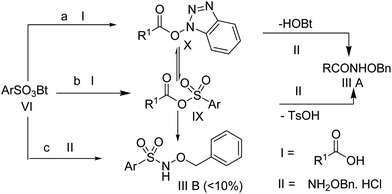
Synthesis of O-benzyl hydroxamates employing the sulfonate esters of N-hydroxybenzotriazole†
Nani Babu Palakurthy,
Dharm Dev,
Sonali Paikaray,
Susmitnarayan Chaudhury and
Bhubaneswar Mandal*
Department of Chemistry, Indian Institute of Technology Guwahati, Guwahati, Assam 781039, India. E-mail: bmandal@iitg.ernet.in
First published on 7th November 2013
Abstract
The direct conversion of various carboxylic acids, that include sterically hindered amino acids and di-peptides, to O-benzyl hydroxamates is demonstrated using sulfonate esters of benzotriazoles under ambient and milder conditions without significant racemization. This simple and efficient protocol is extended to the synthesis of O-benzyl hydroxamates, using in situ generated solid supported TsOBt to facilitate the recovery and re-usability of HOBt and to render the isolation of the products easier. Such in situ generation and further application of a coupling reagent is novel and industrially important.
Introduction
The medicinal and biological importance of hydroxamic acids (R-CONHOH) is overwhelming, as they are the key structural constituents of a wide spectrum of bioactive agents including various antibacterial, antifungal, and anticancer agents.1 As they are very effective metal-ion chelators, they have led to the rational design and discovery of potent inhibitors of metalloenzymes such as thermolysin.2 The internal hydroxamic acid motifs are found in siderophores.3 Biologically active peptides are often structurally manipulated in order to improve the properties of these peptides for research in drug development. An important class of these compounds is the backbone-modified peptides or “pseudopeptides”, such as aza-peptides and oxa-peptides.4 However, a thorough review of synthetic strategies that are available for the synthesis of hydroxamic acids directly from acids, has revealed that the methods available so far are either associated with routine peptide coupling reagents or with expensive reagents.5 Furthermore, the transformation has been carried out from various carboxylate esters and either O or N-protected hydroxylamines.6 Since such methods involve amino acids and peptides, it is important to retain the stereochemistry as well.Additionally, one of the major difficulties encountered during the synthesis of large chemical combinatorial libraries is resolving the need for highly diverse arrays of compounds with heterogeneous behaviour. Organic synthesis by solid phase methods is therefore emerging as a better tool for the clean synthesis of compounds. Tethering starting materials or reagents to an insoluble polymer allows remarkable simplifications in handling steps, rendering the automation of the process feasible. Alternatively, unlike classical solid phase synthesis, the reagents remain attached to the insoluble matrix, while the desired product is easily recovered by merely washing with the respective solvent(s), and the supported reagent is recycled. For example, a similar application of the solid supported N-hydroxybenzotriazole (HOBt) and differently substituted carbodiimides is reported.7 However, synthesis of such solid supported reagents involves cumbersome multi-step procedures. In a few cases, such reagents are commercially available but expensive and sensitive to prolonged storage.
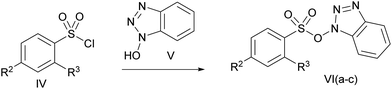
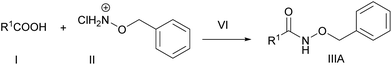
Herein we report a novel synthetic route to synthesize the hydroxamates of various carboxylic acids under milder and ambient conditions (Scheme 1), applying the sulfonate esters of N-hydroxybenzotriazole that can be prepared easily from cheap synthetic sources. In addition, we have introduced the novel concept of in situ generation of the solid supported reagent, the sulfonate ester of N-hydroxybenzotrizole, and demonstrated its utility for coupling carboxylic acid with O-benzylhydroxylamine hydrochloride to obtain the mentioned product. Such reagents can also be easily regenerated and recycled.
 | ||
| Scheme 1 Synthesis of O-benzyl hydroxamates employing the sulfonate esters of N-hydroxybenzotriazole (VI). | ||
Results and discussion
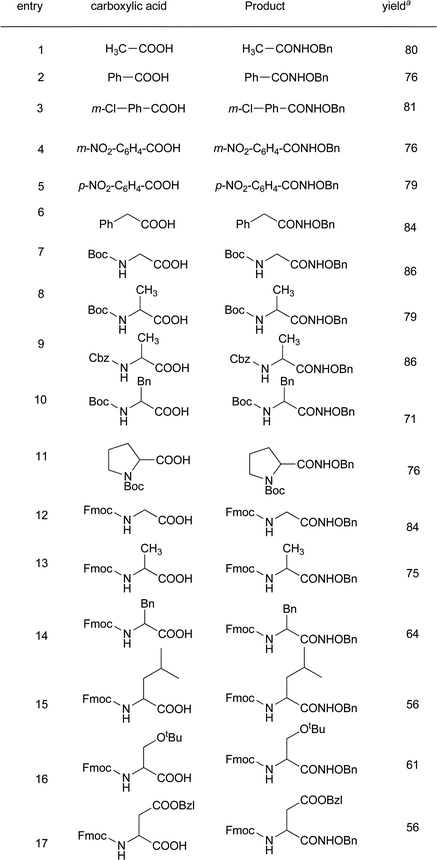
Initially, we stirred phenyl acetic acid (1 mmol), TsOBt (1H benzo[d][1,2,3]triazol-1-yl 4-methanebenzenesulfonate, 1 mmol), DIPEA (Diisopropyl ethyl amine, 2 mmol) and O-benzylhydroxylamine hydrochloride (1 mmol pre-neutralized with 1 mmol DIPEA in 1 mL acetonitrile) at room temperature. Interestingly, the coupling took place within 2 h and gave the desired product (IIIA) in good yield, along with a small amount of the sulfonamide of O-benzylhydroxylamine of p-toulene sulfonic acid (IIIB). In order to explore the chemistry of this particular transformation, we went on to synthesize various sulfonate esters as shown in Scheme 2 and Table 1 using a previously reported procedure.8,9Next, we examined the ability of each of these coupling reagents (VIa–c) to undergo this transformation. Although the insertion of the electron withdrawing group accelerated the reaction, generation of the sulfonamide corresponding to the O-benzylhydroxylamine side product (IIIB) was greater. Therefore, we continued to perform this reaction with TsOBt. The reactivity of TsOBt was moderate and produced the desired product (IIIA) in good to excellent yield. Formation of the side product was also reduced. The base and solvent screening experiments revealed that DIPEA and acetonitrile were the best.
The current methodology was compatible with aliphatic carboxylic acids (entry 1 and 6, Table 2) and aromatic acids (entries 2–5, Table 2). The yields were good to excellent in all the cases and no di- and tri-acylated products were observed in the reaction mixture. The method was also compatible with common N-protecting groups (entries 7–17, Table 2 and entries 1 and 2, Table 3). Various side chain protecting groups such as, tert-butyl ether (entry 16, Table 2) and benzyl ester (entry 17, Table 2) were also tolerated. Apart from the functional group tolerance, the important advantage of this method is the applicability to the sterically hindered amino acids such as alanine (entries 8, 9 and 13, Table 2), phenyl alanine (entries 10 and 14, Table 2) and leucine (entry 15, Table 2). In all these cases, reaction yields were found to be good but lower than their less sterically hindered analogues. Furthermore, sulfonate esters in condensation chemistry were found to cause no/less racemization.9 In our observations, when compared with the optical rotation of the entries (8 and 10, Table 2), the values were found to be close to the reported values6a,10 and it can be concluded that this transformation does not cause significant racemization.
| a Yields refer to the isolated yields after column chromatography. TLC is checked every 30 min. All the unreported products were characterized completely. Reported compounds were characterized with 1H-NMR, IR and ESI-MS, and matched with the reported data. | |||
|---|---|---|---|
 |
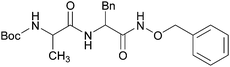
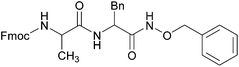
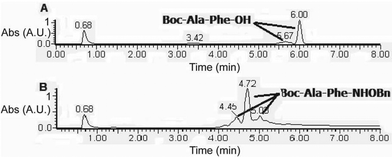
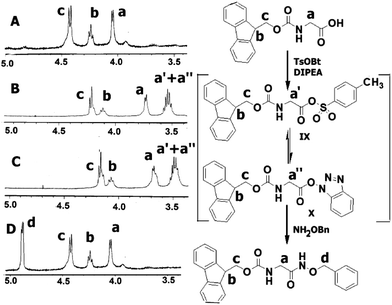
The present methodology was also extended to the synthesis of hydroxamates of di-peptides. Two di-peptides were synthesized using the pentafluorophenol activation strategy and then converted to the corresponding O-benzyl hydroxamates using the current methodology (Table 3). An investigation into the extent of racemization was performed using LCMS. The diastereomeric ratio that was noticed in the case of the C-terminus free di-peptides, was found to be retained even after the application of the present protocol (Fig. 1 and ESI†), which confirmed that this protocol does not cause much racemization.
In order to facilitate the recovery and re-usability of HOBt, we further developed the solid supported reagent, the sulfonate ester of N-hydroxybenzotriazole (VIII), and applied it to hydroxamate synthesis employing the same conditions as that of the solution phase version of the reaction.
Such in situ generated solid supported HOBt esters of p-toulene sulfonic acid (VII, in Scheme 3) enabled the recovery of HOBt which can be recycled effectively. The recyclability of this reagent was initially tested for 3 cycles with phenyl acetic acid and O-benzylhydroxylamine hydrochloride, and each time the isolated yields were very good (entry 1, Table 4). We further applied the same strategy to the synthesis of the O-benzyl hydroxamates of Fmoc-Gly-OH (entry 2, Table 4) and Fmoc-Phe-OH (entry 3, Table 4). Each time, the yield with the solid supported TsOBt was found to be as good as with the TsOBt in solution.
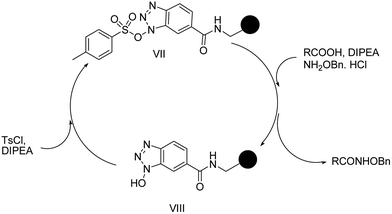 | ||
| Scheme 3 In situ generation of the coupling reagent and its application for hydroxamic acid synthesis. | ||
| Entry | Acid | Cycle | Yield (%) |
|---|---|---|---|
| a The yields refer to the isolated yields after work up (experimental details are provided in the Experimental section). Characterization data of the products were matched with those of the previously synthesized products, Table 2. | |||
| 1 | Phenyl acetic acid | I | 81 |
| II | 80 | ||
| III | 80 | ||
| 2 | Fmoc-Gly-OH | I | 82 |
| II | 82 | ||
| III | 80 | ||
| IV | 76 | ||
| V | 75 | ||
| VI | 71 | ||
| 3 | Fmoc- Phe-OH | I | 58 |
| II | 58 | ||
| III | 57 | ||
| IV | 58 | ||
| V | 54 | ||
| VI | 52 | ||
The reaction mechanism can be depicted as shown in Scheme 4 based on the possible products. The desired O-benzyl hydroxamates (IIIA) can be generated following paths a and b. On the other hand, direct attachment of the O-benzylhydroxylamine (II) on the sulfur center of the reagent VI (path c) and the intermediate IX can lead to the formation of the by-product IIIB. As shown in Scheme 4, the formation of both the intermediates (IX and X) was identified by IR and 1H-NMR spectroscopy. In our model reaction, the peaks at δ 4.04–4.03 were assigned as the glycinic CH2 protons of Fmoc glycine (Fig. 2A). A set of two peaks emerged at δ 3.72–3.71 and δ 3.56–3.49 (B) 2 min after the addition of TsOBt to the Fmoc-Gly-OH, along with the DIPEA in acetonitrile solvent, which persisted as long as the amine component was not added (C). These peaks were probably for the formation of the intermediates IX and X, which is in agreement with the observations of Carpino et al.9a Furthermore, in the IR spectra, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequencies corresponding to the sulfonate ester and the HOBt ester were observed at 1362 cm−1 and 1756 cm−1 respectively (see supporting information†).11
O stretching frequencies corresponding to the sulfonate ester and the HOBt ester were observed at 1362 cm−1 and 1756 cm−1 respectively (see supporting information†).11
Conclusions
In conclusion, we have demonstrated the synthesis of O-benzyl hydroxamate using the sulfonate ester of N-hydroxybenzotriazole, which was successfully applied to a wide variety of acids that include amino acids with various side chain protecting groups, and common N-protecting groups such as Boc, Cbz and Fmoc, under milder and ambient conditions in a green solvent, acetonitrile. This strategy was also successfully applied to the synthesis of the O-benzyl hydroxamates of di-peptides. Gratifyingly, the method enjoys success with in situ generated solid supported TsOBt, which facilitates the recovery of N-hydroxybenzotriazole and was successfully reused for up to six cycles without losing its efficiency. This process even facilitates the isolation of the product without purification using column chromatography. Yields of the isolated products were also found to be good.Experimental
All reagents were purchased from commercial sources and were used without any further purification unless mentioned otherwise. Melting points were uncorrected. Thin layer chromatograms were run on glass plates coated with silica gel G for TLC, using an EtOAc–hexane solvent system. All the compounds were purified by column chromatography using 60–120 mesh silica gel. 1H-NMR (400 MHz for 1H) was recorded using a 400 MHz spectrometer using CDCl3 as the solvent unless mentioned otherwise. Chemical shifts were reported in parts per million (ppm), using tetra methyl silane (0.05% to 1%) as the internal reference. Coupling constants (J) were reported in Hz singlet (s), doublet (d), triplet (t), doublet of doublet (dd), multiplet (m), or broad (br). Low resolution mass spectra were recorded on a Q-TOF ESI-MS instrument, HRMS was recorded on a Q-TOF LC/MS system, IR was recorded on a FT-IR spectrometer and LC-MS was performed on a Q-TOF instrument. Specific rotation was measured on a Perkin Elmer (model 343) polarimeter at 589 nm, 27.1 °C, with the temperature correction off. Data for previously reported compounds (cited therein) matched well with our observed data.General procedure for the synthesis of sulfonate esters of N-hydroxy benzotriazole from sulfonyl chlorides and HOBt
The representative procedure for the synthesis of TsOBt is common to all entries in Table 1 and is as follows. An oven dried 25 mL R.B. flask equipped with a magnetic stirrer bar, was loaded with HOBt (1 mmol, 1 equiv.) which was dissolved in 1 mL of dry CH2Cl2. To this, DIPEA (1 mmol, 1 equiv.) was added under nitrogen. Then, it was cooled to 0 °C, followed by the slow addition (over 30 min) of tosyl chloride using a syringe. Next, the reaction mixture was stirred at room temperature. The progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was washed with saturated NaCl solution (2 × 10 mL) and dried over anhydrous CaCl2. It was then dried in vacuo and purified by recrystallization using hexane and CH2Cl2.General procedure for the synthesis of O-benzyl hydroxamates
The representative procedure for the synthesis of N-benzyloxy-benzamide is common to all entries in Table 2 and is as follows. An oven dried 25 mL R.B. flask equipped with a magnetic stirrer bar, was loaded with benzoic acid (1 mmol, 1 equiv.), TsOBt (1 mmol, 1 equiv.) and DIPEA (2 mmol, 2 equiv.), and was dissolved in 1 mL of acetonitrile at 0 °C, followed by the slow addition of preneutralized NH2OBn with DIPEA (1 equiv. 1 mmol). Then, the reaction mixture was stirred at room temperature. The progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was diluted with EtOAc, then concentrated under reduced pressure, after which it was washed with 5% NaHCO3 (2 × 10 mL), 5% HCl (2 × 10 mL) (for Boc protected acids this acid wash was excluded) and saturated NaCl solution (2 × 10 mL), and dried over anhydrous CaCl2. Next, it was dried in vacuo and purified by column chromatography using hexane and EtOAc.General procedure for the synthesis of O-benzyl hydroxamate over solid phase resin
The solid supported HOBt was swelled for 20 min using dichloromethane as the solvent, with a rotator in a 5 mL sintered syringe. To this, 2 equivalents of DIPEA were added and rotated for 5 min. Then 1.5 equivalents of tosyl chloride were added with continuous rotation using a blood tube rotator. Then the reaction mixture was washed 5 times with CH2Cl2. To this, 2.5 equivalents of the acid were added along with 2 equivalents of DIPEA and rotated for a further 20 min. Finally, the preneutralized mixture of NH2OBn and DIPEA in DCM (1 mL) was added and rotated for 3 h. Then it was washed 5 times with DCM and the washings were collected together. The combined DCM solution was finally washed with 5% NaHCO3 (2 × 3 mL), 5% HCl (2 × 3 mL) and brine (2 × 10 mL), which afforded the desired product for the number of cycles mentioned, while the same solid supported HOBt was repeatedly used in the above mentioned cycle.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) mp 103 °C (Lit. 104 °C), yield (207 mg, 81%), yellow colorless crystals. IR (KBr, ν cm−1) 3099, 2797, 1741, 1561, 769. 1H NMR (400 MHz, CDCl3) δ ppm 8.44 (s, 1H, 1 × ArH), 8.26–8.28 (d, 1H, J = 8.4 Hz, 1 × ArH), 7.98–7.96 (d, 1H, J = 7.6 Hz, 1 × ArH), 7.56–7.52 (t, 1H, J = 8.0 Hz, 1 × ArH), 7.35–7.30 (m, 4H, 4 × ArH), 7.18 (s, 1H, 1 × ArH), 4.94 (s, 2H, CH2); LRMS (ESI) m/z calcd for [M + H]+: 261.05, found: 261.05.
4) mp 103 °C (Lit. 104 °C), yield (207 mg, 81%), yellow colorless crystals. IR (KBr, ν cm−1) 3099, 2797, 1741, 1561, 769. 1H NMR (400 MHz, CDCl3) δ ppm 8.44 (s, 1H, 1 × ArH), 8.26–8.28 (d, 1H, J = 8.4 Hz, 1 × ArH), 7.98–7.96 (d, 1H, J = 7.6 Hz, 1 × ArH), 7.56–7.52 (t, 1H, J = 8.0 Hz, 1 × ArH), 7.35–7.30 (m, 4H, 4 × ArH), 7.18 (s, 1H, 1 × ArH), 4.94 (s, 2H, CH2); LRMS (ESI) m/z calcd for [M + H]+: 261.05, found: 261.05.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) mp 165–166 °C (Lit. 166–167 °C), yield (215 mg, 79%), colorless crystals. IR (KBr, ν cm−1) 2993, 1733, 1574, 971, 758. 1H NMR (400 MHz, CDCl3) δ ppm 8.58 (br s, 1H, NH), 8.42–8.40 (s, 1H, 1 × ArH), 8.36–8.38 (t, 1H, J = 8.0 Hz, 1 × ArH), 7.34–7.33 (m, 4H, 4 × ArH), 7.19 (s, 1H, 1 × ArH), 4.98 (s, 2H, CH2); LRMS (ESI) m/z calcd for [M + H]+: 273.08, found: 273.08.
4) mp 165–166 °C (Lit. 166–167 °C), yield (215 mg, 79%), colorless crystals. IR (KBr, ν cm−1) 2993, 1733, 1574, 971, 758. 1H NMR (400 MHz, CDCl3) δ ppm 8.58 (br s, 1H, NH), 8.42–8.40 (s, 1H, 1 × ArH), 8.36–8.38 (t, 1H, J = 8.0 Hz, 1 × ArH), 7.34–7.33 (m, 4H, 4 × ArH), 7.19 (s, 1H, 1 × ArH), 4.98 (s, 2H, CH2); LRMS (ESI) m/z calcd for [M + H]+: 273.08, found: 273.08.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) Yield (243 mg, 76%), mp 182–185 °C (Lit. 182–185 °C), colorless solid, IR (KBr, ν cm−1) 3093, 2977, 1754, 1591, 942, 764. 1H NMR (400 MHz, CDCl3) δ ppm 9.80 (br s, 1H, NH), 7.29–7.17 (m, 5H, 5 × ArH), 4.89 (s, 2H, CH2), 4.18 (m, 1H, CH), 3.32–3.30 (m, 3H, CH2, CH), 2.04–1.80 (m, 4H, 2 × CH2), 1.41 (s, 9H, C(CH3)3); LRMS (ESI) m/z calcd for [M + H]+:321.18, found: 321.18.
4) Yield (243 mg, 76%), mp 182–185 °C (Lit. 182–185 °C), colorless solid, IR (KBr, ν cm−1) 3093, 2977, 1754, 1591, 942, 764. 1H NMR (400 MHz, CDCl3) δ ppm 9.80 (br s, 1H, NH), 7.29–7.17 (m, 5H, 5 × ArH), 4.89 (s, 2H, CH2), 4.18 (m, 1H, CH), 3.32–3.30 (m, 3H, CH2, CH), 2.04–1.80 (m, 4H, 2 × CH2), 1.41 (s, 9H, C(CH3)3); LRMS (ESI) m/z calcd for [M + H]+:321.18, found: 321.18.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) Yield (296 mg, 75%), Colorless solid, mp 145–147 °C (Lit. 146–148 °C). IR (KBr, ν cm−1) 3093, 2977, 1754, 1591, 942, 764. 1H NMR (400 MHz, CDCl3) δ ppm 7.76–7.75 (d, 2H, J = 7.6 Hz, 2 × ArH), 7.57 (br s, 3H, 3 × ArH), 7.41–7.39 (m, 3H, 3 × ArH), 7.28–7.26 (m, 5H, 5 × ArH), 5.35 (br s, 1H, NH), 4.89 (d, 2H, CH2), 4.36–4.34 (t, J = 7.2 Hz, 1H, 1H, CH), 4.28–4.26(m, 1H, CH), 1.48–1.46 (d, J = 6.8 Hz, 3H, CH3); LRMS (ESI) m/z Calcd. for [M + Na]+: 417.18, found: 417.18.
4) Yield (296 mg, 75%), Colorless solid, mp 145–147 °C (Lit. 146–148 °C). IR (KBr, ν cm−1) 3093, 2977, 1754, 1591, 942, 764. 1H NMR (400 MHz, CDCl3) δ ppm 7.76–7.75 (d, 2H, J = 7.6 Hz, 2 × ArH), 7.57 (br s, 3H, 3 × ArH), 7.41–7.39 (m, 3H, 3 × ArH), 7.28–7.26 (m, 5H, 5 × ArH), 5.35 (br s, 1H, NH), 4.89 (d, 2H, CH2), 4.36–4.34 (t, J = 7.2 Hz, 1H, 1H, CH), 4.28–4.26(m, 1H, CH), 1.48–1.46 (d, J = 6.8 Hz, 3H, CH3); LRMS (ESI) m/z Calcd. for [M + Na]+: 417.18, found: 417.18.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) Yield (315 mg, 64%), white solid, IR (KBr, ν cm−1) 3411, 2928, 1714, 1599, 1396, 1047, 738. 1H NMR (400 MHz, CDCl3) δ ppm 7.73–7.71 (d, J = 7.6 Hz, 3H, 3 × ArH), 7.50–7.7 (t, J = 7.2 Hz, 3H, 3 × ArH), 7.37–7.33 (m, 4H, 4 × ArH), 7.24–7.21 (m, 8H, 8 × ArH), 5.41 (br s, 1H, NH), 4.64 (br s, 1H, NH), 4.39–4.37 (t, J = 6.4 Hz, 2H, CH2), 4.25 (s, 2H, CH2), 4.14–4.08 (m, 2 × 1H, 2 × CH), 3.18–3.17 (m, 1H, CHaHb), 3.06–3.05 (m, 1H, CHaHb); 13C NMR (100 MHz, CDCl3) δ ppm 175.2, 170.8, 143.7, 141.1, 129.4, 128.3, 127.5, 126.9, 126.8, 125.0, 119.8, 66.7, 47.1, 37.8; HRMS (ESI) m/z Cald. for [M + H]+: 493.2127, found: 493.2159.
4) Yield (315 mg, 64%), white solid, IR (KBr, ν cm−1) 3411, 2928, 1714, 1599, 1396, 1047, 738. 1H NMR (400 MHz, CDCl3) δ ppm 7.73–7.71 (d, J = 7.6 Hz, 3H, 3 × ArH), 7.50–7.7 (t, J = 7.2 Hz, 3H, 3 × ArH), 7.37–7.33 (m, 4H, 4 × ArH), 7.24–7.21 (m, 8H, 8 × ArH), 5.41 (br s, 1H, NH), 4.64 (br s, 1H, NH), 4.39–4.37 (t, J = 6.4 Hz, 2H, CH2), 4.25 (s, 2H, CH2), 4.14–4.08 (m, 2 × 1H, 2 × CH), 3.18–3.17 (m, 1H, CHaHb), 3.06–3.05 (m, 1H, CHaHb); 13C NMR (100 MHz, CDCl3) δ ppm 175.2, 170.8, 143.7, 141.1, 129.4, 128.3, 127.5, 126.9, 126.8, 125.0, 119.8, 66.7, 47.1, 37.8; HRMS (ESI) m/z Cald. for [M + H]+: 493.2127, found: 493.2159.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) Yield (299 mg, 61%), white solid, mp 154–157 °C. IR (KBr, ν cm−1) 3309, 2974, 1672, 1537, 1298, 738. 1H NMR (400 MHz, DCl3) δ ppm 9.20 (br s, 1H, NH), 7.74–7.73 (d, J = 7.6 Hz, 2H, 2 × ArH), 7.57–7.55 (d, J = 7.6 Hz, 2H, 2 × ArH), 7.39–7.25 (m, 9H, 9 × ArH), 5.74–7.53 (d, J = 5.6 Hz, 1H, NH), 4.94–4.85 (s, 2H, CH2), 4.37–4.35 (d, J = 6.2 Hz, 2H, CH2), 4.20–4.16 (t, J = 6.8 Hz, 1H, CH), 4.10–4.09 (m, 2H, 2 × CH), 3.70–3.69 (m, 1H, CHaHb), 3.30–3.29 (m, 1H, CHaHb), 1.10 (s, 9H, C(CH3)3); 13C NMR (100 MHz, CDCl3) δ ppm 175.2, 166.2, 143.7, 141.3, 129.1, 128.6, 127.7, 125.1, 120.0, 74.5, 67.2, 61.2, 47.1, 29.7, 27.2 HRMS (ESI) m/z Calcd. for [M + H]+: 489.2389, found: 489.2294.
4) Yield (299 mg, 61%), white solid, mp 154–157 °C. IR (KBr, ν cm−1) 3309, 2974, 1672, 1537, 1298, 738. 1H NMR (400 MHz, DCl3) δ ppm 9.20 (br s, 1H, NH), 7.74–7.73 (d, J = 7.6 Hz, 2H, 2 × ArH), 7.57–7.55 (d, J = 7.6 Hz, 2H, 2 × ArH), 7.39–7.25 (m, 9H, 9 × ArH), 5.74–7.53 (d, J = 5.6 Hz, 1H, NH), 4.94–4.85 (s, 2H, CH2), 4.37–4.35 (d, J = 6.2 Hz, 2H, CH2), 4.20–4.16 (t, J = 6.8 Hz, 1H, CH), 4.10–4.09 (m, 2H, 2 × CH), 3.70–3.69 (m, 1H, CHaHb), 3.30–3.29 (m, 1H, CHaHb), 1.10 (s, 9H, C(CH3)3); 13C NMR (100 MHz, CDCl3) δ ppm 175.2, 166.2, 143.7, 141.3, 129.1, 128.6, 127.7, 125.1, 120.0, 74.5, 67.2, 61.2, 47.1, 29.7, 27.2 HRMS (ESI) m/z Calcd. for [M + H]+: 489.2389, found: 489.2294.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) Yield (349 mg, 79%), white solid, 125–128 °C. IR (KBr, ν cm−1) 3340, 2978, 1725, 1577, 1210, 978. 1H NMR (400 MHz, CDCl3) δ ppm 7.28–7.26 (m, 4H, 4 × ArH), 7.23–7.19 (m, 4H, 4 × ArH), 6.85–6.83 (d, 2H, J = 7.2 Hz, 2 × ArH), 6.92 (br s, 1H, 1 × NH), 5.24 (br s, 1H, NH), 4.50 (s, 2H, CH2), 3.77 (m, 1H, CH), 3.05 (br s, 2H, CHaHb), 1.37 (s, 9H, –C(CH3)3, 1.16–1.15 (d, 3H, J = 6.0 Hz, CH3). 13C NMR (100 MHz, CDCl3) δ ppm 175.2, 170.8, 156.1, 143.7, 143.6, 141.2, 135.1, 128.6, 128.4, 128.3, 127.7, 127.0, 125.0, 119.9, 77.2, 67.4, 67.1, 50.1, 47.0, 36.4; HRMS (ESI) m/z Calcd for [M + K]+: 479.1826 found: 479.1823.
3) Yield (349 mg, 79%), white solid, 125–128 °C. IR (KBr, ν cm−1) 3340, 2978, 1725, 1577, 1210, 978. 1H NMR (400 MHz, CDCl3) δ ppm 7.28–7.26 (m, 4H, 4 × ArH), 7.23–7.19 (m, 4H, 4 × ArH), 6.85–6.83 (d, 2H, J = 7.2 Hz, 2 × ArH), 6.92 (br s, 1H, 1 × NH), 5.24 (br s, 1H, NH), 4.50 (s, 2H, CH2), 3.77 (m, 1H, CH), 3.05 (br s, 2H, CHaHb), 1.37 (s, 9H, –C(CH3)3, 1.16–1.15 (d, 3H, J = 6.0 Hz, CH3). 13C NMR (100 MHz, CDCl3) δ ppm 175.2, 170.8, 156.1, 143.7, 143.6, 141.2, 135.1, 128.6, 128.4, 128.3, 127.7, 127.0, 125.0, 119.9, 77.2, 67.4, 67.1, 50.1, 47.0, 36.4; HRMS (ESI) m/z Calcd for [M + K]+: 479.1826 found: 479.1823.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) Yield (445 mg, 79%), white solid, mp 115–117 °C. IR (KBr, ν cm−1) 3233, 2929, 1693, 1535, 1082, 738. 1H NMR (400 MHz, CDCl3) δ ppm 7.28–7.26 (m, 4H, 4 × ArH), 7.23–7.19 (m, 6H, 6 × ArH), 6.85–6.83 (d, 1H, J = 7.2 Hz, 1 × NH), 5.24 (br s, 1H, 1 × NH), 4.50(s, 2H, CH2), 3.77– 3.74 (m, 1H, α-CH), 3.05 (br s, 2H, CHaHb), 1.37 (s, 9H, –C(CH3)3, 1.16–1.15 (d, 3H, J = 6.0 Hz, CH3); 13C NMR (100 MHz, CDCl3) δ ppm 172.6, 172.3, 156.7, 155.5, 143.8, 143.6, 140.6, 137.2, 135.0, 133.2, 129.1, 129.1, 128.6, 127.9, 127.6, 127.4, 126.9, 126.0, 125.1, 123.7, 120.9, 119.8, 77.2, 74.6, 74.4, 65.6, 60.3, 53.3, 53.2, 49.9, 47.5, 46.6, 36.7, 34.0, 33.3, 31.6, 29.6, 25.3, 25.0, 25.2, 21.0; HRMS (ESI) m/z Calcd. for [M + K]+: 564.2498, found: 564.2456.
3) Yield (445 mg, 79%), white solid, mp 115–117 °C. IR (KBr, ν cm−1) 3233, 2929, 1693, 1535, 1082, 738. 1H NMR (400 MHz, CDCl3) δ ppm 7.28–7.26 (m, 4H, 4 × ArH), 7.23–7.19 (m, 6H, 6 × ArH), 6.85–6.83 (d, 1H, J = 7.2 Hz, 1 × NH), 5.24 (br s, 1H, 1 × NH), 4.50(s, 2H, CH2), 3.77– 3.74 (m, 1H, α-CH), 3.05 (br s, 2H, CHaHb), 1.37 (s, 9H, –C(CH3)3, 1.16–1.15 (d, 3H, J = 6.0 Hz, CH3); 13C NMR (100 MHz, CDCl3) δ ppm 172.6, 172.3, 156.7, 155.5, 143.8, 143.6, 140.6, 137.2, 135.0, 133.2, 129.1, 129.1, 128.6, 127.9, 127.6, 127.4, 126.9, 126.0, 125.1, 123.7, 120.9, 119.8, 77.2, 74.6, 74.4, 65.6, 60.3, 53.3, 53.2, 49.9, 47.5, 46.6, 36.7, 34.0, 33.3, 31.6, 29.6, 25.3, 25.0, 25.2, 21.0; HRMS (ESI) m/z Calcd. for [M + K]+: 564.2498, found: 564.2456.Acknowledgements
PNB and DD thank IIT Guwahati for the fellowship. The authors sincerely thank the Central Instrumentation Facility, IIT Guwahati for the 1H-NMR, 13C-NMR and LRMS facilities. Our funding agency DST (FAST TRACK scheme, sanction no. SR/FT/CS-011/2008) is also greatly acknowledged.Notes and references
- (a) G. Weber, Cancer Res., 1983, 43, 3466 CAS; (b) M. J. Miller, Acc. Chem. Res., 1986, 19, 49 CrossRef CAS.
- For a review, see: M. Whittaker, C. D. Floyd, P. Brown and A. J. H. Gearing, Chem. Rev., 1999, 99, 2735 CrossRef CAS PubMed.
- (a) R. J. Bergeron, J. S. Mcmanis, O. I. V. Phanstiel and J. R. T. Vinson, J. Org. Chem., 1995, 60, 109 CrossRef CAS; (b) R. J. Bergeron and O. I. V. Phanstiel, J. Org. Chem., 1992, 57, 7140 CrossRef CAS.
- D. Sabatino, C. Proulx, S. Klocek, C. B. Bourguet, D. Boeglin, H. Ong and W. D. Lubell, Org. Lett., 2009, 11, 3650 CrossRef CAS PubMed.
- (a) G. Giacomelli, A. Porcheddu and M. Salaris, Org. Lett., 2003, 5, 2715 CrossRef CAS PubMed; (b) M. A. Bailén, R. Chinchilla, D. J. Dodsworth and C. Na`jera, Tetrahedron Lett., 2001, 42, 5013 CrossRef; (c) M. C. Pirrung and J. H. L. Chau, J. Org. Chem., 1995, 60, 8084 CrossRef CAS; (d) E. Thouin and W. D. Lubell, Tetrahedron Lett., 2000, 41, 457 CrossRef CAS; (e) A. R. Tunoori, J. M. White and G. I. George, Org. Lett., 2000, 2, 4091 CrossRef CAS PubMed; (f) J. G. Kim and D. O. Jang, Bull. Korean Chem. Soc., 2010, 31, 171 CrossRef CAS.
- (a) A. Gissot, A. Volonterio and M. Zanda, J. Org. Chem., 2005, 70, 6925 CrossRef CAS PubMed; (b) C. Y. Ho, E. Strobel, J. Ralbovsky, Jr and R. A. Galemmo, J. Org. Chem., 2005, 70, 4873 CrossRef CAS PubMed; (c) J. C. S. Woo, E. Fenster and G. R. Dake, J. Org. Chem., 2004, 69, 8984 CrossRef CAS PubMed; (d) K. Ramasamy, R. K. Olsen and T. Emery, J. Org. Chem., 1981, 46, 5438 CrossRef CAS.
- (a) J. E. Pop, B. P. Déprez and A. L. Tartar, J. Org. Chem., 1997, 62, 2594 CrossRef PubMed; (b) M. C. Desai and L. M. S. Stramiello, Tetrahedron Lett., 1993, 34, 7685 CrossRef CAS; (c) C. F. Sturino and M. Labelle, Tetrahedron Lett., 1998, 39, 5891 CrossRef CAS.
- N. B. Palakurthy and B. Mandal, Tetrahedron Lett., 2011, 52, 7132 CrossRef CAS PubMed.
- (a) L. A. Carpino, J. Xia and A. J. El-Faham, J. Org. Chem., 2004, 69, 62 CrossRef CAS PubMed; (b) B. Devadas, B. Kundu, A. Srivastava and K. B. Mathur, Tetrahedron Lett., 1993, 34, 6455 CrossRef CAS; (c) S. N. Khattab, Chem. Pharm. Bull., 2010, 58, 501 CrossRef CAS.
- (a) [α]D = −55.1° at 25 °C, c =1.1, CHCl3 for entry 8, and [α]D = −6.4° at 25 °C, c = 1.1, CHCl3 for entry 10; (b) M. P. Sibi, H. Hasegawa and S. R. Ghorpade, Org. Lett., 2002, 4, 3343 CrossRef CAS PubMed.
- F. X. Webster and R. M. Silverstein, in Spectrometric identification of Organic Compounds, John Wiley & Sons, 6th Edn., 1997 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: copies of spectra. See DOI: 10.1039/c3ra44294b |
| This journal is © The Royal Society of Chemistry 2014 |