Synthesis and characterization of novel bio-based benzoxazines from eugenol
P. Thirukumaran,
A. Shakila and
Sarojadevi Muthusamy*
Anna University, Chemistry, Chennai, India. E-mail: msrde2000@yahoo.com
First published on 23rd December 2013
Abstract
Polybenzoxazines are phenolic-like materials that possess dimensional and thermal stability, and they release no by-products during their polymerization. In this study, a new class of benzoxazine-containing monomers has been prepared from renewable resource (eugenol, a natural phenol obtained from clove oil), paraformaldehyde and various aromatic diamines. The structures of the monomers were supported by Fourier Transform Infrared (FT-IR), 1H-NMR and 13C-NMR spectral analysis, proving the existence of the benzoxazine ring containing eugenol moiety in its molecular structure. The monomers were polymerized/cured via ring-opening polymerization by heating as indicated by FT-IR and Differential Scanning Calorimetry (DSC). This is confirmed by the disappearance of the peaks due to oxazine ring (942 cm−1). The exothermic peak associated with curing was observed from 170 °C to 250 °C. The thermal and mechanical properties of the polybenzoxazines were investigated through thermogravimetric analysis (TGA) and dynamic mechanical analysis (DMA). The temperatures corresponding to 5% and 10% weight loss is from 240 to 295 °C and from 290 to 340 °C, respectively. The completely cured materials could achieve char yields up to 36.5% at 800 °C in nitrogen atmosphere. DMA revealed that the glass transition temperatures of PBz-SUL and PBz-PHE were higher than that of PBz-DDS and PBz-OXY.
Introduction
Benzoxazine is a molecule having a benzene ring fused to another six-membered heterocycle containing one oxygen atom and a nitrogen atom. There are a number of possible isomeric benzoxazines depending on the relative positions of the two heteroatoms of the oxazine ring system, and 1,3-benzoxazine is the kind of isomer used for polymerization.1 This is a relatively new family of phenolic resin which combines the thermal properties and flame retardance of phenolics, with the mechanical performance and design flexibility of epoxies.2,3 Benzoxazines are generated by the Mannich-like condensation of a phenol, formaldehyde and an amine.4The chemistry of benzoxazines is responsible for a number of inherent processing advantages, including low melt viscosity, no harsh catalyst required, and no volatile release during cure and minimal cure shrinkage.5 Shrinkage does not occur during curing, which is a striking feature of benzoxazine-based polymers.6 They also have good thermal stability that is demonstrated by their high glass transition temperature (170–340 °C), degradation temperature (350–400 °C), and char yield (28–66%).7 Though, polybenzoxazines offer a variety of advantages, pure polybenzoxazine-based polymers also suffer from a number of disadvantages, in terms of (i) high curing temperature (ii) difficulty in processing and (iii) poor mechanical strength [brittleness]. To properly address these issues and overcome the associated disadvantages, various strategies such as (i) preparation of modified monomers with additional functionality (ii) synthesis of novel polymeric precursors and (iii) blending with a high-performance polymer or filler and fiber may be adopted.6
Eugenol is a member of the phenylpropanoids class of chemical compounds. The name is derived from the scientific name for clove, Eugenia aromaticum or Eugenia caryophyllata. Clove is commercially cultivated in India, Madagascar, Sri Lanka, Indonesia and the south of China. Eugenol is responsible for the aroma of cloves. It is the main component in the essential oil extracted from cloves, comprising 72–90% of the total. It is a clear to pale yellow oily liquid extracted from certain essential oils especially from clove oil, nutmeg, cinnamon, basil and bay leaf. It is slightly soluble in water and soluble in organic solvents. It has a spicy, clove-like aroma. Clove oil is widely used for flavouring pastry, special sauces and condiments. It is also used in medicine, especially in the preparations for gum and teeth.8,9 Table 1 shows the relative percentages of all the components in clove leaf and bud oil (Fig. 1).
| S.no. | Monomer | Tm (°C) | Tonset (°C) | Tmax (°C) | Tprocessing window (°C) |
|---|---|---|---|---|---|
| 1 | Bz-DDS | 130 | 195 | 255 | 65 |
| 2 | Bz-SUL | 100 | 205 | 250 | 105 |
| 3 | Bz-OXY | 140 | 190 | 225 | 50 |
| 4 | Bz-PHE | 90 | 225 | 250 | 135 |
The development of new polymers, especially those based on renewable organic raw materials deserve the attention of both academic and industrial research.10 In this study, we focus on the effect of incorporation of eugenol in benzoxazine-based phenolic resins on the thermal properties. Hence, we report the synthesis of benzoxazine-based phenolic resin using eugenol, aromatic diamine and paraformaldehyde as raw materials. The synthesis, characterization, thermal polymerization and the properties of these new benzoxazines are described in this paper.
Experimental
Materials
Eugenol was purchased from Rajkart Enterprises, India; paraformaldehyde, N,N-dimethylformamide (DMF) and ethanol were purchased from Merck Limited, India; potassium carbonate (K2CO3) was purchased from S D Fine-Chem Limited, India; bis (4-aminophenyl)sulphone and bis(4-hydroxyphenyl)sulphone were purchased from Alfa Aesar (Johnson Mathew Company), India, Pvt. Ltd.; hydrazine monohydratewas purchased from Spectrochem Pvt. Ltd., India; hydroquinone was purchased from Fisher Scientific (a part of Thermo Fisher Scientific, India Pvt. Ltd.); p-nitrophenol, p-chloronitrobenzene, dimethylsulphoxide (DMSO) and 10% Pd/C from Sisco Research Laboratories, Pvt. Ltd., India. All chemicals and solvents were used without further purification.Characterization methods
Fourier transform infrared (FTIR) spectra of the samples were obtained using an ABB Bomen (Model MB 3000) Spectrometer. The samples were ground with spectroscopy grade KBr and made into pellets. 1H (500 mHz) & 13C (125 mHz) nuclear magnetic resonance (NMR) spectra were recorded on a Joel Spectrometer with tetramethylsilane (TMS) as the internal standard. Solutions were prepared in DMSO-d6. Differential Scanning Calorimetry was performed in a TA instrument Q10 model using 5–10 mg of the sample at a heating rate of 10 °C min−1 in nitrogen atmosphere. Thermogravimetric analysis (TGA) was performed using a TA Q 600 thermal analyzer. Cured samples were analyzed in open silicon pan at a heating rate of 20 °C min−1 in N2 atmosphere, up to a maximum temperature of 800 °C.Synthesis of diamines
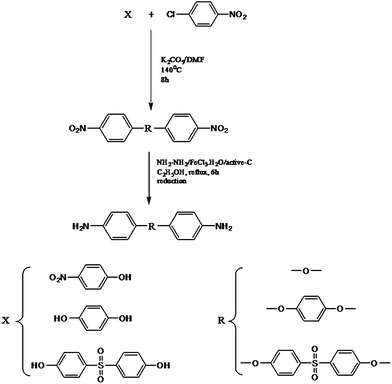
Bis (4-nitrophenyl) ether (0.1 m, 26 g), ferric chloride hexahydrate (1.5 g), activated carbon (1.5 g) and ethanol (300 mL) were taken in a 500 mL three necked round bottomed flask fitted with a reflux condenser and magnetic stirrer.11 To this mixture hydrazine hydrate (60 mL of 85%) was added dropwise over a period of 1 h at reflux temperature. The reaction was continued for about 5 h under reflux conditions. The solution was then filtered to remove the catalysts, cooled to room temperature, and then filtered to obtain white crystals of [bis (4-aminophenyl) ether] [BAPE]. Yield: 75%, m.p. 190 °C.
FT-IR (KBr, cm−1) 3040 (C–H, aromatic), 1512 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, aromatic), 1319 (C–O–C), 3387 and 3325 (N–H, asymmetric and symmetric stretching vibrations); 1H-NMR (DMSO-d6, ppm) 3.5 (s, –NH2) 6.5 (d, –CH
C, aromatic), 1319 (C–O–C), 3387 and 3325 (N–H, asymmetric and symmetric stretching vibrations); 1H-NMR (DMSO-d6, ppm) 3.5 (s, –NH2) 6.5 (d, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N) 6.7 (d, –CH
C–N) 6.7 (d, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O); 13C-NMR (DMSO-d6, ppm) 145 (C
C–O); 13C-NMR (DMSO-d6, ppm) 145 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) *C–NH2) 115 (*C
*C–NH2) 115 (*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH2) 119 (*C
C–NH2) 119 (*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O) 147 (C
C–O) 147 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) *C–O).
*C–O).
IR (KBr, cm−1) 3040 (C–H), 1497 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, aromatic), 1273 (C–O–C), 3310 (N–H, asymmetry), 3225 (N–H, symmetry); 1H NMR (DMSO-d6, ppm) 4.5 (s, –NH2) 7.4 (d, –CH
C, aromatic), 1273 (C–O–C), 3310 (N–H, asymmetry), 3225 (N–H, symmetry); 1H NMR (DMSO-d6, ppm) 4.5 (s, –NH2) 7.4 (d, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N) 7.8 (d, –CH
C–N) 7.8 (d, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O) 8.0 (d, –CH
C–O) 8.0 (d, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O); 13C NMR (DMSO-d6, ppm) 125 (C
C–O); 13C NMR (DMSO-d6, ppm) 125 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) *C–NH2) 100 (*C
*C–NH2) 100 (*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH2) 114 (*C
C–NH2) 114 (*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O) 144 (C
C–O) 144 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) *C–O).
*C–O).
FT-IR (KBr, cm−1) 3040 (C–H, aromatic), 1582 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, aromatic), 1288 (C–O–C), 3456 and 3371 (N–H, asymmetric and symmetric stretching vibrations), 1412 and 1242 (O
C, aromatic), 1288 (C–O–C), 3456 and 3371 (N–H, asymmetric and symmetric stretching vibrations), 1412 and 1242 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, asymmetric and symmetric stretching vibrations); 1H-NMR (DMSO-d6, ppm) 3.6 (s, –NH2), 6.5 (d, –CH
O, asymmetric and symmetric stretching vibrations); 1H-NMR (DMSO-d6, ppm) 3.6 (s, –NH2), 6.5 (d, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N), 6.8 (d, –CH
C–N), 6.8 (d, –CH ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O), 7.1 (d, –CH
C–O), 7.1 (d, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O), 7.9 (d, –CH
C–O), 7.9 (d, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–S); 13C-NMR (DMSO-d6, ppm) 145 (C
C–S); 13C-NMR (DMSO-d6, ppm) 145 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) *C–NH2), 115 (*C
*C–NH2), 115 (*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH2) 119 (*C
C–NH2) 119 (*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O), 147 (C
C–O), 147 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) *C–O), 160 (C
*C–O), 160 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) *C–O), 130 (*C
*C–O), 130 (*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–S), 133 (C
C–S), 133 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) *C–S).
*C–S).
Synthesis of benzoxazine monomers
FT-IR (KBr, cm−1) 3044 (C–H), 1597 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1324 (CH2), 939 (oxazine ring), 1109, 1227 and 1365 (C–O–C), 1186 (C–N–C); 1H-NMR (DMSO-d6, ppm) 4.5 (s, Ar–CH2–O), 5.4 (s, O–CH2–N), 3.7 (s, –OCH3), 3.2 (d, –CH2*–CH
C), 1324 (CH2), 939 (oxazine ring), 1109, 1227 and 1365 (C–O–C), 1186 (C–N–C); 1H-NMR (DMSO-d6, ppm) 4.5 (s, Ar–CH2–O), 5.4 (s, O–CH2–N), 3.7 (s, –OCH3), 3.2 (d, –CH2*–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.9 (m, –CH2–CH*
CH2), 5.9 (m, –CH2–CH*![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.0 (d, –CH2–CH
CH2), 5.0 (d, –CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2*), 6.5–7.5 (m, aromatic protons);13C-NMR (DMSO-d6, ppm) 49.5 (Ar–CH2–O), 79.9 (O–CH2–N), 55.8 (–OCH3), 115.6 (–*CH2–CH
CH2*), 6.5–7.5 (m, aromatic protons);13C-NMR (DMSO-d6, ppm) 49.5 (Ar–CH2–O), 79.9 (O–CH2–N), 55.8 (–OCH3), 115.6 (–*CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 138.2 (–CH2–*CH
CH2), 138.2 (–CH2–*CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 40.5 (–CH2–CH
CH2), 40.5 (–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) *CH2).
*CH2).
FT-IR (KBr, cm−1) 3044 (C–H), 1596 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1327 (CH2), 939 (oxazine ring), 1107, 1224 and 1327 (C–O–C), 1193 (C–N–C); 1H-NMR (DMSO-d6, ppm) 4.5 (s, Ar–CH2–O), 5.3 (s, O–CH2–N), 3.7 (s, –OCH3), 3.2 (d, –CH2*–CH
C), 1327 (CH2), 939 (oxazine ring), 1107, 1224 and 1327 (C–O–C), 1193 (C–N–C); 1H-NMR (DMSO-d6, ppm) 4.5 (s, Ar–CH2–O), 5.3 (s, O–CH2–N), 3.7 (s, –OCH3), 3.2 (d, –CH2*–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.9 (m, –CH2–CH*
CH2), 5.9 (m, –CH2–CH*![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.0 (d, –CH2–CH
CH2), 5.0 (d, –CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2*), 6.5–7.5 (m, aromatic protons);13C-NMR (DMSO-d6, ppm) 49.8 (Ar–CH2–O), 79.5 (O–CH2–N), 56.0 (–OCH3), 116.0 (–*CH2–CH
CH2*), 6.5–7.5 (m, aromatic protons);13C-NMR (DMSO-d6, ppm) 49.8 (Ar–CH2–O), 79.5 (O–CH2–N), 56.0 (–OCH3), 116.0 (–*CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 138.2 (–CH2–*CH
CH2), 138.2 (–CH2–*CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 39.9 (–CH2–CH
CH2), 39.9 (–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) *CH2).
*CH2).
FT-IR (KBr, cm−1) 3040 (C–H), 1593 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1376 (CH2), 942 (oxazine ring), 1103 and 1233 (C–O–C), 1191 (C–N–C), 1144 (O
C), 1376 (CH2), 942 (oxazine ring), 1103 and 1233 (C–O–C), 1191 (C–N–C), 1144 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, ppm) 4.6 (s, Ar–CH2–O), 5.4 (s, O–CH2–N), 3.6 (s, –OCH3), 3.2 (d, –CH2*–CH
O); 1H-NMR (DMSO-d6, ppm) 4.6 (s, Ar–CH2–O), 5.4 (s, O–CH2–N), 3.6 (s, –OCH3), 3.2 (d, –CH2*–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.9 (m, –CH2–CH*
CH2), 5.9 (m, –CH2–CH*![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.0 (d, –CH2–CH
CH2), 5.0 (d, –CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2*), 6.5–7.5 (m, aromatic protons);13C-NMR (DMSO-d6, ppm) 49.5 (Ar–CH2–O), 79.1 (O–CH2–N), 55.9 (–OCH3), 115.8 (–*CH2–CH
CH2*), 6.5–7.5 (m, aromatic protons);13C-NMR (DMSO-d6, ppm) 49.5 (Ar–CH2–O), 79.1 (O–CH2–N), 55.9 (–OCH3), 115.8 (–*CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 138.2 (–CH2–*CH
CH2), 138.2 (–CH2–*CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 40.4 (–CH2–CH
CH2), 40.4 (–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) *CH2).
*CH2).
FT-IR (KBr, cm−1) 3068 (C–H), 1587 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1322 (CH2), 942 (oxazine ring), 1105, 1242 and 1383 (C–O–C), 1166 (C–N–C), 1147 (O
C), 1322 (CH2), 942 (oxazine ring), 1105, 1242 and 1383 (C–O–C), 1166 (C–N–C), 1147 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (DMSO-d6, ppm) 4.6 (s, Ar–CH2–O), 5.4 (s, O–CH2–N), 3.7 (s, –OCH3), 3.2 (d, –CH2*–CH
O); 1H-NMR (DMSO-d6, ppm) 4.6 (s, Ar–CH2–O), 5.4 (s, O–CH2–N), 3.7 (s, –OCH3), 3.2 (d, –CH2*–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.9 (m, –CH2–CH*
CH2), 5.9 (m, –CH2–CH*![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.0 (d, –CH2–CH
CH2), 5.0 (d, –CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2*), 6.5–8.0 (m, aromatic protons); 13C-NMR (DMSO-d6, ppm) 49.4 (Ar–CH2–O), 79.3 (O–CH2–N), 56.1 (–OCH3), 116.0 (–*CH2–CH
CH2*), 6.5–8.0 (m, aromatic protons); 13C-NMR (DMSO-d6, ppm) 49.4 (Ar–CH2–O), 79.3 (O–CH2–N), 56.1 (–OCH3), 116.0 (–*CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 138.5 (–CH2–*CH
CH2), 138.5 (–CH2–*CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 39.9 (–CH2–CH
CH2), 39.9 (–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) *CH2).
*CH2).
Results and discussion
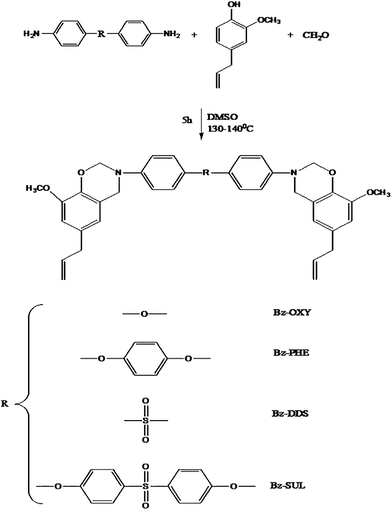
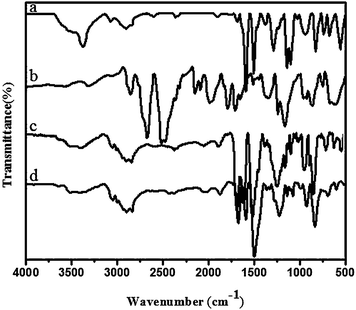
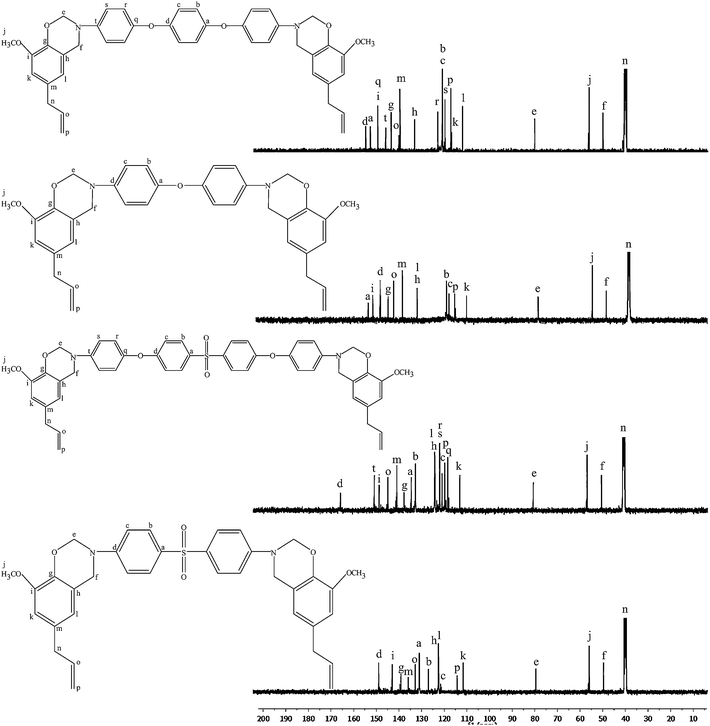
Chemical structure of the monomers was confirmed by FT-IR, 1H NMR & 13C NMR spectral methods and elemental analysis.Eugenol based benzoxazine monomer (Bz-DDS) was synthesised by the condensation reaction of eugenol with formaldehyde and amine as shown in Scheme 2. The FT-IR spectrum (Fig. 2) of this monomer shows characteristic absorption bands at 1233 cm−1 (asymmetric stretching of C–O–C), 1029 cm−1 (symmetric stretching of C–O–C) and 942 cm−1 which is typical of benzoxazine ring structure.12 The spectrum also shows a band at 1376 cm−1 due to tetra substituted benzene ring. Other aromatic vibrations are observed at 1593 and 1504 cm−1. C–H stretching vibration of benzene ring appears at 3068 cm−1 and the peaks at 2934 and 2899 cm−1 are owing to the asymmetric and symmetric stretching vibrations of the methylene group of the oxazine ring as well as alkyl side chain of eugenol.13,14
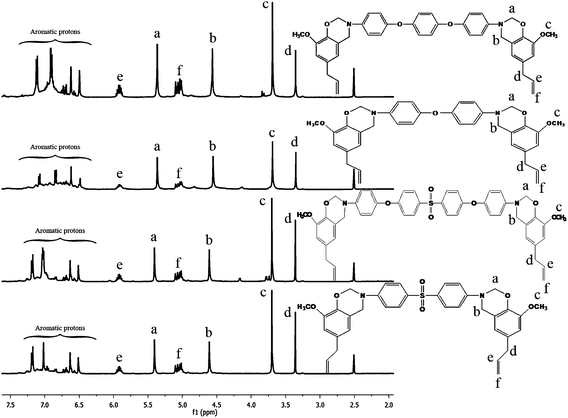
Fig. 3 shows the 1H-NMR spectra of all the benzoxazine monomers. The characteristic oxazine ring protons appear as two singlets at 4.6 and 5.4 ppm which are assigned to C–CH2–N and O–CH2–N respectively.15 The singlet at 3.7 ppm is due to the –OCH3 protons. The doublets at 3.3 and 5.1 ppm are assigned to [–CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH2–] allyl protons. The multiplets between 6.5 and 7.5 ppm are assigned to the aromatic protons.16
CH–CH2–] allyl protons. The multiplets between 6.5 and 7.5 ppm are assigned to the aromatic protons.16
Fig. 4 shows the 13C-NMR spectra of all the monomers. The characteristic carbon resonances of the oxazine ring are found at 49.5 and 79.1 ppm for Ar–CH2–N and N–CH2–O respectively.17 The peak at 55.9 ppm is attributed to the OCH3 carbon and the peaks at 115.8, 138.2 and 40.4 ppm are assigned to CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH2- allyl carbons.
CH–CH2- allyl carbons.
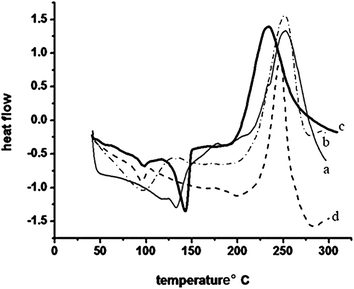
The polymerization behaviour of all the eugenol based benzoxazine monomers were studied using DSC at a heating rate of 10 °C min−1 under nitrogen atmosphere from 25 °C to 325 °C. Fig. 5 shows the DSC thermograms of all the monomers and the results are summarized in Table 2. The endotherm peaks centered around 90–130 °C are attributed to the melting point of the monomers.
| S.no. | Polymer | T5% | T10% | Char yield (%) | LOI values |
|---|---|---|---|---|---|
| 1 | PBz-DDS | 295 | 340 | 32.0 | 30.3 |
| 2 | PBz-SUL | 275 | 325 | 23.4 | 26.9 |
| 3 | PBz-OXY | 240 | 290 | 30.1 | 29.4 |
| 4 | PBz-PHE | 260 | 310 | 36.5 | 32.1 |
The onset of curing is around 195 and 205 °C for Bz-DDS and Bz-SUL, whereas it is around 190 and 225 °C for Bz-OXY and Bz-PHE, respectively. The values are almost 10 to 35 °C lower than that of the monomers containing Bz-SUL and Bz-PHE structures. This is because the oxygen and sulphur linkages allow the molecule to rotate easily and increase the flexibility in Bz-SUL and Bz-PHE, whereas the incorporation of aromatic rings in Bz-DDS and Bz-OXY adds rigidity to the molecule. The exothermic maxima are observed at 255 and 250 °C for Bz-DDS and Bz-SUL, and at 225 and 250 °C for Bz-OXY and Bz-PHE, respectively. The temperature difference between melting point and onset of polymerization is defined as the processing window. The processing window for the monomers Bz-DDS, Bz-SUL, Bz-OXY & Bz-PHE is 65, 105, 50, 135 °C, respectively. All the four monomers have wide processing window, indicating good process-ability for the monomers.16
The curing reactions of the monomers were further confirmed by FTIR analysis. Fig. 6 (a–d) show the FTIR spectra of the monomers after heating at 100, 150, 200 and 250 °C for 1 h at each temperature.
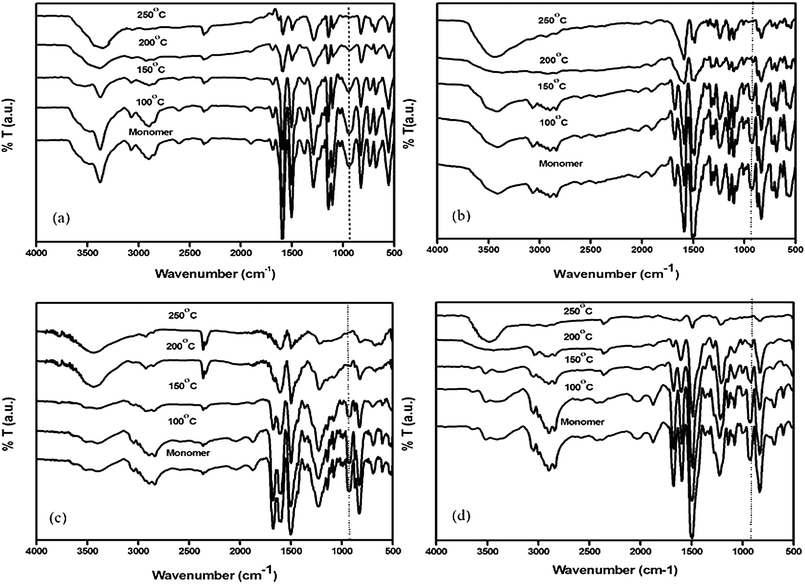 | ||
| Fig. 6 FTIR spectra of (a) Bz-DDS, (b) Bz-SUL, (c) Bz-OXY and (d) Bz-PHE at different curing temperature. | ||
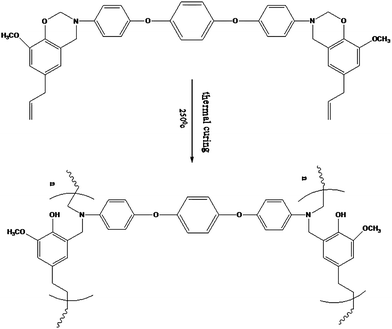
For Bz-DDS monomer, the dramatic decrease of intensity of peaks around 942, 1029, 1233 & 1376 cm−1 shows the curing of the benzoxazine ring components in the monomer.17 During the curing process, the oxazine ring is opened by the breakage of C–O bond. The disappearance of the band at 1686 cm−1 typical of the allylic stretching present in the eugenol part of the benzoxazine monomer confirms the reactivity of the double bonds.18 This occurs at high temperature (200 °C) after the thermal treatment of benzoxazine with the possible production of a network of cross linked material. The formation of hydroxyl groups was observed during the thermal cure of the monomer which was justified by the presence of broad band in the range of 3400 to 3500 cm−1. This result implies that the curing reaction takes place by the ring opening of benzoxazine and addition reaction of allyl group of the eugenol component as shown in the Scheme 3 (curing). Similar curing behaviour was observed in other three benzoxazine monomers.19
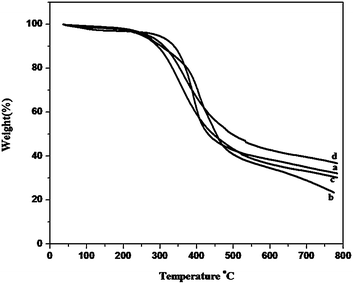
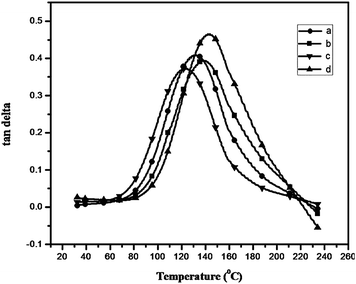
The thermal properties of the polybenzoxazines crosslinked by heating upto 250 °C were studied by thermogravimetric analysis (TGA) (Fig. 7). The data obtained are tabulated in Table 2. It can be seen that the polybenzoxazines show fairly good thermal stability. For all the polymers studied, the 5% and 10% weight loss temperatures (T5 and T10) varies from 240 to 295 °C and 290 to 340 °C. Obviously, the thermal stability of Bz-DDS based polymer is superior to that of the other three polymers. This may be attributed to the fact that the sulfonyl group incorporated in Bz-DDS based polymer had a significant effect on improving the thermal stability, whereas the flexible oxygen linkages in the other three polymers decreased the thermal stability. The char yield at 800 °C in N2 atmosphere varies from 23% to 36% for all the cross linked polybenzoxazines. The highest char yield (36%) of Bz-PHE is due to high aromatic content in its structure compared with the other three polymers (Fig. 8).
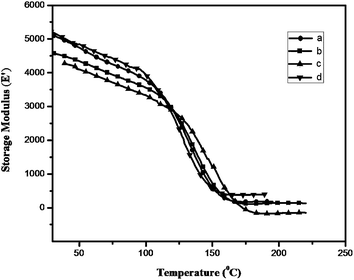 | ||
| Fig. 8 DMA spectra showing storage modulus of (a) PBz-DDS, (b) PBz-SUL, (c) PBz-OXY and (d) PBz-PHE. | ||
The limiting oxygen index (LOI) values, which can be taken as an indicator to evaluate the polymer's flame retardancy, were measured and shown in Table 2. Char yield of a material can be used to estimate LOI according to Van Krevelen and Hoftyzer equation.20 The char yield of the polybenzoxazines was found to be high in the range of 23.4–36.5% indicating high flame retardancy, which is expressed in terms of their LOI value. The LOI values of the polymers should be above the threshold value of 26, to render them self-extinguishing and for their qualification for many applications requiring good flame resistance. It was found that the LOI increases with increasing char yield as expected. The prepared polybenzoxazines show LOI value greater than 26 confirming their good flame retardant properties (Fig. 9).
Conclusion
Bio-based benzoxazine monomers (Bz-OXY, Bz-PHE, Bz-DDS and Bz-SUL) have been successfully synthesized from eugenol, a well known renewable organic resources and by-product of clove oil and polymerized to produce different polybenzoxazines. The structure of the monomers supported by FT-IR, 1H NMR & 13C NMR spectra show the existence of reactive benzoxazine ring, methoxy and allyl groups in its molecular structure. DSC scans of all the benzoxazine monomers indicate good process-ability showing wide processing window. The ring-opening polymerization of monomers is confirmed by cure cycle of FT-IR spectrum. The allyl moiety of benzoxazine also participates during the curing process which improves the cross-linking density of the polymer. This leads to enhancement in thermal stability (340 °C) and char yield (36.5% at 800 °C). Furthermore, all the polymers possess good flame retardancy as shown by their LOI values. From the obtained results, it can be seen that eugenol can be considered as a very attractive renewable bio-source for use in eco-friendly processes.Acknowledgements
The authors acknowledge the Department of Science and Technology, New Delhi and The council of Scientific and Industrial Research for funding this project. The authors also acknowledge DST (FIST) and UGC (SAP) for the financial support extended to procure instrumental facilities.References
- Y. Yusuf, K. Baris and G. Narendra Nath, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 5565 CrossRef.
- B. S. Rao and P. Aruna, React. Funct. Polym., 2011, 71, 148 CrossRef CAS PubMed.
- H. Ishida and D. J. Allen, J. Polym. Sci., Part A: Polym. Chem., 1996, 34, 1019 CAS.
- E. Calo, A. Maffezzoli, G. Mele, F. Martina, S. E. Mazzetto, A. Tarzia and C. Stifani, Green Chem., 2007, 9, 754 RSC.
- C. Zuniga, G. Lligadas, J. C. Ronda, M. Galia and V. Cadiz, Polymer, 2012, 53, 1617 CrossRef CAS PubMed.
- F. Kasapogly, I. Cianga, Y. Yagci and T. Takeichi, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 3320 CrossRef.
- K. S. Santhoshkumar and C. P. Regunadhan Nair, Textbook of Polybenzoxazines: Chemistry and Properties, 2010 Search PubMed.
- D. J. Liaw and W. C. Shen, Polym. Eng. Sci., 1994, 34, 1279 Search PubMed.
- M. R. Kessler and S. R. White, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 2373 CrossRef CAS.
- F. W. Holly and A. C. Cope, J. Am. Chem. Soc., 1944, 66, 1875 CrossRef CAS.
- X. H. Zhang, L. H. Huang, S. Chen and G. R. Qi, eXPRESS Polym. Lett., 2007, 1, 326 CrossRef CAS.
- T. Agag, L. Jiu and H. Ishida, Polymer, 2009, 50, 5940 CrossRef CAS PubMed.
- Y. Liu, C. Yue and J. Gao, Polymer, 2010, 51, 3722 CrossRef CAS PubMed.
- C. V. Mythili, A. M. Retna and S. Gopalakrishnan, Bull. Mater. Sci., 2004, 27, 235 CrossRef CAS.
- T. Agag and T. Takeichi, Macromolecule, 2003, 36, 6010 CrossRef CAS.
- S. Li, S. Yan, J. Yu and B. Yu, J. Appl. Polym. Sci., 2011, 122, 2843 CrossRef CAS.
- J. Opfermann and E. Kaiserberger, Thermochim. Acta, 1992, 203, 167 CrossRef CAS.
- Z. Xiaoqing, M. G. Looney, D. H. Solomon and A. K. Whittaker, Polymer, 1997, 38, 5835 CrossRef.
- P. Kasemsiri, S. Hiziroglu and S. Rimdusit, Thermochim. Acta, 2011, 520, 84 CrossRef CAS PubMed.
- D. W. Van Krevelen, Polymer, 1975, 16, 615–620 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |