Analytical potential of rf-PGD-TOFMS for depth profiling of an oxidized thin film composite
C.
González-Gago
a,
J.
Pisonero
a,
R.
Sandín
b,
J. F.
Fuertes
a,
A.
Sanz-Medel
c and
N.
Bordel
*a
aDepartment of Physics, University of Oviedo, Campus de Mieres, c/Gonzalo Gutierrez Quirós, 33600 Mieres, Spain. E-mail: bordel@uniovi.es
bR&D Department, Acciona Agua, S.A.U., Barcelona, Spain
cDepartment of Physical and Analytical Chemistry, University of Oviedo, c/Julian Clavería 8, 33006 Oviedo, Spain
First published on 14th May 2015
Abstract
The capabilities of radiofrequency pulsed glow discharge time of flight mass spectrometry (rf-pulsed-GD-TOFMS) for the analysis of thin film composite membranes have been investigated in this work. These semi-permeable membranes, used for water purification and desalinization, consist of a thin film of polyamide on top of a polysulfone porous layer deposited on a support sheet of polyester. The analysed samples were four oxidized swatches obtained using NaClO or ClO2 (diluted in seawater) as oxidizing agents, and a non-oxidized virgin membrane. The performance of the rf-pulsed-GD-TOFMS prototype was investigated for the detection of both positive and negative analyte ions. Improved depth profiles could be achieved using an Ar pre-chamber that prevented the entry of air in the discharge. The detection of polyatomic ion signals was needed to monitor properly the transition between the subsequent sputtered membrane layers. In particular, NH3+ and 12C14N− polyatomic ions were mainly found at the polyamide layer (in the positive and negative detection modes, respectively), while 34S+ and 33S1H− ion signals appeared in the polysulfone layer. Qualitative depth profiles of the oxidized samples showed an intense Br ion signal in the polyamide layer. Moreover, the Br ion signal was significantly enhanced when NaClO was used as the oxidizing agent, in agreement with previous studies. It is considered that the incorporation of Br into the membranes is a consequence of its presence in seawater and of the oxidation process.
Introduction
Thin film composite (TFC) membranes are semi-permeable membranes used for water purification or desalinization.1 These membranes used in reverse osmosis (RO) are typically made of a thin film of polyamide on top of a polysulfone porous layer deposited on a support sheet.2 This configuration has the properties needed for the high rejection of undesired compounds (e.g. salts), high filtration rate and good mechanical strength. One of the main drawbacks of TFC membranes is their oxidation since it is an irreversible process, which leads to lower salt rejection. This oxidation is caused by substances commonly employed in water treatment such as sodium hypochlorite (NaClO) and chlorine dioxide (ClO2). RO membrane degradation by chlorine compounds involves polymer deformation (caused by N-chlorination and/or ring chlorination) followed by de-polymerization.3–5 Furthermore, the water matrix composition has a high influence on the oxidation processes. For instance, bromide ions in seawater in the presence of chlorine based oxidants could get converted to bromine. Therefore, bromine can take the role usually played by chlorine when brackish or feed water is employed and can contribute to the degradation of the polyamide membrane.6,7The Fujiwara test has been traditionally used to check polyamide membrane degradation by halogen exposure (e.g. a small quantity of a dye solution is dropped on the membrane surface, the dye will adhere to the support material that has been oxidized, and the damaged areas will appear as bright pink spots).8 Additionally, other techniques, such as ATR-FTIR (Attenuated Total Reflection-Fourier Transform Infrared) and XPS (X-ray photoelectron spectroscopy), have also been employed to determine the oxidation in RO membranes when NaClO acted as the oxidizing agent.5,9 Such studies have demonstrated that these methods are capable of detecting the membrane degradation process even before the Fujiwara test is employed.
In ATR-FTIR, the chemical degradation and structural modifications of the surface active layer can be detected by monitoring changes in the characteristic infrared bands of the polyamide. The ratio between the transmittance bands observed for the degraded membrane and the virgin one can be used as a degradation index. The higher this parameter, the greater is the chemical attack to the polyamide layer.7
The elemental composition and the chemical state of the elements present on the surface of a solid can be measured by XPS. Therefore, it is possible to determine if the halogens present in the oxidized membrane are attached to the polyamide structure evaluating the photoelectron binding energy.6 Previous XPS studies found that the binding energy of the photoelectron Cl(2p) measured in membranes oxidized by NaClO or ClO2 corresponds to chlorine bonded to C.10 Nevertheless, the main inconveniences of the XPS technique are the high instrumental cost, the high vacuum requirements and the long analysis time necessary for in-depth analysis (e.g. several hours for 100 nm). Therefore, the evaluation of alternative methods capable of providing detailed information about the degradation of the polyamide layer in shorter times is of great interest today. In this context, Glow Discharges (GDs) coupled to optical emission spectrometry (OES) or mass spectrometry (MS) are widely employed methods for direct solid chemical analysis. Moreover, the relatively soft and fast sputtering processes achieved by GD sources allow fast depth profile analysis with high depth resolution (∼nm).11,12 A large variety of applications have been developed using radiofrequency glow discharges (rf-GDs), including not just conducting samples but also non-conductive materials. In particular, the recent development of a new rf-pulsed-GD coupled to a time of flight mass spectrometer (TOFMS) has broadened the application fields of this technique, including the analysis of thin polymer films and the determination of anions from halogenated compounds.13–16
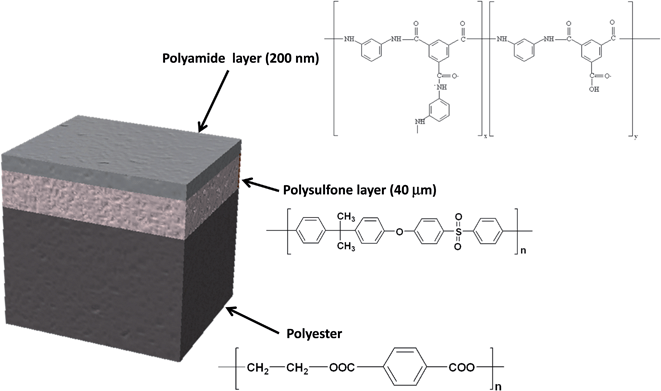
In this work, the analytical potential of rf-pulsed-GD-TOFMS is investigated for the development of a fast analytical method that allows the characterization of the oxidation degree of TFC membranes (see Fig. 1) that were immersed in seawater. In particular, depth profiling of anion and cation species in oxidized TFC membranes is used to evaluate the membrane degradation degree as a function of the employed oxidants for water treatments (e.g. NaClO and ClO2).
Experimental
Sample description
The samples used in this work were provided by Acciona Agua S.A. (Barcelona, Spain). The samples were obtained after treatment of a commercially available TFC membrane (SWHR-380, Dow Filmtec) that consists of a thin film of polyamide (200 nm) on top of a polysulfone porous layer (40 μm), which is deposited on a support sheet of polyester (120 μm). Fig. 1 shows the sample structure and the layer composition. The virgin membrane (sample 1) was immersed in a solution of sodium bromide (50 mg L−1) in Milli-Q water and then dried at room temperature. This non-oxidized sample was used as a reference for bromide ions adsorbed on the polyamide network. The oxidized TFC membranes (samples 2–5) were immersed in a solution of 50 mg L−1 of NaClO (Barcelonesa de Drogas) or ClO2 (generated using the standard method 4500-ClO2-B7,17 and its concentration determined by DPD, standard method 4500-ClO2-D17) in seawater for several hours, in order to achieve accelerated oxidation processes. Table 1 shows the oxidizing agent and the oxidation time for each sample and Table 2 shows the composition of the seawater solution employed for sample oxidation. Once the oxidation process was complete the membrane swatches were gently cleaned with Milli-Q water to stop the degradation process and to ensure that all oxidant species were removed. Further details about sample preparation are given elsewhere.7| Sample | Oxidant solution | Immersion time (hours) |
|---|---|---|
| 1 | — | — |
| 2 | ClONa | 1 |
| 3 | ClONa | 3 |
| 4 | ClO2 | 1 |
| 5 | ClO2 | 7 |
| Seawater composition | |||
|---|---|---|---|
| Ion | Concentration (mg L−1) | Ion | Concentration (mg L−1) |
| Sodium | 12 244 | Chloride | 21 204 |
| Magnesium | 1449 | Sulfate | 2890 |
| Calcium | 400 | Bromide | 72 |
Instrumentation
The thin film composite membranes were analyzed using a rf-pulsed-GD-TOFMS prototype developed in the frame of the European project EMDPA.18 The instrument has been described elsewhere.19,20 The GD ion source is a copper-based modified Grimm-type chamber (EMPA, Switzerland) with a 4 mm diameter anode and a 2.5 mm inner diameter flow tube to direct the gas flow towards the cathode surface. This ion source is mounted on a GD unit (GD Profiler HR instrument, Horiba Jobin Yvon, France) and coupled to a fast orthogonal time-of-flight mass spectrometer (Tofwerk, Switzerland). The rf-GD-TOFMS instrument can record the complete mass spectra with a frequency of up to 100 kHz, and has a mass resolving power of about 3000 (measured at m/z 208).Rf-power is supplied to the plasma through the backside of the sample by an rf-generator operating at 13.56 MHz. This generator can work either in continuous mode or in pulsed mode. However, due to the advantages of the pulsed mode,21 including reduced thermal effects, enhanced atomization, excitation and ionization by application of high short-term power, the experiments included in this work were carried out in the pulsed mode. After optimization a pulse width of 1 ms and a duty cycle of 0.25 were selected for the analysis of TFC membranes.
Constant pressure (200 Pa) and constant forward power (30 W) were used for all the measurements. High-purity argon (99.999% minimum purity) from Air Liquid (Oviedo, Spain) was employed as the discharge gas.
In addition, aiming at minimizing the effect of micro-leaks due to the porous nature of the samples, a sealed pre-chamber surrounding the sample was employed. Ar gas was continuously made to flow through this pre-chamber warranting an Ar atmosphere around the examined sample. A schematic showing the design of this pre-chamber can be seen elsewhere.22
Results and discussion
Thin film composite membranes are flexible and porous materials; therefore, it was necessary to fix them to metallic discs using a double face tape in order to achieve rigidity and to avoid sample deformation during the GD analysis at low pressure. Moreover, the samples were placed in a pre-chamber purged with a continuous flow of Ar to prevent the entry of air into the plasma region. Fig. 2a and b show the mass spectra in the range between m/z 13 and 17 obtained for the analysis of virgin TFC membranes by rf-pulsed-GD-TOFMS, without and with the Ar pre-chamber, respectively. As can be seen, the 16O+ ion signal was significantly reduced when the Ar pre-chamber was used. Similar results were observed for polyatomic ions containing oxygen. For instance, the (16O1H)+ ion signal was also significantly reduced allowing the determination of the (14N1H3)+ ion signal, which was used to identify the external polyamide layer. As a result of this study, the Ar pre-chamber was used in all the measurements.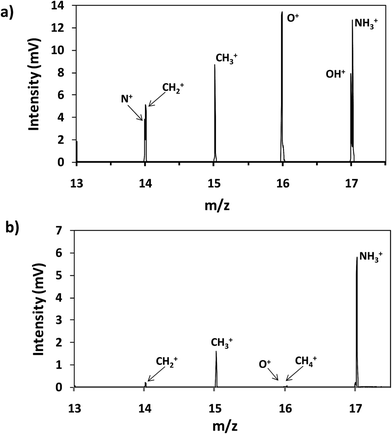 | ||
| Fig. 2 Mass spectrum of the virgin membrane (a) without an Ar pre-chamber and (b) using an Ar-pre-chamber. | ||
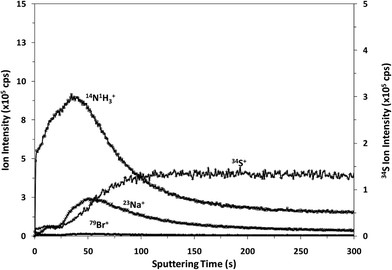
It is well known that rf-pulsed-GD-TOFMS provides a dynamic plasma with different regions (e.g. prepeak, plateau and afterglow). In this sense, Fig. 3 shows ion signal profiles of different analytes along the pulse period. As expected,21 analyte ion signals are significantly enhanced in the afterglow region, just when the GD power pulse finishes. Therefore, this latter integration region was selected to obtain the analytical signal intensities.
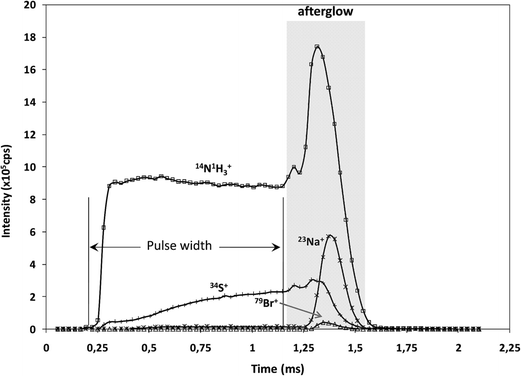 | ||
| Fig. 3 Pulse profiles of 14N1H3+, 23Na+, 34S+ and 79Br+ ion signals along the GD pulse period, obtained at the beginning of the polyamide layer. | ||
Qualitative depth profile of virgin membranes
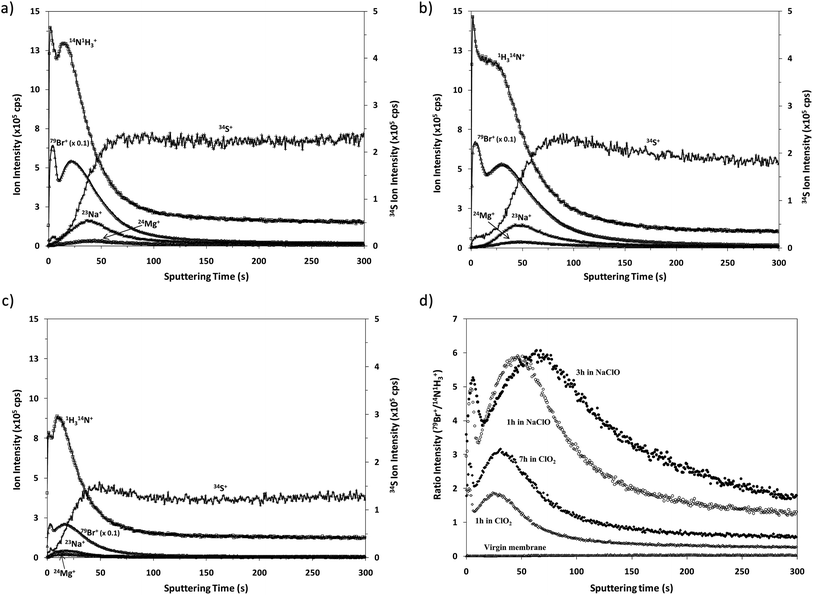
Fig. 4 shows the qualitative depth profile obtained for the virgin TFC membranes by rf-pulsed-GD-TOFMS under the optimized operating conditions. Since the virgin sample had been immersed in a 50 mg L−1 solution of NaBr, 23Na+ and 79Br+ ion signals were selected together with 14N1H3+ and 34S+. It is observed that there is no sharp interface between the polyamide layer (indicated by 14N1H3+) and the polysulfone layer (represented by 34S+). However, an intense 23Na+ ion signal was detected with its maximum just after the one detected for 14N1H3+, indicating that sodium is not adhered on the surface but absorbed in the polyamide layer. Conversely, the 79Br+ ion signal remains at low levels throughout the depth profile, suggesting that the immersion of the virgin membrane in the bromide solution does not seem to induce significant incorporation of this element into the membrane.Qualitative depth profile of oxidized membranes
Fig. 5 shows the qualitative depth profiles obtained for different oxidized samples using rf-pulsed-GD-TOFMS. Fig. 5a and b show the depth profiles of the samples that were immersed for 1 and 3 hours, respectively, in seawater containing NaClO; while Fig. 5c shows the depth profile of the sample that was immersed for 7 hours in seawater containing ClO2. It is observed that all the oxidized samples present depth profiles with an intense 79Br+ ion signal linked to the external polyamide layer. However, the Cl+ ion signal was not detected in spite of immersing the samples in NaClO or ClO2. The appearance of Br instead of Cl in the depth profiles is in agreement with the previous results obtained by R. Sandín et al.,7 using XPS. In that study membrane swatches were immersed in two different solutions containing NaClO as the oxidizing agent. In one of the solutions Milli-Q water was the solvent while seawater was used in the other one. XPS spectra showed a peak corresponding to Cl(2p) for the sample oxidized in the solution with Milli-Q water, but in the other case no peak for Cl was detected and an intense peak corresponding to Br(3d) was observed. These results showed that Br came from the seawater. Additionally, it was concluded that when the degradation process took place in Milli-Q water, chlorination occurred in the polyamide membrane; however, when seawater was used as the solvent, bromination took place due to the stronger halogenation effect of hypobromite in comparison to hypochlorite.23,24As already mentioned, the oxidation of these membranes is typically checked by the Fujiwara test, by dropping a small quantity of a dye solution on the membrane surface. A positive result is obtained if the oxidized material in contact with this dye solution brings about a bright pink colour. Interestingly, Fujiwara tests carried out for the membrane swatches exposed to seawater solutions containing NaClO or ClO2 provided positive results only after exposure times longer than 3 hours in NaClO and after 13 hours in ClO2.25 Conversely, the analysis by GD-TOFMS enabled the corresponding oxidation to be detected much sooner.
Comparative XPS analysis carried out for the virgin membrane and the oxidized samples showed that the longer the oxidation time the higher the observed Br content. Moreover, the Br concentration turned out to be higher for the samples oxidized with NaClO, using the same oxidation exposure time.25 Our results using rf-pulsed-GD-TOFMS also show the apparently faster Br incorporation into the membrane when the oxidation agent is NaClO. To properly compare the 79Br+ depth profiles obtained for the different samples, Fig. 5d shows the normalized depth profiles obtained by plotting the ratio between the intensities measured for 79Br+ and (14N1H3)+versus the sputtering time. These results show clearly the higher oxidation effect of NaClO as well as the increasing Br content in the polyamide layer when the samples are subjected to longer oxidation times. This effect is evident for the profiles of samples oxidized with ClO2 solution for 1 and 7 hours. On the other hand when using NaClO as the oxidizing agent the maximum value of the normalized Br intensity is similar for samples oxidized for one and three hours but it seems that Br penetrates deeper when the oxidation time increases. Although the obtained depth profiles have shown good reproducibility, other effects cannot be completely discarded, such as, for example, small changes in the sputtering rates, which could also influence the resulting qualitative depth profiles. Further analysis of samples oxidized for different times would be necessary to investigate in detail the observed relationship between the Br concentration and oxidation time.
Qualitative depth profile of TFC membranes in negative mode
Although chlorine was the element introduced as the oxidant, the main change observed in the membrane when oxidized was the presence of Br in the polyamide layer coming from the seawater. These two elements have high ionization potentials and therefore their detection as positive ions is not efficient in an Ar plasma (IPCl = 12.97 eV, IPBr = 11.81 eV, EmAr = 11.55 and 11.72 eV). Nevertheless, their detection in negative mode could be analytically useful. Consequently, the performance of the rf-pulsed-GD-TOFMS operating in negative mode for the analysis of the virgin and oxidized membranes was also investigated. To carry out such analyses, the glow discharge was operated under the same optimized experimental conditions (200 Pa, 30 W, 1 ms pulse width and 0.25 duty cycle), in the negative mode.Fig. 6 shows the mass spectra obtained for the virgin membrane in the polyamide layer using both detection modes (e.g. positive and negative) of the rf-pulsed-GD-TOFMS. It is observed that high ion signals of Cl− and Br− are detected in the negative mode, even in the virgin membrane. In the particular case of Cl−, this signal was detected not only in the mass spectrum of the polyamide layer but also when the polysulfone layer was sputtered. These results and previous evidence when analyzing other samples in negative mode indicated that Cl ions were coming from other parts of the GD source (e.g. background from o-rings or spacers used in the instrument). Moreover, it is noticed that different ions dominate the mass spectra in the negative mode when compared to the positive one. For instance, in positive mode the 14N1H3 ion signal was used to identify the polyamide layer. However, in negative mode at m/z = 17 only the presence of 16O1H is observed (the mass spectral resolution of the rf-pulsed-GD-TOFMS system is adequate to distinguish the 14N1H3 ion signal from the 16O1H ion signal (Fig. 2 and inset of Fig. 6)). Therefore, to identify the different layers of the TFC membranes in negative mode, 12C14N− was selected to monitor the polyamide, and 32S1H− was selected to monitor the polysulfone (see the inset of Fig. 6).
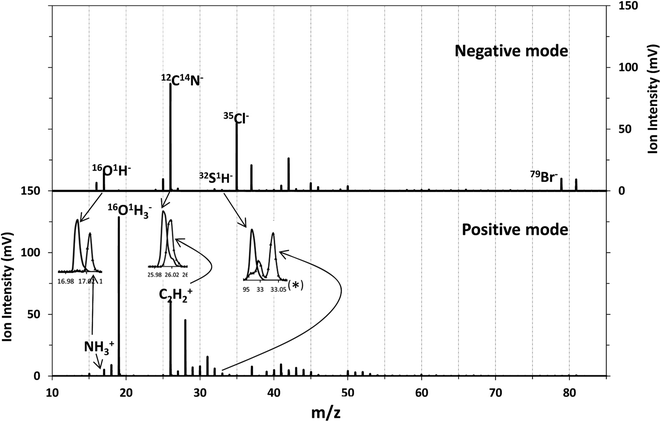 | ||
| Fig. 6 Mass spectrum of the virgin membrane in two detection modes: detection of negative ions (above) and positive ions (below). | ||
Fig. 7a shows the depth profile obtained in the negative mode for the virgin membrane. From this profile it can be observed that the in-depth distribution of the interface between the polyamide and the polysulfone is similar to that obtained in positive mode (see Fig. 4) and, as expected, it presents a low 79Br− intensity. Fig. 7b compares the “normalized” depth profiles of 79Br− (ratio between the intensities measured for 79Br− and (12C14N)−versus the sputtering time) measured for the virgin sample and for the different oxidized membranes. It is noticed that the Br− ion signal is significantly increased for the oxidized membranes and that higher intensities are again observed for the samples oxidized by NaClO. The results are in complete agreement with those obtained by the rf-pulsed-GD-TOFMS in positive mode (Fig. 5d): the Br content increases with the oxidation time, particularly when membrane oxidation took place in ClO2 solution, while longer immersion in NaClO solution gave rise to a deeper penetration of Br inside the polyamide.
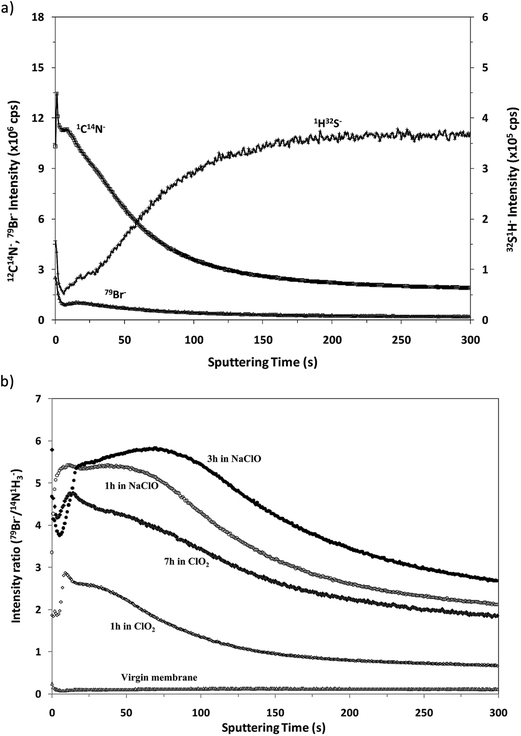 | ||
| Fig. 7 Qualitative depth profiles obtained in negative ion detection mode: (a) depth profile of the virgin membrane and (b) 79Br−/(12C14N)− depth profiles of all the samples under study. | ||
According to XPS analysis, Cl was present on the surface of the virgin membranes probably due to post-treatment that was used to change the hydraulic properties (i.e. permeate flux and salt rejection) of the membrane. Moreover, oxidized samples had similar Cl content independent of the oxidation time or oxidizing agent.25 Unfortunately, reliable information about the origin of Cl could not be extracted from the rf-pulsed-GD-TOFMS analysis due to its strong background (e.g. coming from o-rings or spacers used in the instrument). As a result, discerning if Cl measured in the analysis comes really from the membrane samples or from other contributions is difficult.
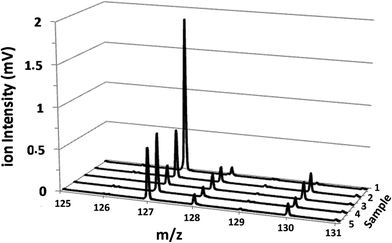
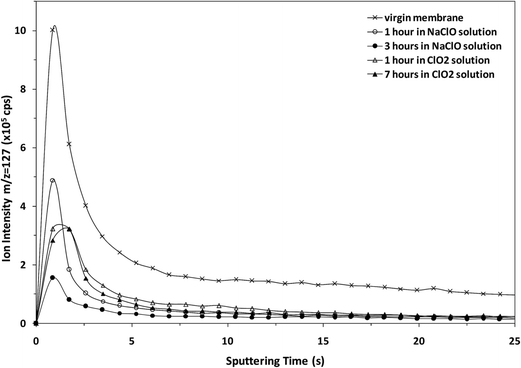
The potential of the rf-pulsed-GD-TOFMS to record the complete mass spectra (up to m/z = 250) at high repetition rates was used to detect the presence of relevant ion signals at high masses. For instance, Fig. 8 shows the mass spectra obtained at the surface of the membranes in the interval between m/z = 125 and m/z = 131 for all the samples. It is observed that, for the virgin membrane, there is a prominent ion signal at m/z = 127. This ion signal has not been assigned to any polyatomic ion because different combinations could be considered: it could correspond to a fragment sputtered from the polyamide layer but also could be the result of the recombination among smaller ion fragments present in the plasma. Interestingly from an analytical point of view, this m/z = 127 ion signal is not so prominent in the mass spectra of the oxidized samples (see Fig. 8). Fig. 9 shows the observed depth profile (e.g. first 25 s) of the m/z = 127 ion signal measured for the virgin sample and for the different oxidized membranes. The signal from m/z = 127 is only present during the first seconds of the analyses while the maximum of the polyamide layer is obtained at around 50 s in the depth profile. Thus, it seems that there is a very thin layer on the surface of the membrane that gets degraded by oxidation. Moreover, this degradation seems to be progressive with the oxidation time and also more effective when the oxidizing agent is NaClO instead of ClO2. The possible presence of a thin coating over the polyamide layer was reported and investigated by C. Y. Tang et al. who concluded that some commercial membranes contained an aliphatic coating layer rich in –COH groups over the polyamide layer.26 In addition, studies carried out by A. Antony et al. showed that this coating was eventually lost during the oxidation.9
Conclusions
This work has shown that rf-pulsed-TOFMS can be a powerful tool for the fast analysis of flexible and porous thin film composite membranes. In particular, appropriate operating conditions can be achieved for the analysis of these membranes using a suitable rigid support and an Ar pre-chamber that prevents the entry of air in the discharge.The positive and negative detection modes of the rf-pulsed-GD-TOFMS system can be used to provide adequate in-depth profiles of the TFC membranes (e.g. virgin and oxidized), showing that Br is incorporated into the whole polyamide layer and not only on the surface. Moreover, a higher content of Br is observed in the samples oxidized with the stronger oxidizing agent (e.g. NaClO versus ClO2).
In both positive and negative modes, it has been possible to confirm a correlation between the oxidation time and the normalized Br intensity in the profile. Moreover, the observed negative polyatomic ions at m/z = 127 indicate the existence of an external thin film that is degraded by oxidation processes. In the latter case, a negative correlation between the oxidation time and the intensity of the m/z = 127 ion signal is observed. This effect turned out to be more pronounced when the oxidizing agent was NaClO, the most powerful oxidant tested. That is, as indicated by Anthony et al.,9 such a thin film could be eventually destroyed after prolonged or stronger oxidation conditions.
Acknowledgements
Financial support from “Plan Nacional de I + D + I” through MAT2010-20921 and CTQ2013-49032-C2-2 R projects (Spanish Ministry of Science, Spanish Ministry of Economy and Competitiveness and Innovation); and through the FC-15-GRUPIN14-040 project (Principality of Asturias, “Plan de Ciencia, Tecnología e innovación 2013–2017 and Feder Program) is acknowledged. We also thank Horiba Jobin Yvon for the loan of the rf-pulsed-GD-TOFMS prototype.References
- K. P. Lee, T. C. Arnot and D. Mattia, A review of reverse osmosis membrane materials for desalination—Development to date and future potential, J. Membr. Sci., 2011, 370, 1–22 CrossRef CAS.
- W. J. Lau, A. F. Ismail, N. Misdan and M. A. Kassim, A recent progress in thin film composite membrane: a review, Desalination, 2012, 287, 190–199 CrossRef CAS.
- S. Avionitis, W. T. Hanbury and T. Hodgkiess, Chlorine degradation of aromatic polyamides, Desalination, 1992, 85, 321–324 CrossRef.
- R. Singh, Polyamide polymer solution behavior under chlorination conditions, J. Membr. Sci., 1994, 88, 285–287 CrossRef CAS.
- G. Kang, C. Gao, W. Chen, X. Jie, Y. Cao and Q. Yuan, Study on hypochlorite degradation of aromatic polyamide reverse osmosis membrane, J. Membr. Sci., 2007, 300, 165–171 CrossRef CAS.
- C. Fritzmann, J. Löwenberg, T. Wintgens and T. Melin, State-of-the-art of reverse osmosis desalination, Desalination, 2007, 216, 1–76 CrossRef CAS.
- R. Sandín, E. Ferrero, C. Repollés, S. Navea, J. Bacardit, J. P. Espinós and J. J. Malfeito, Reverse osmosis membranes oxidation by hypochlorite and chlorine dioxide: spectroscopic techniques vs. Fujiwara test, Desalin. Water Treat., 2013, 51, 318–327 CrossRef.
- T. Uno, K. Okumura and Y. Kuroda, Mechanism of the Fujiwara reaction: structural investigation of reaction products from benzotrichloride, J. Org. Chem., 1981, 46, 3175–3178 CrossRef CAS.
- A. Antony, R. Fudianto, S. Cox and G. Leslie, Assessing the oxidative degradation of polyamide reverse osmosis membrane—Accelerated ageing with hypochlorite exposure, J. Membr. Sci., 2010, 347, 159–164 CrossRef CAS.
- S. Beverly, S. Seal and S. Hong, Identification of surface chemical functional groups correlated to failure of reverse osmosis polymeric membranes, J. Vac. Sci. Technol., A, 2000, 18, 1107–1113 CAS.
- R. Escobar Galindo, R. Gago, A. Lousa and J. M. Albella, Comparative depth-profiling analysis of nanometer-metal multilayers by ion-probing techniques, Trends Anal. Chem., 2009, 28, 494–505 CrossRef CAS.
- J. Pisonero, B. Fernandez, R. Pereiro, N. Bordel and A. Sanz-Medel, Glow-discharge spectrometry for direct analysis of thin and ultra-thin solid films, Trends Anal. Chem., 2006, 25, 11–18 CrossRef CAS.
- N. Tuccitto, L. Lobo, A. Tempez, I. Delfanti, P. Chapon, S. Canulescu, N. Bordel, J. Michler and A. Licciardello, Pulsed radiofrequency glow discharge time-of-flight mass spectrometry for molecular depth profiling of polymer-based films, Rapid Commun. Mass Spectrom., 2009, 23, 549–556 CrossRef PubMed.
- L. Lobo, N. Tuccitto, N. Bordel, R. Pereiro, J. Pisonero, A. Licciardello, A. Tempez, P. Chapon and A. Sanz-Medel, Polymer screening by radiofrequency glow discharge time-of-flight mass spectrometry, Anal. Bioanal. Chem., 2010, 396, 2863–2869 CrossRef CAS PubMed.
- S. Canulescu, J. Whitby, K. Fuhrer, M. Hohl, M. Gonin, T. Horvath and J. Michler, Potential analytical applications of negative ions from a pulsed radiofrequency glow discharge in argon, J. Anal. At. Spectrom., 2009, 24, 178–180 RSC.
- C. González de Vega, L. Lobo, B. Fernandez, N. Bordel, R. Pereiro and A. Sanz-Medel, Pulsed glow discharge time of flight mass spectrometry for the screening of polymer-based coatings containing brominated flame retardants, J. Anal. At. Spectrom., 2012, 27, 318–326 RSC.
- D. Santos, Standard Methods for Drinking Water and Waste-water Analysis, Madrid, 1992 Search PubMed.
- Sixth Framework programme, European Union, Priority [3] (NMP – Nanotechnologies and nano-sciences, knowledge based multifunctional materials and new production processes and devices)- Contract STREP-NMP, n° 032202, 2006.
- A. C. Muñiz, J. Pisonero, L. Lobo, C. Gonzalez, N. Bordel, R. Pereiro, A. Tempez, P. Chapon, N. Tuccitto, A. Licciardello and A. Sanz-Medel, Pulsed radiofrequency glow discharge time of flight mass spectrometer for the direct analysis of bulk and thin coated glasses, J. Anal. At. Spectrom., 2008, 23, 1239–1246 RSC.
- M. Hohl, A. Kanzari, J. Michler, T. Nelis, K. Fuhrer and M. Gonin, Pulsed r.f.-glow-discharge time-of-flight mass spectrometry for fast surface and interface analysis of conductive and non-conductive materials, Surf. Interface Anal., 2006, 38, 292–295 CrossRef.
- L. Lobo, J. Pisonero, N. Bordel, R. Pereiro, A. Tempez, P. Chapon, J. Michler, M. Hohl and A. Sanz-Medel, A comparison of non-pulsed radiofrequency and pulsed radiofrequency glow discharge orthogonal time-of-flight mass spectrometry for analytical purposes, J. Anal. At. Spectrom., 2009, 24, 1373–1381 RSC.
- L. Lobo, N. Bordel, R. Pereiro, A. Tempez, P. Chapon and A. Sanz-Medel, A purged argon pre-chamber for analytical glow discharge—time of flight mass spectrometry applications, J. Anal. At. Spectrom., 2011, 26, 798–803 RSC.
- K. Ichihashi, K. Teranishi and A. Ichimura, Brominated trihalomethane formation in halogenation of humic acid in the coexistence of hypochlorite and hypobromiteions, Water Res., 1999, 33, 477–483 CrossRef CAS.
- E. A. Voudriast and M. Reinhard, Reactivities of Hypochlorous and Hypobromous Acid, Chlorine Monoxide, Hypobromous Acidium Ion, Chlorine, Bromine, and Bromine Chloride in Electrophilic Aromatic Substitution Reactions with p -Xylene in Water, Environ. Sci. Technol., 1988, 22, 1049–1056 CrossRef PubMed.
- R. Sandín, E. Ferrero, C. Repollés, J. P. Espinós, N. Bordel, C. González-Gago and J. J. Malfeito, Oxidación de membranas de ósmosis inversa. Mecanismo de degradación en agua de mar, XAEDyR International Congress, Seville, Spain, 26-28 November, 2014 Search PubMed.
- C. Y. Tang, Y.-N. Kwon and J. O. Leckie, Probing the nano- and micro-scales of reverse osmosis membranes—A comprehensive characterization of physiochemical properties of uncoated and coated membranes by XPS, TEM, ATR-FTIR, and streaming potential measurements, J. Membr. Sci., 2007, 287, 146–156 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |