Copper-catalyzed N-(hetero)arylation of amino acids in water†
Krishna K. Sharma,
Meenakshi Mandloi,
Neha Rai and
Rahul Jain*
Department of Medicinal Chemistry, National Institute of Pharmaceutical Education and Research, Sector 67, S. A. S. Nagar, Punjab 160 062, India. E-mail: rahuljain@niper.ac.in
First published on 5th October 2016
Abstract
An environmentally benign, mild, cost-effective and gram-scalable copper-catalyzed method for the N-(hetero)arylation of zwitterionic natural and unnatural amino acids using 2-isobutyrylcyclohexanone as a β-diketone ligand and aryl bromides as coupling partners in water for 50 min at 90 °C under microwave irradiation is reported. Electronically and sterically diverse aryl coupling partners were inserted efficiently, including challenging heteroaryl electrophilic partners in high yields without affecting the enantiopurity of the product.
During the past two decades, functionalized amino acids have received great attention in synthetic organic chemistry, and drug discovery emanating out of peptide-based structural design.1,2 The presence of synthetically modified natural and unnatural amino acids in biologically relevant structural scaffolds makes them a potential lynchpin for functionalization.
Natural peptides and proteins show high activity, but have limited therapeutic potential because of their large size, conformational flexibility and proteolysis-initiated low metabolic stability. The introduction of functionalized amino acids in the peptide backbone is one of the promising approaches to address these issues.3 Specifically designed N-alkylated amino acids provide potential chiral building blocks for incorporation in peptides, peptidomimetics and proteins leading to modulated conformational freedom, altered binding site interactions, and enhanced pharmacokinetic properties.4 Due to difficult accessibility, the application of N-arylated amino acids in the synthesis of bioactive peptides is an interesting but rather an unexplored area of research.
The transition metal copper-catalyzed Ullmann and palladium-catalyzed Buchwald–Hartwig amination reactions are common and efficient routes to synthesize N-arylated compounds from aryl halides.5,6 Higher cost of palladium catalysts and phosphine-based ligands, air and moisture sensitivity and toxic nature of palladium renders them environmentally-unfriendly catalytic systems for chemical transformations.6 The classical Ullmann reaction also suffers due to harsh conditions, high temperature, sluggish rate, stoichiometric use of metal, low yield, and use of high boiling toxic solvents such as DMF, DMSO or toluene dampening its applicability in the benign synthesis of enantiomerically pure products.7 Over the years, copper-catalyzed modified Ullmann-type reactions have received great attention primarily due to the advent of inexpensive and non-toxic copper salts and a wide array of commercially available low molecular weight bidentate ligands.8 Significant progress in copper-catalyzed reactions is witnessed due to the application of a plethora of nitrogen- and oxygen-based bidentate ligands such as phenanthrolines, aliphatic 1,2-diamines, ethylene glycol, diethyl salicylamide, thiophene 2-carboxylate, amino acid derivatives, β-diketones, β-ketoesters, BINOL and oxalic diamides on aliphatic and aromatic amine substrates.9 Despite of such advances in copper-catalyzed reactions, direct application of the reaction in the synthesis of N-arylated amino acids under benign conditions is absent or rare due to toxic reaction solvent and loss of enantiomeric purity in the desired chiral products.10
Few reports describe the use of water as solvent in amination reactions on aliphatic amines11 and amino acids,12 but with limitations such as use of designer ligands, lack of diverse substrate scope and harsh reaction conditions, making them unsuitable for the synthesis of enantiomerically pure products. More recently, some reports on the N-arylation of amino acid esters using designer and expensive palladium-ligand precatalytic systems in conventional organic medium have emerged.13 Therefore, an environmentally benign and generalized protocol for the N-arylation and challenging N-heteroarylation leading to the synthesis of enantiopure N-(hetero)arylated amino acids using inexpensive coupling partners is highly desirable. We planned N-(hetero)arylation reactions on the zwitterionic amino acids due to their cost-effective and cheap access. Functionalization of fully unprotected amino acid due to its zwitterionic character is one of the most difficult reaction; some exceptions includes amide bond formation, Kent ligation and Staudinger ligation.14 To overcome these limitations, we set our task in developing a racemization-free method for the N-arylation of unprotected amino acids under aqueous conditions.
In continuation of our work on the synthesis of functionalized amino acids and heterocycles and their exploration in search of potent peptide- and peptidomimetic-based chemical entities,15 we required a variety of N-(hetero)arylated amino acids. Herein, we report that inexpensive electrophilic aryl bromides undergo highly efficient cross-coupling with unprotected amino acids under environmentally benign conditions using a copper(I)-catalyzed protocol in the presence of β-diketone ligand in water mediated by microwave (MW) irradiation. The method is applicable to reactions scalable up to multi-gram of substrates. To the best of our knowledge, this is the first racemization-free report on the copper(I)-catalyzed N-(hetero)-arylation of natural and unnatural zwitterionic amino acids in water using inexpensive aryl bromides as coupling partners and β-diketone as ligand.
We began model experiments by reacting L-valine (1) and coupling partner bromobenzene to identify a suitable N-arylation protocol under aqueous conditions. First, we investigated cross-coupling reaction of bromobenzene with 1 in the presence of copper(I) iodide (5 mol%), ligand (20 mol%) and K2CO3 as a base (2.5 equiv.) under conventional heating at 90 °C for 24 h in water (1 mL), to observe no product formation. We then switched heating mode to more safe and faster microwave (MW) irradiation and were delighted to note that the use of well-known amino acid ligand, L-proline (L1) afforded the desired N-arylated product 2a in 33% yield, thereby providing necessary encouragement to further investigate N-arylation reaction in water. Under the reaction conditions described above, the use of ligands such as constrained aliphatic amines 1,2-diaminocyclohexane (L2) and trans-N,N′-dimethylcyclohexane-1,2-diamine (L3), and non-constrained amines N,N′-dimethylethylenediamine (L4) and N,N,N′,N′-tetramethyl-ethylenediamine (L5) produced 2a ranged between 17 and 24% yield (Scheme 1). Next, we explored the applicability of nitrogen containing aromatic ligands such as 2,2′-bipyridine (L6) and 1,10-phenanthroline (L7), herein ligand L6 afforded only trace amount of product while no product formation was noted with ligand L7 (Scheme 1). We next explored various acyclic and cyclic β-diketones ligands (L8–L11) under aqueous conditions and noted that L11 afforded 53% yield of the product, and clearly emerged as the most suited ligand for N-arylation reaction in water.
With the success of the most appropriate ligand identification study, we turned our attention to the optimization of other N-arylation reaction parameters (Table 1). We attempted a reaction by increasing the reaction time to 70 min; however no improvement in the product yield was noted (Table 1, entry 1). The attempts towards temperature variations did not offer any advantage by means of enhancement in product formation (Table 1, entries 2 and 3). The use of alternate bases such as KOH, K3PO4, and Cs2CO3 did not show any improvement in yield of product (Table 1, entries 4–6). The use of other catalytic sources such as Cu2O, CuBr, CuCl and CuOAc did not enhance yield of N-arylated product (Table 1, entries 7–10).
| Entry | [Cu] (5 mol%) | Base | Temp. | Additive | 2ab (% yield) |
|---|---|---|---|---|---|
| a All reactions were performed with L-valine (1.2 equiv.), PhBr (1 equiv. 0.5 mmol), base (2.5 equiv.), H2O (1 mL), L11 (20 mol%).b Isolated yield.c Reaction time (70 min).d PEG-400 (1 equiv.).e PEG-400 (1.5 equiv.).f CuI (10 mol%), ligand (40 mol%).g Ligand was not used.h Conventional heating for 24 h. | |||||
| 1 | CuI | K2CO3 | 90 °C | — | 50c |
| 2 | CuI | K2CO3 | 80 °C | — | 41 |
| 3 | CuI | K2CO3 | 110 °C | — | 47 |
| 4 | CuI | KOH | 90 °C | — | 15 |
| 5 | CuI | K3PO4 | 90 °C | — | 7 |
| 6 | CuI | Cs2CO3 | 90 °C | — | 11 |
| 7 | Cu2O | K2CO3 | 90 °C | — | 17 |
| 8 | CuBr | K2CO3 | 90 °C | — | 19 |
| 9 | CuCl | K2CO3 | 90 °C | — | 46 |
| 10 | CuOAc | K2CO3 | 90 °C | — | 26 |
| 11 | CuI | K2CO3 | 90 °C | Ethylene glycol | 10 |
| 12 | CuI | K2CO3 | 90 °C | Glycerol | Trace |
| 13 | CuI | K2CO3 | 90 °C | PEG | 45 |
| 14 | CuI | K2CO3 | 90 °C | PEG-400 | 82d |
| 15 | CuI | K2CO3 | 90 °C | PEG-400 | 87e |
| 16 | CuI | K2CO3 | 90 °C | PEG-400 | 82f |
| 17 | CuI | K2CO3 | 90 °C | PEG-200 | 61 |
| 18 | CuI | K2CO3 | 90 °C | PEG-600 | 56 |
| 19 | CuI | K2CO3 | 90 °C | PEG-400 | 11g |
| 20 | CuI | K2CO3 | 90 °C | PEG-400 | Traceh |
We reasoned that attempts to improve yield failed possibly because of the partial solubility of the reactive state complexes in the solvent medium. An examination of the literature reports revealed that the use of additives results in enhanced product formation, possibly by increasing the solubility of transition state intermediates without affecting reaction sustainability.16 This prompted us to examine the applicability of various additives such as ethylene glycol, glycerol, PEG, PEG-200, PEG-400 and PEG-600 in the N-arylation reaction. The use of strongly hydrophilic PEG-400 (1 equiv.) afforded 2a in 82% yield (Table 1, entry 14) while increasing its quantity to 1.5 equivalents afforded remarkably higher 87% yield of 2a under aqueous conditions (Table 1, entry 15). The use of ethylene glycol, glycerol, PEG, PEG-200, and PEG-600 afforded 2a ranged in trace-61% yield (Table 1, entries 11–13, 17 and 18). It was noted that omitting the ligand from the reaction under optimized conditions afforded product in 11% yield, clearly confirming that β-diketone ligand L11 is critical for reaction (Table 1, entry 19). No product formation was observed in an attempt to conduct reaction under conventional heating conditions reflecting on the desirability of MW irradiation in the N-arylation reaction in water (Table 1, entry 20).
To demonstrate the utility of newly developed sustainable in-water protocol, aryl substrate scope of electronically and sterically diverse aryl bromides was investigated with 1 as nucleophilic partner. Less electrophilic electron-donating aryl partners easily cross-coupled under the developed protocol in high yields (Table 2, entries 2b–d). Electron-withdrawing group substituted aryl bromides also coupled very efficiently with 1 to afford product in up to 91% yield (Table 2, entries 2e–r). The N-arylation method was found highly robust in coupling sterically bulky electrophilic partners such as tert-butylphenyl bromide, biphenyl bromide and naphthyl bromide to produce arylated amino acids in 80–83% yields (Table 2, entries 2d–f). The reaction is noted to exhibit excellent functional group tolerance as a range of functional group containing aryl bromide easily coupled in high yields (Table 2, entries 2g–r). Noticeable examples are ortho-substituted derivative (Table 2, entry 2m) and disubstituted derivatives (Table 2, entries 2j and 2q–r). Chemically reactive functional groups containing aryl bromides also coupled efficiently with complete chemoselectivity due to mild nature of the developed protocol (Table 2, entries 2g–h, and 2n–o). Heteroaryl bromides such as 3-bromopyridine, 3-bromoquinoline and 6-bromoquinoline also coupled with the zwitterionic L-valine (1) in excellent yields to provide entry to difficult to access N-heteroarylated amino acid derivatives (Table 2, entries 2s–u).
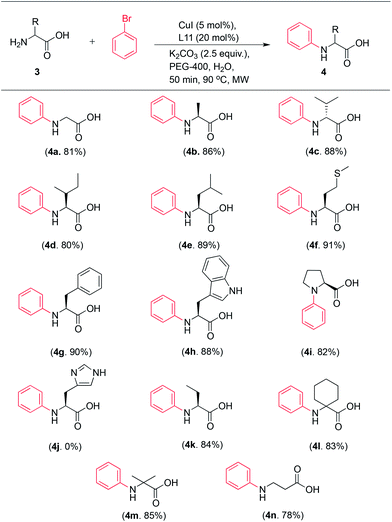
We then examined the amino acid scope of the reaction by coupling of bromobenzene with a large number of natural and unnatural amino acids and the results of the study are summarized in Table 3. The reaction was noted to have wide applicability amply demonstrated by successful N-arylation of various α-, β- and even cyclic amino acids in high yields, irrespective of the size and the nature of the side chain.
Natural α-amino acids were N-arylated in up to 91% yield with bromobenzene (Table 3, entries 4a–b and 4d–i). The unnatural amino acids also coupled readily with bromobenzene to produce the N-arylated products in yields ranged between 78–84% (Table 3, entries 4k–n). We noted although not surprisingly, N-arylation of unprotected L-histidine was not allowed under this protocol (Table 3, entry 4j). We envision that it is due to the formation of Cu–histidine complex during the reaction, besides its tautomeric character and H-bond donor and acceptor properties rendering it difficult to functionalize without protection.17
To examine the wider application of the protocol, the N-arylation of amino acids was scaled up to 10 mmol. As noted from Scheme 2, almost identical yields of 2e, 2t and 4h were obtained under the optimized mild and benign reaction conditions (Scheme 2).
A plausible mechanistic cycle of the copper(I)-catalyzed N-arylation reaction of zwitterionic amino acids with electrophilic aryl bromides proceeds through a modified Ullmann-type mechanism via an oxidative-addition–reductive elimination cycle involving Cu(I)/Cu(III) intermediates (Scheme 3).18 It is envisioned that N-arylation reaction proceeds via complexation of copper(I) iodide with β-ketone ligand L11, which is followed by nucleophile coordination by amino group, resulting in the formation of the transition state TS-1. Next, deprotonation by the base results in the Cu(I)-centred intermediate stage TS-2, which subsequently via electrophilic coordination with bromobenzene gave Cu(I)-centred complex TS-3. The oxidative addition transformation results in the Cu(III)-centered transition state TS-4. In the final step of the catalytic cycle, TS-4 rearranges to form the desired N-phenyl-L-valine via reductive elimination and regeneration of the Cu(I)-L11 complex in the reaction medium through the expulsion of bromide anion.
The racemization study of the synthesized N-arylated amino acids was performed on HPLC using a ChiralPak WH column and a mobile phase of 0.25 mM copper(II) sulfate in water (93%) and 2-propanol (7%) for 180 min. The column temperature was kept at 50 °C, and the flow rate of 1 mL min−1 was fixed for analysis. For the purpose of comparison, we also undertook the synthesis of N-phenyl-DL-valine. The chiral purity of N-phenyl-L-valine (2a), N-phenyl-D-valine (4c), and N-phenyl-DL-valine under the optimized conditions was examined, and it was found that enantiomeric purity of N-phenyl-L-valine and N-phenyl-D-valine remained intact during the copper(I)-catalyzed transformation (please see, ESI†). It is evident that the use of zwitterionic amino acids prevents the base-mediated α-proton abstraction, resulting in intact enantiomeric integrity of the arylated amino acids.
In conclusion, we have successfully developed an efficient, rapid, environmentally benign, gram-scalable, practical and experimentally safe approach for the N-(hetero)arylation of amino acids under mild conditions using inexpensive aryl bromides as coupling partners. This protocol allow low catalyst and ligand loading for N-arylation transformation in 50 min under MW irradiation and uses copper(I) iodide as catalyst, 2-isobutyrylcyclohexanone (L11) as ligand and PEG-400 as additive in water. This catalytic transformation readily coupled ortho-, meta-, and para-positions reactive functional group containing aryl bromides in high yields. This protocol is equally applicable to access challenging N-heteroarylated amino acids under the identical reaction conditions. By using this protocol, various α- and β-natural and unnatural amino acids were N-arylated in high yields. The application of method on fully unprotected zwitterionic amino acids provides scaffolds that could be easily modified at either of the terminus rendering them suitable for immediate assembly in a peptide-based structure. Chiral HPLC study shows that enantiomeric purity of the product during N-arylation of zwitterionic amino acid remains intact. Most importantly, this methodology opens up new perspectives for the synthesis of N-arylated amino acids under environmentally benign conditions in a cost-effective manner. Further application of N-(hetero)arylated amino acids in search of the bioactive peptides and peptidomimetics is currently under investigation.
Acknowledgements
Krishna K. Sharma thanks University Grant Commission (UGC), New Delhi, India for the award of Senior Research Fellowship.Notes and references
- A. F. Noisier and M. A. Brimble, Chem. Rev., 2014, 114, 8775 CrossRef CAS PubMed.
- (a) A. P. Kozikowski, S. Wang, D. Ma, J. Yao, S. Ahmad, R. I. Glazer, K. Bogi, P. Acs, S. Modarres, N. E. Lewin and P. M. Blumberg, J. Med. Chem., 1997, 40, 1316 CrossRef CAS PubMed; (b) G. A. Doherty, T. Kamenecka, E. McCauley, G. Van Riper, R. A. Mumford, S. Tong and W. K. Hagmann, Bioorg. Med. Chem. Lett., 2002, 12, 729 CrossRef CAS PubMed; (c) J. Lee, C. Reynolds, M. C. Jetter, M. A. Youngman, D. J. Hlasta, S. L. Dax, D. J. Stone, S.-P. Zhang and E. E. Codd, Bioorg. Med. Chem. Lett., 2003, 13, 1879 CrossRef CAS PubMed; (d) W. G. Thomas and F. A. Mendelsohn, Int. J. Biochem. Cell Biol., 2003, 35, 774 CrossRef CAS PubMed; (e) Y. Endo, K. Shudo, K. Furuhata, H. Ogura, S. Sakai, N. Aimi, Y. Hitotsuyanagi and Y. Koyama, Chem. Pharm. Bull., 1984, 32, 358 CrossRef CAS.
- (a) B. E. I. Ramakers, J. C. M. van Hest and D. W. P. M. Löwik, Chem. Soc. Rev., 2014, 43, 2743 RSC; (b) D. J. Craik, D. P. Fairlie, S. Liras and D. Price, Chem. Biol. Drug Des., 2013, 81, 136 CrossRef CAS PubMed; (c) F. Albericio and H. G. Kruger, Future Med. Chem., 2012, 4, 1527 CrossRef CAS PubMed; (d) D. A. Dougherty, Curr. Opin. Chem. Biol., 2000, 4, 645 CrossRef CAS PubMed.
- (a) S. Royo-Gracia, K. Gaus and N. Sewald, Future Med. Chem., 2009, 1, 1289 CrossRef PubMed; (b) A. Grauer and B. Konig, Eur. J. Org. Chem., 2009, 5099 CrossRef CAS.
- (a) I. P. Beletskaya and A. V. Cheprakov, Coord. Chem. Rev., 2004, 248, 2337 CrossRef CAS; (b) C. Fischer and B. Koenig, Beilstein J. Org. Chem., 2011, 7, 59 CrossRef CAS PubMed; (c) J. F. Hartwig, Angew. Chem., Int. Ed., 1998, 37, 2046 CrossRef CAS; (d) L. Jiang and S. L. Buchwald, Palladium-Catalyzed Aromatic Carbon–Nitrogen Bond Formation, Wiley-VCH, Weinheim, 2nd edn, 2004, p. 699 Search PubMed; (e) E. M. Beccalli, G. Broggini, M. Martinelli and S. Sottocornola, Chem. Rev., 2007, 107, 5318 CrossRef CAS PubMed; (f) B. H. Yang and S. L. Buchwald, J. Organomet. Chem., 2009, 576, 125 CrossRef.
- H. Zhang, Q. Cai and D. Ma, J. Org. Chem., 2005, 70, 5164 CrossRef CAS PubMed.
- (a) F. Ullmann, Chem. Ber., 1903, 36, 2382 CrossRef; (b) F. Ullmann and P. Sponagel, Chem. Ber., 1905, 38, 2211 CrossRef CAS.
- For selected reviews on copper-catalyzed cross coupling reactions, see: (a) I. P. Beletskaya and A. V. Cheprakov, Coord. Chem. Rev., 2004, 248, 2337 CrossRef CAS; (b) S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400 CrossRef CAS PubMed; (c) K. Kunz, U. Scholz and D. Ganzer, Synlett, 2003, 2428 CrossRef CAS.
- (a) R. K. Gujadhur, C. G. Bates and D. Venkataraman, Org. Lett., 2001, 3, 4315 CrossRef CAS PubMed; (b) J. C. Antilla, J. M. Baskin, T. E. Barder and S. L. Buchwald, J. Org. Chem., 2004, 69, 5578 CrossRef CAS PubMed; (c) L. Jiang, G. E. Job, A. Klapars and S. L. Buchwald, Org. Lett., 2003, 5, 3667 CrossRef CAS PubMed; (d) J. C. Antilla, A. Klapars and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 11684 CrossRef CAS PubMed; (e) F. Y. Kwong, A. Klapars and S. L. Buchwald, Org. Lett., 2002, 4, 581 CrossRef CAS PubMed; (f) F. Y. Kwong and S. L. Buchwald, Org. Lett., 2003, 5, 793 CrossRef CAS PubMed; (g) H.-J. Cristau, P. P. Cellier, J.-F. Spindler and M. Taillefer, Eur. J. Org. Chem., 2004, 695 CrossRef CAS; (h) H.-J. Cristau, P. P. Cellier, J.-F. Spindler and M. Taillefer, Chem.–Eur. J., 2004, 10, 5607 CrossRef CAS PubMed; (i) S. Zhang, D. Zhang and L. S. Liebeskind, J. Org. Chem., 1997, 62, 2312 CrossRef CAS PubMed; (j) D. Ma, Y. Zhang, J. Yao, S. Wu and F. Tao, J. Am. Chem. Soc., 1998, 120, 12459 CrossRef CAS; (k) D. Ma, Q. Cai and H. Zhang, Org. Lett., 2003, 5, 2453 CrossRef CAS PubMed; (l) D. Jiang, H. Fu, Y. Jiang and Y. Zhao, J. Org. Chem., 2007, 72, 672 CrossRef CAS PubMed; (m) X. Lv and W. Bao, J. Org. Chem., 2007, 72, 3863 CrossRef CAS PubMed; (n) A. Shafir and S. L. Buchwald, J. Am. Chem. Soc., 2006, 128, 8742 CrossRef CAS PubMed; (o) W. Zhou, M. Fan, J. Yin, Y. Jiang and D. Ma, J. Am. Chem. Soc., 2015, 137, 11942 CrossRef CAS PubMed.
- J. P. Greenstein and M. Wintz, Chemistry of the amino acids, John Wiley & Sons Inc., New York, 1961, vol. 1, p. 569 Search PubMed.
- (a) Y. Wang, Z. Wu, L. Wang, Z. Li and X. Zhou, Chem.–Eur. J., 2009, 15, 8971 CrossRef CAS PubMed; (b) D. Wang, F. Zhang, D. Kuang, J. Yu and J. Li, Green Chem., 2012, 14, 1268 RSC; (c) L. Liang, Z. Li and X. Zhou, Org. Lett., 2009, 11, 3294 CrossRef CAS PubMed; (d) J. Engel-Andreasen, B. Shimpukade and T. Ulven, Green Chem., 2013, 15, 336 RSC.
- (a) Z. Lu and R. J. Twieg, Tetrahedron Lett., 2005, 46, 2997 CrossRef CAS; (b) S. Rottger, P. J. Sjoberg and M. Larhed, J. Comb. Chem., 2007, 9, 204 CrossRef PubMed.
- (a) S. M. King and S. L. Buchwald, Org. Lett., 2016, 18, 4128 CrossRef CAS PubMed; (b) S. Sharif, M. G. Organ, D. Mitchell, M. J. Rodriguez and J. L. Farmer, Chem.–Eur. J., 2016, 22, 14860 Search PubMed.
- (a) R. K. Sharma and R. Jain, Syntlett, 2007, 603 CAS; (b) P. E. Dawson, T. W. Muir, I. Clark-Lewis and S. B. Kent, Science, 1994, 266, 776 CAS; (c) P. E. Dawson, M. J. Churchill, M. R. Ghadiri and S. B. Kent, J. Am. Chem. Soc., 1997, 119, 4325 CrossRef CAS; (d) P. E. Dawson and S. B. Kent, Annu. Rev. Biochem., 2000, 69, 923 CrossRef CAS PubMed; (e) M. Köhn and R. Breinbauer, Angew. Chem., Int. Ed., 2004, 43, 3106 CrossRef PubMed; (f) F. L. Lin, H. M. Hoyt, H. Halbeek, R. G. Bergman and C. R. Bertozzi, J. Am. Chem. Soc., 2005, 127, 2686 CrossRef CAS PubMed; (g) M. B. Soellner, A. Tam and R. T. Raines, J. Org. Chem., 2006, 71, 9824 CrossRef CAS PubMed.
- (a) A. Mahindra and R. Jain, Synlett, 2012, 23, 1759 CrossRef CAS; (b) A. Mahindra, N. Bagra and R. Jain, J. Org. Chem., 2013, 78, 10954 CrossRef CAS PubMed; (c) A. Mahindra and R. Jain, Org. Biomol. Chem., 2014, 12, 3792 RSC; (d) K. K. Sharma, S. Sharma, A. Kudwal and R. Jain, Org. Biomol. Chem., 2015, 13, 4637 RSC; (e) K. K. Sharma, D. I. Patel and R. Jain, Chem. Commun., 2015, 51, 15129 RSC; (f) A. Mahindra, K. K. Sharma, D. Rathore, S. I. Khan, M. R. Jacob and R. Jain, Med. Chem. Commun., 2014, 5, 671 RSC; (g) A. Mahindra, N. Bagra, N. Wangoo, S. I. Khan, M. R. Jacob and R. Jain, ACS Med. Chem. Lett., 2014, 5, 315 CrossRef CAS PubMed; (h) K. K. Sharma, M. Mandloi and R. Jain, Org. Biomol. Chem., 2016, 14, 8937 RSC.
- (a) M. Kidwai, N. K. Mishra, S. Bhardwaj, A. Jahan, A. Kumar and S. Mozumdar, ChemCatChem, 2010, 2, 1312 CrossRef CAS; (b) S. Chandrasekhar, S. S. Sultana, S. R. Yaragorla and N. R. Reddy, Synthesis, 2006, 5, 839 CrossRef; (c) Q. Zhang, J. Luo and Y. Wei, Synth. Commun., 2012, 42, 114 CrossRef CAS.
- (a) B. Sreedhar, K. S. Kumar, P. Srinivas, V. Balasubrahmanyam and G. T. Venkanna, J. Mol. Catal. A: Chem., 2007, 265, 183 CrossRef CAS; (b) J. Ohata, M. B. Minus, M. E. Abernathy and Z. T. Ball, J. Am. Chem. Soc., 2016, 138, 7472 CrossRef CAS PubMed.
- (a) R. A. Altman, E. D. Koval and S. L. Buchwald, J. Org. Chem., 2007, 72, 6190 CrossRef CAS PubMed; (b) A. Casitas, A. E. King, T. Parella, M. Costas, S. S. Stahl and X. Ribas, Chem. Sci., 2010, 1, 326 RSC; (c) G. Lefevre, G. Franc, C. Adamo, A. Jutand and I. Ciofini, Organometallics, 2012, 31, 914 CrossRef CAS; (d) G. Lefevre, G. Franc, A. Tlili, C. Adamo, M. Taillefer, I. Ciofini and A. Jutand, Organometallics, 2012, 31, 7694 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, product characterization, copies of 1H and 13C NMR and chiral purity studies. See DOI: 10.1039/c6ra23364c |
| This journal is © The Royal Society of Chemistry 2016 |