A new method for improving the ion conductivity of anion exchange membranes by using TiO2 nanoparticles coated with ionic liquid
Yuhao Chu,
Yuenan Chen,
Nanjun Chen,
Fanghui Wang and
Hong Zhu*
State Key Laboratory of Chemical Resource Engineering, Institute of Modern Catalysis, Department of Organic Chemistry, School of Science, Beijing University of Chemical Technology, Beijing, 100029, P. R. China. E-mail: zhuho128@126.com; Tel: +86-10-64444919
First published on 6th October 2016
Abstract
Recently a new method for increasing the ion conductivity of anion exchange membranes (AEM) was developed based on the novel materials ionic liquids (ILs). We mixed the ILs into the membrane directly instead of immobilizing onto the polymer backbone as in the traditional way. Nano-TiO2 was introduced to stabilize the ILs in the membrane. The ILs were immobilized by the nano-TiO2, acting as the “active sites” in the membrane, to enhance the mobility of the hydroxyl groups so as to increase the ion conductivity. Both pure ILs composite membranes and ILs–TiO2 composite membranes were synthesized, and their properties were compared. 1H nuclear magnetic resonance spectroscopy and Fourier transform infrared spectroscopy were used to analyze the structures of the composite membranes. The mechanical properties, thermal stabilities, ion conductivities, water uptakes, swelling ratios, and ion exchange capacities of the membranes were investigated. The interaction between the TiO2 and ionic liquids was confirmed by X-ray diffraction. The stability of the ILs in the membrane was measured comprehensively. All these results show that this novel method is effective and promising for AEM applications.
Introduction
Recently, fuel cells have become a popular clean energy technology because of their effectiveness in energy conversion.1–5 Proton exchange membrane fuel cells (PEMFCs) have been widely used in many fields like portable, stationary power and even electromobile.6 However, the high costs of the materials in PEMFCs and complex water management have limited the applications of PEMFCs.7,8 In the past few years, anion exchange membrane fuel cells (AEMFCs) have been developed to overcome the disadvantages of PEMFCs such as the cost and stability of the catalyst.9 Instead of the platinum catalysts used in PEMFCs, the catalysts used in AEMFCs can be non-noble metal catalysts, which can vastly reduce the cost and improve the stability of the noble catalysts.10,11 However, the low ion conductivity and poor dimensional stability of the anion exchange membranes (AEMs) have hindered the development of AEMFCs.12,13 Thus, the development of efficient AEMs has become more and more urgent.Ion conductivity has proved to be the most important issue in the development of AEMs.14–16 Many developments have been made in recent years to improve the ion conductivity of AEMs. Zha et al. have developed a novel metal-cation-based AEM in which the traditional quaternary ammonium groups were replaced by the metal-cation complex17 to improve the ion conductivity. This work provided effectively method for developing the novel cation, however, the cost of developing novel cation is relatively high and the synthesis route is complex. Many studies are focused on modifying the microstructures of the membranes, and hydrophilic/hydrophobic microphase separation is investigated so as to display well-defined ion conductive channels. Zhuang et al. have shown that hydrophilic/hydrophobic microphase separation can be obtained by changing the length of the carbon chains grafted with conductive groups,18 introducing alkyl chains into the polymer backbone,19 and varying the distance between the conductive groups and alkyl chains.20 These three methods can build well-organized ion conductive channels in the membranes and greatly improve the ion conductivity. Another way to obtain microphase separation is the use of block copolymers. Many research groups have demonstrated that microphase separation through the formation of diblock copolymers can improve the properties of the membrane effectively. Multiple copolymers have been done following this method, such as PPO diblock copolymers,21 aromatic block copolymers,22 and phenolphthalein-based multiblock copolymers.23 AEMs based on these copolymers achieved well properties.
Herein, we developed a relatively simple method focus on improving the ion conductivity of AEMs without changing the microstructure of the membranes. Dissociated ionic liquids were first introduced into the membranes in AEMs. In our design, the ionic liquids act as the “active sites” in the membrane and can accelerate the movement of the hydroxyl groups. When the hydroxyl groups attempt to pass through the membrane, the electrostatic attraction between the ILs and the hydroxyl groups can improve the mobility of the hydroxyl groups so as to form the ion conductive channel in the membrane, thus leading to the improvement in ion conductivity. In avoiding the run off the ILs under high relative humidity, we introduced nano-TiO2 to stabilize the ILs in the membrane. The interaction between the nano-TiO2 and ILs has been confirmed in the literature.24–26 We dispersed the nano-TiO2 into the polymer chains sufficiently by the ultrasound method, so the ILs can be “immobilized” in the membrane owing to the interaction between the ILs and nano-TiO2. The ILs dispersed in the membrane offer ion conductive sites which help form the conductive channels.
In this work, we chose the common polymer backbone PPO and the conventional conductive group triethylamine so that the ability of ILs to improve the ion conductivity of AEMs can be investigated comprehensively. The PPO was firstly brominated into the bromine form and then substituted by the triethylamine after the Menshutkin reaction. Subsequently, different contents of ILs were mixed into the membrane to prepare a series of triethylamine–ionic liquid composite AEMs. A certain amount of nano-TiO2 was added into the membranes to form a series of triethylamine–nano TiO2–ionic liquid composite AEMs. The interactions between nano-TiO2 and ILs in the free states and in the membranes were measured by XRD respectively. The structure of the AEMs was studied by FTIR, NMR, and SEM. The AEM properties ion exchange capacity (IEC), water uptake, swelling ratio, hydroxide ion conductivity, mechanical and thermal properties, and chemical stability were analyzed.
Experimental
Materials
Nano-TiO2, N-methylimidazolium and methyl iodide were purchased from Aladdin Chemical (Shanghai, China) and used as received. PPO was purchased from Nanya Chemical Company (Nanjing, China). All the solutions were stored over 4 Å molecular sieves. All other chemicals were purchased from the Beijing Chemical Reagent Store (China) and used without further purification. Deionized water was used throughout this work.Preparation of 1-methy-3-methylimidazolium ionic liquid (MeIL)
N-Methylimidazolium (1.5 g, 0.018 mol) and iodomethane (3.12 g, 0.021 mol) were mixed in a flask under electromagnetic stirring. The mixture was kept at room temperature for two hours. The product was washed by ethyl acetate for three times and evaporated under decompression to remove the residual solvent. The melting point was measured to be 75 °C (literature data 79 °C). The structure of the product was confirmed by NMR.Preparation of composites of ionic liquid and nano-TiO2
Nano-TiO2 (0.8 g, 0.01 mol) was first diluted in 5 ml of 50% ethanol/water solution, and then 1-methy-3-methylimidazolium ionic liquid (2.24 g, 0.01 mol) was added into the solution to form a uniform system. The solution was evaporated at room temperature under magnetic stirring for 30 min. The product was then dried in the oven at 40 °C for 24 h. Subsequently, the product was maintained under vacuum for 5 days at 40 °C to remove any residual solvent. The resultant powders were used for powder X-ray diffraction and TEM measurements.Preparation of triethylamine-grafted membrane (BPPO–TEA)
0.25 g of bromomethylated poly(2,6-dimethyl-1,4-phenylene oxide) (BPPO) with a degree of bromination of 50% was dissolved in 10 ml of dimethyl formamide (DMF). Excess triethylamine was added into the solution to complete the quaternization. Then the solution was cast on a flat glass plate and dried for 24 h in an oven at 120 °C to form the BPPO–TEA membrane.Preparation of triethylamine–ionic liquid composite membrane (BPPO–TEA–IL)
0.25 g of bromomethylated poly(2,6-dimethyl-1,4-phenylene oxide) (BPPO) with a degree of bromination of 50% was dissolved in 10 ml of DMF. Then triethylamine was added into the solution to complete the quaternization. The reaction was maintained at room temperature for 4–5 h, and then different amounts (5%, 10%, 15%, 20%, and 25% weight of the polymer) of the ionic liquid were dispersed into the polymer solution to form solutions with different concentrations of ionic liquid. Subsequently, the solutions were cast on flat glass plates and dried in an oven for 24 h to form BPPO–TEA–IL composite membranes. The membranes were soaked in 1 M sodium hydroxide aqueous solution for 48 h to finish the anion exchange. Finally, the obtained composite membranes were washed several times with deionized water and immersed in deionized water before testing.Preparation of triethylamine–nano TiO2–ionic liquid composite membranes (BPPO–TEA–1TiO2–IL)
0.0025 g of nano-TiO2 (1% of the weight of the polymer) was dispersed into 10 ml of DMF. The solution was kept in an ultrasonic bath for 12 h to make sure that the nano-TiO2 was dispersed adequately. Then 0.25 g of polymer was dissolved into the solution for 12 h to ensure that the polymer structure was uniformly filled with the nano-TiO2. Subsequently, the quaternization was achieved according to the above method, and different amounts of the ionic liquid were added. The system was maintained at room temperature for 8 h to form nano-TiO2–ionic liquid–polymer composite solutions with different concentrations of ionic fluids. The solutions were cast on flat glass plates and dried in an oven for 24 h to form the BPPO–TEA–1TiO2–IL composite membranes. The membranes were soaked in 1 M sodium hydroxide aqueous solution before taken from the alkali solution. And then, the membranes were washed by deionized water for several times and immersed in deionized water before testing.Measurements
| IEC = (VHCl × C1 − VNaOH × C2)/M | (1) |
| WU (%) = (mw − md)/md × 100% | (2) |
The SR of the membrane was measured in three directions (length, width, and thickness) by analyzing the variation of the parameters after the membrane has been soaked in deionized water for 24 h.
| Swelling ratio (%) = (Lw − Ld)/Ld × 100% | (3) |
The WU and SR measurements were made at room temperature and 60 °C.
| σ = d/(RA) | (4) |
Results and discussion
Synthesis of methyl ILs and nano-TiO2 composite materials
The synthesis procedure for the methyl IL (MeIL) is given in Scheme 1. The structure of MeIL is confirmed by the NMR spectrum shown in Fig. 1. The peaks at 9.0 and 7.6 are assigned to the hydrogens on the imidazole ring. The single peak around 4.0 is assigned to the hydrogens on the two methyl groups. The peak between 3.0 and 3.5 belongs to the residual water, and the peak at 2.5 is the solvent peak.The nano-TiO2 composite materials were prepared according to the self-assembly method. XRD was used to investigate the interactions between the TiO2 nanoparticles and ILs. The patterns for pure TiO2 nanoparticles, pure MeIL, and mixtures of TiO2 and MeIL are shown in Fig. 2. The results show that the mixture retains most of the crystalline structures of both component materials, while the new peaks and peak displacements indicate interactions between the TiO2 nanoparticles and ILs at the atomic level. The TEM was analysed to investigate the size of composite materials. The result is shown in Fig. 3. The size of pure nano-TiO2 bought from Aladdin Chemical is 25 nm and the size of composite materials is calculated to be between 50–70 nm. The larger size of the composite materials due to the presence of IL layers and aggregation of nano-TiO2.
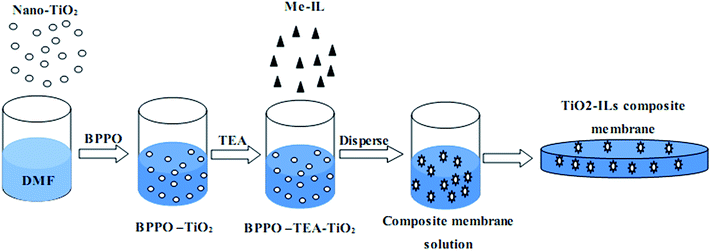
Synthesis of triethylamine–nano TiO2–ionic liquid composite membranes
The composite membranes were prepared according to the route shown in Scheme 2. The route is composed of grafting, dispersing, and self-assembly. The FTIR spectra are shown in Fig. 4. The peaks around 3500 cm−1 are associated with the residual water in the membrane, which indicates the well ability of store water of the membranes. The peaks at 2850 cm−1 is associated with the C–H bond and the peaks between 1500 cm−1 to 1600 cm−1 are ascribed to the characteristic peaks on the benzene. There hardly any peaks appear at 620 cm−1, the successful displace of C–Br is thus confirmed. The NMR test results are shown in Fig. 5. The peaks at 9.0, 7.6, and 3.9 indicate the successful introduction of the methyl ILs. The peaks around 7.0 and 6.5 are assigned to the hydrogens on the benzene rings. The hydrogens on the groups –CH2– and –CH2CH3 can be observed between 1.2 and 3.5. The peak around 3.0 is assigned to the residual water.XRD was used to study the interactions between MeIL and nano-TiO2 in the membranes. The patterns are shown in Fig. 6. The patterns for BPPO–TEA and BPPO–TEA–IL are similar in general, showing that the IL is mixed into the polymer directly without any interactions with the polymer chains. After the doping of the nano-TiO2, some peaks have disappeared, and the characteristic peaks of TiO2 are observed, indicating that the microstructure of the polymer has changed. Therefore, the interactions between the TiO2, IL, and polymer chains in the membrane is confirmed.
Membrane morphology
Scanning electron microscopy (SEM) was used to analyze the structure of the membranes. BPPO–TEA–IL and BPPO–TEA–1TiO2–15IL were observed severally. The micrographs are shown in Fig. 7. The BPPO–TEA–IL membrane is homogeneous in general, but some impurity particles can be observed. After the addition of the nano TiO2, the self-assembly interaction between the MeILs and the surface of the nano TiO2 enhances the dispersion of the nano TiO2–MeIL composite particles because the nano TiO2 particles are well dispersed in the membrane. No obvious agglomeration of the composite particles can be observed from the images. These results imply that the prepared composite membranes have proper structure.The EDX-mapping test was selected to investigate the elements and their dispersion in the BPPO–TEA–1TiO2–15IL membrane. As shown in Fig. 8, the elements C, N, O, Br, Ti, and I are observed in the membrane, confirming the successfully introduction of nano TiO2 and MeIL. Fig. 9 shows the dispersion of the elements. The blue points stand for the element Ti, the green points represent the element I, and the red points stand for the element N. All the elements are uniformly distributed in the membrane. The elements N and I aggregate around the element Ti, indicating the self-assembly structure between the MeIL and the surface of the nano TiO2.
 | ||
| Fig. 9 (a) Dispersion of N in membrane BPPO–TEA–1TiO2–15MeIL, (b) dispersion of Ti in membrane, (c) dispersion of I in membrane, and (d) dispersion of all three elements. | ||
Ionic conductivity
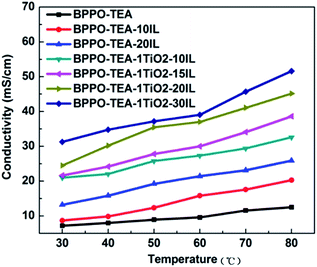
The ion conductivities of the BPPO–TEA–ILs composite membranes were measured under 100% relative humidity (RH). A membrane with only TEA was also synthesized and measured. The results are shown in Fig. 10. The results indicate that the introduction of even a small amount of IL can improve the ion conductivity of the membrane. The ion conductivity of the membrane increases with increasing IL content. Considering that the polymer structure becomes loosened at high temperatures, the IL molecules can run off from the membranes. As a result, the ion conductivity of the membrane increases more slowly at high IL contents and high temperatures, as shown in Fig. 10.TiO2 was added to stabilize the IL in the membrane. The ion conductivities of the membranes with TiO2 were measured detailedly to compare with those of the corresponding membranes without TiO2. The results were listed in Fig. 11. The significant increase in ion conductivity with the addition of TiO2 indicates that the IL is effectively stabilized by the nano-TiO2 dispersed in the polymer. Like those of the corresponding membranes without TiO2, the ion conductivities of the TiO2–IL composite membranes also increase with increasing IL content. At an IL content of 30 wt%, the ion conductivity can reach 51.6 mS cm−1 at 80 °C, which is a great improvement over that of the BPPO–TEA membrane. These results indicate that the addition of nano-TiO2 can reduce the wastage of IL because the IL is stored in the membrane. The self-assembly between the nano-TiO2 and ILs can stabilize the ILs in the membrane so that more ILs can play their roles in improving the ion conductivities of the membranes.
The Arrhenius behaviors of the corresponding membranes without TiO2 and nano-TiO2–ILs composite membranes were investigated respectively. The transport activation energy Ea of an AEM is calculated as follows:
| Ea = −b × R | (5) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) σ–1000/T, and R is the gas constant. The Ea values of the corresponding membranes without TiO2 range from 11.47 to 14.81 kJ mol−1, which are close to the literature values of common AEMs.27–29 The Ea values of the nano-TiO2–ILs composite membranes, which range from 7.36 to 9.47 kJ mol−1, are much smaller indicating the ion conductive channels in these membranes are less sensitive to the temperature and more stable in the solution. The introduction of nano-TiO2 stabilizes the ILs and the interaction between nano-TiO2, IL and polymer backbones make the polymer chains combine closer so that the ion conductive channels created by the active sites of IL are enhanced.30 The results are shown in Fig. 12.
σ–1000/T, and R is the gas constant. The Ea values of the corresponding membranes without TiO2 range from 11.47 to 14.81 kJ mol−1, which are close to the literature values of common AEMs.27–29 The Ea values of the nano-TiO2–ILs composite membranes, which range from 7.36 to 9.47 kJ mol−1, are much smaller indicating the ion conductive channels in these membranes are less sensitive to the temperature and more stable in the solution. The introduction of nano-TiO2 stabilizes the ILs and the interaction between nano-TiO2, IL and polymer backbones make the polymer chains combine closer so that the ion conductive channels created by the active sites of IL are enhanced.30 The results are shown in Fig. 12.
IEC, water uptake, and swelling ratio of membranes
The water uptake (WU) and swelling ratio (SR) are important properties of anion exchange membranes. These properties are closely connected to the mechanical properties of AEMs. A low swelling ratio often leads to fine mechanical properties. The WUs and SRs of the membranes were measured at room temperature and 60 °C. The results are shown in Table 1. The WUs and SRs are higher at 60 °C than at room temperature because the loose structure of the polymer will absorb more water molecules at the higher temperature. When come to the total results, we found an interesting phenomenon. The SRs of the membranes vary within a small range with increasing of IL content in the composite membrane. An explanation is given as follows: the swelling ratio is mostly decided by the structure of the polymer backbone and the content of ion conductive groups, and the added ILs just act as the “transporters” in the ion conduction process without changing the content of conductive group trimethylamine or breaking the structure of the polymer backbones. Thus, the addition of the ILs has no obvious influence on the SRs of the membranes because the content of conductive groups and the structure of the polymer backbones, the decisive factors for the SR, are the same each membrane. The ion conductivity of the BPPO–TEA–1TiO2–30IL membrane is 30% higher than that of the BPPO–TEA–1TiO2–10IL membrane, but the SR is about the same for both membranes. These results indicate that the addition of nano-TiO2 and ILs creates some efficient ion conductive channels in the membrane for increasing the ion conductivity without breaking the original compact structure of the BPPO–TEA–IL membrane. The properties of BPPO–TEA and BPPO–TEA–IL membranes are also measured under the same condition. The WUs and SRs of the two types membranes are higher than BPPO–TEA–TiO2–IL composite membranes, which indicates the positive effect on mechanical properties of membranes after the addition of inorganic nano material.| Membrane | Water uptake (%) | Swelling ratio (%) | Ion conductivity (mS cm−1) | |||
|---|---|---|---|---|---|---|
| 30 °C | 60 °C | 30 °C | 60 °C | 30 °C | 60 °C | |
| BPPO–TEA | 18.67 | 28.37 | 9.32 | 12.67 | 7.83 | 8.16 |
| BPPO–TEA–15IL | 18.95 | 27.36 | 9.14 | 12.89 | 8.12 | 14.27 |
| BPPO–TEA–20IL | 19.41 | 29.29 | 9.37 | 12.36 | 13.27 | 21.37 |
| BPPO–TEA–30IL | 19.56 | 28.76 | 9.35 | 12.96 | 14.96 | 24.01 |
| BPPO–TEA–1TiO2–15IL | 8.95 | 14.37 | 4.14 | 7.89 | 21.64 | 30 |
| BPPO–TEA–1TiO2–20IL | 9.31 | 14.29 | 4.37 | 7.33 | 24.5 | 37.02 |
| BPPO–TEA–1TiO2–30IL | 9.56 | 14.57 | 4.35 | 7.96 | 31.25 | 39.02 |
The IECs of the BPPO–TEA–1TiO2–15IL, BPPO–TEA–1TiO2–20IL and BPPO–TEA–1TiO2–30IL membranes were measured by back titration. The IEC values of the three membranes are close because the ion conductive groups in the membranes are equal in content. In order to investigate the role in increasing the ion conductivity of the ILs in the membrane in depth, the ion density (α) of the membranes and the ion mobility (μ) of the hydroxyl were calculated by the following equations:
| α = mdry × IEC/Vwet | (6) |
| μ = σ/Fα | (7) |
| Membrane | IEC (mmol g−1) | Ion density (mol L−1) | Ion mobility (×10−4 cm2 s−1 V−1) |
|---|---|---|---|
| BPPO–TEA–1TiO2–15IL | 1.47 | 2.16 | 1.04 |
| BPPO–TEA–1TiO2–20IL | 1.62 | 2.06 | 1.23 |
| BPPO–TEA–1TiO2–30IL | 1.76 | 2.14 | 1.51 |
Thermal stability
Thermogravimetric analysis (TGA) was used to investigate the thermal stability of the composite membranes. The pure BPPO–TEA membrane, BPPO–TEA–IL composite membrane, and BPPO–TEA–1TiO2–IL composite membrane were measured respectively. The IL content in the membrane BPPO–TEA–1TiO2–10IL is 10%.The results are shown in Fig. 13. The curves show that each membrane shows a three-step decomposition. The first step between 50 °C and 120 °C is assigned to the decomposition of the bonded water and the residual solvent, which indicates the appropriate water storage ability of the membrane. The second decomposition between 200 °C and 390 °C is assigned to the degradation of the quaternary ammonium groups and IL. The third stage after 420 °C is ascribed to the degradation of the polymer backbone. After the addition of the nano-TiO2, the curve becomes more flat, which confirms an improvement in the thermal stability of the membrane. The test results show that the composite membranes have thermal properties suitable for fuel cell applications.
Mechanical properties
The mechanical properties of the membranes are also very important in the practical applications of AEMs. The mechanical properties of the BPPO–TEA membrane, the two ratios of the BPPO–TEA–IL membranes, and the three ratios BPPO–TEA–1TiO2–IL composite membranes were measured at room temperature (RT) and 60% RH, and the results are shown in Table 3. The pure BPPO–TEA membrane has a tensile strength of 28.412 MPa and an elongation at break of 4.04%. After the addition of the IL, the tensile strength decreases to 25.885 MPa. The tensile strength decreases with increasing IL content because the introduction of the ILs loosens the structure of the polymer chains in the membranes and reduces the crystallinity of the membranes. The mechanical properties are higher in the BPPO–TEA–1TiO2–IL composite membranes because the nucleating effects of the inorganic filler nano-TiO2 enhance the interactions between the polymer chains. The tensile strengths of the BPPO–TEA–1TiO2–IL composite membranes are in the range from 20.52 to 30.48 MPa, the elongation at break is in the range from 4.54% to 6.15%, and the tensile modulus is in the range from 646.92 to 921.13 MPa. The three properties decrease with increasing IL content in the membranes, probably because more IL in the membranes lead to more water absorption of the membranes to influence the mechanical properties of the membranes and the excess IL will reduce the nucleating effect of nano TiO2. In summary, the mechanical properties of the BPPO–TEA–1TiO2–IL membranes are proper compared with those of other membranes reported before.| Sample | Tensile strength (MPa) | Tensile modulus (MPa) | Elongation at break (%) |
|---|---|---|---|
| BPPO–TEA | 28.412 | 889.10 | 4.04 |
| BPPO–TEA–15IL | 25.885 | 696.59 | 4.11 |
| BPPO–TEA–20IL | 22.438 | 765.71 | 4.09 |
| BPPO–TEA–1TiO2–15IL | 30.483 | 921.13 | 6.15 |
| BPPO–TEA–1TiO2–20IL | 22.588 | 772.20 | 5.34 |
| BPPO–TEA–1TiO2–30IL | 20.524 | 646.92 | 4.54 |
| PPO-g-Q72 (ref. 31) | 35.9 | 800 | 7.2 |
| sQPBI-X32 | 20.4 | 570 | 12.5 |
| PSAN90-[DAMIm][OH]20 (ref. 33) | 14.3 | 621.8 | 89.8 |
Alkaline stability
The long-term chemical stability of the composite membranes can be divided into two parts: the degradation of the cation and wastage rate of the ILs. The pure BPPO–TEA membrane, BPPO–TEA–ILs composite membrane and BPPO–TEA–1TiO2–IL composite membrane were immersed into a 4 M NaOH solution at room temperature, and the ion conductivities of these membranes were measured several times. After 280 h, the membranes remained tough and the dimensions of the membranes were proper, indicating the good stability of the polymer backbones. The variations of ion conductivity with time for various membranes are shown in Fig. 14. The degradation rate of the ion conductivity of the pure BPPO–TEA membrane is 78.4% because of the Hofmann elimination of the quaternary ammonium salt under alkaline condition. Meanwhile, the degradation rates of the ion conductivities of the BPPO–TEA–IL composite membranes are as high as 74.3%, 80.2% and 84.5%, indicating that the ILs run off from the membranes during the test time. However, the run off of the ILs in the membranes were effectively controlled after the addition of the nano-TiO2. The degradation rates of the ion conductivities of BPPO–TEA–1TiO2–IL composite membranes were reduced to 30.3%, 31.8% and 35.1%, confirming the role of the nano TiO2 in stabilizing the ILs in the membranes.Conclusions
A new method was developed to improve the ion conductivity of AEMs by introducing IL and nano-TiO2 into the membranes. MeIL was immobilized by nano-TiO2 to prevent waste. The ion conductivity tests showed that the introduction of the MeIL can observably improve the ion conductivities of the membranes and the introduction of the nano-TiO2 can greatly stabilize the MeIL in the membranes. The theoretical calculations showed that the improvement of the ion conductivities of the membranes is due to the improvement of ion mobility. The ion conductivities of BPPO–TEA–1TiO2–IL composite membranes range from 21.64 to 31.25 mS cm−1 at room temperature and 38.6 to 51.6 mS cm−1 at 80 °C. However, the water uptakes and swelling ratios of the composite membranes are relatively low. The thermal stabilities and mechanical properties of the composite membranes are sufficiently high for AEM applications. These results showed that the new method for improving the ion conductivity is promising for AEM applications.Acknowledgements
The authors gratefully acknowledge the financial support from the National High Technology Research and Development Program of China (no. 2011AA11A273), the National Natural Science Foundation of China (no. 21176022, 21176023), the International S&T Cooperation Program of China (no. 2013DFA51860).Notes and references
- J. Fan, H. Zhu, R. Li, N. Chen and K. Han, J. Mater. Chem. A, 2014, 2, 9376–9385 Search PubMed.
- C. Xue, J. Zou, Z. Sun, F. Wang, K. Han and H. Zhu, Int. J. Hydrogen Energy, 2014, 39, 7931–7939 CrossRef CAS.
- K. Han, J. Shen, C. Hayner, H. Ye, M. Kung and H. Kung, J. Power Sources, 2014, 251, 331–337 CrossRef CAS.
- A. H. N. Rao, R. L. Thankamony, H.-J. Kim, S. Nam and T.-H. Kim, Polymer, 2013, 54, 111–119 CrossRef CAS.
- G. Merle, M. Wessling and K. Nijmeijer, J. Membr. Sci., 2011, 377, 1–35 CrossRef CAS.
- K. D. Kreuer, Chem. Mater., 2014, 26, 361–380 CrossRef CAS.
- H. W. Zhang and P. K. Shen, Chem. Rev., 2012, 112, 2780–2832 CrossRef CAS PubMed.
- G. Couture, A. Alaaeddine, F. Boschet and B. Ameduri, Prog. Polym. Sci., 2011, 36, 1521–1557 CrossRef CAS.
- A. Lai, L. Wang, X. Lin, Y. Zhuo, Q. Zhang, A. Zhu and Q. Liu, J. Membr. Sci., 2015, 481, 9–18 CrossRef CAS.
- N. Li, J. Yong, A. H. Michel and C. Wang, J. Am. Chem. Soc., 2013, 135, 10124–10133 CrossRef CAS PubMed.
- Y. Zhuo, A. Lai, Q. Zhang, A. Zhu, M. Ye and Q. Liu, J. Membr. Sci., 2015, 491, 138–148 CrossRef CAS.
- G. Nie, X. Li, J. Tao, W. Wu and S. Liao, J. Membr. Sci., 2015, 474, 187–195 CrossRef CAS.
- Y. Zhao, H. Yu, F. Xie, Y. Liu, Z. Shao and B. Yi, J. Power Sources, 2014, 269, 1–6 CrossRef CAS.
- S. Yun, J. Parrondo and V. Ramani, Derivatized Cardo, J. Mater. Chem. A, 2014, 2, 6605–6615 CAS.
- D. Y. Chen and M. A. Hickner, ACS Appl. Mater. Interfaces, 2012, 4, 5775–5781 CAS.
- N. W. Li and M. D. Guiver, Macromolecules, 2014, 47, 2175–2198 CrossRef CAS.
- Y. Zha, L. Melanie, M. Disabb, D. Zachary, A. H. Michael and N. Gregory, J. Am. Chem. Soc., 2012, 134, 4493–4496 CrossRef CAS PubMed.
- J. Pan, Y. Li, J. Han, G. Li, L. Tan, C. Chen, J. Lu and L. Zhuang, Energy Environ. Sci., 2013, 6, 2912–2915 CAS.
- J. Pan, C. Chen, L. Zhuang and J. Lu, Acc. Chem. Res., 2012, 45, 473–481 CrossRef CAS PubMed.
- J. Pan, C. Chen, Y. Li, L. Wang, L. Tan, G. Li, X. Tang, L. Xiao, J. Lu and L. Zhuang, Energy Environ. Sci., 2014, 7, 354–360 CAS.
- Y. Yamg and M. K. Daniel, Macromolecules, 2015, 48, 4471–4480 CrossRef.
- Y. Naoki, O. Hideaki, M. Junpei, N. Eriko, A. Koichiro, W. Masahiro and M. Kenji, ACS Appl. Mater. Interfaces, 2014, 6, 17044–17052 Search PubMed.
- A. Lai, L. Wang, C. Lin, Y. Zhuo, Q. Zhang, A. Zhu and Q. Liu, ACS Appl. Mater. Interfaces, 2015, 7, 8284–8292 CAS.
- M. G. Izabelle, P. F. Clarissa, R. B. Caroline, Z. T. Aniele, A. P. Marcos, A. V. Marcos, M. Giovanna, C. R. Lucas and C. R. Danieli, ACS Appl. Mater. Interfaces, 2014, 6, 11536–11543 Search PubMed.
- H. Chang, S. Chang, T. Hung, J. Jiang, J. Kuo and S. H. A. Lin, J. Phys. Chem. C, 2011, 115, 23778–23783 CAS.
- T. Alammar, H. Noei, Y. Wang and A.-V. Mudring, Mild Yet, Nanoscale, 2013, 5, 8045–8055 RSC.
- X. Lin, Y. Liu, D. P. Simon, L. Ai, R. V. John, L. Wu, Y. Li, X. Liang, Q. Li and T. Xu, J. Power Sources, 2013, 233, 259–268 CrossRef CAS.
- B. Qiu, B. Lin, Z. Si, L. Oiu, F. Chu, J. Zhao and F. Yan, J. Power Sources, 2012, 217, 329–335 CrossRef CAS.
- X. Li, J. Tao, G. Nie, L. Wang, L. Li and S. Liao, RSC Adv., 2014, 4, 41398–41410 RSC.
- F. Song, Y. Fu, Y. Gao, J. Li, J. Qiao, X. Zhou and Y. Liu, Electrochim. Acta, 2015, 177, 137–144 CrossRef CAS.
- J. Ran, L. Wu, Q. Ge, Y. Chen and T. Xu, J. Membr. Sci., 2014, 470, 229–236 CrossRef CAS.
- J. Li-cheng, L.-c. Steve, L. Bi-yun and H. Yi-lun, J. Membr. Sci., 2014, 460, 160–170 CrossRef.
- B. Lin, F. Chu, Y. Ren, B. Jia, N. Yuan, H. Shang, T. Feng, Y. Zhu and J. Ding, J. Power Sources, 2014, 266, 186–192 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |