.6 | 6.14 | 20.8 | 10.6 | 249 |
| Pt reference | I−/I3− | N719 | 16.53 | 0.730 | 63.9 | 7.71 | 16.2 | 6.7 | 249 |
| SnS2@RGO hybrid | I−/I3− | N719 | 14.80 | 0.718 | 67.02 | 7.12 | 17.96 | 7.24 | 250 |
| SnS2 | I−/I3− | N719 | 13.60 | 0.770 | 53.28 | 5.58 | 39.73 | 11.24 | 250 |
| RGO | I−/I3− | N719 | 10.08 | 0.661 | 52.29 | 3.73 | 34.20 | 50.25 | 250 |
| Pt reference | I−/I3− | N719 | 14.00 | 0.720 | 67.36 | 6.79 | 24.21 | 5.08 | 250 |
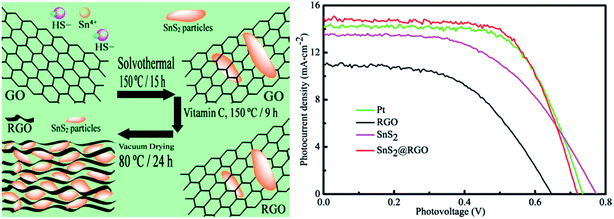 | ||
| Fig. 18 (Left) illustration depicting preparation of tin sulfide nanoparticles/reduced graphene oxide (SnS2@RGO) nanocomposites. (Right) photocurrent density–photovoltage (J–V) curves for DSSCs having Pt, RGO, SnS2, and SnS2@RGO composite CEs. Reprinted with permission from ref. 250, B. Yang, X. Zuo, P. Chen, L. Zhou, X. Yang, H. Zhang, G. Li, M. Wu, Y. Ma, S. Jin and X. Chen, nanocomposite of tin sulfide nanoparticles with reduced graphene oxide in high-efficiency dye-sensitized solar cells. ACS Appl. Mater. Interfaces, 2015, 7, 137–143. Copyright© American Chemical Society. | ||
4. Transition-metal diselenides based counter electrodes
4.1 MoSe2 counter electrodes
Molybdenum dichalcogenides, such as MoS2, MoSe2, and MoTe2, are a very interesting class of materials that has attracted a great attention because of their unique properties.251–257 Molybdenum diselenide (MoSe2) is a semiconductor that has been studied for device applications.92,258,259 The nanosheets of MoSe2 have weak van der Waals forces, therefore, poorly adhere to the surface of substrates. To resolve this problem, Lee et al.260 and Chen et al.261 applied a CVD technique to grow catalytic MoSe2 films on Mo foils. Lee et al.260 used few-layer MoSe2 in a DSSC as CE for the reduction of I3− to I−. Fig. 19 shows a schematic representation for the preparation of few-layer MoSe2, and their SEM and TEM images. The few-layer MoSe2 on Mo film was developed by selenizing the Mo-coated soda-lime glass in a tube furnace. X-ray diffraction analysis exhibits body-centered cubic (bcc) crystal structures of the Mo film. The Mo film thickness was 1 μm and selenization was performed at 550 °C for 5 minutes, and produced the best performance for the DSSC. The MoSe2 layer was about 70 nm as confirmed by the cross-sectional SEM image. The surface of the selenized Mo/glass contains nanoparticles, and the HRTEM image shows the few-layer structures. The interlayer spacing was measured as 0.63–0.64 nm. The few-layer MoSe2 was prepared by surface selenization of Mo-coated soda-lime glass, which yielded a PCE of 9.0%, compared to a PCE of 8.68% for the Pt/FTO-based photoanode, thus showing the Pt/FTO free MoSe2 CE outperformed the conventional CE. An MoS2/Mo combination as a CE in a DSSC shows a PCE of 8.69%. Fig. 20 shows a comparison of J–V curves of the DSSCs having MoSe2/Mo and MoS2/Mo CEs with different temperatures and times of selenization and sulfurization, and the Pt/FTO CE. The PCE of the DSSCs decreased from 7.14% for MoSe2 CEs selenized at 580 °C for 60 minutes, to a PCE of 4.26% for 120 minutes. The edge sites were also found to be important for the high catalytic activity. The Mo substrate plays an important role in significantly reducing the sheet resistance of the CE, which eventually leads to a high DSSC performance. The low sheet resistance of 0.29 Ω sq−1 for MoSe2/Mo, compared to 12.60 Ω sq−1 for Pt/FTO, indicates that MoSe2/Mo is better as a CE in a DSSC than a Pt/FTO. The RCT of 0.87 Ω cm2 for MoSe2 and 0.61 Ω cm2 for MoS2 is lower compared to Pt (6.26 Ω cm2) due to high conductivity of the Mo substrate, therefore the Mo substrate seems superior to the FTO substrate. Chen et al.261 also used a MoSe2/Mo based CE in a DSSC to develop a Pt/FTO-free CE, which showed a PCE of 8.13%, slightly higher than the Pt/FTO CE (PCE of 8.06%). The Rs and RCT values of the MoSe2/Mo CEs were found to be 16.5% and 3.35% of the Pt/FTO CE, indicating a higher electrocatalytic activity of the MoSe2/Mo.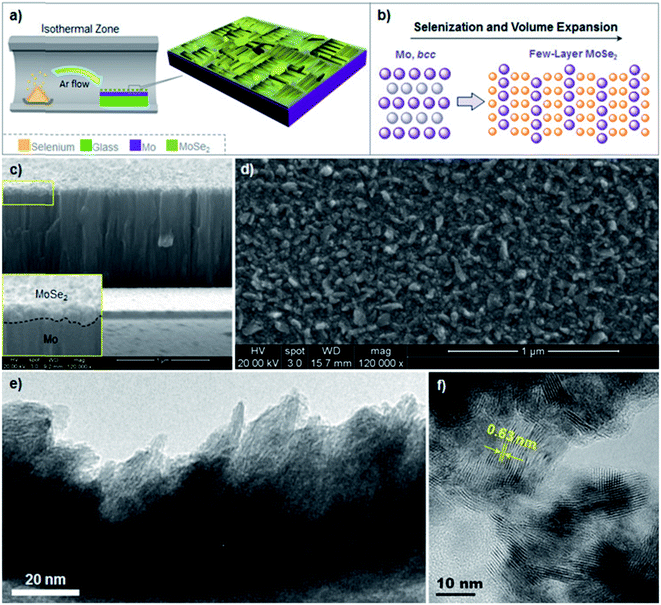 | ||
| Fig. 19 (a) Schematic representation for the selenization of Mo-coated soda-lime glass in a tube furnace for few-layer MoSe2; (b) schematic illustration showing the formation of few-layer MoSe2 from body-centered cubic (bcc) crystal structures of Mo (c) cross-sectional SEM image of the MoSe2 on Mo surface, inset shows the borderline between MoSe2 and Mo substrate; (d) SEM image of the as-synthesized MoSe2 nanostructures; (e) HRTEM image of the few-layer MoSe2; (f) high magnification HRTEM image of the few-layer MoSe2 with interlayer spacing of 0.63–0.64 nm. Reprinted with permission from ref. 260, L. T. L. Lee, J. He, B. Wang, Y. Ma, K. Y. Wong, Q. Li, X. Xiao and T. Chen, few-layer MoSe2 possessing high catalytic activity towards iodide/tri-iodide redox shuttles. Sci. Rep., 2014, 4, 4063–4069. Copyright© Nature Publishing Group. | ||
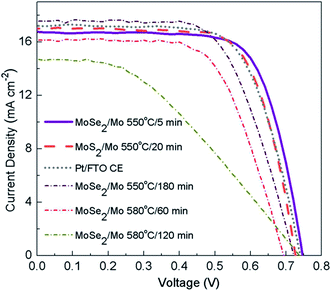 | ||
| Fig. 20 Photocurrent density–voltage (J–V) curves of DSSCs having MoSe2/Mo and MoS2/Mo counter electrodes with different temperatures and time of selenization and sulfurization and a comparison with conventional Pt/FTO counter electrodes. Reprinted with permission from ref. 260, L. T. L. Lee, J. He, B. Wang, Y. Ma, K. Y. Wong, Q. Li, X. Xiao and T. Chen, few-layer MoSe2 possessing high catalytic activity towards iodide/tri-iodide redox shuttles. Sci. Rep., 2014, 4, 4063–4069. Copyright© Nature Publishing Group. | ||
Thin films of the metal selenides NiSe2, CoSe2, and MoSe2 were used as CEs for DSSCs for I3− reduction by Ji et al.262 NiSe2 was found to be equally efficient to conventional Pt CEs. In comparison, NiSe2 also showed a higher PCE than its sulfide analog (NiS2) due to lower resistance to charge transfer. Fig. 21 shows SEM images of NiSe2, CoSe2, and MoSe2, and Tafel polarization curves and J–V curves of the metal selenides NiSe2, CoSe2, MoSe2, WSe2, Bi2Se3, MnSe, PbSe, as well as Pt based CEs used in DSSCs. The metal selenides were deposited on FTO glass. The NiSe2, CoSe2, and MoSe2 showed higher exchange current densities compared to other selenides. The J–V curve measurements of the DSSCs indicated better performance of the NiSe2 CE than that of the Pt CE, however the performance of DSSCs having CoSe2 and MoSe2 CEs was lower compared to the Pt CE.
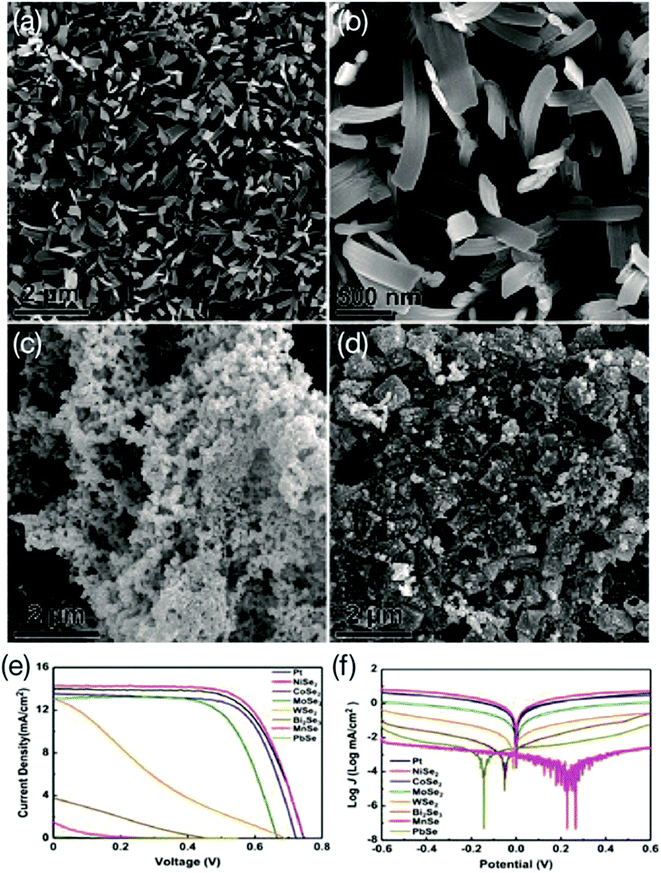 | ||
| Fig. 21 SEM images of NiSe2 (a, b) and SEM images of CoSe2 (c) and MoSe2 (d). J–V characteristic curves (e) and Tafel polarization curves (f) of DSSCs based on CEs of made of metal selenides; NiSe2, CoSe2, MoSe2, WSe2, Bi2Se3, MnSe, PbSe, and Pt. Reprinted with permission from ref. 262, I. A. Ji, H. M. Choi and J. H. Bang, metal selenide films as the counter electrode in dye-sensitized solar cell. Mater. Lett., 2014, 123, 51–54. Copyright© Elsevier. | ||
In another study, MoSe2 nanosheets were prepared using a solvothermal method which shows microsphere hierarchical architecture.263 MoSe2 nanosheets used as CE in fabricating a DSSC device showed a PCE of 9.80%, exceeding the PCE of a Pt CE based DSSC (η = 8.17%) for the triiodide/iodide (I3−/I−) redox reaction. Bi et al.264 anchored fullerene-structured MoSe2 hollow spheres on highly nitrogen-doped graphene (HNG) as a CE for a DSSC. Diethylenetriamine (DETA) was used as a dopant for nitrogen of graphene. The hollow spheres consisted of 12–15 layers of MoSe2 which formed the conductive network for facilitating rapid electron transfer in the DSCC. The MoSe2 hollow spheres of 60–100 nm diameter and 8–12 nm thickness were dispersed on a HNG surface. The HNG–MoSe2 hybrid has 52.4 wt% of MoSe2 content. The N content increased from 2.5% in MoSe2/graphene hybrid to 12.5% in the HNG–MoSe2 hybrid, which also showed high stability after 200 consecutive cycles of CV measurements. The HNG–MoSe2 hybrid CE showed a PCE of 10.01%, slightly lower than a Pt CE (η = 10.55%) under similar conditions, while the MoSe2/graphene hybrid CE had a PCE of 7.34% arising from a poor fill factor of 0.60.
Composites of MoSe2 nanosheets (NS) and poly(3,4 ethylenedioxythiophene):poly(styrenesulfonate) were investigated as the CE of a DSSC by Huang et al.265 MoSe2 NS acts as an electrocatalyst, while the PEDOT:PSS plays a role of a conductive binder to facilitate electron transfer between the MoSe2 and the substrate. The weight ratios of MoSe2 and PEDOT:PSS (MP) in the composites varied from 0.25 to 2.00. Each composite film contained 50 mg of MoSe2 powder and 200 mg, 100 mg, 50 mg, and 25 mg of PEDOT:PSS, which are respectively referred to as MP-0.25, MP-0.50, MP-1.00, and MP-2.00, as per the ratios. The DSSC with a MP-1.00 composite film (equal weights of MoSe2 and PEDOT:PSS) CE exhibits the highest electrocatalytic activity for the reduction of I3−. The DSSC containing the MoSe2 NS/PEDOT:PSS composite film based CE shows a PCE of 7.58%, compared to the Pt CE exhibiting a PCE of 7.81% under similar experimental conditions. When MoSe2 NS/PEDOT:PSS composite films coated on a titanium (Ti) foil flexible substrate was used as CE, the DSSC showed a PCE of 8.51%, compared to a PCE of 8.21% for the Pt-coated Ti foil CE. The CEs in the DSSCs show the relative order of the electrocatalytic activity as Pt > MP-1.00 > bare MoSe2 > bare PEDOT:PSS, which is the same as observed by the IPCE spectra. The MP-1.00 composite film shows larger values of the heterogeneous rate constant, and the effective catalytic surface area as compared to bare MoSe2 and bare PEDOT:PSS, because of MoSe2 NS contents. The RCT–Tafel values of 3.23 Ω cm2 for Pt, 181.46 Ω cm2 for bare PEDOT:PSS, 3.77 Ω cm2 for MP-1.00, and 3.11 Ω cm2 for bare MoSe2 were observed. The electrocatalytic ability of the CEs follow a trend seen in the RCT values measured from Tafel polarization curves and EIS. The MoSe2 NS/PEDOT:PSS composite film based CEs thus show potential to replace a costly Pt electrode.
A cobalt selenide (Co0.85Se)/MoSe2/molybdenum oxide (MoO3) ternary hybrid was evaluated as a CE for DSSCs.266 Co0.85Se/MoSe2/MoO3 ternary hybrids consist of nanorods, nanosheets, and nanoparticles, as confirmed by FESEM. CV showed larger current density for the Co0.85Se/MoSe2/MoO3 hybrid compared with a sputtered Pt CE. The Co0.85Se/MoSe2/MoO3 CE based DSSCs showed a PCE of 7.10%, much higher than that of a DSSC with a Pt CE (η = 6.03%). For comparison, the Co0.85Se hollow nanoparticles as a CE for DSSCs showed a PCE of 6.03%, lower compared to the Pt CE based DSSC (η = 6.45%).267 The transparent CEs using metal selenides alloys (M-Se; M = Co, Ni, Cu, Fe, Ru) were studied for the electrocatalytic activity for DSSCs and triiodide (I3−) reduction.268 The DSSCs containing CEs consisting of a metal selenide alloy showed PCEs of 8.30% for Co0.85Se, 7.85% for Ni0.85Se, 6.43% for Cu0.50Se, 7.64% for FeSe, and 9.22% for Ru0.33Se. A Pt CE based DSSC exhibited PCE of 6.18%. Also, a nickel cobalt sulfide (NiCo2S4) nanoneedle array269 used as a CE for a DSSC showed a PCE value of 6.9%, which is comparable to a Pt CE (η = 7.7%).
4.2 NbSe2 counter electrodes
Niobium diselenide (NbSe2) has attracted attention due to its superconducting properties270–275 and can be easily processed into nanosheets, nanoflakes, nanowires and nanotubes usable for applications in field-effect transistors,99a light-emitting diodes,276 superconductors,277 and lithium batteries.278NbSe2 nanosheets, nanorods, and NbSe2/C composites were used as CEs for DSSCs.279 The morphology and structure of the NbSe2 materials were characterized by SEM, TEM, and XRD while their electrochemical properties were evaluated by CV, EIS, and Tafel polarization curve measurements. The CEs based on NbSe2 nanorods and NbSe2 nanosheets showed lower charge transfer resistance and ionic diffusion. DSSCs having NbSe2 nanosheet-based CEs achieved a PCE of 7.34%, which further increased to 7.80% for the NbSe2/C composite-based CEs due to reduced series resistance, which is a PCE of 98.7% of the conventional Pt-based CEs (η = 7.90%). Also, NbSe2 nanostructures deposited via spray-coating were used to develop Pt-free CEs for DSSCs by Ibrahem et al.280 Fig. 22 shows SEM images and AFM height profiles of NbSe2 nanosheets, nanorods, and nanoparticles, CV, J–V curves, IPCE spectra, and EIS (presented in Nyquist plots) of Pt and NbSe2 nanostructures based CEs used in DSSCs. The morphology of the synthesized NbSe2 nanostructures was analyzed by SEM and AFM techniques. SEM analysis indicated the pristine NbSe2 2D sheets were 100 μm thick. The separate NbSe2 nanosheets were between 100–500 nm in lateral dimension. The length of NbSe2 nanorods were up to 1.2 μm with diameters ranging between 20 to 100 nm. The average size of the NbSe2 nanoparticles was between 50–100 nm. The AFM images revealed an average thickness of <8 nm for the NbSe2 nanosheets, <5 nm for the NbSe2 nanorods, and <3 nm for the NbSe2 nanoparticles. HRTEM of the NbSe2 nanosheets on their edge showed a spacing of 6.3 Å. HRTEM revealed the crystalline nature of individual NbSe2 nanorods and nanoparticles. The NbSe2 nanosheets, nanorods and nanoparticles were studied as CEs in DSSCs as a replacement to a conventional Pt CE. The dye-absorbed TiO2 electrodes were prepared by dipping electrodes into ruthenium dye 719 solution for 24 hours at room temperature. The dye solution contained 0.5 mM dye N719, [cis-di(thiocyanato)-N-N0-bis(2,20-bipyridyl-4-carboxylic acid-40-tetrabutyl-ammonium carboxylate) ruthenium(II)], and 0.5 mM chenodeoxycholic acid in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of tert-butanol and acetonitrile. The electrolyte solution was composed of 1-butyl-3-methylimidazolium iodide (BMII, 0.6 M), 4-tert-butylpyridine (0.5 M), iodine (0.03 M), and guanidinium thiocyanate (0.1 M) in a acetonitrile–valeronitrile mixture. The NbSe2 nanosheet CEs achieved a PCE of 7.73%, compared to a PCE of 7.01% for Pt-based CEs for DSSCs. The DSSCs with NbSe2 nanoparticles and nanorods based CEs show PCE values of 6.27% and 5.05%, respectively, due to low FF arising from relatively smaller surface areas, as well as low exposure on the FTO glass substrates. DSSCs having NbSe2 nanosheets based CE show the best IPCE spectral response, where the peak increases from 84% for a Pt CE to 89% for a NbSe2 nanosheets based CE. On the other hand, lower IPCE peak values were observed for NbSe2 nanoparticles and nanorods based CEs. The charge-transfer processes at the interface of TiO2/dye/electrolyte were analyzed by EIS. The NbSe2 nanosheet based CEs for the DSSCs shows a middle-frequency semicircle, implying it had the highest electro-catalytic activity for the reduction of triiodide ions (I3−), and efficient generation of electrons, therefore, occurring of larger electrons at the TiO2/dye/electrolyte interface. This study suggests that NbSe2 nanosheets could be used as alternative CEs to conventional Pt CEs in DSSCs because of their large surface area.
1 mixture of tert-butanol and acetonitrile. The electrolyte solution was composed of 1-butyl-3-methylimidazolium iodide (BMII, 0.6 M), 4-tert-butylpyridine (0.5 M), iodine (0.03 M), and guanidinium thiocyanate (0.1 M) in a acetonitrile–valeronitrile mixture. The NbSe2 nanosheet CEs achieved a PCE of 7.73%, compared to a PCE of 7.01% for Pt-based CEs for DSSCs. The DSSCs with NbSe2 nanoparticles and nanorods based CEs show PCE values of 6.27% and 5.05%, respectively, due to low FF arising from relatively smaller surface areas, as well as low exposure on the FTO glass substrates. DSSCs having NbSe2 nanosheets based CE show the best IPCE spectral response, where the peak increases from 84% for a Pt CE to 89% for a NbSe2 nanosheets based CE. On the other hand, lower IPCE peak values were observed for NbSe2 nanoparticles and nanorods based CEs. The charge-transfer processes at the interface of TiO2/dye/electrolyte were analyzed by EIS. The NbSe2 nanosheet based CEs for the DSSCs shows a middle-frequency semicircle, implying it had the highest electro-catalytic activity for the reduction of triiodide ions (I3−), and efficient generation of electrons, therefore, occurring of larger electrons at the TiO2/dye/electrolyte interface. This study suggests that NbSe2 nanosheets could be used as alternative CEs to conventional Pt CEs in DSSCs because of their large surface area.
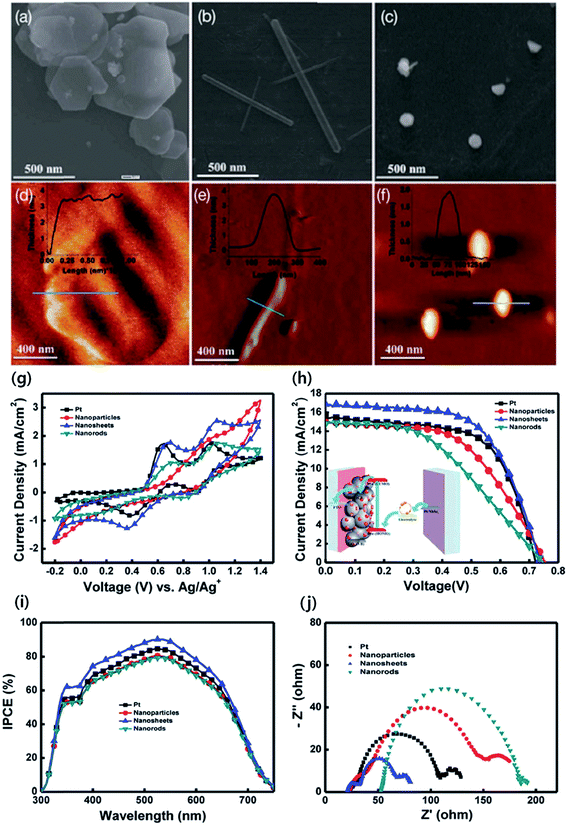 | ||
| Fig. 22 SEM images of NbSe2 nanostructures that include nanosheets (a), nanorods (b), and nanoparticles (c), (d–f) AFM height images of NbSe2 nanosheets (d), nanorods (e), and nanoparticles (f). The insets show height profiles measured by AFM images. (g) Cyclic voltammograms (CV) of Pt CE and NbSe2 nanostructures based CEs. (h) J–V curves of Pt and NbSe2 nanostructures. The inset represents DSSC structure with an energy level diagram of the materials. (i) Photon-to-current conversion efficiency (IPCE) curves of Pt and NbSe2 nanostructures, and (j) EIS Nyquist plots of Pt and NbSe2 nanostructures based CEs used in DSSCs. Reprinted with permission from ref. 280, M. A. Ibrahem, W. C. Huang, T. W. Lan, K. M. Boopathi, Y. C. Hsiao, C. H. Chen, W. Budiawan, Y. Y. Chen, C. S. Chang, L. J. Li, C. H. Tsai, C. C. Chu, controlled mechanical cleavage of bulk niobium diselenide to nanoscaled sheet, rod, and particle structures for Pt-free dye-sensitized solar cells. J. Mater. Chem. A, 2014, 2, 11382–11390. Copyright© Royal Society of Chemistry. | ||
4.3 TaSe2 counter electrodes
Three transition-metal selenides (MoSe2, WSe2, and TaSe2) were prepared by Guo et al.281 and compared as potential CEs for fabricating DSSC devices using a solvothermal method. These transition-metal selenides show high electrocatalytic activity for the reduction of triiodide (I3−), except for MoSe2, due to low adsorption and charge-transfer. Fig. 23 shows SEM and TEM images of MoSe2 (a and d), WSe2 (b and e), and TaSe2; the Nyquist plots of EIS measurements recorded at −0.75 V bias of the DSSCs based on MoSe2, WSe2, and Pt CEs for the triiodide/iodide (I3−/I−) redox couple in the electrolyte; and their J–V curves measured under simulated sunlight illumination. The MoSe2 nanosheets had thickness of about 10 nm with lateral dimensions ranging 100–150 nm. WSe2 consists of interlaced nanoplates with 15 nm average thickness and a width varying between 60 and 100 nm. The SEM image of TaSe2 shows fluffy nanoparticles with a wide size distribution. The HRTEM images of MoSe2, WSe2, and TaSe2 indicated spacings of 0.282 nm, 0.283 nm, and 0.291 nm, respectively. The WSe2 shows a pore size distribution (PSD) curve at about 43 nm, MoSe2 at 30 nm, and TaSe2 at 10 nm. The mesoporous structure of WSe2 facilitates the adsorption of triiodide (I3−) and transport during the electrocatalytic activity. The BET surface areas were 95.6, 104.4 and 78.8 m2 g−1, and the total pore volumes were 0.32 cm3 g−1, 0.41 cm3 g−1 and 0.14 cm3 g−1, for WSe2, MoSe2, TaSe2, respectively. The significant difference in BET surface area and the pore size distribution led to different adsorption properties and electrocatalytic activity of the DSSCs. The RCT of the transition-metal selenides at the electrolyte/electrode interface indicates the level of electrocatalytic activity. The RCT value of 0.78 Ω cm2 for the WSe2 CE is smaller compared with a Pt CE (1.32 Ω cm2) and a TaSe2 (1.89 Ω cm2) CE, while the RCT value of the MoSe2 (2.43 Ω cm2) CE was larger than that of the Pt CE. Therefore, WSe2 shows better electrocatalytic activity for triiodide (I3−) reduction. The ionic ZN value of WSe2 is 1.15 Ω cm2, lower than that of the MoSe2, and TaSe2 CEs, and comparable to the Pt CE, which demonstrates a larger diffusion coefficient for triiodide (I3−) within the CEs. The conductivities measured by linear sweep voltammetry decrease in the relative order TaSe2 > MoSe2 > WSe2. The DSSCs based on TaSe2 and WSe2 CEs showed larger Jsc than that of MoSe2 CE. WSe2 has larger pores and high electrocatalytic activity suitable for fast regeneration and transfer of triiodide (I3−). PCE values of 7.32% for TaSe2 and 7.48% for Wse2 were measured, which are comparable to a sputtered Pt CE (η = 7.91%). The lower PCE value of 6.70% for MoSe2 arises from a lower Voc and Jsc for the DSSC.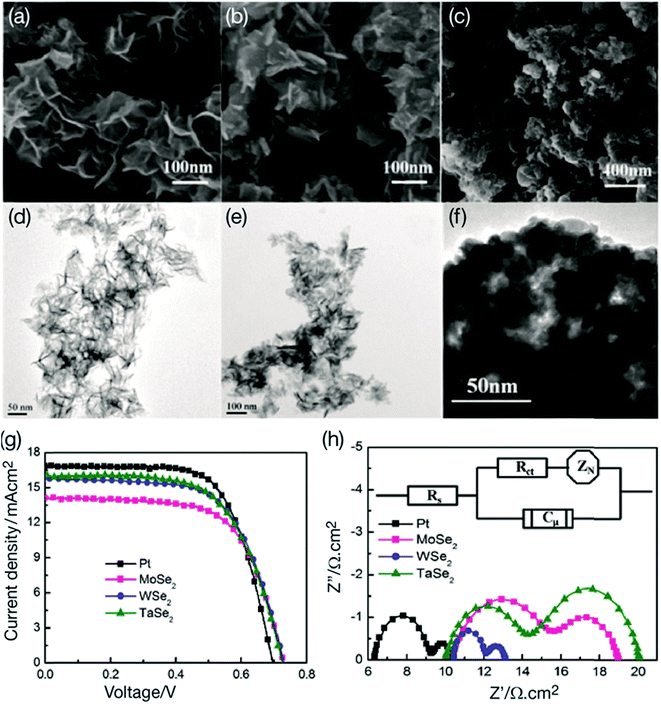 | ||
| Fig. 23 SEM and TEM images of MoSe2 (a and d), WSe2 (b and e), and TaSe2 (c and f). (g) Photocurrent–voltage (J–V) curves of the DSSCs based on MoSe2, WSe2, and Pt CEs, measured under simulated sunlight illumination (100 mW cm−2, 1.5 AM G). (h) Nyquist plots of electrochemical impedance spectroscopy (EIS) measurements for DSSCs fabricated with two identical electrodes in the triiodide/iodide (I3−/I−) redox couple in the electrolyte. The inset represents equivalent circuit of DSSCs for fitting Nyquist plots. Reprinted with permission from ref. 281, J. Guo, S. Liang, Y. Shi, C. Hao, X. Wang and T. Ma, transition metal selenides as efficient counter-electrode materials for dye-sensitized solar cells. Phys. Chem. Chem. Phys., 2015, 17, 28985–28992. Copyright© Royal Society of Chemistry/Owner Societies. | ||
4.4 NiSe2 counter electrodes
Nickel diselenide (NiSe2) has been studied as a CE of DSSCs for the reduction of I3−, which yielded higher PCE of 8.69% than that of a conventional Pt CE (8.04%) under the same experimental conditions.282 Zhang et al.283 developed two types of NiSe2 CEs on RGO; namely, microsphere NiSe2/RGO, and octahedron NiSe2/RGO through a hydrothermal process. The microsphere NiSe2/RGO CE exhibited higher electrocatalytic performance than a Pt CE for the reduction of triiodide (I3−) because of better carrier transfer induced by graphene nanosheets.CEs of ternary Ni–Co compounds having different morphological structures such as nanoparticles, nanotubes, nanowires, nanoflakes, follower-like, and urchin-like have been studied for DSSCs.284,285 For example, the CE made of flower-like NiCo2S4/NiS microspheres286 exhibited a PCE of 8.8%, much higher than a standard Pt CE (η = 8.1%). A similar concept was employed by Qian et al.287 for developing a very interesting class of CEs for DSSCs from nickel cobalt (Ni–Co) selenides having different morphological structures, due to the tuning of the Ni/Co molar ratios. The morphological structure and electrocatalytic performance of ternary Ni–Co selenides was optimized by using different Ni/Co molar ratios. Fig. 24 shows the SEM images of Co3Se4, Ni0.33Co0.67Se precursor, Ni0.33Co0.67Se, Ni0.5Co0.5Se, Ni0.67Co0.33Se, and NiSe, and also shows CV and J–V curves of their DSSCs. The different morphological structures were obtained by tuning the Ni/Co molar ratio, NixCo1−xSe, where, x was 0, 0.33, 0.5, 0.67, and 1.0. The 3D dandelion-like precursor of Ni0.33Co0.67Se assembled into nanotubes having about 100 nm diameter, Ni0.5Co0.5Se into a floccus-like microsphere structure with a diameter of 4 microns, Ni0.67Co0.33Se microspheres compiled into nanosheets, and Co3Se4 built up rough-surface nanotubes. The specific surface areas determined from the BET method were 5.0, 10.1, 26.1, 28.9, and 35.6 m2 g−1, for NiSe, Co3Se4, Ni0.5Co0.5Se, Ni0.67Co0.33Se and Ni0.33Co0.67Se, respectively. Among all metal selenides, Ni0.33Co0.67Se has the highest specific surface area, which is favorable for providing more active sites for catalysis and increasing the contact area between its CE and the electrolyte, which results in better electrochemical and photovoltaic properties of its DSSCs. The RCT values of CEs follows the relative order of NiSe > Co3Se4 > Pt > Ni0.67Co0.33Se > Ni0.5Co0.5Se > Ni0.33Co0.67Se, which implies that the electrocatalytic activity increases in a reverse order. Thus, NiSe was of the lowest activity and Ni0.33Co0.67Se was of the highest catalytic activity for the reduction of triiodide (I3−). Therefore, higher contents of Ni in the Ni–Co selenides CEs are not favorable for electrocatalytic activity. The values of cathodic peak current density (red-1) and the Epp can also help understand the electrocatalytic activities of these CEs. The authors noted the following relative order of cathodic peak current density: NiSe (1.044 mA cm−2) < Co3Se4 (1.849 mA cm−2) < Pt (2.373 mA cm−2) < Ni0.67Co0.33Se (2.878 mA cm−2) < Ni0.5Co0.5Se (2.917 mA cm−2) < Ni0.33Co0.67Se (3.120 mA cm−2). They also noted Epp values of NiSe (602 mV) > Co3Se4 (565 mV) > Pt (460 mV) > Ni0.67Co0.33Se (365 mV) > Ni0.5Co0.5Se (349 mV) > Ni0.33Co0.67Se (329 mV), which were in an agreement with EIS measurements of the CEs. The 3D dandelion-like Ni0.33S0.67Se microspheres based CEs exhibited the highest PCE of 9.01%, exceeding that of the Pt CE (η = 8.30%). The DSSC with a Co3Se4 CE showed a PCE of 7.95%, higher than that of NiSe CE (η = 7.23%). These results support the notion that the ternary Ni–Co selenides possess higher electrocatalytic activities and photovoltaic properties than those of binary selenides NiSe and Co3Se4 as well as Pt CEs for the triiodide (I3−) reduction, due to their unique morphology and chemical composition.
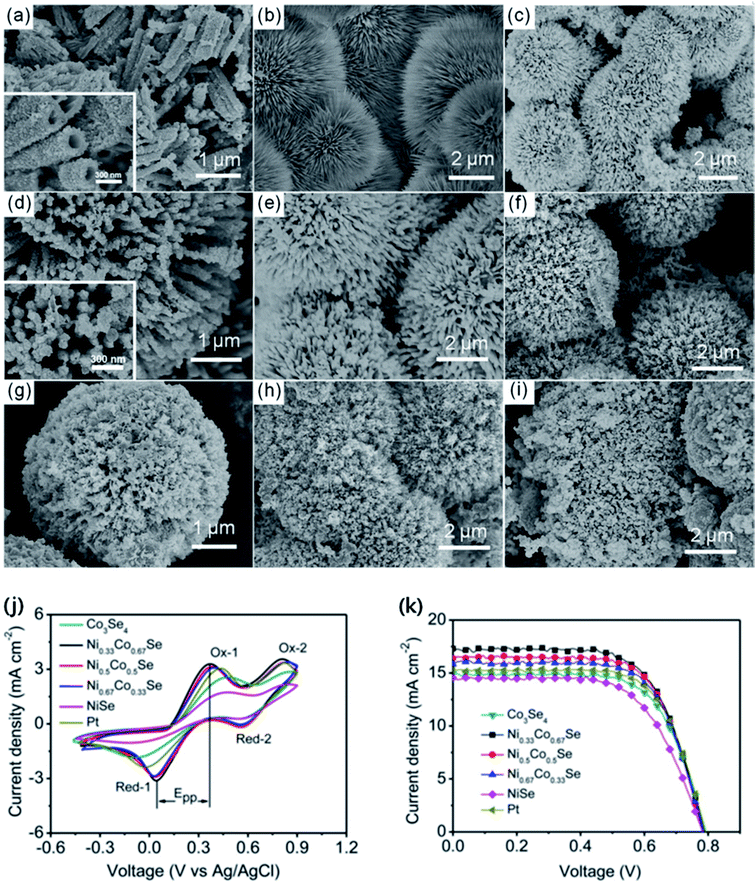 | ||
| Fig. 24 SEM images of (a) Co3Se4, (b) Ni0.33Co0.67Se precursor, (c–e) Ni0.33Co0.67Se, (f and g) Ni0.5Co0.5Se, (h) Ni0.67Co0.33Se, and (i) NiSe. (j) CVs of DSSCs with Co3Se4, Ni0.33Co0.67Se, Ni0.5Co0.5Se, Ni0.67Co0.33Se, NiSe, and Pt-based CEs at a scan rate of 50 mV s−1. (k) Photocurrent density–voltage (J–V) curves of DSSCs with Co3Se4, Ni0.33Co0.67Se, Ni0.5Co0.5Se, Ni0.67Co0.33Se, NiSe, and Pt CEs under AM 1.5G illumination. Reprinted with permission from ref. 287, X. Qian, H. Li, L. Shao, X. Jiang and L. Hou, morphology-tuned synthesis of nickel cobalt selenides as highly efficient Pt-free counter electrode catalysts for dye-sensitized solar cells. ACS Appl. Mater. Interfaces, 2016, 8, 29486–29495. Copyright© American Chemical Society. | ||
4.5 FeSe2 counter electrodes
Iron diselenide (FeSe2) can be processed into nanosheets, nanocubes, flower-like structures, and nanorods, rod clusters, and microspheres,288–294 and has applications in catalysis,295 batteries,296,297 and photovoltaic devices.298 The first-row (3d) transition metal dichalcogenides (MX2) with pyrite structure (where, M = Fe, Co, Ni, and X= S, Se) also exhibit electronic, optoelectronic, and magnetic properties comparable to other TMDs.299–302 Huang et al.302 used 2D FeSe2 nanosheets with 7 nm average thickness as a CE for a DSSC. Fig. 25 shows the SEM and TEM images of the synthesized FeSe2 nanosheets. The thickness of FeSe2 nanosheets was found to be in the 4–7 nm range, as evaluated by TEM images. High-resolution TEM (HRTEM) indicated the crystalline nature of the nanosheets, having 0.168 nm interplanar spacing. The FeSe2 nanosheets had specific surface area of 35.02 m2 g−1 as calculated by the BET analysis. The FeSe2 nanosheets exhibit a low charge-transfer resistance (RCT) and a high electrocatalytic activity for triiodide (I3−) reduction in DSSCs. The FeSe2 nanosheets CE based DSSC showed a PCE of 7.53%, comparable to a Pt CE, while the PCE of FeSe2 microparticles CE based DSSC was slightly lower (η = 6.88%). The large surface area of the FeSe2 nanosheets contributed to a higher PCE by providing more active sites for electrocatalytic activity, as well as a larger electrode/electrolyte interface that of the FeSe2 microparticles. The electrochemical stability of the CEs of FeSe2 nanosheets and Pt were investigated with sequential CV scanning. The current densities of the FeSe2 CE showed no change after 1000 cycles, confirming corrosion resistance to the electrolyte. The PCEs of FeSe2 nanosheets under nitrogen protection (N–FeSe2) and exposure to air for one week (O–FeSe2) were also studied. The RCT of the N–FeSe2 CE (0.53 Ω cm2) was much lower that the O–FeSe2 CE (10.13 Ω cm2) and a Pt CE (1.68 Ω cm2), indicating poorer electrocatalytic activity of the O–FeSe2 CE compared to the N–FeSe2 and Pt CEs. The Tafel polarization curves decreased in the order N–FeSe2 > Pt > O–FeSe2, having the same order as of exchange current density, CV and EIS measurements. The O–FeSe2 nanosheets CE based DSSC has a PCE of 6.15%, much lower than that of the N–FeSe2 (η = 7.53%) CE and Pt CE (η = 7.47%) under similar conditions.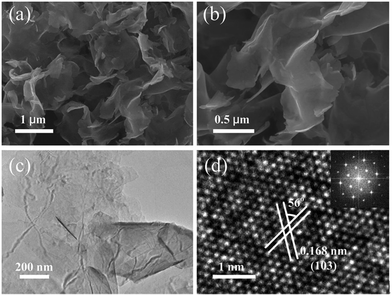 | ||
| Fig. 25 (a, b) SEM images and (c) TEM image of the as-synthesized FeSe2 nanosheets, and (d) HRTEM image of a small portion of FeSe2 nanosheet (inset shows SAED pattern of the FeSe2 nanosheets). Reprinted with permission from ref. 302, S. Huang, Q. He, W. Chen, Q. Qiao, J. Zai and X. Qian, ultrathin FeSe2 nanosheets: controlled synthesis and application as a heterogeneous catalyst in dye-sensitized solar cells. Chem.–Eur. J., 2015, 21, 4085–4091. Copyright© Wiley-VCH. | ||
3D hierarchical FeSe2 microspheres using a hot-injection method were prepared and studied by Huang et al.303 The morphologies of the FeSe2 nanomaterials was controlled by the use of alkyl thiols; 1-dodecanethiol (1-DDT) or tert-dodecanethiol (t-DDT) and their contents were used in synthesis, which varied from irregular FeSe2 micro/nanoparticles to 3D hierarchical FeSe2 microspheres and consisted of ultrathin FeSe2 nanosheets or urchin-like microspheres made of crystalline FeSe2 nanorods having an average diameter of 650 nm. The FeSe2 nanomaterials were used as CEs for DSSCs. 3D hierarchical FeSe2 microspheres made of ultrathin FeSe2 nanosheets showed the lowest RCT of 0.49 Ω cm2 at the electrolyte/electrode interface, a lower ZN value of 0.39 Ω cm2, and faster reaction kinetics for the reduction of I3− to I− than that of a Pt CE (RCT of 1.15 Ω cm2 and ZN value of 0.91 Ω cm2). RCT values followed the relative order of FeSe2 microparticles < FeSe2 nanorods < Pt < FeSe2 nanosheets, as supported by EIS measurements. A DSSC with a FeSe2 nanosheets CE exhibited a PCE of 8.39%, slightly better than that of a Pt CE (8.20%) under simulated solar illumination of 100 mW cm−2 (AM 1.5). FeSe2 nanorods showed a PCE value of 8.03%, higher than that of FeSe2 microparticles (7.68%). The PCE value of DSSCs is morphology dependent, where the FeSe2 nanosheets CE has a high electrocatalytic activity and a larger specific surface area (30.03 m2 g−1) than that of FeSe2 nanorods (19.82 m2 g−1). The FeSe2 nanosheets CE based DSSC retained 99.5% of its initial photocurrent density, compared to 98.3% retained by the Pt CE, after simulated solar illumination of 100 mW cm−2 for 1 hour, and this indicates better stability of the FeSe2 nanosheets CE than that of standard Pt CE. Also, the 3D flower-like and sphere-shaped FeSe2 films were used as CEs for DSSCs.304 The 3D flower-like FeSe2-based CE exhibited a comparable PCE to a Pt CE (η = 8.00% versus 7.87%).
4.6 CoSe2 counter electrodes
Cobalt diselenide (CoSe2) has been extensively studied as a catalyst for oxygen reduction reactions.305–308 The CoSe2 nanorods synthesized by a hydrothermal method was studied as CEs for DSSCs.309 FESEM revealed a CoSe2 nanorod morphology of the CoSe2, which supports carrier transport from the surface of the nanorods to the redox electrolyte. The CoSe2 CE also exhibited larger current density compared with a Pt CE, as measured by CV. EIS shows an Rs of 8.034 Ω cm2 and a low RCT of 0.097 Ω cm2 for the CoSe2 CE. The DSSC with a CoSe2 CE showed a PCE value of 8.38%, higher than the DSSC based on a Pt CE (η = 7.83%), under simulated sunlight illumination of 100 mW cm−2 (AM 1.5G).A comprehensive and detailed study was conducted by Chiu et al.310 on composite films of CoSe2/carbon (CoSe2/C) deposited on FTO substrates having three different morphologies, developed using electro-deposition, followed by an annealing process at 500 °C for 30 minutes in vacuum. In the first stage, three types of CoSe2/carbon films containing nanowalls were deposited with an electro-deposition process employing different pH baths, while in the second stage, the morphology of the films was transformed after the annealing. The N719 dye-adsorbed TiO2 film was used as a photoanode for DSSCs. CoSe2/C films had three different morphologies, including nanograin (NG), nanorock (NR), and nanoclimbing-wall (NCW), which were used as CEs for DSSCs. The electrocatalytic activity of these three CEs was analyzed by CV, RDE, Tafel polarization curves, and EIS, which showed a relative order of CoSe2/C–NCW > CoSe2/C–NG > Pt > CoSe2/C–NR as CEs in the DSSCs, a similar order as was observed for the RCT values obtained by EIS and Tafel measurements. The CoSe2/C–NCW showed higher electrical conductivity and a large effective surface area and, therefore, the best electrocatalytic ability for triiodide (I3−) reduction. The DSSCs with CoSe2/C–NG, CoSe2/C–NCR, CoSe2/C–NCW, and Pt CEs exhibited IPCE values between 80–95% in the 400 to 600 nm wavelength region, where the highest IPCE value of 95% was observed for the CoSe2/C–NCW CE. The DSSC having a CoSe2/C–NCW CE showed the highest PCE value of 8.92%, even higher than compared with the Pt CE (8.25%). The CoSe2/C–NCW CEs were electro-deposited onto low-cost, flexible, and highly porous substrates, such as carbon cloth (CC, sheet resistance = 0.63 Ω sq−1) and nickel foam (NF, porosity = 95%, sheet resistance = 0.45 Ω sq−1). Fig. 26 shows photographs of the flexible nickel foam (NF) and carbon cloth (CC), SEM images of CoSe2/C–NCW film on the flexible NF and CC substrates, and J–V curves of the DSSCs, with CoSe2/C–NCW on NF and CC CEs measured at different light intensities (20–100 mW cm−2). The CoSe2/carbon–NCW CEs shell covered all the minute parts of the carbon cloth and nickel foam core shell structures. The DSSC containing the CE with CoSe2/C–NCW deposited on nickel foam exhibited the highest PCE of 10.46% at 100 mW cm−2 (1 Sun) and 7.90% at 20 mW cm−2 (0.2 Sun). The DSSC with the CE of CoSe2/C–NCW deposited on low-weight carbon cloth showed a PCE of 9.87% at 1 Sun and 7.83% at 0.2 Sun. The low cost and flexible CoSe2/C–NCW CEs seem to be promising materials to replace expensive Pt CEs for DSSCs for indoor, outdoor or wearable applications.
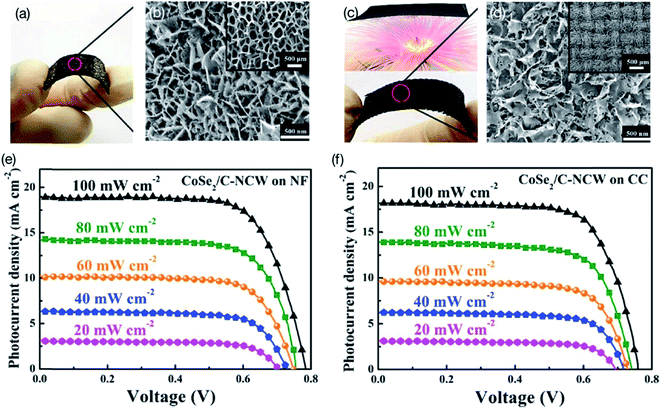 | ||
| Fig. 26 (a) Photograph of the nickel foam (NF) and (b) SEM image of CoSe2/C–NCW film on the nickel foam (NF). (c) Photograph of the carbon cloth (CC) and thin CC substrate on a soft flower having needle-like petals and (d) SEM image of CoSe2/C–NCW film on carbon cloth (CC). Inset shows field-emission scanning electron microscopy (FE-SEM) images in a magnified version. (e) Photocurrent–voltage (J–V) curves of the DSSCs with CoSe2/C–NCW on flexible nickel foam (NF) CE, measured at light intensities varying from 20 mW cm−2 (0.2 Sun) to 100 mW cm−2 (1 Sun). (f) Photocurrent–voltage (J–V) curves of the DSSCs with CoSe2/C–NCW on flexible carbon cloth (CC) CE, under similar light intensities. Reprinted with permission from ref. 310, I. T. Chiu, C. T. Li, C. P. Lee, P. Y. Chen, Y. H. Tseng, R. Vittal and K. C. Ho, nanoclimbing-wall-like CoSe2/carbon composite film for the counter electrode of a highly efficient dye-sensitized solar cell: a study on the morphology control. Nano Energy, 2016, 22, 594–606. Copyright© Elsevier. | ||
CoSe2 and RGO composites were also explored as CEs in DSSCs, which showed a PCE of 7.01% versus a Pt CE (η = 6.77%).311 Co0.85Se and Ni0.85Se was deposited on FTO glass substrate by a low-temperature hydrothermal process and were used as CEs for DSSCs by Gong et al.312 Co0.85Se has a graphene-like nanostructure and possesses a large surface area, while Ni0.85Se is composed of aggregated particles. The graphene-like Co0.85Se CE showed higher electrocatalytic activity than that of the Pt CE for the reduction of triiodide (I3−). DSSCs with Co0.85Se CEs showed a PCE of 9.40%, significantly higher than that of a Pt CE (8.64%), under simulated solar light of 100 mW cm−2 (AM 1.5G). In the case of the Ni0.85Se CE, the PCE of 8.32% was slightly lower than a Pt CE. Both Jsc and PCE values showed a relative order of Ni0.85Se < Pt < Co0.85Se. The RCT value was found to increase in the relative order Co0.85Se (0.6 Ω cm2) < Pt (1.1 Ω cm2) < Ni0.85Se (1.8 Ω cm2), suggesting an inverse order of electrocatalytic activity of these CEs in the DSSCs. Table 4 summarizes the photovoltaic parameters of MoSe2, WSe2, TaSe2, NbSe2, FeSe2, CoSe2 and Bi2Se3 based CEs for DSSCs, and their comparison with a standard Pt CE.
| Counter electrodes | Redox couple | Dye | Jsc (mA cm−2) | Voc (V) | FF (%) | PCE (η, %) | Rs (Ω cm2) | RCT (Ω cm2) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| a Nanograin (NG), nanorock (NR), nanoclimbing-wall (NCW), nickel foam (NF), carbon cloth (CC), rGO = reduced graphene oxide. In the case of Rs and RCT: some of the authors used Ω instead of Ω cm2 for the resistances without mentioning the size of the electrode. | |||||||||
| MoSe2/Mo (in situ sulfurization) | I−/I3− | N719 | 16.71 | 0.746 | 72.2 | 9.00 | 1.74 | 1.39 | 260 |
| MoS2/Mo (in situ sulfurization) | I−/I3− | N719 | 16.95 | 0.726 | 70.6 | 8.69 | 1.21 | 5.25 | 260 |
| Pt reference | I−/I3− | N719 | 17.19 | 0.740 | 68.3 | 8.68 | 12.52 | 0.22 | 260 |
| MoSe2/Mo (in situ selenization) | I−/I3− | N719 | 15.07 | 0.805 | 67 | 8.13 | 2.64 | 0.30 | 261 |
| Pt reference | I−/I3− | N719 | 16.11 | 0.794 | 63 | 8.06 | 15.98 | 8.95 | 261 |
| NiSe2 | I−/I3− | N719 | 14.3 | 0.75 | 68 | 7.3 | 20.9 | 45.0 | 262 |
| NiS2 | I−/I3− | N719 | 14.7 | 0.72 | 52 | 5.5 | 28.4 | 50.4 | 262 |
| CoSe2 | I−/I3− | N719 | 13.5 | 0.72 | 68 | 6.6 | 14.8 | 102.7 | 262 |
| MoSe2 | I−/I3− | N719 | 13.0 | 0.67 | 68 | 5.9 | 16.5 | 229.8 | 262 |
| Pt reference | I−/I3− | N719 | 14.0 | 0.75 | 69 | 7.2 | 18.5 | 34.2 | 262 |
| MoSe2 (hollow spheres) | I−/I3− | N749 | 16.06 | 0.704 | 38.67 | 4.46 | — | 10.24 | 264 |
| MoSe2/graphene (12.5% N2-doped) | I−/I3− | N749 | 19.73 | 0.724 | 70.07 | 10.01 | 7.18 | 3.04 | 264 |
| MoSe2/graphene | I−/I3− | N749 | 17.12 | 0.710 | 60.41 | 7.34 | — | 8.49 | 264 |
| Graphene | I−/I3− | N749 | 16.67 | 0.535 | 54.12 | 4.83 | — | 16.27 | 264 |
| Pt reference | I−/I3− | N749 | 19.93 | 0.723 | 73.22 | 10.55 | 7.14 | 2.81 | 264 |
| MoSe2/PEDOT:PSS | I−/I3− | N719 | 15.97 | 0.70 | 67 | 7.58 | 18.08 | 5.43 | 265 |
| Pt reference | I−/I3− | N719 | 16.38 | 0.74 | 65 | 7.81 | 20.19 | 4.60 | 265 |
| MoSe2/PEDOT:PSS@Ti | I−/I3− | N719 | 16.41 | 0.75 | 69 | 8.51 | — | — | 265 |
| Pt@Ti reference | I−/I3− | N719 | 16.31 | 0.74 | 68 | 8.21 | — | — | 265 |
| Bare MoSe2 | I−/I3− | N719 | 12.65 | 0.66 | 28 | 2.29 | 20.50 | 39.74 | 265 |
| Bare PEDOT:PSS | I−/I3− | N719 | 9.32 | 0.67 | 46 | 2.90 | 17.36 | 190.91 | 265 |
| Co0.85Se | I−/I3− | N719 | 13.41 | 0.763 | 62.7 | 6.42 | — | — | 266 |
| 3Co0.85Se/0.5MoSe2/0.5MoO3 | I−/I3− | N719 | 13.43 | 0.765 | 65.7 | 6.75 | — | — | 266 |
| 2Co0.85Se/MoSe2/MoO3 | I−/I3− | N719 | 13.80 | 0.768 | 67.1 | 7.10 | — | — | 266 |
| Co0.85Se/1.5MoSe2/1.5MoO3 | I−/I3− | N719 | 13.06 | 0.760 | 64.4 | 6.39 | — | — | 266 |
| MoSe2/MoO3 | I−/I3− | N719 | 12.95 | 0.754 | 62.7 | 6.12 | — | — | 266 |
| Pt reference | I−/I3− | N719 | 13.05 | 0.759 | 60.9 | 6.03 | — | — | 266 |
| Co0.85Se | I−/I3− | N719 | 13.44 | 0.66 | 68 | 6.03 | 55.83 | 9.28 | 267 |
| Pt reference | I−/I3− | N719 | 14.37 | 0.67 | 67 | 6.45 | 30.64 | 13.89 | 267 |
| Co0.85Se (front irradiation) | I−/I3− | N719 | 16.74 | 0.742 | 66.8 | 8.30 | — | 2.84 | 268 |
| Co0.85Se (rear irradiation) | I−/I3− | N719 | 9.92 | 0.721 | 64.7 | 4.63 | — | — | 268 |
| Ni0.85Se (front irradiation) | I−/I3− | N719 | 16.67 | 0.740 | 63.6 | 7.85 | — | 2.96 | 268 |
| Ni0.85Se (rear irradiation) | I−/I3− | N719 | 9.26 | 0.731 | 64.6 | 4.37 | — | — | 268 |
| Cu0.50Se (front irradiation) | I−/I3− | N719 | 14.55 | 0.713 | 62.0 | 6.43 | — | 5.44 | 268 |
| Cu0.50Se (rear irradiation) | I−/I3− | N719 | 10.01 | 0.666 | 63.6 | 4.24 | — | — | 268 |
| FeSe (front irradiation) | I−/I3− | N719 | 17.10 | 0.733 | 61.0 | 7.64 | — | 4.90 | 268 |
| FeSe (rear irradiation) | I−/I3− | N719 | 10.49 | 0.732 | 65.8 | 5.05 | — | — | 268 |
| Ru0.33Se (front irradiation) | I−/I3− | N719 | 18.93 | 0.715 | 68.1 | 9.22 | — | 2.77 | 268 |
| Ru0.33Se (rear irradiation) | I−/I3− | N719 | 11.89 | 0.714 | 69.5 | 5.90 | — | — | 268 |
| Pt (front irradiation) | I−/I3− | N719 | 13.09 | 0.712 | 66.3 | 6.18 | — | 7.23 | 268 |
| Pt (rear irradiation) | I−/I3− | N719 | 9.48 | 0.652 | 57.6 | 3.56 | — | — | 268 |
| NiCo2S4 (hydrothermal method) | I−/I3− | N719 | 13.38 | 0.76 | 63.2 | 6.9 | — | — | 269 |
| NiCo2O4 (hydrothermal method) | I−/I3− | N719 | 8.2 | 0.67 | 26.7 | 1.5 | — | — | 269 |
| Pt reference | I−/I3− | N719 | 14.20 | 0.8 | 63.4 | 7.7 | — | — | 269 |
| NbSe2 (nanosheets) | I−/I3− | N719 | 15.04 | 0.77 | 63 | 7.34 | 27.72 | 2.59 | 279 |
| NbSe2 (nanorods) | I−/I3− | N719 | 13.94 | 0.76 | 64 | 6.78 | 19.38 | 6.21 | 279 |
| NbSe2/C | I−/I3− | N719 | 15.58 | 0.77 | 65 | 7.80 | 24.07 | 3.52 | 279 |
| Pt reference | I−/I3− | N719 | 15.88 | 0.72 | 69 | 7.90 | 8.15 | 2.35 | 279 |
| NbSe2 (nanosheets) | I−/I3− | N719 | 16.85 | 0.74 | 62 | 7.73 | — | — | 280 |
| NbSe2 (nanorods) | I−/I3− | N719 | 14.85 | 0.74 | 46 | 5.05 | — | — | 280 |
| NbSe2 (microparticles) | I−/I3− | N719 | 14.93 | 0.75 | 55 | 6.27 | — | — | 280 |
| Pt reference | I−/I3− | N719 | 15.59 | 0.72 | 62 | 7.01 | — | — | 280 |
| MoSe2 (solvothermal reaction) | I−/I3− | N719 | 14.11 | 0.73 | 65 | 6.70 | 10.32 | 2.43 | 281 |
| WSe2 (solvothermal reaction) | I−/I3− | N719 | 15.50 | 0.73 | 66 | 7.48 | 10.70 | 0.78 | 281 |
| TaSe2 (solvothermal reaction) | I−/I3− | N719 | 15.81 | 0.73 | 64 | 7.32 | 10.00 | 1.89 | 281 |
| Pt reference | I−/I3− | N719 | 16.84 | 0.70 | 67 | 7.91 | 6.32 | 1.32 | 281 |
| NiSe2 (hydrothermal reaction) | I−/I3− | N719 | 15.94 | 0.734 | 74.3 | 8.69 | 2.57 | 0.81 | 282 |
| Pt reference | I−/I3− | N719 | 15.26 | 0.731 | 72.1 | 8.04 | 2.50 | 0.97 | 282 |
| NiCo2S4/NiS (spin casting) | I−/I3− | N719 | 17.7 | 0.744 | 67 | 8.8 | — | — | 286 |
| NiCo2S4 (spin casting) | I−/I3− | N719 | 17.4 | 0.743 | 66 | 8.5 | — | — | 286 |
| Co9S8 (spin casting) | I−/I3− | N719 | 16.2 | 0.741 | 64 | 7.7 | — | — | 286 |
| NiS (spin casting) | I−/I3− | N719 | 14.9 | 0.735 | 63 | 6.9 | — | — | 286 |
| Pt reference | I−/I3− | N719 | 16.5 | 0.736 | 67 | 8.1 | — | — | 286 |
| Ni0.33Co0.67Se | I−/I3− | N719 | 17.29 | 0.789 | 67 | 9.01 | 30.40 | 1.11 | 287 |
| Ni0.5Co0.5Se | I−/I3− | N719 | 16.42 | 0.783 | 69 | 8.80 | 30.38 | 1.50 | 287 |
| Ni0.67Co0.33Se | I−/I3− | N719 | 15.89 | 0.784 | 69 | 8.59 | 30.04 | 1.96 | 287 |
| Co3Se4 | I−/I3− | N719 | 14.96 | 0.793 | 67 | 7.95 | 29.94 | 7.66 | 287 |
| NiSe | I−/I3− | N719 | 14.54 | 0.783 | 64 | 7.23 | 29.87 | 13.88 | 287 |
| Pt reference | I−/I3− | N719 | 15.33 | 0.791 | 69 | 8.30 | 30.30 | 2.88 | 287 |
| FeSe2 (nanosheets, under N2) | I−/I3− | N719 | 17.49 | 0.718 | 60 | 7.53 | 8.07 | 0.53 | 302 |
| FeSe2 (nanosheets, air exposed) | I−/I3− | N719 | 15.51 | 0.708 | 56 | 6.15 | 10.13 | 6.26 | 302 |
| FeSe2 (microparticles) | I−/I3− | N719 | 16.32 | 0.715 | 59 | 6.88 | 8.51 | 4.10 | 302 |
| Pt reference | I−/I3− | N719 | 17.77 | 0.725 | 58 | 7.47 | 7.94 | 1.68 | 302 |
| FeSe2 microparticles (MPs) | I−/I3− | N719 | 15.63 | 0.745 | 66 | 7.68 | 9.03 | 2.32 | 303 |
| FeSe2 nanosheets (NSs) | I−/I3− | N719 | 16.14 | 0.744 | 70 | 8.39 | 8.78 | 0.49 | 303 |
| FeSe2 nanorods (NRs) | I−/I3− | N719 | 15.79 | 0.748 | 68 | 8.03 | 8.71 | 1.62 | 303 |
| Pt reference | I−/I3− | N719 | 15.87 | 0.750 | 69 | 8.20 | 8.62 | 1.15 | 303 |
| FeSe2 (3D flower-like) | I−/I3− | N719 | 14.93 | 0.744 | 72.1 | 8.00 | 16.82 | 0.53 | 304 |
| FeSe2 (sphere-shaped) | I−/I3− | N719 | 14.60 | 0.724 | 69.8 | 7.38 | 27.05 | 0.96 | 304 |
| Pt reference | I−/I3− | N719 | 15.13 | 0.741 | 70.2 | 7.87 | 17.01 | 0.78 | 304 |
| CoSe2 (hydrothermal, 140 °C) | I−/I3− | N719 | 16.65 | 0.750 | 64.4 | 8.04 | 8.783 | 0.132 | 309 |
| CoSe2 (hydrothermal, 160 °C) | I−/I3− | N719 | 17.04 | 0.743 | 66.2 | 8.38 | 8.034 | 0.097 | 309 |
| CoSe2 (hydrothermal, 180 °C) | I−/I3− | N719 | 15.44 | 0.750 | 63.9 | 7.40 | 15.17 | 0.932 | 309 |
| Pt reference | I−/I3− | N719 | 16.88 | 0.743 | 62.4 | 7.83 | 12.86 | 1.923 | 309 |
| CoSe2/C–NG | I−/I3− | N719 | 17.51 | 0.73 | 67 | 8.41 | 20.6 | 0.85 | 310 |
| CoSe2/C–NR | I−/I3− | N719 | 15.98 | 0.73 | 67 | 7.83 | 20.6 | 1.16 | 310 |
| CoSe2/C–NCW | I−/I3− | N719 | 18.03 | 0.73 | 67 | 8.92 | 20.6 | 0.52 | 310 |
| CoSe2/C–NCW on nickel foam | I−/I3− | N719 | 18.86 | 0.78 | 71 | 10.46 | — | — | 310 |
| CoSe2/C–NCW on carbon cloth | I−/I3− | N719 | 18.16 | 0.76 | 71 | 9.87 | — | — | 310 |
| Pt reference | I−/I3− | N719 | 16.43 | 0.74 | 67 | 8.25 | 20.6 | 1.04 | 310 |
| CoSe2 | I−/I3− | N719 | 12.95 | 0.773 | 65 | 6.47 | — | 0.50 | 311 |
| CoSe2@RGO | I−/I3− | N719 | 12.24 | 0.792 | 72 | 7.01 | — | 0.20 | 311 |
| Reduced graphene oxide (RGO) | I−/I3− | N719 | 12.11 | 0.761 | 40 | 3.66 | — | 64.75 | 311 |
| Pt reference | I−/I3− | N719 | 13.12 | 0.765 | 67 | 6.77 | — | 0.61 | 311 |
| Ni0.85Se | I−/I3− | N719 | 15.63 | 0.739 | 72 | 8.32 | 1.8 | 1.8 | 312 |
| Co0.85Se | I−/I3− | N719 | 16.98 | 0.738 | 75 | 9.40 | 2.1 | 0.6 | 312 |
| Pt reference | I−/I3− | N719 | 16.03 | 0.738 | 73 | 8.64 | 2.6 | 1.1 | 312 |
| Bi2Se3 nanoparticles | I−/I3− | N719 | 7.02 | 0.55 | 46 | 1.86 | — | — | 325 |
| Bi2Se3/graphene (40 mg) | I−/I3− | N719 | 15.42 | 0.78 | 50 | 6.35 | — | — | 325 |
| Bi2Se3/graphene (60 mg) | I−/I3− | N719 | 16.36 | 0.75 | 57 | 7.09 | — | — | 325 |
| Bi2Se3/graphene (80 mg) | I−/I3− | N719 | 16.01 | 0.76 | 53 | 6.66 | — | — | 325 |
| Pt reference | I−/I3− | N719 | 15.65 | 0.68 | 59 | 6.47 | — | — | 325 |
| ZnO (photoanode) | I−/I3− | N719 | 8.189 | 0.656 | 55.2 | 2.96 | 16.6 | 7.23 | 335 |
| Bi2Te3/ZnO (photoanode) | I−/I3− | N719 | 11.767 | 0.637 | 57.0 | 4.27 | 15.8 | 3.75 | 335 |
4.7 Bi2Se3 counter electrodes
Bismuth selenide (Bi2Se3) and bismuth telluride (Bi2Te3) have been previously studied as topological insulators.313,314 Bi2Se3, a semiconducting and thermoelectric material, can be processed into single layers, nanosheets, nanotubes, nanoribbons, and nanowires315–318 and has applications in field-effect transistors,319 sensors,320 non-volatile memory devices,321 photovoltaic devices,322 and drug delivery and anti-cancer therapy.323,324 Bi2Se3/RGO nanocomposites were prepared by a microwave-assisted hydrothermal method as a CE for a DSSC by Zhu et al.325 Fig. 27 shows the TEM images of the graphene nanosheets, Bi2Se3 nanospheres and Bi2Se3/graphene nanocomposites containing 60 mg graphene contents. The TEM images showed that 10–15 nm Bi2Se3 nanoparticles were attached onto graphene nanosheets. SEM images showed that the graphene had a flake-like structure while the Bi2Se3 nanoparticles were large size spheres. Furthermore, the surface of the graphene oxide was found to be very smooth in comparison with graphene nanosheets doped with Bi2Se3 nanoparticles. The inclusion of Bi2Se3 nanoparticles onto graphene nanosheets was controlled to achieve high electrocatalytic activity of the CE for the reduction of triiodide (I3−). The DSSC with a Bi2Se3/graphene (60 mg) CE yielded a high PCE of 7.09%, which is comparable to a Pt CE (η = 6.23%). The Bi2Se3/graphene nanocomposite CEs with 40 mg and 80 mg graphene content showed PCE values of 6.35% and 6.66% for the DSSCs, respectively, which was much higher than that of pure Bi2Se3 CE (η = 1.86%), but still comparable to a Pt CE.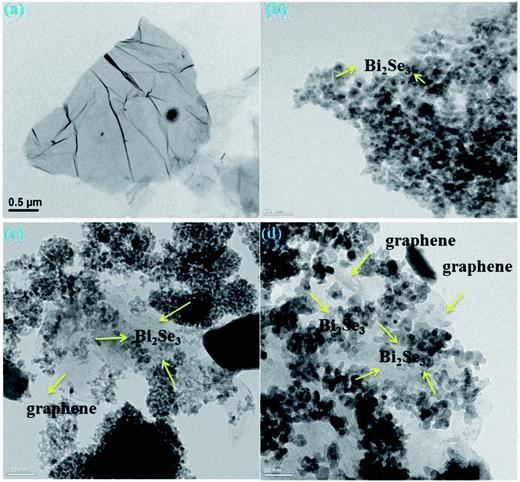 | ||
| Fig. 27 TEM images of (a) graphene nanosheet, (b) Bi2Se3 nanospheres (c) and (d) Bi2Se3/graphene nanocomposite (60 mg graphene). Reprinted with permission from ref. 325, L. Zhu, K. Y. Cho and W. C. Oh, microwave-assisted synthesis of Bi2Se3/reduced graphene oxide nanocomposite as efficient catalytic counter electrode for dye-sensitized solar cell, Fullerenes, Nanotubes, Carbon Nanostruct., 2016, 24, 622–629. Copyright© Taylor & Francis Group. | ||
5. Bi2Te3 based photoanodes
Bismuth telluride (Bi2Te3) has been studied for thermoelectric applications and can be processed into nanowires arrays,326–329 nanotubes,330 nanoplates,331 nanosheets,332,333 and thin films.334 Dou et al.335 developed hybrid photoanodes by dispersing Bi2Te3 nanotubes into ZnO nanoparticles. The 0.5, 1.0, 1.5, 2.0, and 2.5 wt% of highly crystalline Bi2Te3 nanotubes were mixed with ZnO nanoparticles to fabricate photoanodes for DSSCs. Pt films deposited on FTO glass was used as a CE. The electrolyte consisted of 0.05 M I2, 0.5 M LiI, and 0.1 M 4-tert-butylpyridine in acetonitrile-propylene carbonate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution. In the Bi2Te3/ZnO hybrid, Bi2Te3 nanotubes provided a conduction pathway which facilitated electron transfer. The Jsc value increased gradually with increasing Bi2Te3 nanotubes content up to 1.5 wt% (11.767 mA cm−2) and then started decreasing as the Bi2Te3 nanotubes content exceeded 2.0 wt% (9.957 mA cm−2), due to the low dye loading on the surface of ZnO nanoparticles. A similar trend was observed for the PCE values. The DSSCs with a Bi2Te3/ZnO composite photoanode containing 1.5 wt% Bi2Te3 nanotubes in the ZnO photoanode showed a PCE of 4.27%, which is 44.3% higher compared with a pure ZnO photoanode. The PCE values were 2.96% for the bare ZnO photoanode, and 3.74, 3.96, 4.27, 3.41 and 3.01% for the 0.5, 1.0, 1.5, 2.0 and 2.5 wt% Bi2Te3 nanotubes content photoanodes, respectively. This study indicated that loading of thermoelectric Bi2Te3 in a ZnO photoanode improves the photovoltaic performance of DSSCs. Wan et al.336 prepared hexagonal Bi2Te3 nanosheets of 300–400 nm length by a hydrothermal method. The nanocomposites of the Bi2Te3 nanosheets and ZnO nanoparticles were used as photoanodes for DSSCs, where the Bi2Te3 nanosheet concentration was varied from 0 to 0.25 wt%. The DSSCs with 0.15 wt% of Bi2Te3 nanosheets in the Bi2Te3 nanosheet/ZnO nanoparticle composite photoanode showed a PCE of 4.10%, which was improved by 46.95% compared with the bare ZnO photoanode. The thermoelectric Bi2Te3 nanosheets in the composite photoanode helped in enhancing the electron density and reducing the temperature of the DSSCs. The Bi2Te3 nanosheet/ZnO nanoparticles composite photoanode significantly improved the DSSC performance. A thermoelectric Bi2Te3/TiO2 composite based photoanode was also used for DSSCs, where Bi2Te3 nanoplates help in converting heat into electricity and enhanced the rate of charge transfer.337 This resulted in a 28% increase of the PCE of the DSSC. The Bi2Te3 nanoplates have also been used for doping TiO2 photoanodes.338 The effect of Bi2Te3 nanoplates size was evaluated for DSSCs, where a decrease in the size of the Bi2Te3 nanoplates led to and higher PCE. The performance of DSSCs with a Bi2Te3/TiO2 photoanode increased by 15.3% compared to the undoped TiO2 photoanode.
1) solution. In the Bi2Te3/ZnO hybrid, Bi2Te3 nanotubes provided a conduction pathway which facilitated electron transfer. The Jsc value increased gradually with increasing Bi2Te3 nanotubes content up to 1.5 wt% (11.767 mA cm−2) and then started decreasing as the Bi2Te3 nanotubes content exceeded 2.0 wt% (9.957 mA cm−2), due to the low dye loading on the surface of ZnO nanoparticles. A similar trend was observed for the PCE values. The DSSCs with a Bi2Te3/ZnO composite photoanode containing 1.5 wt% Bi2Te3 nanotubes in the ZnO photoanode showed a PCE of 4.27%, which is 44.3% higher compared with a pure ZnO photoanode. The PCE values were 2.96% for the bare ZnO photoanode, and 3.74, 3.96, 4.27, 3.41 and 3.01% for the 0.5, 1.0, 1.5, 2.0 and 2.5 wt% Bi2Te3 nanotubes content photoanodes, respectively. This study indicated that loading of thermoelectric Bi2Te3 in a ZnO photoanode improves the photovoltaic performance of DSSCs. Wan et al.336 prepared hexagonal Bi2Te3 nanosheets of 300–400 nm length by a hydrothermal method. The nanocomposites of the Bi2Te3 nanosheets and ZnO nanoparticles were used as photoanodes for DSSCs, where the Bi2Te3 nanosheet concentration was varied from 0 to 0.25 wt%. The DSSCs with 0.15 wt% of Bi2Te3 nanosheets in the Bi2Te3 nanosheet/ZnO nanoparticle composite photoanode showed a PCE of 4.10%, which was improved by 46.95% compared with the bare ZnO photoanode. The thermoelectric Bi2Te3 nanosheets in the composite photoanode helped in enhancing the electron density and reducing the temperature of the DSSCs. The Bi2Te3 nanosheet/ZnO nanoparticles composite photoanode significantly improved the DSSC performance. A thermoelectric Bi2Te3/TiO2 composite based photoanode was also used for DSSCs, where Bi2Te3 nanoplates help in converting heat into electricity and enhanced the rate of charge transfer.337 This resulted in a 28% increase of the PCE of the DSSC. The Bi2Te3 nanoplates have also been used for doping TiO2 photoanodes.338 The effect of Bi2Te3 nanoplates size was evaluated for DSSCs, where a decrease in the size of the Bi2Te3 nanoplates led to and higher PCE. The performance of DSSCs with a Bi2Te3/TiO2 photoanode increased by 15.3% compared to the undoped TiO2 photoanode.
6. Long-term stability of TMDs based DSSCs
The long-term stability of DSSCs is one of the most important parameters for commercial applications. The stability of DSSC devices depends upon a number of factors and the components used in their fabrication. The decrease in power conversion efficiency of a DSSC can be associated with the stability of different electrolyte components, photosensitizing dyes, aging of the TiO2 photoanode, degradation of the CE (cathode), corrosion of components by electrolytes, sealant, leakage, exposure to solar irradiation, high humidity, and elevated temperature.339–349A few research reports have been published on the long-term stability of TMDs based DSSCs which are briefly discussed here. Infant et al.131 studied the stability of CVD-deposited vertically oriented MoS2 thin films on an FTO surface used as a CE in a DSSC. The electrochemical stability of a MoS2 CE based DSSC was analyzed by CV measurements, where electrodes were repeatedly subjected to 20 cycles at a 10 mV s−1 scan rate for the I−/I3− redox couple in the electrolyte. The MoS2 based CE showed no significant change up to 20th consecutive cycle, whereas the Pt CE exhibited some changes between the cycles (Fig. 28). This confirms that CVD deposited MoS2 strongly adhered onto the surface of the FTO substrate. The stability of MoS2 CEs was measured under ambient conditions by storing them for 15 days, where the PCE remained at 94% of its initial efficiency value, which was much higher than that of the Pt CE.
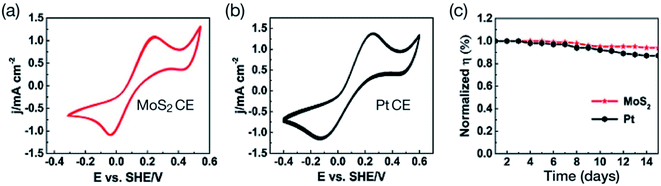 | ||
| Fig. 28 Electrochemical properties of thin films of MoS2 prepared at 600 °C for 15 minutes. 20 consecutive cyclic voltammogram (CV) curve of MoS2 (a) and Pt (b) counter electrodes recorded at the scan rate of 10 mV s−1 and (c) PCE of MoS2 and Pt CEs based DSSCs measured for 15 days under ambient conditions. Reprinted with permission from ref. 131, R. S. Infant, X. Xu, W. Yang, F. Yang, L. Hou and Y. Li, highly active and reflective MoS2 counter electrode for enhancement of photovoltaic efficiency of dye sensitized solar cells. Electrochim. Acta, 2016, 212, 614–620. Copyright© Elsevier. | ||
For preparing Pt-free dye-sensitized solar cells, Liu et al.350 fabricated DSSCs using MoS2 and RGO composite as a CE for the reduction of triiodide (I3−) to iodide (I−). AFM, XPS, and XRD confirmed the deposition of MoS2 nanoparticles onto the RGO surface. The CV measurement showed a higher current density for the MoS2/RGO nanocomposite based CE compared to RGO, MoS2, and Pt-sputtered CEs due to an increased surface area. The MoS2/RGO CE also exhibited a low RCT of 0.57 Ω cm2 for the reduction of triiodide (I3−) to iodide (I−). The MoS2/RGO nanocomposite CE based DSSC showed a PCE of 6.04%, comparable to a PCE of 6.38% for the conventional Pt CE. MoS2/RGO nanocomposites based CEs also have better electrochemical stability, as no degradation in current densities was observed up to 100 repeated CV tests. The stability test conducted on a DSSC having MoS2/RGO nanocomposites as CEs showed over 10% degradation in PCE over a period of 20 days, as depicted in (Fig. 29a). Therefore, MoS2/RGO nanocomposites based CEs were found to be stable both for environmental and consecutive electrochemical tests. Li et al.200 prepared a composite film of TiS2/PEDOT:PSS on an ITO substrate as a CE of DSSCs for the I−/I3− redox system, which exhibited a PCE as high as 7.04% and is comparable to a Pt CE. Fig. 29b shows dark current density–voltage curves of DSSCs with Pt, bare TiS2, bare PEDOT:PSS, and 10 wt% TiS2/PEDOT:PSS composite CEs, and also the long-term stability of a 10 wt% TiS2/PEDOT:PSS composite CE based DSSC. This another example of long-term stability of DSSCs based on TiS2/PEDOT:PSS CEs.
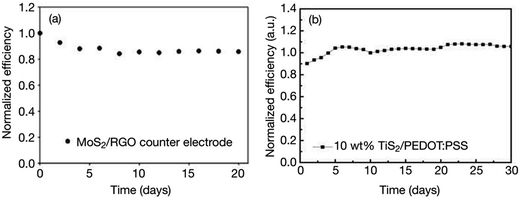 | ||
| Fig. 29 (a) Stability of a DSSC with MoS2/RGO based counter electrode recorded for 20 days. Reprinted with permission from ref. 350, C. J. Liu, S. Y. Tai, S. W. Chou, Y. C. Yu, K. D. Chang, S. Wang, F. S. S. Chien, J. Y. Lin and T. W. Lin, facile synthesis of MoS2/graphene nanocomposite with high catalytic activity toward triiodide reduction in dye-sensitized solar cells. J. Mater. Chem., 2015, 22, 21057–21064. Copyright© Royal Society of Chemistry. (b) The long-term stability of the DSSCs with 10 wt% TiS2/PEDOT:PSS composite CE. Reprinted with permission from ref. 200, C. T. Li, C. P. Lee, Y. Y. Li, M. H. Yeh and K. C. Ho, a composite film of TiS2/PEDOT:PSS as the electrocatalyst for the counter electrode in dye-sensitized solar cells. J. Mater. Chem. A, 2013, 1, 14888–14896. Copyright© Royal Society of Chemistry. | ||
The stability of DSSCs with a TiS2/graphene hybrid CE was studied by Meng et al.199 (Fig. 30). The TiS2–graphene hybrid CE maintained 96% of its initial PCE value after 500 hours in air, also exhibiting higher electrochemical stability. The Rs and ZN values for the TiS2–graphene hybrid CE based DSSC did not change after 10 cyclic measurements. The RCT value of a DSSC with Pt CE significantly increased with increasing cycling number, while there was no change in the RCT value of the TiS2–graphene hybrid after 10 cycles. This indicates better electrochemical stability of the TiS2–graphene hybrid CE than the Pt CE. The stability of photovoltaic parameters of DSSCs having mesoporous CoS2 nanotube arrays as CEs was recorded for 10 days by Tsai et al.233 a slight drop in the Voc and FF values were observed, which resulted in a 2.2% decrease in the PCE of the CoS2 nanotube array CEs. The photovoltaic parameters of the DSSC were quite stable up to 10 days, indicating good stability of DSSCs with mesoporous CoS2 nanotube array CEs (Fig. 31).
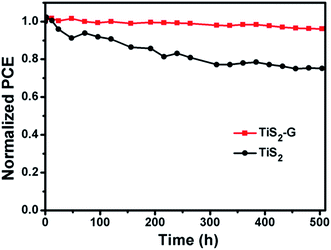 | ||
| Fig. 30 Stability of DSSCs with TiS2–graphene hybrid and Pt CEs. Reprinted with permission from ref. 199, X. Meng, C. Yu, B. Lu, J. Yang and J. Qiu, dual integration system endowing two-dimensional titanium disulfide with enhanced triiodide reduction performance in dye-sensitized solar cells. Nano Energy, 2016, 22, 59–69. Copyright© Elsevier. | ||
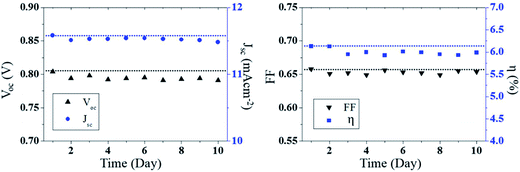 | ||
| Fig. 31 Stability of photovoltaic parameters; open-circuit voltage (Voc), short-circuit photocurrent density (Jsc), fill factor (FF), and power conversion efficiency (η) of DSSCs with mesoporous CoS2 nanotube array CE as a function of time. Reprinted with permission from ref. 233, J. C. Tsai, M. H. Hon and I. C. Leu, fabrication of mesoporous CoS2 nanotube arrays as the counter electrodes of dye-sensitized solar cells. Chem.–Asian J., 2015, 10, 1932–1939. Copyright© Wiley-VCH. | ||
The electrochemical stability of DSSCs with FeSe2 nanosheets and Pt CEs in an iodine-based electrolyte was studied by Huang et al.302 Both CEs were subjected to consecutively CV scanning. The FeSe2 nanosheets-based CE showed no change in the current densities and Epp values up to 1000 cycles, whereas the Epp value for the Pt-based CE was observed to increase after 1000 consecutive cycles (Fig. 32). This study confirmed a better corrosion resistance of FeSe2 nanosheets CE to the iodine-based electrolyte than a Pt CE. Both CEs were also subjected to sequential EIS scanning, where negligible changes in RCT were noticed after 10 cycles, again indicating an excellent electrochemical stability of both CEs. Furthermore, Rs and ZN values also showed no change upon repeated scanning cycles.
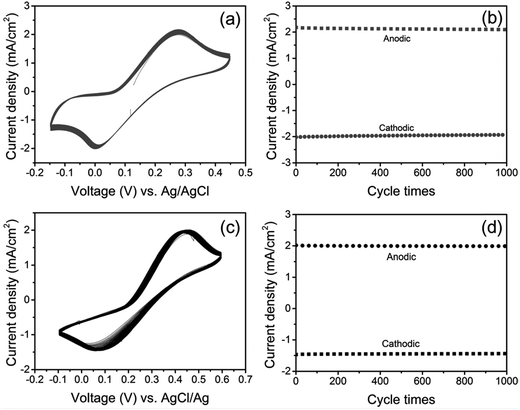 | ||
| Fig. 32 1000 consecutive cycles of CVs of FeSe2 nanosheets-based CE (a) and Pt-based CE (c) at a scan rate of 50 mV s−1 and the anodic as well as cathodic peak current densities up to 1000 cycles for FeSe2- (b) and Pt-based CEs (d), respectively. Reprinted with permission from ref. 302, S. Huang, Q. He, W. Chen, Q. Qiao, J. Zai and X. Qian, ultrathin FeSe2 nanosheets: controlled synthesis and application as a heterogeneous catalyst in dye-sensitized solar cells. Chem.–Eur. J., 2015, 21, 4085–4091. Copyright© Wiley-VCH. | ||
The stability of a FeS2 nanorod based CE was also measured in an iodide (I−) electrolyte up to 10 days, and CV plots showed slight change at different times of aging.222 The hydrothermally synthesized CoSe2 nanorods were used as an electrocatalyst for a DSSC for the reduction of I3− using N719 dye by Sun et al.351 The single crystalline CoSe2 nanorods based CE showed a PCE of 10.20%, compared with a PCE of 8.17% for a Pt CE, under 1 Sun illumination. The DSSCs having CoSe2 CEs were stored in daylight and their photovoltaic properties were measured every day, and showed long-term stability. These studies show an excellent electrochemical stability of TMDs-based CEs for DSSCs in iodine-based electrolyte, with no corrosion. The TMDs CEs are also quite stable when stored under ambient conditions of up to 2–3 weeks, as no significant changes were observed in the PCEs of the DSSC devices. Furthermore, TMDs based CEs should be further investigated to exhibit better electrochemical stability and environmental stability than that of standard Pt CEs, and endurance tests should be carried out to study the their stability. The DSSS devices should have a service life of at least 20 years under ambient conditions as pointed out by Grätzel.342 The long-term stability of DSSCs is feasible by carefully selecting components and solar cell structure. The TMDs based CEs may overcome the concerns associated with scarcity, high production cost, and corrosion of Pt CEs in electrolyte solutions. Researchers in this field should address the stability for of TMDs based CEs to evaluate the performance of Pt-free DSSCs.
Some achievements and strategies to overcome the long-term stability of Pt-free DSSCs are summarized in the following section. Kato et al.352 used Raman spectroscopy and EIS to evaluate the durability of DSSCs for 2.5 years in outdoor conditions. Both N719 dye-adsorbed TiO2 CEs and carbon CEs were found to be stable. The Voc and FF values were slightly decreased because of increased ZN of triiodide (I3−), arising from the change in electrolyte components. Matsui et al.353 achieved stability over 1000 hours for DSSCs at 85 °C and under 85% relative humidity, and recorded no degradation of the photovoltaic performance between −40 and 90 °C for 200 cycles. Xue et al.354 measured thermal stability of DSSCs between −20 to 25 °C temperature range for 1080 hours. DSSCs with N719 dye absorbed on the TiO2 photoanode retained 80% of their initial PCE values after aging for 1080 hours. The deterioration of the N719 dye was found to be the main cause for a decrease the PCE and degradation of DSSC devices. Harikisun and Desilvestro355 evaluated photovoltaic performance of Z907-based DSSCs after continuous light-soaking at 55–60 °C for 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 hours, where a slight degradation was observed. The accelerated aging tests predicted a life time of 40 years for Middle European conditions while 25 years for Southern European conditions. The 10% and 20% decrease of photovoltaic performance was measured for ionic liquid and solvent based electrolytes over 1000 hours at 180 °C, respectively.
600 hours, where a slight degradation was observed. The accelerated aging tests predicted a life time of 40 years for Middle European conditions while 25 years for Southern European conditions. The 10% and 20% decrease of photovoltaic performance was measured for ionic liquid and solvent based electrolytes over 1000 hours at 180 °C, respectively.
Strategies to improve the long-term stability of DSSCs include developing new photosensitizing dyes, new non-volatile electrolytes, encapsulation, and of course new photoanodes and CEs. Like the heterojunction solar cells,27 several strategies for improving electrochemical and thermal stability of DSSC devices have been proposed. The role of photosensitizing dyes containing π-conjugated organic systems have been studied for the stability of DSSCs. Wu et al.356 suggested a novel concept of molecular engineering of donor–acceptor–π–acceptor (D–A–π–A) based photosensitizers, not only to improve the stability of DSSCs but also to enhance the photovoltaic performance. Katoh et al.357 compared the stability of five sensitizing dyes in DSSCs with and without π-conjugated oligothiophene moiety, which indicated that dyes with π-conjugated oligothiophene exhibit higher stability than those of without oligothiophene moiety. Joly et al.358 fabricated DSSCs with a new organic sensitizer (RK1) which showed a Jsc of 18.26 mA cm−2, Voc of 0.76 V, and FF of 0.74, resulting in a PCE value of 10.2% under 1 Sun illumination for the triiodide/iodide (I3−/I−) redox couple. A similar PCE of 10.19% was achieved for the ruthenium N719 dye. When RK1 dye was used with a viscous ionic liquid electrolyte, the DSSC yielded a Jsc of 15.40 mA cm−2, Voc of 0.665 V, FF of 0.69, and a PCE of 7.36%, with outstanding stability. The DSSC exhibited no degradation of photovoltaic performance after visible-light soaking at 65 °C for 2200 hours, but, thereafter, DSSC started degrading and retained 75% of its initial PCE at 65 °C after 5000 hours.
A new coumarin dye, namely 2-cyano-3-{5′-[1-cyano-2-(1,1,6,6-tetramethyl-10-oxo-2,3,5,6-tetrahydro-1H,4H,10H-11-oxa-3a-aza-benzo[de]anthracen-9-yl)-vinyl]-[2,2′]bithiophenyl-5-yl}-acrylic acid (NKX-2883) was developed by Wang et al.359 to examine the stability of DSSCs in a nonvolatile electrolyte made of 0.1 M I2, 0.6 M 1,2-dimethyl-3-n-propylimidazolium iodide (DMPImI), and 0.1 M N-methylbenzimidazole (NMBI) in 3-methoxypropionitrile. The NKX-2883 dye-based DSSC showed a Jsc value of 18.8 mA cm−2 and a PCE of 6.5% with 6 micron thick TiO2 film. The DSSCs maintained a PCE of 6% under continuous light soaking of 100 mW cm−2 (1 Sun illumination) at 50–55 °C for 1000 hours. Organic photosensitizing dyes having long alkyl chains were also proposed to improve long-term stability for both liquid and quasi-solid-state DSSCs.360 In another study, quinoxaline based metal-free organic sensitizing dyes were utilized to introduce long-term stability in DSSCs.361 The length of the alkyl chains on the donor unit was found to affect the performance of DSSC devices. The quasi-solid-state DSSCs with a quinoxaline-based organic dye showed a PCE of 7.14%, and maintained 100% of its initial PCE value after continuous sunlight irradiation for 1000 hours, indicating that molecular engineering of dye molecules can lead to both high PCE and long-term stability of DSSC devices.
The electrochemical stability of CEs in corrosive triiodide/iodide (I3−/I−) electrolyte is of significant concern because it restricts commercial applications of DSSCs. To overcome this disadvantage of the I−/I3− redox couple, research activities have been focused on finding alternative iodine-free non-corrosive redox electrolytes.19,362–366 Cell-sealing conditions are also important when using liquid electrolytes. The DSSCs can be made suitable for outdoor applications by using encapsulation. The role of electrolytes has been studied in the stability of DSSCs by Sauvage et al.367 suggesting a new electrolyte based on butyronitrile solvent with low volatility, along with thiophene-based sensitizer Na–Ru(4,4′-bis(5-(hexylthio)thiophen-2-yl)-2,2′-bipyridine)(4-carboxylic acid-4′-carboxylate-2,2′-bipyridine)(thiocyanate)2, coded C106, for DSSCs which showed >95% retention of PCE value after 1000 hours at 60 °C for an exposure to 100 mW cm−2 light illumination. Yoon et al.368 fabricated DSSCs with 1-propyl-3-methyl imidazolium iodide (PMII) ion-gel electrolyte with a poly(styrene-block-ethyleneoxide-block-styrene) (SEOS) triblock copolymer. The DSSC with ion-gel electrolyte retained 92% of its initial PCE up to 1440 hours, compared to 78% for the ionic liquid electrolyte. Lee et al.369 reported long-term stability of DSSCs with organic tetrabutylammonium iodide (TBAI) or 1-methyl 3-propyl imidazolium iodide (PMII) in methoxypropionitrile-based electrolytes. The DSSCs having TBAI retained 96.9% of their initial efficiency after being stored for 1000 hours under 1 Sun light irradiation at 60 °C. Yang et al.370 proposed the use of poly(ethylene oxide)–poly(vinylidene fluoride) (PEO–PVDF) polymer-blend electrolytes with water and ethanol for improving stability of DSSCs. The electrical conductivity was found to increase after adding water and ethanol to the PEO–PVDF polymer-blend electrolytes. The cross-linking capability of hydroxyl-rich additives for modified electrolytes was found to have a positive impact. Chen et al.371 demonstrated the long-time durability of DSSCs by using a succinonitrile, silica nanoparticles and 1-butyl-3-methylimidazolium tetrafluoroborate (BMI·BF4) gel system which maintained 93% of its initial PCE after aging at 60 °C for 1000 hours. Dembele et al.372 also demonstrated that adding 1.0 wt% concentration of MWCNTs to TiO2 photoanodes can improve both PCE and stability of a DSSC, where the PCE value increased to 4.1% compared with 3.7% for pure TiO2 photoanodes. The performance of the DSSC devices was measured for 10 consecutive days under ambient light exposure. The PCE decreased about 10% for the MWCNTs/TiO2 photoanodes, compared to 35% decrease in pure TiO2 photoanodes.
The long-term stability of Pt-free DSSCs is of significant importance for both indoor and outdoor applications and different approaches can be examined to improve environmental stability.356–361,367–377 These studies show that long-term stability may be introduced in TMDs based DSSCs by similar strategies of using long alkyl chain organic dyes, ion-gel and polymer-based electrolytes, silica nanoparticles, or modifying TiO2 photoanodes. Similar electrochemical and thermal stability studies should be conducted for TMD CEs for DSSC devices.
7. Conclusion and perspective
The electrochemical and photovoltaic properties of DSSC devices employing CEs (CEs) of 2D layered transition metal dichalcogenides (MoS2, MoSe2, WS2, TiS2, NbSe2, TaSe2, NiSe2, FeSe2, CoSe2, SnS2, Bi2Se3 and their based composites) have been summarized and discussed. This data indicates that the PCEs of TMDs-based CEs surpasses conventional Pt CEs in dye-sensitized solar cell (DSSC) devices. The composites of TMDs with graphene, CNTs, carbon, carbon nanofibers (CNFs), and PEDOT:PSS also show a great potential as CEs for DSSCs. The DSSCs having CEs made of chemical vapor deposition (CVD)-grown vertically inclined MoS2 films,131 hydrothermally prepared MoS2 films,135,141 MoS2 and MoSe2 thin films deposited on Mo foil,260,261 MoS2/graphene, MoS2/CNTs,164 MoS2/carbon,171 and MoSe2/PEDOT:PSS composites exhibit higher PCE values than that of Pt CEs for the reduction of triiodide (I3−) to iodide (I−). Furthermore, the CEs of TiS2/graphene hybrids,199 NiS2/reduced graphene oxide (RGO) composites,210 FeS2,221 NbSe2,280 and FeSe2 nanosheets302 also show a better photovoltaic performance than that of Pt CEs in DSSCs. The low cost and flexible CoSe2/carbon-nanoclimbing-wall CE deposited on nickel foam shows the highest PCE of 10.46% versus a Pt CE (η = 8.25%) at 1 Sun illumination (100 mW cm−2 (AM 1.5G)).310 It has been observed that the morphology of CEs also plays an important role in determining the electrocatalytic activity of DSSC devices, where CEs with large surface area nanostructures tend to show larger PCE values and a higher electrocatalytic activity of the DSSCs.282 The DSSCs with TiS2/graphene hybrids199 and TiS2/PEDOT:PSS composites200 based CEs showed environmental stability of up to 20 and 30 days, respectively, with no degradation in the photovoltaic performance. Interestingly, thermoelectric Bi2Te3 nanosheet/ZnO nanoparticles composite based photoanodes335 exhibited a 46.95% increase in PCE value compared with a bare ZnO photoanode based DSSC. Also, the DSSC with the Bi2Te3/TiO2 composite based photoanode336 showed a 28% increase in PCE than that of pure TiO2 photoanode.Transition metal dichalcogenides (TMDs) based materials which are analogues of 2D graphene are emerging as a great alternative to fabricating low-cost Pt-free DSSC devices. TMDs-based CEs have demonstrated better electrochemical stability than that of standard Pt CEs in iodine-based electrolyte and also under ambient conditions. In addition to long-term stability, TMDs may also cause cytotoxicity to humans, as has been observed for other nanostructured materials,378–383 therefore, aspects of toxicity should be investigated in studies with DSSC devices.
2D TMDs based CEs are a cheap alternative to Pt CEs for DSSCs. In this review, we have summarized recent developments of TMDs used as CE materials for DSSCs which are still in their infancy. The low-cost 2D TMDs are abundantly available in nature, and can easily be processed into thin films and hybridized with other inorganic and organic materials for fabricating DSSC devices. The vast majority of 2D TMDs have yet to be studied, even as 2D graphene-based materials, but at this early stage they offer a low-cost alternative and outperform their Pt counterparts. Tremendous possibilities exist for developing new TMDs based CEs for I3−/I−, Co2+/Co3+ and T2/T− redox couples. The important requirements for commercial applications are ease of processing, low-cost manufacturing, high PCE value, and long-term electrochemical stability. Like graphene-based materials, more edge active sites can be created in TMDs-based CEs to facilitate more dye adsorption. Research on the use of TMDs CEs are in very early stage of developing Pt-free DSSCs. TMDs based CEs offer increased charge transfer capability and fast reaction kinetics for the reduction of triiodide (I3−) to iodide (I−) in electrolyte for DSSCs and their potential can be realized in parallel to graphene. The large family of TMDs, such as MoS2, MoSe2, MoTe2, WS2, WSe2, WTe2, FeSe2, TaS2, NbSe2, etc., should also be explored with other inorganic and organic materials, as the family of 2D materials is enormously large and are expected to play an important role in developing low-cost highly efficient DSSCs for commercial applications.
Disclaimer
The authors cannot accept liability for any kind of scientific data contained in this review article whatsoever for the accuracy of contents or any omissions or any errors or a claim of completeness.Acknowledgements
Eric Singh is thankful to Prof. Dr G. Y. Yeom for offering him a summer research internship in his group at the School of Advanced Materials Science and Engineering, Sungkyunkwan University (SKKU), South Korea. This work was supported by the Nano Material Technology Development Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2016M3A7B4910429).References
- B. O'Regan and M. Grätzel, A low-cost, high-efficiency solar cell based on dye-sensitized, Nature, 1991, 353, 737–740 CrossRef.
- M. K. Nazeeruddin, A. Kay, I. Rodicio, R. Humphry-Baker, E. Müller, P. Liska, N. Vlachopoulos and M. Grätzel, Conversion of light to electricity by cis-X2 bis(2,2′-bipyridyl-4,4′-dicarboxylate) ruthenium(II) charge-transfer sensitizers (X = Cl–, Br–, I–, CN–, and SCN–) on nanocrystalline titanium dioxide electrodes, J. Am. Chem. Soc., 1993, 115, 6382–6390 CrossRef CAS.
- M. K. Nazeeruddin, P. Pechy and M. Grätzel, Efficient panchromatic sensitization of nanocrystalline TiO2 films by a black dye based on a trithiocyanato–ruthenium complex, Chem. Commun., 1997, 1705–1706 RSC.
- S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, B. F. Curchod, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, M. K. Nazeeruddin and M. Grätzel, Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers, Nat. Chem., 2014, 6, 242–247 CrossRef CAS PubMed.
- M. Grätzel, Perspectives for dye-sensitized nanocrystalline solar cells, Prog. Photovoltaics, 2000, 8, 171–185 Search PubMed.
- M. K. Hara and H. Arakawa, Dye-sensitized solar cells, Handbook of Photovoltaic Science and Engineering, ed. A. Luque and S. Hegedus, Wiley, New York, 2003, pp. 663–700 Search PubMed.
- M. Grätzel, Dye-sensitized solar cells, J. Photochem. Photobiol., C, 2003, 4, 145–153 CrossRef.
- B. E. Hardin, H. J. Snaith and M. D. McGehee, The renaissance of dye-sensitized solar cells, Nat. Photonics, 2012, 6, 162–169 CrossRef CAS.
- J. H. Yum, E. Baranoff, S. Wenger, M. K. Nazeeruddin and M. Grätzel, Panchromatic engineering for dye-sensitized solar cells, Energy Environ. Sci., 2011, 4, 842–857 CAS.
- T. Prakash, Review on nanostructured semiconductors for dye sensitized solar cells, Electron. Mater. Lett., 2012, 8, 231–243 CrossRef CAS.
- G. Boschloo and A. Hagfeldt, Characteristics of the iodide/triiodide redox mediator in dye-sensitized solar cells, Acc. Chem. Res., 2009, 42, 1819–1826 CrossRef CAS PubMed.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Dye-sensitized solar cells, Chem. Rev., 2010, 110, 6595–6663 CrossRef CAS PubMed.
- Q. Zhang and G. Cao, Nanostructured photoelectrodes for dye-sensitized solar cells, Nano Today, 2011, 6, 91–109 CrossRef CAS.
- S. Zhang, X. Yang, Y. Numata and L. Han, Highly efficient dye-sensitized solar cells: progress and future challenges, Energy Environ. Sci., 2013, 6, 1443–1464 CAS.
- N. Tétreault and M. Grätzel, Novel nanostructures for next generation dye-sensitized solar cells, Energy Environ. Sci., 2012, 5, 8506–8516 Search PubMed.
- A. Listorti, B. O'Regan and J. R. Durrant, Electron transfer dynamics in dye-sensitized solar cells, Chem. Mater., 2011, 23, 3381–3399 CrossRef CAS.
- M. Ye, X. Wen, M. Wang, J. Iocozzia, N. Zhang, C. Lin and Z. Lin, Recent advances in dye-sensitized solar cells: from photoanodes, sensitizers and electrolytes to counter electrodes, Mater. Today, 2015, 18, 155–162 CrossRef CAS.
- (a) M. Wu, X. Lin, Y. Wang, L. Wang, W. Guo, D. Qi, X. Peng, A. Hagfeldt, M. Grätzel and T. Ma, Economical Pt-free catalysts for counter electrodes of dye-sensitized solar cells, J. Am. Chem. Soc., 2012, 134, 3419–3428 CrossRef CAS PubMed; (b) F. Hao, P. Dong, Q. Luo, J. Li, J. Lou and H. Lin, Recent advances in alternative cathode materials for iodine-free dye-sensitized solar cells, Energy Environ. Sci., 2013, 6, 2003–2019 RSC.
- (a) A. C. Cakir and S. Erten-Ela, Comparison between synthesis techniques to obtain ZnO nanorods and its effect on dye sensitized solar cells, Adv. Powder Technol., 2012, 23, 655–660 CrossRef CAS; (b) Y. Çakmak, S. Kolemen, M. Buyuktemiz, Y. Dede and S. Erten-Ela, Synthesis and dye sensitized solar cell applications of Bodipy derivatives with bis-dimethylfluorenyl amine donor groups, New J. Chem., 2015, 39, 4086–4092 RSC.
- (a) S. Erten-Ela, S. Cogal, G. C. Cogal and A. U. Oksuz, Highly conductive polymer materials based multi-walled carbon nanotubes as counter electrodes for dye-sensitized solar cells, Fullerenes, Nanotubes, Carbon Nanostruct., 2016, 24, 380–384 CrossRef CAS; (b) M. Wu and T. Ma, Recent progress of counter electrode catalysts in dye-sensitized solar cells, J. Phys. Chem. C, 2014, 118, 16727–16742 CrossRef CAS.
- S. Thomas, T. G. Deepak, G. S. Anjusree, T. A. Arun, S. V. Nair and A. S. Nair, A review on counter electrode materials in dye-sensitized solar cells, J. Mater. Chem. A, 2014, 2, 4474–4490 CAS.
- (a) Encyclopedia of Nanoscience and Nanotechnology; 10-volume set, ed. H. S. Nalwa, American Scientific Publishers, Los Angeles, 2004 Search PubMed; (b) Handbook of Nanostructured Materials and Nanotechnology; 5-volume set, ed. H. S. Nalwa, Academic Press, San Diego, 2000 Search PubMed; (c) Nanomaterials for Energy Storage Applications, ed. H. S. Nalwa, American Scientific Publishers, Los Angeles, 2009 Search PubMed.
- T. E. Graedel, E. M. Harper, N. T. Nassar, P. Nuss and B. K. Reck, Criticality of metals and metalloids, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 4257–4262, DOI:10.1073/pnas.1500415112.
- Minerals, Critical Minerals, and the U.S. Economy, National Research Council, National Academies Press, Washington D.C., U.S., 2008 Search PubMed; Assessments of Criticality, National Research Council (US) Chemical Sciences Roundtable, 2012 Search PubMed.
- E. Singh and H. S. Nalwa, Graphene-based dye-sensitized solar cells: a review, Sci. Adv. Mater., 2015, 7, 1863–1912 CrossRef CAS.
- E. Singh and H. S. Nalwa, Graphene-based bulk-heterojunction solar cells: a review, J. Nanosci. Nanotechnol., 2015, 15, 6237–6278 CrossRef CAS PubMed.
- E. Singh and H. S. Nalwa, Stability of graphene-based heterojunction solar cells, RSC Adv., 2015, 5, 73575–73600 RSC.
- J. A. Wilson and A. D. Yoffe, The transition metal dichalcogenides discussion and interpretation of the observed optical, electrical and structural properties, Adv. Phys., 1969, 18, 193–335 CrossRef CAS.
- X. Huang, Z. Zeng and H. Zhang, Metal dichalcogenide nanosheets: preparation, properties and applications, Chem. Soc. Rev., 2013, 42, 1934–1946 RSC.
- M. Chhowalla, H. S. Shin, G. Eda, L. J. Li, K. P. Loh and H. Zhang, The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets, Nat. Chem., 2013, 5, 263–275 CrossRef PubMed.
- C. Ataca, H. Sahin and S. Ciraci, Stable, single-layer MX2 transition-metal oxides and dichalcogenides in a honeycomb-like structure, J. Phys. Chem. C, 2012, 116, 8983–8999 CAS.
- A. K. Geim and I. V. Grigorieva, van der Waals heterostructures, Nature, 2013, 499, 419–425 CrossRef CAS PubMed.
- B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti and A. Kis, Single-layer MoS2 transistors, Nat. Nanotechnol., 2011, 6, 147–150 CrossRef CAS PubMed.
- W. S. Yun, S. W. Han, S. C. Hong, I. G. Kim and J. D. Lee, Thickness and strain effects on electronic structures of transition metal dichalcogenides: 2H–MX2 semiconductors (M= Mo, W; X= S, Se, Te), Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 033305 CrossRef.
- G. Eda and S. A. Maier, Two-Dimensional Crystals: Managing Light for Optoelectronics, ACS Nano, 2013, 7, 5660–5665 CrossRef CAS PubMed.
- S. Tongay, J. Suh, C. Ataca, W. Fan, A. Luce, J. S. Kang, J. Liu, C. Ko, R. Raghunathanan, J. Zhou, F. Ogletree, J. Li, J. C. Grossman and J. Wu, Defects activated photoluminescence in two-dimensional semiconductors: interplay between bound, charged, and free excitons, Sci. Rep., 2013, 3, 2657 CrossRef PubMed.
- P. Tonndorf, R. Schmidt, P. Böttger, X. Zhang, J. Borner, A. Liebig, M. Albrecht, C. Kloc, O. Gorgan, D. R. T. Zahn, S. M. de Vasconcellos and R. Bratschitsch, Photoluminescence Emission and Raman Response of Monolayer MoS2, MoSe2, and WSe2, Opt. Express, 2013, 21, 4908–4916 CrossRef CAS PubMed.
- K. M. McCreary, A. T. Hanbicki, G. G. Jernigan, J. C. Culbertson and B. T. Jonker, Synthesis of large-area WS2 monolayers with exceptional photoluminescence, Sci. Rep., 2016, 6, 19159 CrossRef CAS PubMed.
- K. Ueno, K. Saiki, T. Shimada and A. Koma, Epitaxial growth of transition metal dichalcogenides on cleaved faces of mica, J. Vac. Sci. Technol., A, 1990, 8, 68–72 CAS.
- K. S. Novoselov, D. Jiang, F. Schedin, T. J. Booth, V. V. Khotkevich, S. V. Morozov and A. K. Geim, Two-dimensional atomic crystals, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10451–10453 CrossRef CAS PubMed.
- H. Li, G. Lu, Y. Wang, Z. Yin, C. Cong, Q. He, L. Wang, F. Ding, T. Yu and H. Zhang, Mechanical Exfoliation and Characterization of Single-and Few-Layer Nanosheets of WSe2, TaS2, and TaSe2, Small, 2013, 9, 1974–1981 CrossRef CAS PubMed.
- A. A. Al-Hilli and B. L. Evans, The preparation and properties of transition metal dichalcogenide single crystals, J. Cryst. Growth, 1972, 15, 93–101 CrossRef CAS.
- Y. H. Lee, L. Yu, H. Wang, W. Fang, X. Ling, Y. Shi, C. T. Lin, J. K. Huang, M. T. Chang, C. S. Chang, M. Dresselhaus, T. Palacios, L. J. Li and J. Kong, Synthesis and transfer of single-layer transition metal disulfides on diverse surfaces, Nano Lett., 2013, 13, 1852–1857 CrossRef CAS PubMed.
- Y. Shi, H. Li and L. J. Li, Recent advances in controlled synthesis of two-dimensional transition metal dichalcogenides via vapour deposition techniques, Chem. Soc. Rev., 2015, 44, 2744–2756 RSC.
- R. Gatensby, N. McEvoy, K. Lee, T. Hallam, N. C. Berner, E. Rezvani, S. Winters, M. O'Brien and G. S. Duesberg, Controlled synthesis of transition metal dichalcogenide thin films for electronic applications, Appl. Surf. Sci., 2015, 297, 139–146 CrossRef.
- S. Jeong, D. Yoo, J. T. Jang, M. Kim and J. Cheon, Well-defined colloidal 2-D layered transition-metal chalcogenide nanocrystals via generalized synthetic protocols, J. Am. Chem. Soc., 2012, 134, 18233–18236 CrossRef CAS PubMed.
- M. S. Whittingham and F. R. Gamble, The lithium intercalates of the transition metal dichalcogenides, Mater. Res. Bull., 1975, 10, 363–371 CrossRef CAS.
- Z. Zeng, T. Sun, J. Zhu, X. Huang, Z. Yin, G. Lu, Z. Fan, Q. Yan, H. H. Hng and H. Zhang, An Effective Method for the Fabrication of Few-Layer-Thick Inorganic Nanosheets, Angew. Chem., Int. Ed., 2012, 51, 9052–9056 CrossRef CAS PubMed.
- A. Y. S. Eng, A. Ambrosi, Z. Sofer, P. Simek and M. Pumera, Electrochemistry of transition metal dichalcogenides: strong dependence on the metal-to-chalcogen composition and exfoliation method, ACS Nano, 2014, 8, 12185–12198 CrossRef CAS PubMed.
- R. A. Gordon, D. Yang, E. D. Crozier, D. T. Jiang and R. F. Frindt, Structures of exfoliated single layers of WS2, MoS2, and MoSe2 in aqueous suspension, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 125407 CrossRef.
- G. Cunningham, M. Lotya, C. S. Cucinotta, S. Sanvito, S. D. Bergin, R. Menzel, M. S. Shaffer and J. N. Coleman, Solvent exfoliation of transition metal dichalcogenides: dispersibility of exfoliated nanosheets varies only weakly between compounds, ACS Nano, 2012, 6, 3468–3480 CrossRef CAS PubMed.
- R. J. Smith, P. J. King, M. Lotya, C. Wirtz, U. Khan, S. De, A. O'Neill, G. S. Duesberg, J. C. Grunlan, G. Moriarty and J. Chen, Large-scale exfoliation of inorganic layered compounds in aqueous surfactant solutions, Adv. Mater., 2011, 23, 3944–3948 CrossRef CAS PubMed.
- V. Nicolosi, M. Chhowalla, M. G. Kanatzidis, M. S. Strano and J. N. Coleman, Liquid exfoliation of layered materials, Science, 2013, 340, 1226419 CrossRef.
- J. N. Coleman, M. Lotya, A. O'Neill, S. D. Bergin, P. J. King, U. Khan, K. Young, A. Gaucher, S. De, R. J. Smith, I. V. Shvets, S. K. Arora, G. Stanton, H. Y. Kim, K. Lee, G. Kim, G. S. Duesberg, T. Hallam, J. J. Boland, J. J. Wang, J. F. Donegan, J. C. Grunlan, G. Moriarty, A. Shmeliov, R. J. Nicholls, J. M. Perkins, E. M. Grieveson, K. Theuwissen, D. W. McComb, P. D. Nellist and V. Nicolosi, Two-dimensional nanosheets produced by liquid exfoliation of layered materials, Science, 2011, 331, 568–571 CrossRef CAS PubMed.
- L. Niu, K. Li, H. Zhen, Y. S. Chui, W. Zhang, F. Yan and Z. Zheng, Salt-Assisted High-Throughput Synthesis of Single-and Few-Layer Transition Metal Dichalcogenides and Their Application in Organic Solar Cells, Small, 2014, 10, 4651–4657 CrossRef CAS PubMed.
- X. Zhang, X. F. Qiao, W. Shi, J. B. Wu, D. S. Jiang and P. H. Tan, Phonon and Raman scattering of two-dimensional transition metal dichalcogenides from monolayer, multilayer to bulk material, Chem. Soc. Rev., 2015, 44, 2757–2785 RSC.
- A. S. Pawbake, M. S. Pawar, S. R. Jadkar and D. J. Late, Large area chemical vapor deposition of monolayer transition metal dichalcogenides and their temperature dependent Raman spectroscopy studies, Nanoscale, 2016, 8, 3008–3018 RSC.
- J. L. Verble and T. J. Wieting, Lattice Mode Degeneracy in MoS2 and Other Layer Compounds, Phys. Rev. Lett., 1970, 25, 362 CrossRef CAS.
- G. Frey, R. Tenne, M. Matthews, M. Dresselhaus and G. Dresselhaus, Raman and resonance Raman investigation of MoS2 nanoparticles, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 60, 2883–2892 CrossRef CAS.
- A. Castellanos-Gomez, J. Quereda, H. P. van der Meulen, N. Agraït and G. Rubio-Bollinger, Spatially resolved optical absorption spectroscopy of single-and few-layer MoS2 by hyperspectral imaging, Nanotechnology, 2016, 27, 115705 CrossRef PubMed.
- S. K. Balasingam, J. S. Lee and Y. Jun, Few-layered MoSe2 nanosheets as an advanced electrode material for supercapacitors, Dalton Trans., 2015, 44, 15491–15498 RSC.
- W. Zhao, Z. Ghorannevis, K. K. Amara, J. R. Pang, M. Toh, X. Zhang, C. Kloc, P. H. Tan and G. Eda, Lattice dynamics in mono-and few-layer sheets of WS2 and WSe2, Nanoscale, 2013, 5, 9677–9683 RSC.
- C. Lee, H. Yan, L. E. Brus, T. F. Heinz, J. Hone and S. Ryu, Anomalous Lattice Vibrations of Single- and Few-Layer MoS2, ACS Nano, 2010, 4, 2695–2700 CrossRef CAS PubMed.
- A. Molina-Sanchez and L. Wirtz, Phonons in single-layer and few-layer MoS2 and WS2, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 155413 CrossRef.
- S. Tongay, J. Zhou, C. Ataca, K. Lo, T. S. Matthews, J. Li, J. C. Grossman and J. Wu, Thermally Driven Crossover from Indirect toward Direct Bandgap in 2D Semiconductors: MoSe2 versus MoS2, Nano Lett., 2012, 12, 5576–5580 CrossRef CAS PubMed.
- H. Li, Q. Zhang, C. C. R. Yap, B. K. Tay, T. H. T. Edwin, A. Olivier and D. Baillargeat, From Bulk to Monolayer MoS2: Evolution of Raman Scattering, Adv. Funct. Mater., 2012, 22, 1385–1390 CrossRef CAS.
- A. Berkdemir, H. R. Gutierrez, A. R. Botello-Mendez, N. Perea-Lopez, A. L. Elias, C. I. Chia, B. Wang, V. H. Crespi, F. Lopez-Urias, J. C. Charlier, H. Terrones and M. Terrones, Identification of individual and few layers of WS2 using Raman Spectroscopy, Sci. Rep., 2013, 3, 1755, DOI:10.1038/srep01755.
- H. Sahin, S. Tongay, S. Horzum, W. Fan, J. Zhou, J. Li, J. Wu and F. M. Peeters, Anomalous Raman spectra and thickness-dependent electronic properties of WSe2, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 165409 CrossRef.
- R. Saito, Y. Tatsumi, S. Huang, X. Ling and M. S. Dresselhaus, Raman spectroscopy of transition metal dichalcogenides, J. Phys.: Condens. Matter, 2016, 28, 353002 CrossRef CAS PubMed.
- H. Terrones, E. Del Corro, S. Feng, J. M. Poumirol, D. Rhodes, D. Smirnov, N. R. Pradhan, Z. Lin, M. A. T. Nguyen, A. L. Elias and T. E. Mallouk, New first order Raman-active modes in few layered transition metal dichalcogenides, Sci. Rep., 2014, 4, 4215 CrossRef CAS PubMed.
- S. Y. Chen, C. Zheng, M. S. Fuhrer and J. Yan, Helicity-resolved Raman scattering of MoS2, MoSe2, WS2, and WSe2 atomic layers, Nano Lett., 2015, 15, 2526–2532 CrossRef CAS PubMed.
- P. D. Fleischauer and R. Bauer, Chemical and structural effects on the lubrication properties of sputtered MoS2 films, Tribol. Trans., 1988, 31, 239–250 CrossRef CAS.
- T. Spalvins, Lubrication with sputtered MoS2 films: principles, operation, and limitations, J. Mater. Eng. Perform., 1992, 1, 347–351 CrossRef CAS.
- M. R. Hilton, R. Bauer, S. V. Didziulis, M. T. Dugger, J. M. Keem and J. Scholhamer, Structural and tribological studies of MoS2 solid lubricant films having tailored metal-multilayer nanostructures, Surf. Coat. Technol., 1992, 53, 13–23 CrossRef CAS.
- A. Savan, E. Pflüger, P. Voumard, A. Schröer and M. Simmonds, Modern solid lubrication: recent developments and applications of MoS2, Lubr. Sci., 2000, 12, 185–203 CrossRef CAS.
- L. Rapoport, Y. Bilik, Y. Feldman, M. Homyonfer, S. R. Cohen and R. Tenne, Hollow nanoparticles of WS2 as potential solid-state lubricants, Nature, 1997, 387, 791–793 CrossRef CAS.
- L. Rapoport, N. Fleischer and R. Tenne, Fullerene-like WS2 Nanoparticles: Superior Lubricants for Harsh Conditions, Adv. Mater., 2003, 15, 651–655 CrossRef CAS.
- Y. Q. Liu, C. S. Li, J. H. Yang, Y. M. Yu and X. K. Li, Synthesis and tribological properties of tubular NbS2 and TaS2 nanostructures, Chin. J. Chem. Phys., 2007, 20, 768–772 CrossRef CAS.
- T. Polcar and A. Cavaleiro, Review on self-lubricant transition metal dichalcogenide nanocomposite coatings alloyed with carbon, Surf. Coat. Technol., 2011, 206, 686–695 CrossRef CAS.
- J. F. Yang, B. Parakash, J. Hardell and Q. F. Fang, Tribological properties of transition metal di-chalcogenide based lubricant coatings, Front. Mater. Sci., 2012, 6, 116–127 CrossRef.
- D. Krasnozhon, D. Lembke, C. Nyffeler, Y. Leblebici and A. Kis, MoS2 transistors operating at gigahertz frequencies, Nano Lett., 2014, 14, 5905–5911 CrossRef CAS PubMed.
- H. Fang, S. Chuang, T. C. Chang, K. Takei, T. Takahashi and A. Javey, High-performance single layered WSe2 p-FETs with chemically doped contacts, Nano Lett., 2012, 12, 3788–3792 CrossRef CAS PubMed.
- H. Wang, L. Yu, Y. H. Lee, Y. Shi, A. Hsu, M. L. Chin, L. J. Li, M. Dubey, J. Kong and T. Palacios, Integrated circuits based on bilayer MoS2 transistors, Nano Lett., 2012, 12, 4674–4680 CrossRef CAS PubMed.
- H. Li, J. Wu, Z. Yin and H. Zhang, Preparation and applications of mechanically exfoliated single-layer and multilayer MoS2 and WSe2 nanosheets, Acc. Chem. Res., 2014, 47, 1067–1075 CrossRef CAS PubMed.
- C. Gong, H. Zhang, W. Wang, L. Colombo, R. M. Wallace and K. Cho, Band alignment of two-dimensional transition metal dichalcogenides: application in tunnel field effect transistors, Appl. Phys. Lett., 2013, 103, 053513 CrossRef.
- D. Jariwala, V. K. Sangwan, L. J. Lauhon, T. J. Marks and M. C. Hersam, Emerging device applications for semiconducting two-dimensional transition metal dichalcogenides, ACS Nano, 2014, 8, 1102–1120 CrossRef CAS PubMed.
- F. Schwierz, J. Pezoldt and R. Granzner, Two-dimensional materials and their prospects in transistor electronics, Nanoscale, 2015, 7, 8261–8283 RSC.
- H. Y. Chang, S. Yang, J. Lee, L. Tao, W. S. Hwang, D. Jena, N. Lu and D. Akinwande, High-performance, highly bendable MoS2 transistors with high-k dielectrics for flexible low-power systems, ACS Nano, 2013, 7, 5446–5452 CrossRef CAS PubMed.
- R. Cheng, S. Jiang, Y. Chen, Y. Liu, N. Weiss, H. C. Cheng, H. Wu, Y. Huang and X. Duan, Few-layer molybdenum disulfide transistors and circuits for high-speed flexible electronics, Nat. Commun., 2014, 5, 5143 CrossRef CAS PubMed.
- W. S. Hwang, M. Remskar, R. Yan, V. Protasenko, K. Tahy, S. D. Chae, P. Zhao, A. Konar, H. G. Xing, A. Seabaugh and D. Jena, Transistors with chemically synthesized layered semiconductor WS2 exhibiting 105 room temperature modulation and ambipolar behavior, Appl. Phys. Lett., 2012, 101, 013107 CrossRef.
- M. Tosun, S. Chuang, H. Fang, A. B. Sachid, M. Hettick, Y. Lin, Y. Zeng and A. Javey, High-gain inverters based on WSe2 complementary field-effect transistors, ACS Nano, 2014, 8, 4948–4953 CrossRef CAS PubMed.
- S. Larentis, B. Fallahazad and E. Tutuc, Field-effect transistors and intrinsic mobility in ultra-thin MoSe2 layers, Appl. Phys. Lett., 2012, 101, 223104 CrossRef.
- B. Chamlagain, Q. Li, N. J. Ghimire, H. J. Chuang, M. M. Perera, H. Tu, Y. Xu, M. Pan, D. Xaio, J. Yan and D. Mandrus, Mobility improvement and temperature dependence in MoSe2 field-effect transistors on parylene-C substrate, ACS Nano, 2014, 8, 5079–5088 CrossRef CAS PubMed.
- S. Fathipour, N. Ma, W. S. Hwang, V. Protasenko, S. Vishwanath, H. G. Xing, H. Xu, D. Jena, J. Appenzeller and A. Seabaugh, Exfoliated multilayer MoTe2 field-effect transistors, Appl. Phys. Lett., 2014, 105, 192101 CrossRef.
- Y. F. Lin, Y. Xu, S. T. Wang, S. L. Li, M. Yamamoto, A. Aparecido-Ferreira, W. Li, H. Sun, S. Nakaharai, W. B. Jian and K. Ueno, Ambipolar MoTe2 transistors and their applications in logic circuits, Adv. Mater., 2014, 26, 3263–3269 CrossRef CAS PubMed.
- E. Liu, Y. Fu, Y. Wang, Y. Feng, H. Liu, X. Wan, W. Zhou, B. Wang, L. Shao, C. H. Ho and Y. S. Huang, Integrated digital inverters based on two-dimensional anisotropic ReS2 field-effect transistors, Nat. Commun., 2015, 6, 6991 CrossRef CAS PubMed.
- D. Gao, Q. Xue, X. Mao, W. Wang, Q. Xu and D. Xue, Ferromagnetism in ultrathin VS2 nanosheets, J. Mater. Chem. C, 2013, 1, 5909–5916 RSC.
- T. Kanazawa, T. Amemiya, A. Ishikawa, V. Upadhyaya, K. Tsuruta, T. Tanaka and Y. Miyamoto, Few-layer HfS2 transistors, Sci. Rep., 2016, 6, 22277 CrossRef CAS PubMed.
- (a) M. S. El-Bana, D. Wolverson, S. Russo, G. Balakrishnan, D. M. Paul and S. J. Bending, Superconductivity in two-dimensional NbSe2 field effect transistors, Supercond. Sci. Technol., 2013, 26, 125020 CrossRef; (b) S. Yang, Q. Yue, H. Cai, K. Wu, C. Jiang and S. Tongay, Highly efficient gas molecule-tunable few-layer GaSe phototransistors, J. Mater. Chem. C, 2016, 4, 248–253 RSC.
- X. Geng, Y. Yu, X. Zhou, C. Wang, K. Xu, Y. Zhang, C. Wu, L. Wang, Y. Jiang and Q. Yang, Design and construction of ultra-thin MoSe2 nanosheet-based heterojunction for high-speed and low-noise photodetection, Nano Res., 2016, 9, 2641–2651 CrossRef CAS.
- E. Zhang, Y. Jin, X. Yuan, W. Wang, C. Zhang, L. Tang, S. Liu, P. Zhou, W. Hu and F. Xiu, ReS2-Based Field-Effect Transistors and Photodetectors, Adv. Funct. Mater., 2015, 25, 4076–4082 CrossRef CAS.
- S. Yang, C. Wang, C. Ataca, Y. Li, H. Chen, H. Cai, A. Suslu, J. C. Grossman, C. Jiang, Q. Liu and S. Tongay, Self-Driven Photodetector and Ambipolar Transistor in Atomically Thin GaTe-MoS2 p–n vdW Heterostructure, ACS Appl. Mater. Interfaces, 2016, 8, 2533–2539 CAS.
- J. S. Ross, P. Klement, A. M. Jones, N. J. Ghimire, J. Yan, D. G. Mandrus, T. Taniguchi, K. Watanabe, K. Kitamura, W. Yao and D. H. Cobden, Electrically tunable excitonic light-emitting diodes based on monolayer WSe2 pn junctions, Nat. Nanotechnol., 2014, 9, 268–272 CrossRef CAS PubMed.
- Z. Yin, X. Zhang, Y. Cai, J. Chen, J. I. Wong, Y. Y. Tay, J. Chai, J. Wu, Z. Zeng, B. Zheng and H. Y. Yang, Preparation of MoS2–MoO3 Hybrid Nanomaterials for Light-Emitting Diodes, Angew. Chem., Int. Ed., 2014, 53, 12560–12565 CAS.
- A. Lipatov, P. Sharma, A. Gruverman and A. Sinitskii, Optoelectrical Molybdenum Disulfide (MoS2)-Ferroelectric Memories, ACS Nano, 2015, 9, 8089–8098 CrossRef CAS PubMed.
- J. Lee, Z. Wang, K. He, J. Shan and P. X. L. Feng, High frequency MoS2 nanomechanical resonators, ACS Nano, 2013, 7, 6086–6091 CrossRef CAS PubMed.
- F. Clerici, M. Fontana, S. Bianco, M. Serrapede, F. Perrucci, S. Ferrero, E. Tresso and A. Lamberti, In situ MoS2 Decoration of Laser-Induced Graphene as Flexible Supercapacitor Electrodes, ACS Appl. Mater. Interfaces, 2016, 8, 10459–10465 CAS.
- X. Zhou, B. Xu, Z. Lin, D. Shu and L. Ma, Hydrothermal Synthesis of Flower-Like MoS2 Nanospheres for Electrochemical Supercapacitors, J. Nanosci. Nanotechnol., 2014, 14, 7250–7254 CrossRef CAS PubMed.
- G. Du, Z. Guo, S. Wang, R. Zeng, Z. Chen and H. Liu, Superior Stability and High Capacity of Restacked Molybdenum Disulfide as Anode Material for Lithium Ion Batteries, Chem. Commun., 2010, 46, 1106–1108 RSC.
- Z. Zhang, X. Shi, X. Yang, Y. Fu, K. Zhang, Y. Lai and J. Li, Nanooctahedra particles assembled FeSe2 microspheres embedded into sulfur-doped reduced graphene oxide sheets as a promising anode for sodium ion batteries, ACS Appl. Mater. Interfaces, 2016, 8, 13849–13856 CAS.
- X. Xu, C. S. Rout, J. Yang, R. Cao, P. Oh, H. S. Shin and J. Cho, Freeze-dried WS2 composites with low content of graphene as high-rate lithium storage materials, J. Mater. Chem. A, 2013, 1, 14548–14554 CAS.
- O. Lopez-Sanchez, E. Alarcon Llado, V. Koman, A. Fontcuberta i Morral, A. Radenovic and A. Kis, Light Generation and Harvesting in a van der Waals Heterostructure, ACS Nano, 2014, 8, 3042–3048 CrossRef CAS PubMed.
- F. K. Perkins, A. L. Friedman, E. Cobas, P. M. Campbell, G. G. Jernigan and B. T. Jonker, Chemical vapor sensing with monolayer MoS2, Nano Lett., 2013, 13, 668–673 CrossRef CAS PubMed.
- D. J. Late, T. Doneux and M. Bougouma, Single-layer MoSe2 based NH3 gas sensor, Appl. Phys. Lett., 2014, 105, 233103 CrossRef.
- T. Wang, R. Zhu, J. Zhuo, Z. Zhu, Y. Shao and M. Li, Direct detection of DNA below ppb level based on thionin-functionalized layered MoS2 electrochemical sensors, Anal. Chem., 2014, 86, 12064–12069 CrossRef CAS PubMed.
- M. Pumera and A. H. Loo, Layered transition-metal dichalcogenides (MoS2 and WS2) for sensing and biosensing, TrAC, Trends Anal. Chem., 2014, 61, 49–53 CrossRef CAS.
- Z. Li and S. L. Wong, Functionalization of 2D transition metal dichalcogenides for biomedical applications, Mater. Sci. Eng., C, 2016, 70, 1095–1106 CrossRef PubMed.
- E. Singh, K. S. Kim, G. Y. Yeom and H. S. Nalwa, Atomically thin-layered molybdenum disulfide (MoS2) for bulk-heterojunction solar cells, ACS Appl. Mater. Interfaces, 2017, 9, 3223–3245 CAS.
- (a) K. S. Kim, K. H. Kim, Y. Nam, J. Jeon, S. Yim, E. Singh, J. Y. Lee, S. Lee, Y. S. Jung, G. Y. Yeom and D. W. Kim, Atomic Layer Etching Mechanism of MoS2 for Nanodevices, ACS Appl. Mater. Interfaces, 2017, 9, 11967–11976 CrossRef CAS PubMed; (b) Q. H. Wang, K. Kalantar-Zadeh, A. Kis, J. N. Coleman and M. S. Strano, Electronics and optoelectronics of two-dimensional transition metal dichalcogenides, Nat. Nanotechnol., 2012, 7, 699–712 CrossRef CAS PubMed.
- K. F. Mak and J. Shan, Photonics and optoelectronics of 2D semiconductor transition metal dichalcogenides, Nat. Photonics, 2016, 10, 216–226 CrossRef CAS.
- J. U. Lee, K. Kim, S. Han, G. H. Ryu, Z. Lee and H. Cheong, Raman Signatures of Polytypism in Molybdenum Disulfide, ACS Nano, 2016, 10, 1948–1953 CrossRef CAS PubMed.
- A. R. Beal, J. C. Knights and W. Y. Liang, Transmission spectra of some transition metal dichalcogenides II. Group VIA: trigonal prismatic coordination, J. Phys. C: Solid State Phys., 1972, 5, 3540 CrossRef CAS.
- J. A. Woollam and R. B. Somoano, Superconducting critical fields of alkali and alkaline-earth intercalates of MoS2, Phys. Rev. B: Solid State, 1976, 13, 3843 CrossRef CAS.
- F. Wypych and R. Schöllhorn, 1T–MoS2, a new metallic modification of molybdenum disulfide, J. Chem. Soc., Chem. Commun., 1992, 1386–1388 RSC.
- L. Wang, Z. Xu, W. Wang and X. Bai, Atomic mechanism of dynamic electrochemical lithiation processes of MoS2 nanosheets, J. Am. Chem. Soc., 2014, 136, 6693–6697 CrossRef CAS PubMed.
- S. N. Shirodkar and U. V. Waghmare, Emergence of Ferroelectricity at a Metal-Semiconductor Transition in a 1T Monolayer of MoS2, Phys. Rev. Lett., 2014, 112, 157601 CrossRef PubMed.
- M. Acerce, D. Voiry and M. Chhowalla, Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials, Nat. Nanotechnol., 2015, 10, 313–318 CrossRef CAS PubMed.
- P. Cheng, K. Sun and Y. H. Hu, Memristive Behavior and Ideal Memristor of 1T Phase MoS2 Nanosheets, Nano Lett., 2015, 16, 572–576 CrossRef PubMed.
- R. Kappera, D. Voiry, S. E. Yalcin, B. Branch, G. Gupta, A. D. Mohite and M. Chhowalla, Phase-engineered low-resistance contacts for ultrathin MoS2 transistors, Nat. Mater., 2014, 13, 1128–1134 CrossRef CAS PubMed.
- W. Wei, K. Sun and Y. H. Hu, An efficient counter electrode material for dye-sensitized solar cells—flower-structured 1T metallic phase MoS2, J. Mater. Chem. A, 2016, 4, 12398–12401 CAS.
- R. S. Infant, X. Xu, W. Yang, F. Yang, L. Hou and Y. Li, Highly active and reflective MoS2 counter electrode for enhancement of photovoltaic efficiency of dye sensitized solar cells, Electrochim. Acta, 2016, 212, 614–620 CrossRef.
- S. Jiang, X. Yin, J. Zhang, X. Zhu, J. Li and M. He, Vertical ultrathin MoS2 nanosheets on a flexible substrate as an efficient counter electrode for dye-sensitized solar cells, Nanoscale, 2015, 7, 10459–10464 RSC.
- B. Lei, G. R. Li and X. P. Gao, Morphology dependence of molybdenum disulfide transparent counter electrode in dye-sensitized solar cells, J. Mater. Chem. A, 2014, 2, 3919–3925 CAS.
- J. Zhang, S. Najmaei, H. Lin and J. Lou, MoS2 atomic layers with artificial active edge sites as transparent counter electrodes for improved performance of dye-sensitized solar cells, Nanoscale, 2014, 6, 5279–5283 RSC.
- M. Al-Mamun, H. Zhang, P. Liu, Y. Wang, J. Cao and H. Zhao, Directly hydrothermal growth of ultrathin MoS2 nanostructured films as high performance counter electrodes for dye-sensitised solar cells, RSC Adv., 2014, 4, 21277–21283 RSC.
- C. K. Cheng and C. K. Hsieh, Electrochemical deposition of molybdenum sulfide thin films on conductive plastic substrates as platinum-free flexible counter electrodes for dye-sensitized solar cells, Thin Solid Films, 2015, 584, 52–60 CrossRef CAS.
- C. H. Lin, C. H. Tsai, F. G. Tseng, Y. Y. Yu, H. C. Wu and C. K. Hsieh, Low-Temperature Thermally Reduced Molybdenum Disulfide as a Pt-Free Counter Electrode for Dye-Sensitized Solar Cells, Nanoscale Res. Lett., 2015, 10, 1–10 CrossRef PubMed.
- S. A. Patil, P. Y. Kalode, R. S. Mane, D. V. Shinde, A. Doyoung, C. Keumnam, M. M. Sung, S. B. Ambade and S. H. Han, Highly efficient and stable DSSCs of wet-chemically synthesized MoS2 counter electrode, Dalton Trans., 2014, 43, 5256–5259 RSC.
- W. H. Jhang and Y. J. Lin, Overpotential modification at the MoS2 counter electrode/electrolyte interfaces by thermal annealing resulting improvement in photovoltaic performance of dye-sensitized solar cells, J. Mater. Sci.: Mater. Electron., 2015, 26, 3739–3743 CrossRef CAS.
- A. Antonelou, G. Syrrokostas, L. Sygellou, G. Leftheriotis, V. Dracopoulos and S. N. Yannopoulos, Facile, substrate-scale growth of mono-and few-layer homogeneous MoS2 films on Mo foils with enhanced catalytic activity as counter electrodes in DSSCs, Nanotechnology, 2015, 27, 045404 CrossRef PubMed.
- M. Wu, Y. Wang, X. Lin, N. Yu, L. Wang, L. Wang, A. Hagfeldt and T. Ma, Economical and effective sulfide catalysts for dye-sensitized solar cells as counter electrodes, Phys. Chem. Chem. Phys., 2011, 13, 19298–19301 RSC.
- H. Zhang, J. Choi, A. Ramani, D. Voiry, S. N. Natoli, M. Chhowalla, D. R. McMillin and J. H. Choi, Engineering Chemically Exfoliated Large-Area Two-Dimensional MoS2 Nanolayers with Porphyrins for Improved Light Harvesting, ChemPhysChem, 2016, 17, 2854–2862 CrossRef CAS PubMed.
- W. Liu, S. He, T. Yang, Y. Feng, G. Qian, J. Xu and S. Miao, TEOS-assisted synthesis of porous MoS2 with ultra-small exfoliated sheets and applications in dye-sensitized solar cells, Appl. Surf. Sci., 2014, 313, 498–503 CrossRef CAS.
- S. S. Kim, J. W. Lee, J. M. Yun and S. I. Na, 2-Dimensional MoS2 nanosheets as transparent and highly electrocatalytic counter electrode in dye-sensitized solar cells: effect of thermal treatments, J. Ind. Eng. Chem., 2015, 29, 71–77 CrossRef CAS.
- S. Hussain, S. F. Shaikh, D. Vikraman, R. S. Mane, O. S. Joo, M. Naushad and J. Jung, High-Performance Platinum-Free Dye-Sensitized Solar Cells with Molybdenum Disulfide Films as Counter Electrodes, ChemPhysChem, 2015, 16, 3959–3965 CrossRef CAS PubMed.
- H. Jeong, J. Y. Kim, B. Koo, H. J. Son, D. Kim and M. J. Ko, Rapid sintering of MoS2 counter electrode using near-infrared pulsed laser for use in highly efficient dye-sensitized solar cells, J. Power Sources, 2016, 330, 104–110 CrossRef CAS.
- J. Liang, J. Li, H. Zhu, Y. Han, Y. Wang, C. Wang, Z. Jin, G. Zhang and J. Liu, One-step fabrication of large-area ultrathin MoS2 nanofilms with high catalytic activity for photovoltaic devices, Nanoscale, 2016, 8, 16017–16025 RSC.
- C. Tan, W. Zhao, A. Chaturvedi, Z. Fei, Z. Zeng, J. Chen, Y. Huang, P. Ercius, Z. Luo, X. Qi and B. Chen, Preparation of Single-Layer MoS2xSe2(1 − x) and MoxW1−xS2 Nanosheets with High-Concentration Metallic 1T Phase, Small, 2016, 12, 1866–1874 CrossRef CAS PubMed.
- O. Eksik, J. Gao, S. A. Shojaee, A. Thomas, P. Chow, S. F. Bartolucci, D. A. Lucca and N. Koratkar, Epoxy nanocomposites with two-dimensional transition metal dichalcogenide additives, ACS Nano, 2014, 8, 5282–5289 CrossRef CAS PubMed.
- C. Tan and H. Zhang, Two-dimensional transition metal dichalcogenide nanosheet-based composites, Chem. Soc. Rev., 2015, 44, 2713–2731 RSC.
- J. Y. Lin, C. Y. Chan and S. W. Chou, Electrophoretic deposition of transparent MoS2–graphene nanosheet composite films as counter electrodes in dye-sensitized solar cells, Chem. Commun., 2013, 49, 1440–1442 RSC.
- G. Yue, J. Y. Lin, S. Y. Tai, Y. Xiao and J. Wu, A catalytic composite film of MoS2/graphene flake as a counter electrode for Pt-free dye-sensitized solar cells, Electrochim. Acta, 2012, 85, 162–168 CrossRef CAS.
- C. Yu, X. Meng, X. Song, S. Liang, Q. Dong, G. Wang, C. Hao, X. Yang, T. Ma, P. M. Ajayan and J. Qiu, Graphene-mediated highly-dispersed MoS2 nanosheets with enhanced triiodide reduction activity for dye-sensitized solar cells, Carbon, 2016, 100, 474–483 CrossRef CAS.
- P. Lynch, U. Khan, A. Harvey, I. Ahmed and J. N. Coleman, Graphene–MoS2 nanosheet composites as electrodes for dye sensitised solar cells, Mater. Res. Express, 2016, 3, 035007 CrossRef.
- S. Li, H. Min, F. Xu, L. Tong, J. Chen, C. Zhu and L. Sun, All electrochemical fabrication of MoS2/graphene counter electrodes for efficient dye-sensitized solar cells, RSC Adv., 2016, 6, 34546–34552 RSC.
- C. K. Cheng, C. H. Lin, H. C. Wu, C. C. M. Ma, T. K. Yeh, H. Y. Chou, C. H. Tsai and C. K. Hsieh, The Two-Dimensional Nanocomposite of Molybdenum Disulfide and Nitrogen-Doped Graphene Oxide for Efficient Counter Electrode of Dye-Sensitized Solar Cells, Nanoscale Res. Lett., 2016, 11, 1–9 CrossRef CAS PubMed.
- M. S. Fan, C. P. Lee, C. T. Li, Y. J. Huang, R. Vittal and K. C. Ho, Nitrogen-doped graphene/molybdenum disulfide composite as the electrocatalytic film for dye-sensitized solar cells, Electrochim. Acta, 2016, 211, 164–172 CrossRef CAS.
- J. Y. Lin, G. Yue, S. Y. Tai, Y. Xiao, H. M. Cheng, F. M. Wang and J. Wu, Hydrothermal synthesis of graphene flake embedded nanosheet-like molybdenum sulfide hybrids as counter electrode catalysts for dye-sensitized solar cells, Mater. Chem. Phys., 2013, 143, 53–59 CrossRef CAS.
- G. Yue, X. Ma, Q. Jiang, F. Tan, J. Wu, C. Chen, F. Li and Q. Li, PEDOT:PSS and glucose assisted preparation of molybdenum disulfide/single-wall carbon nanotubes counter electrode and served in dye-sensitized solar cells, Electrochim. Acta, 2014, 142, 68–75 CrossRef CAS.
- S. Y. Tai, C. J. Liu, S. W. Chou, F. S. S. Chien, J. Y. Lin and T. W. Lin, Few-layer MoS2 nanosheets coated onto multi-walled carbon nanotubes as a low-cost and highly electrocatalytic counter electrode for dye-sensitized solar cells, J. Mater. Chem., 2012, 22, 24753–24759 RSC.
- C. H. Lin, C. H. Tsai, F. G. Tseng, C. C. M. Ma, H. C. Wu and C. K. Hsieh, Three-dimensional vertically aligned hybrid nanoarchitecture of two-dimensional molybdenum disulfide nanosheets anchored on directly grown one-dimensional carbon nanotubes for use as a counter electrode in dye-sensitized solar cells, J. Alloys Compd., 2017, 692, 941–949 CrossRef CAS.
- M. Zheng, J. Huo, Y. Tu, J. Wu, L. Hu and S. Dai, Flowerlike molybdenum sulfide/multi-walled carbon nanotube hybrid as Pt-free counter electrode used in dye-sensitized solar cells, Electrochim. Acta, 2015, 173, 252–259 CrossRef CAS.
- W. Liu, S. He, Y. Wang, Y. Dou, D. Pan, Y. Feng, G. Qian, J. Xu and S. Miao, PEG-assisted synthesis of homogeneous carbon nanotubes–MoS2–carbon as a counter electrode for dye-sensitized solar cells, Electrochim. Acta, 2014, 144, 119–126 CrossRef CAS.
- G. Yue, W. Zhang, J. Wu and Q. Jiang, Glucose aided synthesis of molybdenum sulfide/carbon nanotubes composites as counter electrode for high performance dye-sensitized solar cells, Electrochim. Acta, 2013, 112, 655–662 CrossRef CAS.
- J. Y. Lin, A. L. Su, C. Y. Chang, K. C. Hung and T. W. Lin, Molybdenum Disulfide/Reduced Graphene Oxide–Carbon Nanotube Hybrids as Efficient Catalytic Materials in Dye-Sensitized Solar Cells, ChemElectroChem, 2015, 2, 720–725 CrossRef CAS.
- (a) S. Wang, X. Jiang, H. Zheng, H. Wu, S. J. Kim and C. Feng, Solvothermal synthesis of MoS2/carbon nanotube composites with improved electrochemical performance for lithium ion batteries, Nanosci. Nanotechnol. Lett., 2012, 4, 378–383 CrossRef CAS; (b) J. Z. Wang, L. Lu, M. Lotya, J. N. Coleman, S. L. Chou, H. K. Liu, A. I. Minett and J. Chen, Development of MoS2–CNT composite thin film from layered MoS2 for lithium batteries, Adv. Energy Mater., 2013, 3, 798–805 CrossRef CAS.
- T. Du, N. Wang, H. Chen, H. He, H. Lin and K. Liu, TiO2-based solar cells sensitized by chemical-bath-deposited few-layer MoS2, J. Power Sources, 2015, 275, 943–949 CrossRef CAS.
- W. H. Jhang and Y. J. Lin, Interface modification of MoS2 counter electrode/electrolyte in dye-sensitized solar cells by incorporating TiO2 nanoparticles, Curr. Appl. Phys., 2015, 15, 906–909 CrossRef.
- H. C. Hung, Y. J. Lin and Z. Y. Ke, Interface modification of MoS2:TiO2 counter electrode/electrolyte in dye-sensitized solar cells by doping with different Co contents, J. Mater. Sci.: Mater. Electron., 2016, 27, 5059–5063 CrossRef CAS.
- Z. He, W. Que, Y. Xing and X. Liu, Reporting performance in MoS2–TiO2 bilayer and heterojunction films based dye-sensitized photovoltaic devices, J. Alloys Compd., 2016, 672, 481–488 CrossRef CAS.
- G. Yue, J. Wu, Y. Xiao, M. Huang, J. Lin and J. Y. Lin, High performance platinum-free counter electrode of molybdenum sulfide–carbon used in dye-sensitized solar cells, J. Mater. Chem. A, 2013, 1, 1495–1501 CAS.
- W. B. Li, M. X. Sun, J. He, S. F. Sun, Q. Zhang and Y. Y. Shi, Preparation of MoS2/Carbon Fiber Counter Electrodes and Its Application in DSSCs, J. Mater. Sci. Eng., 2015, 3, 020 CrossRef.
- J. Theerthagiri, R. A. Senthil, P. Arunachalam, J. Madhavan, M. H. Buraidah, A. Santhanam and A. K. Arof, Synthesis of various carbon incorporated flower-like MoS2 microspheres as counter electrode for dye-sensitized solar cells, J. Solid State Electrochem., 2016, 1–10 Search PubMed.
- D. Song, M. Li, Y. Jiang, Z. Chen, F. Bai, Y. Li and B. Jiang, Facile fabrication of MoS2/PEDOT–PSS composites as low-cost and efficient counter electrodes for dye-sensitized solar cells, J. Photochem. Photobiol., A, 2014, 279, 47–51 CrossRef CAS.
- X. Liang, H. W. Zheng, X. J. Li, Y. H. Yu, G. T. Yue, W. Zhang, J. J. Tian and T. F. Li, Nanocomposites of Bi5FeTi3O15 with MoS2 as Novel Pt-free Counter Electrode in Dye-Sensitized Solar Cells, Ceram. Int., 2016, 42, 12888–12893 CrossRef CAS.
- J. G. Song, J. Park, W. Lee, T. Choi, H. Jung, C. W. Lee, S. H. Hwang, J. M. Myoung, J. H. Jung, S. H. Kim and C. Lansalot-Matras, Layer-controlled, wafer-scale, and conformal synthesis of tungsten disulfide nanosheets using atomic layer deposition, ACS Nano, 2013, 7, 11333–11340 CrossRef CAS PubMed.
- A. L. Elias, N. Perea-López, A. Castro-Beltrán, A. Berkdemir, R. Lv, S. Feng, A. D. Long, T. Hayashi, Y. A. Kim, M. Endo and H. R. Gutiérrez, Controlled synthesis and transfer of large-area WS2 sheets: from single layer to few layers, ACS Nano, 2013, 7, 5235–5242 CrossRef CAS PubMed.
- S. I. Nikitenko, Y. Koltypin, Y. Mastai, M. Koltypin and A. Gedanken, Sonochemical synthesis of tungsten sulfide nanorods, J. Mater. Chem., 2002, 12, 1450–1452 RSC.
- Y. Q. Zhu, W. K. Hsu, H. Terrones, N. Grobert, B. H. Chang, M. Terrones, B. Q. Wei, H. W. Kroto, D. R. M. Walton, C. B. Boothroyd and I. Kinloch, Morphology, structure and growth of WS2 nanotubes, J. Mater. Chem., 2000, 10, 2570–2577 RSC.
- R. Rosentsveig, A. Margolin, Y. Feldman, R. Popovitz-Biro and R. Tenne, WS2 nanotube bundles and foils, Chem. Mater., 2002, 14, 471–473 CrossRef CAS.
- Y. Wang, S. Li, Y. Bai, Z. Chen, Q. Jiang, T. Li and W. Zhang, Dye-sensitized solar cells based on low cost carbon-coated tungsten disulphide counter electrodes, Electrochim. Acta, 2013, 114, 30–34 CrossRef CAS.
- S. Hussain, S. F. Shaikh, D. Vikraman, R. S. Mane, O. S. Joo, M. Naushad and J. Jung, Sputtering and sulfurization-combined synthesis of a transparent WS2 counter electrode and its application to dye-sensitized solar cells, RSC Adv., 2015, 5, 103567–103572 RSC.
- S. H. Ahn and A. Manthiram, Edge-Oriented Tungsten Disulfide Catalyst Produced from Mesoporous WO3 for Highly Efficient Dye-Sensitized Solar Cells, Adv. Energy Mater., 2016, 6, 1501814, DOI:10.1002/aenm.201501814.
- S. Li, Z. Chen and W. Zhang, Dye-sensitized solar cells based on WS2 counter electrodes, Mater. Lett., 2012, 72, 22–24 CrossRef CAS.
- G. Yue, J. Wu, J. Y. Lin, Y. Xiao, S. Y. Tai, J. Lin, M. Huang and Z. Lan, A counter electrode of multi-wall carbon nanotubes decorated with tungsten sulfide used in dye-sensitized solar cells, Carbon, 2013, 55, 1–9 CrossRef CAS.
- J. Wu, G. Yue, Y. Xiao, M. Huang, J. Lin, L. Fan, Z. Lan and J. Y. Lin, Glucose aided preparation of tungsten sulfide/multi-wall carbon nanotube hybrid and use as counter electrode in dye-sensitized solar cells, ACS Appl. Mater. Interfaces, 2012, 4, 6530–6536 CAS.
- J. A. Wilson, Concerning the semimetallic characters of TiS2 and TiSe2, Solid State Commun., 1977, 22, 551–553 CrossRef CAS.
- G. A. Benesh, A. M. Woolley and C. Umrigar, The pressure dependences of TiS2 and TiSe2 band structures, J. Phys. C: Solid State Phys., 1985, 18(8), 1595 CrossRef CAS.
- C. Bourgès, T. Barbier, G. Guélou, P. Vaqueiro, A. V. Powell, O. I. Lebedev, N. Barrier, Y. Kinemuchi and E. Guilmeau, Thermoelectric properties of TiS2 mechanically alloyed compounds, J. Eur. Ceram. Soc., 2016, 36, 1183–1189 CrossRef.
- M. Inoue, H. P. Hughes and A. D. Yoffe, The electronic and magnetic properties of the 3d transition metal intercalates of TiS2, Adv. Phys., 1989, 38, 565–604 CrossRef CAS.
- C. Wan, X. Gu, F. Dang, T. Itoh, Y. Wang, H. Sasaki, M. Kondo, K. Koga, K. Yabuki, G. J. Snyder and R. Yang, Flexible n-type thermoelectric materials by organic intercalation of layered transition metal dichalcogenide TiS2, Nat. Mater., 2015, 14, 622–627 CrossRef CAS PubMed.
- C. S. Cucinotta, K. Dolui, H. Pettersson, Q. M. Ramasse, E. Long, S. E. O'Brian, V. Nicolosi and S. Sanvito, Electronic Properties and Chemical Reactivity of TiS2 Nanoflakes, J. Phys. Chem. C, 2015, 119, 15707–15715 CAS.
- J. Chen, S. L. Li, Z. L. Tao, Y. T. Shen and C. X. Cui, Titanium disulfide nanotubes as hydrogen-storage materials, J. Am. Chem. Soc., 2003, 125, 5284–5285 CrossRef CAS PubMed.
- S. Prabakar, C. W. Bumby and R. D. Tilley, Liquid-phase synthesis of flower-like and flake-like titanium disulfide nanostructures, Chem. Mater., 2009, 21, 1725–1730 CrossRef CAS.
- A. Margolin, R. Popovitz-Biro, A. Albu-Yaron, A. Moshkovich, L. Rapoport and R. Tenne, Fullerene-like nanoparticles of titanium disulfide, Curr. Nanosci., 2005, 1, 253–262 CrossRef CAS.
- K. H. Park, J. Choi, H. J. Kim, D. H. Oh, J. R. Ahn and S. U. Son, Unstable Single-Layered Colloidal TiS2 Nanodisks, Small, 2008, 4, 945–950 CrossRef CAS PubMed.
- X. Sun, P. Bonnick and L. F. Nazar, Layered TiS2 Positive Electrode for Mg Batteries, ACS Energy Lett., 2016, 1, 297–301 CrossRef CAS.
- C. Lin, X. Zhu, J. Feng, C. Wu, S. Hu, J. Peng, Y. Guo, L. Peng, J. Zhao, J. Huang and J. Yang, Hydrogen-incorporated TiS2 ultrathin nanosheets with ultrahigh conductivity for stamp-transferrable electrodes, J. Am. Chem. Soc., 2013, 135, 5144–5151 CrossRef CAS PubMed.
- X. Meng, C. Yu, B. Lu, J. Yang and J. Qiu, Dual integration system endowing two-dimensional titanium disulfide with enhanced triiodide reduction performance in dye-sensitized solar cells, Nano Energy, 2016, 22, 59–69 CrossRef CAS.
- C. T. Li, C. P. Lee, Y. Y. Li, M. H. Yeh and K. C. Ho, A composite film of TiS2/PEDOT:PSS as the electrocatalyst for the counter electrode in dye-sensitized solar cells, J. Mater. Chem. A, 2013, 1, 14888–14896 CAS.
- S. L. Yang, H. B. Yao, M. R. Gao and S. H. Yu, Monodisperse cubic pyrite NiS2 dodecahedrons and microspheres synthesized by a solvothermal process in a mixed solvent: thermal stability and magnetic properties, CrystEngComm, 2009, 11, 1383–1390 RSC.
- X. Song, W. Shen, Z. Sun, C. Yang, P. Zhang and L. Gao, Size-engineerable NiS2 hollow spheres photo co-catalysts from supermolecular precursor for H2 production from water splitting, Chem. Eng. J., 2016, 290, 74–81 CrossRef CAS.
- C. Tang, Z. Pu, Q. Liu, A. M. Asiri and X. Sun, NiS2 nanosheets array grown on carbon cloth as an efficient 3D hydrogen evolution cathode, Electrochim. Acta, 2015, 153, 508–514 CrossRef CAS.
- F. Gautier, G. Krill, M. F. Lapierre and C. Robert, Influence of non-stoichiometry on the electrical and magnetic properties of NiS2, Solid State Commun., 1972, 11, 1201–1203 CrossRef CAS.
- A. Jamil, S. S. Batool, F. Sher and M. A. Rafiq, Determination of density of states, conduction mechanisms and dielectric properties of nickel disulfide nanoparticles, AIP Adv., 2016, 6, 055120 CrossRef.
- Z. Wan, C. Jia and Y. Wang, In situ growth of hierarchical NiS2 hollow microspheres as efficient counter electrode for dye-sensitized solar cell, Nanoscale, 2015, 7, 12737–12742 RSC.
- X. Zuo, S. Yan, B. Yang, G. Li, M. Wu, Y. Ma, S. Jin and K. Zhu, Hollow spherical NiS/NiS2, J. Mater. Sci.: Mater. Electron., 2016, 27, 7974 CrossRef CAS.
- X. Zuo, S. Yan, B. Yang, G. Li, H. Zhang, H. Tang, M. Wu, Y. Ma, S. Jin and K. Zhu, Template-free synthesis of nickel sulfides hollow spheres and their application in dye-sensitized solar cells, Sol. Energy, 2016, 132, 503–510 CrossRef CAS.
- J. Zheng, W. Zhou, Y. Ma, W. Cao, C. Wang and L. Guo, Facet-dependent NiS2 polyhedrons on counter electrodes for dye-sensitized solar cells, Chem. Commun., 2015, 51, 12863–12866 RSC.
- Z. Li, F. Gong, G. Zhou and Z. S. Wang, NiS2/reduced graphene oxide nanocomposites for efficient dye-sensitized solar cells, J. Phys. Chem. C, 2013, 117, 6561–6566 CAS.
- L. Zhu, B. J. Richardson and Q. Yu, Anisotropic growth of iron pyrite FeS2 nanocrystals via oriented attachment, Chem. Mater., 2015, 27, 3516–3525 CrossRef CAS.
- J. Puthussery, S. Seefeld, N. Berry, M. Gibbs and M. Law, Colloidal iron pyrite (FeS2) nanocrystal inks for thin-film photovoltaics, J. Am. Chem. Soc., 2010, 133, 716–719 CrossRef PubMed.
- Y. Bi, Y. Yuan, C. L. Exstrom, S. A. Darveau and J. Huang, Air stable, photosensitive, phase pure iron pyrite nanocrystal thin films for photovoltaic application, Nano Lett., 2011, 11, 4953–4957 CrossRef CAS PubMed.
- M. Cabaán-Acevedo, M. S. Faber, Y. Tan, R. J. Hamers and S. Jin, Synthesis and properties of semiconducting iron pyrite (FeS2) nanowires, Nano Lett., 2012, 12, 1977–1982 CrossRef PubMed.
- S. Shukla, G. Xing, H. Ge, R. R. Prabhakar, S. Mathew, Z. Su, V. Nalla, T. Venkatesan, N. Mathews, T. Sritharan and T. C. Sum, Origin of photocarrier losses in iron pyrite (FeS2) nanocubes, ACS Nano, 2016, 10, 4431–4440 CrossRef CAS PubMed.
- M. Barawi, I. J. Ferrer, E. Flores, S. Yoda, J. R. Ares and C. Sánchez, Hydrogen photoassisted generation by visible light and an earth abundant photocatalyst: pyrite (FeS2), J. Phys. Chem. C, 2016, 120, 9547–9552 CAS.
- I. G. Orletskii, P. D. Mar'yanchuk, E. V. Maistruk, M. N. Solovan and V. V. Brus, Low-temperature spray-pyrolysis of FeS2 films and their electrical and optical properties, Phys. Solid State, 2016, 58, 37–41 CrossRef CAS.
- F. Jiang, L. T. Peckler and A. J. Muscat, Phase pure pyrite FeS2 nanocubes synthesized using oleylamine as ligand, solvent, and reductant, Cryst. Growth Des., 2015, 15, 3565–3572 CAS.
- X. Zhang, T. Scott, T. Socha, D. Nielsen, M. Manno, M. Johnson, Y. Yan, Y. Losovyj, P. Dowben, E. S. Aydil and C. Leighton, Phase stability and stoichiometry in thin film iron pyrite: impact on electronic transport properties, ACS Appl. Mater. Interfaces, 2015, 7, 14130–14139 CAS.
- L. Xu, Y. Hu, H. Zhang, H. Jiang and C. Li, Confined synthesis of FeS2 nanoparticles encapsulated in carbon nanotube hybrids for ultrastable lithium-ion batteries, ACS Sustainable Chem. Eng., 2016, 4, 4251–4255 CrossRef CAS.
- S. Shukla, N. H. Loc, P. P. Boix, T. M. Koh, R. R. Prabhakar, H. K. Mulmudi, J. Zhang, S. Chen, C. F. Ng, C. H. A. Huan and N. Mathews, Iron pyrite thin film counter electrodes for dye-sensitized solar cells: high efficiency for iodine and cobalt redox electrolyte cells, ACS Nano, 2014, 8, 10597–10605 CrossRef CAS PubMed.
- Q. H. Huang, T. Ling, S. Z. Qiao and X. W. Du, Pyrite nanorod arrays as an efficient counter electrode for dye-sensitized solar cells, J. Mater. Chem. A, 2013, 1, 11828–11833 CAS.
- C. Song, S. Wang, W. Dong, X. Fang, J. Shao, J. Zhu and X. Pan, Hydrothermal synthesis of iron pyrite (FeS2) as efficient counter electrodes for dye-sensitized solar cells, Sol. Energy, 2016, 133, 429–436 CrossRef CAS.
- Y. C. Wang, D. Y. Wang, Y. T. Jiang, H. A. Chen, C. C. Chen, K. C. Ho, H. L. Chou and C. W. Chen, FeS2 Nanocrystal Ink as a Catalytic Electrode for Dye-Sensitized Solar Cells, Angew. Chem., Int. Ed., 2013, 52, 6694–6698 CrossRef CAS PubMed.
- B. Kilic, S. Turkdogan, A. Astam, O. C. Ozer, M. Asgin, H. Cebeci, D. Urk and S. P. Mucur, Preparation of Carbon Nanotube/TiO2 Mesoporous Hybrid Photoanode with Iron Pyrite (FeS2) Thin Films Counter Electrodes for Dye-Sensitized Solar Cell., Sci. Rep., 2016, 6, 27052 CrossRef CAS PubMed.
- S. Miyahara and T. Teranishi, Magnetic properties of FeS2 and CoS2, J. Appl. Phys., 1968, 39, 896–897 CrossRef CAS.
- N. Wu, Y. B. Losovyj, D. Wisbey, K. Belashchenko, M. Manno, L. Wang, C. Leighton and P. A. Dowben, The electronic band structure of CoS2, J. Phys.: Condens. Matter, 2007, 19, 156224 CrossRef.
- W. Fang, D. Liu, Q. Lu, X. Sun and A. M. Asiri, Nickel promoted cobalt disulfide nanowire array supported on carbon cloth: an efficient and stable bifunctional electrocatalyst for full water splitting, Electrochem. Commun., 2016, 63, 60–64 CrossRef CAS.
- Q. Wang, L. Jiao, Y. Han, H. Du, W. Peng, Q. Huan, D. Song, Y. Si, Y. Wang and H. Yuan, CoS2 hollow spheres: fabrication and their application in lithium-ion batteries, J. Phys. Chem. C, 2011, 115, 8300–8304 CAS.
- L. Yu, J. F. Yang and X. W. D. Lou, Formation of CoS2 nanobubble hollow prisms for highly reversible lithium storage, Angew. Chem., Int. Ed., 2016, 55, 13422–13426 CrossRef CAS PubMed.
- D. Zhang, H. Liu, J. Zhang, X. Wang, R. Zhang, J. Zhou, J. Zhong and B. Yuan, Synthesis of novel CoS2 nanodendrites with high performance supercapacitors, Int. J. Electrochem. Sci., 2016, 11, 6791–6798 CrossRef CAS.
- J. Jin, X. Zhang and T. He, Self-assembled CoS2 nanocrystal film as an efficient counter electrode for dye-sensitized solar cells, J. Phys. Chem. C, 2014, 118, 24877–24883 CAS.
- J. C. Tsai, M. H. Hon and I. C. Leu, Fabrication of mesoporous CoS2 nanotube arrays as the counter electrodes of dye-sensitized solar cells, Chem.–Asian J., 2015, 10, 1932–1939 CrossRef CAS PubMed.
- J. C. Tsai, M. H. Hon and C. Leu, Preparation of CoS2 nanoflake arrays through ion exchange reaction of Co(OH)2 and their application as counter electrodes for dye-sensitized solar cells, RSC Adv., 2015, 5, 4328–4333 RSC.
- M. Congiu, L. G. S. Albano, F. Decker and C. F. O. Graeff, Single precursor route to efficient cobalt sulphide counter electrodes for dye sensitized solar cells, Electrochim. Acta, 2015, 151, 517–524 CrossRef CAS.
- S. S. Rao, C. V. Gopi, S. K. Kim, M. K. Son, M. S. Jeong, A. D. Savariraj, K. Prabakar and H. J. Kim, Cobalt sulfide thin film as an efficient counter electrode for dye-sensitized solar cells, Electrochim. Acta, 2014, 133, 174–179 CrossRef.
- X. Cui, Z. Xie and Y. Wang, Novel CoS2 embedded carbon nanocages by direct sulfurizing metal–organic frameworks for dye-sensitized solar cells, Nanoscale, 2016, 8, 11984–11992 RSC.
- H. J. Kim, C. W. Kim, D. Punnoose, C. V. Gopi, S. K. Kim, K. Prabakar and S. S. Rao, Nickel doped cobalt sulfide as a high performance counter electrode for dye-sensitized solar cells, Appl. Surf. Sci., 2015, 328, 78–85 CrossRef CAS.
- X. Duan, Z. Gao, J. Chang, D. Wu, P. Ma, J. He, F. Xu, S. Gao and K. Jiang, CoS2–graphene composite as efficient catalytic counter electrode for dye-sensitized solar cell, Electrochim. Acta, 2013, 114, 173–179 CrossRef CAS.
- L. Sun, Y. Bai, N. Zhang and K. Sun, The facile preparation of a cobalt disulfide–reduced graphene oxide composite film as an efficient counter electrode for dye-sensitized solar cells, Chem. Commun., 2015, 51, 1846–1849 RSC.
- B. Hai, K. Tang, C. Wang, C. An, Q. Yang, G. Shen and Y. Qian, Synthesis of SnS2 nanocrystals via a solvothermal process, J. Cryst. Growth, 2001, 225, 92–95 CrossRef CAS.
- C. Zhai, N. Du and H. Z. D. Yang, Large-scale synthesis of ultrathin hexagonal tin disulfide nanosheets with highly reversible lithium storage, Chem. Commun., 2011, 47, 1270–1272 RSC.
- Y. T. Lin, J. B. Shi, Y. C. Chen, C. J. Chen and P. F. Wu, Synthesis and characterization of tin disulfide (SnS2) nanowires, Nanoscale Res. Lett., 2009, 4, 694 CrossRef CAS PubMed.
- J. Wang, J. Liu, H. Xu, S. Ji, J. Wang, Y. Zhou, P. Hodgson and Y. Li, Gram-scale and template-free synthesis of ultralong tin disulfide nanobelts and their lithium ion storage performances, J. Mater. Chem. A, 2013, 1, 1117–1122 CAS.
- Y. Huang, H. Zang, J. S. Chen, E. A. Sutter, P. W. Sutter, C. Y. Nam and M. Cotlet, Hybrid quantum dot-tin disulfide field-effect transistors with improved photocurrent and spectral responsivity, Appl. Phys. Lett., 2016, 108, 123502 CrossRef.
- A. Giberti, A. Gaiardo, B. Fabbri, S. Gherardi, V. Guidi, C. Malagù, P. Bellutti, G. Zonta, D. Casotti and G. Cruciani, Tin(IV) sulfide nanorods as a new gas sensing material, Sens. Actuators, B, 2016, 223, 827–833 CrossRef CAS.
- W. Du, D. Deng, Z. Han, W. Xiao, C. Bian and X. Qian, Hexagonal tin disulfide nanoplatelets: a new photocatalyst driven by solar light, CrystEngComm, 2011, 13, 2071–2076 RSC.
- N. T. N. Truong and C. Park, Synthesis and characterization of tin disulfide nanocrystals for hybrid bulk hetero-junction solar cell applications, Electron. Mater. Lett., 2016, 12, 308–314 CrossRef.
- Y. Bai, X. Zong, H. Yu, Z. G. Chen and L. Wang, Scalable low-cost SnS2 nanosheets as counter electrode building blocks for dye-sensitized solar cells, Chem.–Eur. J., 2014, 20, 8670–8676 CrossRef CAS PubMed.
- B. Yang, X. Zuo, P. Chen, L. Zhou, X. Yang, H. Zhang, G. Li, M. Wu, Y. Ma, S. Jin and X. Chen, Nanocomposite of tin sulfide nanoparticles with reduced graphene oxide in high-efficiency dye-sensitized solar cells, ACS Appl. Mater. Interfaces, 2015, 7, 137–143 CAS.
- S. Sugai and T. Ueda, High-pressure Raman spectroscopy in the layered materials 2H–MoS2, 2HMoTe2, and 2H–MoTe2, Phys. Rev. B: Condens. Matter Mater. Phys., 1982, 26, 6554 CrossRef CAS.
- T. Böker, R. Severin, A. Müller, C. Janowitz, R. Manzke, D. Voß, P. Krüger, A. Mazur and J. Pollmann, Band structure of MoS2, MoSe2, and α-MoTe2: angle-resolved photoelectron spectroscopy and ab initio calculations, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 235305 CrossRef.
- N. L. Heda, A. Dashora, A. Marwal, Y. Sharma, S. K. Srivastava, G. Ahmed, R. Jain and B. L. Ahuja, Electronic properties and Compton profiles of molybdenum dichalcogenides, J. Phys. Chem. Solids, 2010, 71, 187–193 CrossRef CAS.
- S. H. Rhim, Y. S. Kim and A. J. Freeman, Strain-induced giant second-harmonic generation in monolayered 2H–MoX2 (X = S, Se, Te), Appl. Phys. Lett., 2015, 107, 241908 CrossRef.
- S. Caramazza, C. Marini, L. Simonelli, P. Dore and P. Postorino, Temperature dependent EXAFS study on transition metal dichalcogenides MoX2 (X = S, Se, Te), J. Phys.: Condens. Matter, 2016, 28, 325401 CrossRef CAS PubMed.
- H. Jiang, Electronic band structures of molybdenum and tungsten dichalcogenides by the GW approach, J. Phys. Chem. C, 2012, 116, 7664–7671 CAS.
- U. Ahuja, R. Joshi, D. C. Kothari, H. Tiwari and K. Venugopalan, Optical response of mixed molybdenum dichalcogenides for solar cell applications using the modified becke–johnson potential, Z. Naturforsch., A: Phys. Sci., 2016, 71, 213–223 CAS.
- A. Abderrahmane, P. J. Ko, T. V. Thu, S. Ishizawa, T. Takamura and A. Sandhu, High photosensitivity few-layered MoSe2 back-gated field-effect phototransistors, Nanotechnology, 2014, 25, 365202 CrossRef CAS PubMed.
- H. J. Chuang, B. Chamlagain, M. Koehler, M. M. Perera, J. Yan, D. Mandrus, D. Tomaánek and Z. Zhou, Low-resistance 2D/2D ohmic contacts: a universal approach to high-performance WSe2, MoS2, and MoSe2 transistors, Nano Lett., 2016, 16, 1896–1902 CrossRef CAS PubMed.
- L. T. L. Lee, J. He, B. Wang, Y. Ma, K. Y. Wong, Q. Li, X. Xiao and T. Chen, Few-layer MoSe2 possessing high catalytic activity towards iodide/tri-iodide redox shuttles, Sci. Rep., 2014, 4, 4063–4069 CrossRef PubMed.
- H. Chen, Y. Xie, H. Cui, W. Zhao, X. Zhu, Y. Wang, X. Lü and F. Huang, In situ growth of a MoSe2/Mo counter electrode for high efficiency dye-sensitized solar cells, Chem. Commun., 2014, 50, 4475–4477 RSC.
- I. A. Ji, H. M. Choi and J. H. Bang, Metal selenide films as the counter electrode in dye-sensitized solar cell, Mater. Lett., 2014, 123, 51–54 CrossRef CAS.
- J. Jia, J. Wu, J. Dong, Y. Tu, Z. Lan, L. Fan and Y. Wei, High-performance molybdenum diselenide electrodes used in dye-sensitized solar cells and supercapacitors, IEEE J. Photovoltaics, 2016, 6, 1196–1202 CrossRef.
- E. Bi, H. Chen, X. Yang, F. Ye, M. Yin and L. Han, Fullerene-structured MoSe2 hollow spheres anchored on highly nitrogen-doped graphene as a conductive catalyst for photovoltaic applications, Sci. Rep., 2015, 5, 13214 CrossRef CAS PubMed.
- Y. J. Huang, M. S. Fan, C. T. Li, C. P. Lee, T. Y. Chen, R. Vittal and K. C. Ho, MoSe2 nanosheet/poly(3,4-ethylenedioxythiophene):poly (styrenesulfonate) composite film as a Pt-free counter electrode for dye-sensitized solar cells, Electrochim. Acta, 2016, 211, 794–803 CrossRef CAS.
- J. Dong, J. Wu, J. Jia, L. Hu and S. Dai, Cobalt/molybdenum ternary hybrid with hierarchical architecture used as high efficient counter electrode for dye-sensitized solar cells, Sol. Energy, 2015, 122, 326–333 CrossRef CAS.
- Q. Jiang and G. Hu, Co0.85Se hollow nanoparticles as Pt-free counter electrode materials for dye-sensitized solar cells, Mater. Lett., 2015, 153, 114–117 CrossRef CAS.
- Y. Duan, Q. Tang, J. Liu, B. He and L. Yu, Transparent metal selenide alloy counter electrodes for high-efficiency bifacial dye-sensitized solar cells, Angew. Chem., Int. Ed., 2014, 53, 14569–14574 CrossRef CAS PubMed.
- A. Banerjee, K. K. Upadhyay, S. Bhatnagar, M. Tathavadekar, U. Bansode, S. Agarkar and S. B. Ogale, Nickel cobalt sulfide nanoneedle array as an effective alternative to Pt as a counter electrode in dye sensitized solar cells, RSC Adv., 2014, 4, 8289–8294 RSC.
- D. Jerome, A. J. Grant and A. D. Yoffe, Pressure enhanced superconductivity in NbSe2, Solid State Commun., 1971, 9, 2183–2185 CrossRef CAS.
- D. H. Galvan, J. H. Kim, M. B. Maple, M. Avalos-Borja and E. Adem, Formation of NbSe2 nanotubes by electron irradiation, Fullerene Sci. Technol., 2000, 8, 143–151 CrossRef CAS.
- N. E. Staley, J. Wu, P. Eklund, Y. Liu, L. Li and Z. Xu, Electric field effect on superconductivity in atomically thin flakes of NbSe2, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 184505 CrossRef.
- V. V. Ivanovskaya, A. N. Enyashin, N. I. Medvedeva and A. L. Ivanovskii, Electronic properties of superconducting NbSe2 nanotubes, Phys. Status Solidi B, 2003, 238, R1–R4 CrossRef CAS.
- T. Tsuneta, T. Toshima, K. Inagaki, T. Shibayama, S. Tanda, S. Uji, M. Ahlskog, P. Hakonen and M. Paalanen, Formation of metallic NbSe2 nanotubes and nanofibers, Curr. Appl. Phys., 2003, 3, 473–476 CrossRef.
- E. Hitz, J. Wan, A. Patel, Y. Xu, L. Meshi, J. Dai, Y. Chen, A. Lu, A. V. Davydov and L. Hu, Electrochemical intercalation of lithium ions into NbSe2 nanosheets, ACS Appl. Mater. Interfaces, 2016, 8, 11390–11395 CAS.
- K. J. Reynolds, G. L. Frey and R. H. Friend, Solution-processed niobium diselenide as conductor and anode for polymer light-emitting diodes, Appl. Phys. Lett., 2003, 82, 1123–1125 CrossRef CAS.
- (a) M. Yoshida, J. Ye, T. Nishizaki, N. Kobayashi and Y. Iwasa, Electrostatic and electrochemical tuning of superconductivity in two-dimensional NbSe2 crystals, Appl. Phys. Lett., 2016, 108, 202602 CrossRef; (b) X. Zhu, Y. Guo, H. Cheng, J. Dai, X. An, J. Zhao, K. Tian, S. Wei, X. C. Zeng, C. Wu and Y. Xie, Signature of coexistence of superconductivity and ferromagnetism in two-dimensional NbSe2 triggered by surface molecular adsorption, Nat. Commun., 2016, 7, 11210 CrossRef CAS PubMed.
- D. W. Murphy, F. A. Trumbore and J. N. Carides, A new niobium selenide cathode for nonaqueous lithium batteries, J. Electrochem. Soc., 1977, 124, 325–329 CrossRef CAS.
- J. Guo, Y. Shi, C. Zhu, L. Wang, N. Wang and T. Ma, Cost-effective and morphology-controllable niobium diselenides for highly efficient counter electrodes of dye-sensitized solar cells, J. Mater. Chem. A, 2013, 1, 11874–11879 CAS.
- M. A. Ibrahem, W. C. Huang, T. W. Lan, K. M. Boopathi, Y. C. Hsiao, C. H. Chen, W. Budiawan, Y. Y. Chen, C. S. Chang, L. J. Li, C. H. Tsai and C. C. Chu, Controlled mechanical cleavage of bulk niobium diselenide to nanoscaled sheet, rod, and particle structures for Pt-free dye-sensitized solar cells, J. Mater. Chem. A, 2014, 2, 11382–11390 CAS.
- J. Guo, S. Liang, Y. Shi, C. Hao, X. Wang and T. Ma, Transition metal selenides as efficient counter-electrode materials for dye-sensitized solar cells, Phys. Chem. Chem. Phys., 2015, 17, 28985–28992 RSC.
- F. Gong, X. Xu, Z. Li, G. Zhou and Z. S. Wang, NiSe2 as an efficient electrocatalyst for a Pt-free counter electrode of dye-sensitized solar cells, Chem. Commun., 2013, 49, 1437–1439 RSC.
- X. Zhang, T. Z. Jing, S. Q. Guo, G. D. Gao and L. Liu, Synthesis of NiSe2/reduced graphene oxide crystalline materials and their efficient electrocatalytic activity in dye-sensitized solar cells, RSC Adv., 2014, 4, 50312–50317 RSC.
- J. Xiao, L. Wan, S. Yang, F. Xiao and S. Wang, Design hierarchical electrodes with highly conductive NiCo2S4 nanotube arrays grown on carbon fiber paper for high-performance pseudocapacitors, Nano Lett., 2014, 14, 831–838 CrossRef CAS PubMed.
- Q. Wang, B. Liu, X. Wang, S. Ran, L. Wang, D. Chen and G. Shen, Morphology evolution of urchin-like NiCo2O4 nanostructures and their applications as psuedocapacitors and photoelectrochemical cells, J. Mater. Chem., 2012, 22, 21647–21653 RSC.
- J. Huo, J. Wu, M. Zheng, Y. Tu and Z. Lan, Flower-like nickel cobalt sulfide microspheres modified with nickel sulfide as Pt-free counter electrode for dye-sensitized solar cells, J. Power Sources, 2016, 304, 266–272 CrossRef CAS.
- X. Qian, H. Li, L. Shao, X. Jiang and L. Hou, Morphology-tuned synthesis of nickel cobalt selenides as highly efficient Pt-free counter electrode catalysts for dye-sensitized solar cells, ACS Appl. Mater. Interfaces, 2016, 8, 29486–29495 CAS.
- R. G. Coleman, New occurrences of ferroselite (FeSe2), Geochim. Cosmochim. Acta, 1959, 16, 296–301 CrossRef CAS.
- H. D. Lutz and B. Müller, Lattice vibration spectra LXVIII. Single-crystal Raman spectra of marcasite-type iron chalcogenides and pnictides, FeX2 (X = S, Se, Te; P, As, Sb), Phys. Chem. Miner., 1991, 18, 265–268 CrossRef CAS.
- E. Bastola, K. P. Bhandari, A. J. Matthews, N. Shrestha and R. J. Ellingson, Elemental anion thermal injection synthesis of nanocrystalline marcasite iron dichalcogenide FeSe2 and FeTe2, RSC Adv., 2016, 6, 69708–69714 RSC.
- X. Mao, J. G. Kim, J. Han, H. S. Jung, S. G. Lee, N. A. Kotov and J. Lee, Phase-pure FeSex (x = 1, 2) nanoparticles with one-and two-photon luminescence, J. Am. Chem. Soc., 2014, 136, 7189–7192 CrossRef CAS PubMed.
- B. Yuan, W. Luan and S. T. Tu, One-step synthesis of cubic FeS2 and flower-like FeSe2 particles by a solvothermal reduction process, Dalton Trans., 2012, 41, 772–776 RSC.
- J. Xu, K. Jang, J. Lee, H. J. Kim, J. Jeong, J. G. Park and S. U. Son, Phase-selective growth of assembled FeSe2 nanorods from organometallic polymers and their surface magnetism, Cryst. Growth Des., 2011, 11, 2707–2710 CAS.
- W. Shi, X. Zhang, G. Che, W. Fan and C. Liu, Controlled hydrothermal synthesis and magnetic properties of three-dimensional FeSe2 rod clusters and microspheres, Chem. Eng. J., 2013, 215, 508–516 CrossRef.
- X. Chang, J. Jian, G. Cai, R. Wu and J. Li, Three-dimensional FeSe2 microflowers assembled by nanosheets: synthesis, optical properties, and catalytic activity for the hydrogen evolution reaction, Electron. Mater. Lett., 2016, 12, 237–242 CrossRef CAS.
- G. D. Park, J. H. Kim and Y. C. Kang, Large-scale production of spherical FeSe2–amorphous carbon composite powders as anode materials for sodium-ion batteries, Mater. Charact., 2016, 120, 349–356 CrossRef CAS.
- B. G. Ganga, C. Ganeshraj, A. G. Krishna and P. N. Santhosh, Electronic and optical properties of FeSe2 polymorphs: solar cell absorber, arXiv preprint arXiv:1303.1381, 2013.
- R. Jin, K. Zhao, X. Pu, M. Zhang, F. Cai, X. Yang, H. Kim and Y. Zhao, Structural and photovoltaic properties of FeSe2 films prepared by radio frequency magnetron sputtering, Mater. Lett., 2016, 179, 179–181 CrossRef CAS.
- D. Kong, J. J. Cha, H. Wang, H. R. Lee and Y. Cui, First-row transition metal dichalcogenide catalysts for hydrogen evolution reaction, Energy Environ. Sci., 2013, 6, 3553–3558 CAS.
- J. Yang, G. H. Cheng, J. H. Zeng, S. H. Yu, X. M. Liu and Y. T. Qian, Shape control and characterization of transition metal diselenides MSe2 (M = Ni, Co, Fe) prepared by a solvothermal-reduction process, Chem. Mater., 2001, 13, 848–853 CrossRef CAS.
- X. Chen and R. Fan, Low-temperature hydrothermal synthesis of transition metal dichalcogenides, Chem. Mater., 2001, 13, 802–805 CrossRef CAS.
- S. Huang, Q. He, W. Chen, Q. Qiao, J. Zai and X. Qian, Ultrathin FeSe2 nanosheets: controlled synthesis and application as a heterogeneous catalyst in dye-sensitized solar cells, Chem.–Eur. J., 2015, 21, 4085–4091 CrossRef CAS PubMed.
- S. Huang, Q. He, W. Chen, J. Zai, Q. Qiao and X. Qian, 3D hierarchical FeSe2 microspheres: controlled synthesis and applications in dye-sensitized solar cells, Nano Energy, 2015, 15, 205–215 CrossRef CAS.
- W. Wang, X. Pan, W. Liu, B. Zhang, H. Chen, X. Fang, J. Yao and S. Dai, FeSe2 films with controllable morphologies as efficient counter electrodes for dye-sensitized solar cells, Chem. Commun., 2014, 50, 2618–2620 RSC.
- D. Kong, H. Wang, Z. Lu and Y. Cui, CoSe2 nanoparticles grown on carbon fiber paper: an efficient and stable electrocatalyst for hydrogen evolution reaction, J. Am. Chem. Soc., 2014, 136, 4897–4900 CrossRef CAS PubMed.
- Y. Liu, H. Cheng, M. Lyu, S. Fan, Q. Liu, W. Zhang, Y. Zhi, C. Wang, C. Xiao, S. Wei and B. Ye, Low overpotential in vacancy-rich ultrathin CoSe2 nanosheets for water oxidation, J. Am. Chem. Soc., 2014, 136, 15670–15675 CrossRef CAS PubMed.
- R. Wu, Y. Xue, B. Liu, K. Zhou, J. Wei and S. H. Chan, Cobalt diselenide nanoparticles embedded within porous carbon polyhedra as advanced electrocatalyst for oxygen reduction reaction, J. Power Sources, 2016, 330, 132–139 CrossRef CAS.
- C. L. McCarthy, C. A. Downes, E. C. Schueller, K. Abuyen and R. L. Brutchey, Method for the solution deposition of phase-pure CoSe2 as an efficient hydrogen evolution reaction electrocatalyst, ACS Energy Lett., 2016, 1, 607–611 CrossRef CAS.
- J. Dong, J. Wu, J. Jia, S. Wu, P. Zhou, Y. Tu and Z. Lan, Cobalt selenide nanorods used as a high efficient counter electrode for dye-sensitized solar cells, Electrochim. Acta, 2015, 168, 69–75 CrossRef CAS.
- I. T. Chiu, C. T. Li, C. P. Lee, P. Y. Chen, Y. H. Tseng, R. Vittal and K. C. Ho, Nanoclimbing-wall-like CoSe2/carbon composite film for the counter electrode of a highly efficient dye-sensitized solar cell: a study on the morphology control, Nano Energy, 2016, 22, 594–606 CrossRef CAS.
- W. Y. Choi, Synthesis of CoSe2/RGO composites and their application in a counter electrode of dye-sensitized solar cells, Master thesis, Department of Chemical Engineering, Graduate school of Ulsan National Institute of Science and Technology (UNIST), South Korea, 2015, http://scholarworks.unist.ac.kr/handle/201301/10604.
- F. Gong, H. Wang, X. Xu, G. Zhou and Z. S. Wang, In situ growth of Co0.85Se and Ni0.85Se on conductive substrates as high-performance counter electrodes for dye-sensitized solar cells, J. Am. Chem. Soc., 2012, 134, 10953–10958 CrossRef CAS PubMed.
- H. Zhang, C. X. Liu, X. L. Qi, X. Dai, Z. Fang and S. C. Zhang, Topological insulators in Bi2Se3, Bi2Te3 and Sb2Te3 with a single Dirac cone on the surface, Nat. Phys., 2009, 5, 438–442 CrossRef CAS.
- P. A. Sharma, A. L. Sharma, M. Hekmaty, K. Hattar, V. Stavila, R. Goeke, K. Erickson, D. L. Medlin, M. Brahlek, N. Koirala and S. Oh, Ion beam modification of topological insulator bismuth selenide, Appl. Phys. Lett., 2014, 105, 242106 CrossRef.
- Y. Sun, H. Cheng, S. Gao, Q. Liu, Z. Sun, C. Xiao, C. Wu, S. Wei and Y. Xie, Atomically thick bismuth selenide freestanding single layers achieving enhanced thermoelectric energy harvesting, J. Am. Chem. Soc., 2012, 134, 20294–20297 CrossRef CAS PubMed.
- S. K. Batabyal, C. Basu, A. R. Das and G. S. Sanyal, Solvothermal synthesis of bismuth selenide nanotubes, Mater. Lett., 2006, 60, 2582–2585 CrossRef CAS.
- H. Tang, X. Wang, Y. Xiong, Y. Zhao, Y. Zhang, Y. Zhang, J. Yang and D. Xu, Thermoelectric characterization of individual bismuth selenide topological insulator nanoribbons, Nanoscale, 2015, 7, 6683–6690 RSC.
- Y. Zou, Z. G. Chen, Y. Huang, L. Yang, J. Drennan and J. Zou, Anisotropic electrical properties from vapor–solid–solid grown Bi2Se3 nanoribbons and nanowires, J. Phys. Chem. C, 2014, 118, 20620–20626 CAS.
- H. Zhu, E. Zhao, C. A. Richter and Q. Li, Topological insulator Bi2Se3 nanowire field effect transistors, ECS Trans., 2014, 64, 51–59 CrossRef CAS.
- Y. Fan, G. Hao, S. Luo, X. Qi and J. Zhong, Photoresponse properties of Bi2Se3 nanoplates, Sci. Adv. Mater., 2015, 7, 1589–1593 CrossRef CAS.
- X. Zhang, F. Wen, J. Xiang, X. Wang, L. Wang, W. Hu and Z. Liu, Wearable non-volatile memory devices based on topological insulator Bi2Se3/Pt fibers, Appl. Phys. Lett., 2015, 107, 103109 CrossRef.
- Z. Yuan, Z. Wu, S. Bai, W. Cui, J. Liu, T. Song and B. Sun, Layered bismuth selenide utilized as hole transporting layer for highly stable organic photovoltaics, Org. Electron., 2015, 26, 327–333 CrossRef CAS.
- Z. Li, Y. Hu, K. A. Howard, T. Jiang, X. Fan, Z. Miao, Y. Sun, F. Besenbacher and M. Yu, Multifunctional bismuth selenide nanocomposites for antitumor thermo-chemotherapy and imaging, ACS Nano, 2015, 10, 984–997 CrossRef PubMed.
- Z. Li, J. Liu, Y. Hu, K. A. Howard, Z. Li, X. Fan, M. Chang, Y. Sun, F. Besenbacher, C. Chen and M. Yu, Multimodal imaging-guided antitumor photothermal therapy and drug delivery using bismuth selenide spherical-sponge, ACS Nano, 2016, 10, 9646–9658 CrossRef CAS PubMed.
- L. Zhu, K. Y. Cho and W. C. Oh, Microwave-assisted synthesis of Bi2Se3/reduced graphene oxide nanocomposite as efficient catalytic counter electrode for dye-sensitized solar cell, Fullerenes, Nanotubes, Carbon Nanostruct., 2016, 24, 622–629 CrossRef CAS.
- A. L. Prieto, M. S. Sander, M. S. Martín-González, R. Gronsky, T. Sands and A. M. Stacy, Electrodeposition of ordered Bi2Te3 nanowire arrays, J. Am. Chem. Soc., 2001, 123, 7160–7161 CrossRef CAS PubMed.
- L. Ma, Q. Zhang, Q. Zhao, Z. Li, C. Ji and X. J. Xu, Synthesis and characterization of Bi2Te3/Te superlattice nanowire arrays, J. Nanosci. Nanotechnol., 2016, 16, 1207–1210 CrossRef CAS PubMed.
- J. Lee, J. Kim, W. Moon, A. Berger and J. Lee, Enhanced seebeck coefficients of thermoelectric Bi2Te3 nanowires as a result of an optimized annealing process, J. Phys. Chem. C, 2012, 116, 19512–19516 CAS.
- J. Gooth, R. Zierold, P. Sergelius, B. Hamdou, J. Garcia, C. Damm, B. Rellinghaus, H. J. Pettersson, A. Pertsova, C. Canali and M. Borg, Local magnetic suppression of topological surface states in Bi2Te3 nanowires, ACS Nano, 2016, 10, 7180–7188 CrossRef CAS PubMed.
- R. Du, H. C. Hsu, A. C. Balram, Y. Yin, S. Dong, W. Dai, W. Zhao, D. Kim, S. Y. Yu, J. Wang and X. Li, Robustness of topological surface states against strong disorder observed in Bi2Te3 nanotubes, Phys. Rev. B, 2016, 93, 195402 CrossRef.
- J. S. Son, M. K. Choi, M. K. Han, K. Park, J. Y. Kim, S. J. Lim, M. Oh, Y. Kuk, C. Park, S. J. Kim and T. Hyeon, n-Type nanostructured thermoelectric materials prepared from chemically synthesized ultrathin Bi2Te3 nanoplates, Nano Lett., 2012, 12, 640–647 CrossRef CAS PubMed.
- H. W. Tsai, T. H. Wang, T. C. Chan, P. J. Chen, C. C. Chung, A. Yaghoubi, C. N. Liao, E. W. G. Diau and Y. L. Chueh, Fabrication of large-scale single-crystal bismuth telluride (Bi2Te3) nanosheet arrays by a single-step electrolysis process, Nanoscale, 2014, 6, 7780–7785 RSC.
- L. Miao, J. Yi, Q. Wang, D. Feng, H. He, S. Lu, C. Zhao, H. Zhang and S. Wen, Broadband third order nonlinear optical responses of bismuth telluride nanosheets, Opt. Mater. Express, 2016, 6, 2244–2251 CrossRef.
- N. W. Park, J. Y. Ahn, A. Umar, S. G. Yoon and S. K. Lee, Thermoelectric properties of n-type bismuth telluride (Bi2Te3) thin films prepared by RF sputtering, Sci. Adv. Mater., 2016, 8, 1172–1176 CrossRef CAS.
- Y. Dou, F. Wu, L. Fang, G. Liu, C. Mao, K. Wan and M. Zhou, Enhanced performance of dye-sensitized solar cell using Bi2Te3 nanotube/ZnO nanoparticle composite photoanode by the synergistic effect of photovoltaic and thermoelectric conversion, J. Power Sources, 2016, 307, 181–189 CrossRef CAS.
- K. Wan, F. Wu, Y. Dou, L. Fang and C. Mao, Enhance the performance of dye-sensitized solar cells by Bi2Te3 nanosheet/ZnO nanoparticle composite photoanode, J. Alloys Compd., 2016, 680, 373–380 CrossRef CAS.
- T. Chen, G. H. Guai, C. Gong, W. Hu, J. Zhu, H. Yang, Q. Yan and C. M. Li, Thermoelectric Bi2Te3-improved charge collection for high-performance dye-sensitized solar cells, Energy Environ. Sci., 2012, 5, 6294–6298 CAS.
- L. Hu, L. Fang, F. Wu and J. Wang, Effect of the size of Bi2Te3 nanoplates on the performance of TiO2-based novel hybrid DSSC combined PV and TE effect, in MRS Proceedings (Vol. 1640, mrsf13–1640), Cambridge University Press, 2014 Search PubMed.
- A. Hinsch, J. M. Kroon, R. Kern, I. Uhlendorf, J. Holzbock, A. Meyer and J. Ferber, Long-term stability of dye-sensitised solar cells, Prog. Photovoltaics, 2001, 9, 425–438 CAS.
- H. Pettersson and T. Gruszecki, Long-term stability of low-power dye-sensitised solar cells prepared by industrial methods, Sol. Energy Mater. Sol. Cells, 2001, 70, 203–212 CrossRef CAS.
- P. M. Sommeling, M. Späth, H. J. P. Smit, N. J. Bakker and J. M. Kroon, Long-term stability testing of dye-sensitized solar cells, J. Photochem. Photobiol., A, 2004, 164, 137–144 CrossRef CAS.
- M. Grätzel, Photovoltaic performance and long-term stability of dye-sensitized meosocopic solar cells, C. R. Chim., 2006, 9, 578–583 CrossRef.
- S. Dai, J. Weng, Y. Sui, S. Chen, S. Xiao, Y. Huang, F. Kong, X. Pan, L. Hu, C. Zhang and K. Wang, The design and outdoor application of dye-sensitized solar cells, Inorg. Chim. Acta, 2008, 361, 786–791 CrossRef CAS.
- Y. Takeda, N. Kato, K. Higuchi, A. Takeichi, T. Motohiro, S. Fukumoto, T. Sano and T. Toyoda, Monolithically series-interconnected transparent modules of dye-sensitized solar cells, Sol. Energy Mater. Sol. Cells, 2009, 93, 808–811 CrossRef CAS.
- M. I. Asghar, K. Miettunen, J. Halme, P. Vahermaa, M. Toivola, K. Aitola and P. Lund, Review of stability for advanced dye solar cells, Energy Environ. Sci., 2010, 3, 418–426 CAS.
- A. G. Kontos, T. Stergiopoulos, V. Likodimos, D. Milliken, H. Desilvesto, G. Tulloch and P. Falaras, Long-term thermal stability of liquid dye solar cells, J. Phys. Chem. C, 2013, 117, 8636–8646 CAS.
- R. Jiang, A. Anderson, P. R. Barnes, L. Xiaoe, C. Law and B. C. O'Regan, 2000 hours photostability testing of dye sensitised solar cells using a cobalt bipyridine electrolyte, J. Mater. Chem. A, 2014, 2, 4751–4757 CAS.
- F. Sauvage, A review on current status of stability and knowledge on liquid electrolyte-based dye-sensitized solar cells, Adv. Chem., 2014, 939525, DOI:/10.1155/2014/939525.
- S. Yun, P. D. Lund and A. Hinsch, Stability assessment of alternative platinum free counter electrodes for dye-sensitized solar cells, Energy Environ. Sci., 2015, 8, 3495–3514 CAS.
- C. J. Liu, S. Y. Tai, S. W. Chou, Y. C. Yu, K. D. Chang, S. Wang, F. S. S. Chien, J. Y. Lin and T. W. Lin, Facile synthesis of MoS2/graphene nanocomposite with high catalytic activity toward triiodide reduction in dye-sensitized solar cells, J. Mater. Chem., 2015, 22, 21057–21064 RSC.
- H. Sun, L. Zhang and Z. S. Wang, Single-crystal CoSe2 nanorods as an efficient electrocatalyst for dye-sensitized solar cells, J. Mater. Chem. A, 2014, 2, 16023–16029 CAS.
- N. Kato, Y. Takeda, K. Higuchi, A. Takeichi, E. Sudo, H. Tanaka, T. Motohiro, T. Sano and T. Toyoda, Degradation analysis of dye-sensitized solar cell module after long-term stability test under outdoor working condition, Sol. Energy Mater. Sol. Cells, 2009, 93, 893–897 CrossRef CAS.
- H. Matsui, K. Okada, T. Kitamura and N. Tanabe, Thermal stability of dye-sensitized solar cells with current collecting grid, Sol. Energy Mater. Sol. Cells, 2009, 93, 1110–1115 CrossRef CAS.
- G. Xue, Y. Guo, T. Yu, J. Guan, X. Yu, J. Zhang, J. Liu and Z. Zou, Degradation mechanisms investigation for long-term thermal stability of dye-sensitized solar cells, Int. J. Electrochem. Sci., 2012, 7, 1496–1511 CAS.
- R. Harikisun and H. Desilvestro, Long-term stability of dye solar cells, Sol. Energy, 2011, 85, 1179–1188 CrossRef CAS.
- Y. Wu, W. H. Zhu, S. M. Zakeeruddin and M. Grätzel, Insight into D–A–π–A structured sensitizers: a promising route to highly efficient and stable dye-sensitized solar sells, ACS Appl. Mater. Interfaces, 2015, 7, 9307–9318 CAS.
- R. Katoh, A. Furube, S. Mori, M. Miyashita, K. Sunahara, N. Koumura and K. Hara, Highly stable sensitizer dyes for dye-sensitized solar cells: role of the oligothiophene moiety, Energy Environ. Sci., 2009, 2, 542–546 CAS.
- D. Joly, L. Pellejà, S. Narbey, F. Oswald, J. Chiron, J. N. Clifford, E. Palomares and R. Demadrille, A robust organic dye for dye sensitized solar cells based on iodine/iodide electrolytes combining high efficiency and outstanding stability, Sci. Rep., 2014, 4, 4033 CrossRef CAS PubMed.
- Z. S. Wang, Y. Cui, K. Hara, Y. Dan-oh, C. Kasada and A. Shinpo, A high-light-harvesting-efficiency coumarin dye for stable dye-sensitized solar cells, Adv. Mater., 2007, 19, 1138–1141 CrossRef CAS.
- S. Kim, D. Kim, H. Choi, M. S. Kang, K. Song, S. O. Kang and J. Ko, Enhanced photovoltaic performance and long-term stability of quasi-solid-state dye-sensitized solar cells via molecular engineering, Chem. Commun., 2008, 4951–4953 RSC.
- X. Lu, Q. Feng, T. Lan, G. Zhou and Z. S. Wang, Molecular engineering of quinoxaline-based organic sensitizers for highly efficient and stable dye-sensitized solar cells, Chem. Mater., 2012, 24, 3179–3187 CrossRef CAS.
- S. Yanagida, Y. Yu and K. Manseki, Iodine/iodide-free dye-sensitized solar cells, Acc. Chem. Res., 2009, 42, 1827–1838 CrossRef CAS PubMed.
- H. Tian and L. Sun, Iodine-free redox couples for dye-sensitized solar cells, J. Mater. Chem., 2011, 21, 10592–10601 RSC.
- M. Wang, C. Grätzel, S. M. Zakeeruddin and M. Grätzel, Recent developments in redox electrolytes for dye-sensitized solar cells, Energy Environ. Sci., 2012, 5, 9394–9405 CAS.
- S. Ahmad, T. Bessho, F. Kessler, E. Baranoff, J. Frey, C. Yi, M. Grätzel and M. K. Nazeeruddin, A new generation of platinum and iodine free efficient dye-sensitized solar cells, Phys. Chem. Chem. Phys., 2012, 14, 10631–10639 RSC.
- J. Cong, X. Yang, L. Kloo and L. Sun, Iodine/iodide-free redox shuttles for liquid electrolyte-based dye-sensitized solar cells, Energy Environ. Sci., 2012, 5, 9180–9194 CAS.
- F. Sauvage, S. Chhor, A. Marchioro, J. E. Moser and M. Grätzel, Butyronitrile-based electrolyte for dye-sensitized solar cells, J. Am. Chem. Soc., 2011, 133, 13103–13109 CrossRef CAS PubMed.
- J. Yoon, D. Kang, J. Won, J. Y. Park and Y. S. Kang, Dye-sensitized solar cells using ion-gel electrolytes for long-term stability, J. Power Sources, 2012, 201, 395–401 CrossRef CAS.
- K. M. Lee, W. H. Chiu, M. D. Lu and W. F. Hsieh, Improvement on the long-term stability of flexible plastic dye-sensitized solar cells, J. Power Sources, 2011, 196, 8897–8903 CrossRef CAS.
- Y. Yang, C. H. Zhou, S. Xu, H. Hu, B. L. Chen, J. Zhang, S. J. Wu, W. Liu and X. Z. Zhao, Improved stability of quasi-solid-state dye-sensitized solar cell based on poly (ethylene oxide)–poly(vinylidene fluoride) polymer-blend electrolytes, J. Power Sources, 2008, 185, 1492–1498 CrossRef CAS.
- Z. Chen, H. Yang, X. Li, F. Li, T. Yi and C. Huang, Thermostable succinonitrile-based gel electrolyte for efficient, long-life dye-sensitized solar cells, J. Mater. Chem., 2007, 17, 1602–1607 RSC.
- K. T. Dembele, R. Nechache, L. Nikolova, A. Vomiero, C. Santato, S. Licoccia and F. Rosei, Effect of multi-walled carbon nanotubes on the stability of dye sensitized solar cells, J. Power Sources, 2013, 233, 93–97 CrossRef CAS.
- S. R. Kim, M. K. Parvez, I. In, H. Y. Lee and J. M. Park, Novel photo-crosslinkable polymeric electrolyte system based on poly(ethylene glycol) and trimethylolpropane triacrylate for dye-sensitized solar cell with long-term stability, Electrochim. Acta, 2009, 54, 6306–6311 CrossRef CAS.
- F. Sauvage, M. K. Fischer, A. Mishra, S. M. Zakeeruddin, M. K. Nazeeruddin, P. Bäuerle and M. Grätzel, A dendritic oligothiophene ruthenium sensitizer for stable dye-sensitized solar cells, ChemSusChem, 2009, 2, 761–768 CrossRef CAS PubMed.
- M. K. Parvez, I. In, J. M. Park, S. H. Lee and S. R. Kim, Long-term stable dye-sensitized solar cells based on UV photo-crosslinkable poly(ethylene glycol) and poly(ethylene glycol) diacrylate based electrolytes, Sol. Energy Mater. Sol. Cells, 2011, 95, 318–322 CrossRef CAS.
- F. Bella, D. Pugliese, J. R. Nair, A. Sacco, S. Bianco, C. Gerbaldi, C. Barolo and R. Bongiovanni, A UV-crosslinked polymer electrolyte membrane for quasi-solid dye-sensitized solar cells with excellent efficiency and durability, Phys. Chem. Chem. Phys., 2013, 15, 3706–3711 RSC.
- F. Bella, G. Griffini, M. Gerosa, S. Turri and R. Bongiovanni, Performance and stability improvements for dye-sensitized solar cells in the presence of luminescent coatings, J. Power Sources, 2015, 283, 195–203 CrossRef CAS.
- S. Singh and H. S. Nalwa, Nanotechnology and health safety–toxicity and risk assessments of nanostructured materials on human health, J. Nanosci. Nanotechnol., 2007, 7, 3048–3070 CrossRef CAS PubMed.
- Nanotoxicology-Interactions of Nanomaterials with Biological Systems, ed. Y. L. Zhao and H. S. Nalwa, American Scientific Publishers, Los Angeles, 2007 Search PubMed.
- X. Hu and Q. Zhou, Health and Ecosystem Risks of Graphene, Chem. Rev., 2013, 113, 3815–3835 CrossRef CAS PubMed.
- E. L. K. Chng and M. Pumera, Toxicity of graphene related materials and transition metal dichalcogenides, RSC Adv., 2015, 5, 3074–3080 RSC.
- J. Hao, G. Song, T. Liu, X. Yi, K. Yang, L. Cheng and Z. Liu, In vivo long-term biodistribution, excretion, and toxicology of PEGylated transition-metal dichalcogenides MS2 (M = Mo, W, Ti) nanosheets, Adv. Sci., 2017, 4, 1600160, DOI:10.1002/advs.201600160.
- W. Z. Teo, E. L. K. Chng, Z. Sofer and M. Pumera, Cytotoxicity of exfoliated transition-metal dichalcogenides (MoS2, WS2, and WSe2) is lower than that of graphene and its analogues, Chem.–Eur. J., 2014, 20, 9627–9632 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |