Novel Ru(II)-photo-antibiotics for the development of infection-resistant mask coatings
Arif Ali
Mandal†
a,
Saurav
Kumar†
b,
Srijan
Singh
a,
Apurba
Mandal
a,
Samya
Banerjee
 *a and
Prodyut
Dhar
*a and
Prodyut
Dhar
 *b
*b
aDepartment of Chemistry, Indian Institute of Technology (BHU), Varanasi, Uttar Pradesh 221005, India. E-mail: samya.chy@iitbhu.ac.in
bSchool of Biochemical Engineering, Indian Institute of Technology (BHU), Varanasi, Uttar Pradesh 221005, India. E-mail: prodyut.bce@iitbhu.ac.in
First published on 8th December 2025
Abstract
Four novel Ru(II)-photoantibiotics (Ru1–Ru4) were developed to produce infection-resistant self-cleaning mask coatings. These photoantibiotics eliminate bacterial infections by generating oxidative stress and photothermal effects upon exposure to visible light (400–700 nm) with tuneable surface hydrophobicity.
“Antimicrobial resistance is outpacing advances in modern medicine, threatening the health of families worldwide,” said Dr Ghebreyesus, WHO Director-General, highlighting that infectious diseases caused by drug-resistant bacteria are a major cause of death globally.1 According to the WHO, drug-resistant infections are projected to increase by 70%, with an estimated 40 million deaths during 2025–2050.1 The World Bank further predicts that antimicrobial resistance (AMR) could lead to an increase of ca. 1 trillion US$ healthcare costs by 2050 and a global GDP loss of ca. 1–3 trillion US$ annually by 2030.2 Air is a primary medium for bacterial diffusion and transmission.3 Thus, face masks with high filtration efficiency and antimicrobial protection have a crucial role in limiting airborne infections.3 Approximately 3.4 billion masks were used during the COVID-19 pandemic worldwide, which helped to reduce the risk of infection transfer from 17.4% to 3.4%.4 Beyond pandemics, masks remain essential in preventing hospital-acquired infections.4 Surgical masks are made of polymers such as polyethylene and polypropylene that are functionally non-degradable.5 Therefore, continuous or improper reuse, poor washing, and exposure to heat and humidity promote microbial colonization on mask surfaces, increasing the risk of secondary infections.5 Moreover, the large-scale disposal of used masks, categorized as biohazardous waste, has created a significant environmental burden.6 Although several disinfection strategies can enable mask reuse, these methods require specialized facilities and are not easily accessible to the general public.6 An alternative and innovative approach involves developing a coating of an antimicrobial agent onto the mask for reuse.7 Metal and metal oxide coatings have been widely studied for their antimicrobial efficacy.6 However, the coating of visible light-responsive metallo-photo-antibiotics, which can introduce an antibacterial photodynamic (aPDT) mechanism, onto mask surfaces remains totally unexplored. aPDT offers a promising strategy to combat AMR, as it operates via ROS generation to damage multiple bacterial targets, minimizing the risk of resistance development.8–10 Additionally, aPDT enables localized, light-triggered disinfection, providing an effective, safe, and sustainable solution for self-sterilizing mask technologies.8–10 Thus, for the development of infection-resistant mask coatings, metallo-photo-antibiotics could be a viable and sustainable solution. But this aspect of metallo-photo-antibiotics is completely unexplored. This exciting possibility and research gap in infection-resistant mask coating development pushed us to develop a series of novel visible light sensitive Ru(II)-photo-antibiotics viz., [Ru(CH3-Phtpy)2](PF6)2 (Ru1), [Ru(NMe2-Phtpy)2](PF6)2 (Ru2), [Ru(OH-Phtpy)2](PF6)2 (Ru3), and [Ru(CF3-Phtpy)2](PF6)2 (Ru4) (where, CH3-Phtpy = 4′-(4-methylphenyl)-2,2′:6′,2″ terpyridine, NMe2-Phtpy = 4-([2,2′:6′,2″-terpyridin]-4′-yl)-N,N-dimethylaniline, OH-Phtpy = 4′-(hydroxyphenyl)-2,2′:6′,2″-terpyridine, and CF3-Phtpy = 4′-(4 trifluoromethylphenyl)-2,2’:6′,2′′-terpyridine) (Fig. 1a). Ru1–Ru4 were screened for their visible light (400–700 nm, 10 J cm−2) triggered antibacterial activities against various G(+) and G(−) bacteria and disinfection capability upon coating onto the mask. Collectively, the in-solution and in vitro evidence suggested that Ru1–Ru4 offer microbial decontamination through surface modification and active photodynamic bacteria killing through ROS generation, making them a superior candidate for next-generation antimicrobial coatings for infection-resistant masks (Fig. 1b). Ru1–Ru4 were synthesised, and their structural characterisation was performed using HRMS, FT-IR, SC-XRD, 1H and 13C NMR and UV-Vis spectroscopy, and elemental analysis (Fig. 1c, d, and Fig. S1–S14, SI). In 1H NMR of Ru1–Ru4, the peaks at ca. 7.00–10.40 ppm correspond to the aromatic protons of the complexes (Fig. S2, S4, S6, S8, SI). Ru1 and Ru2, respectively, showed characteristic peaks of 6 protons (two –CH3 groups) at ca. 2.52 ppm and 12 protons (two -NMe2 groups) at ca. 3.14 ppm. The HRMS of Ru1–Ru4 in acetonitrile showed a major peak of [M-2PF6]2+ (Fig. S10–S13, SI). Ru4 had a distorted octahedral geometry in which the Ru centre is surrounded by six N centres from the two CF3-Phtpy units in a meridional manner (Fig. 1c and Table S1, SI). Two molecules were present in one unit cell of Ru4 (Fig. S15, SI). The selected bond angles (°) and bond lengths (Å) of Ru4 are listed in Table S2, SI. The Ru–N bond within the central pyridine ring (Ru1–N1 and Ru1–N3) measures approximately 1.98 Å, which is shorter than the Ru–N bond involving the terminal pyridine nitrogen (∼2.08 Å). The bite angles are within the range of 78.5°–102.6°, whereas the trans angles are within the range of 157.6°–177.0°. The UV-Vis spectra of Ru1–Ru4 in DMSO showed a broad band between 450 nm and 600 nm due to metal-to-ligand charge transfer (MLCT).11 The minimal UV-Vis. spectral changes of Ru1–Ru4 in DMSO:H2O (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
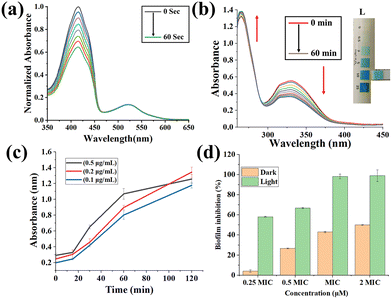
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99, v/v) medium under light conditions (10 J cm−2, 400–700 nm) showed good photo stability up to 7.0 h (Fig. S16, SI). Ru1–Ru4 did not show any notable absorption change in a GSH (1 mM) solution under light, indicating their robustness to GSH (Fig. S17, SI). The photochemical/physical properties of Ru1–Ru4 were investigated using computational methods. The optimized structures of Ru1–Ru4 were in good agreement with the crystal structure of Ru4 (Fig. S18 and Tables S3–S6, SI). For Ru1–Ru4, the HOMO was partially on the Ru(II) centre, and the LUMO was concentrated mainly on the terpyridine moieties around the Ru(II) centre. TD-DFT calculation for Ru1–Ru4 predicted the electronic transitions within the complexes. Key findings obtained from these calculations are presented in Table S7, SI. TD-DFT calculations indicated the 1O2 generation ability of Ru1–Ru4. A minimum energy gap (ΔES–T) of 0.98 eV is required between the lowest singlet state (S0) and the lowest triplet state (T1) to generate 1O2.12 The ΔES–T values of Ru1–Ru4 were 2.20 eV, 1.95 eV, 2.17 eV, and 2.21 eV, respectively, which are much higher than 0.98 eV, validating the 1O2 generation ability of Ru1–Ru4 (Fig. S19, SI). This fact inspired us to investigate the 1O2 generation ability of Ru1–Ru4 using DPBF as a 1O2 probe8–10 in PBS–DMSO (99
99, v/v) medium under light conditions (10 J cm−2, 400–700 nm) showed good photo stability up to 7.0 h (Fig. S16, SI). Ru1–Ru4 did not show any notable absorption change in a GSH (1 mM) solution under light, indicating their robustness to GSH (Fig. S17, SI). The photochemical/physical properties of Ru1–Ru4 were investigated using computational methods. The optimized structures of Ru1–Ru4 were in good agreement with the crystal structure of Ru4 (Fig. S18 and Tables S3–S6, SI). For Ru1–Ru4, the HOMO was partially on the Ru(II) centre, and the LUMO was concentrated mainly on the terpyridine moieties around the Ru(II) centre. TD-DFT calculation for Ru1–Ru4 predicted the electronic transitions within the complexes. Key findings obtained from these calculations are presented in Table S7, SI. TD-DFT calculations indicated the 1O2 generation ability of Ru1–Ru4. A minimum energy gap (ΔES–T) of 0.98 eV is required between the lowest singlet state (S0) and the lowest triplet state (T1) to generate 1O2.12 The ΔES–T values of Ru1–Ru4 were 2.20 eV, 1.95 eV, 2.17 eV, and 2.21 eV, respectively, which are much higher than 0.98 eV, validating the 1O2 generation ability of Ru1–Ru4 (Fig. S19, SI). This fact inspired us to investigate the 1O2 generation ability of Ru1–Ru4 using DPBF as a 1O2 probe8–10 in PBS–DMSO (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, pH = 7.0) solution (Fig. 2a, and Fig. S20, SI). Upon light irradiation, Ru1–Ru4 (2 µM) showed a decrease in DPBF (30 µM)-based absorbance. The 1O2 quantum yields (ΦΔ) of Ru1–Ru4 ranged from 0.09 to 0.15, using [Ru(bpy)3]Cl2 as a standard (ΦΔ = 0.22). Furthermore, the introduction of sodium azide (NaN3), a known 1O2 scavenger,13 inhibited the decrement of the DPBF-based peak, confirming the 1O2 generation ability of Ru1–Ru4 in the presence of light (Fig. S21, SI). 1O2 is known to damage bacterial cellular components like DNA, proteins, and cell membranes, ultimately leading to bacterial death.8–10 Upon light irradiation, Ru1–Ru4 (2 µM) showed a gradual decrease in the characteristic absorbance of MB (10 µM) (∼700 nm) (ca., 41%–58% within 50 s) in PBS–DMSO (99
1 v/v, pH = 7.0) solution (Fig. 2a, and Fig. S20, SI). Upon light irradiation, Ru1–Ru4 (2 µM) showed a decrease in DPBF (30 µM)-based absorbance. The 1O2 quantum yields (ΦΔ) of Ru1–Ru4 ranged from 0.09 to 0.15, using [Ru(bpy)3]Cl2 as a standard (ΦΔ = 0.22). Furthermore, the introduction of sodium azide (NaN3), a known 1O2 scavenger,13 inhibited the decrement of the DPBF-based peak, confirming the 1O2 generation ability of Ru1–Ru4 in the presence of light (Fig. S21, SI). 1O2 is known to damage bacterial cellular components like DNA, proteins, and cell membranes, ultimately leading to bacterial death.8–10 Upon light irradiation, Ru1–Ru4 (2 µM) showed a gradual decrease in the characteristic absorbance of MB (10 µM) (∼700 nm) (ca., 41%–58% within 50 s) in PBS–DMSO (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, pH = 7.0) solution, revealing the ˙OH production ability of Ru1–Ru4 (Fig. S22, SI). The ˙OH generation ability of Ru1–Ru4 was validated using MB degradation in the presence of tert-butanol, a ˙OH scavenger.14 In the presence of tert-butanol, Ru1–Ru4 + light showed no change in absorbance of MB, confirming that the MB degradation in Ru1–Ru4 + light is specifically due to ˙OH generation (Fig. S23, SI). NADH plays a crucial role as an electron donor in cellular redox processes in bacteria, producing ATP for survival.15 Its oxidation to NAD+ disturbs the bacterial redox equilibrium and metabolic activity, impairing energy generation.15 Therefore, the NADH oxidation ability of Ru2 in the presence of light was investigated using UV-Vis. spectroscopy by monitoring characteristic bands of NADH and NAD+ in a PBS-DMSO (99
1 v/v, pH = 7.0) solution, revealing the ˙OH production ability of Ru1–Ru4 (Fig. S22, SI). The ˙OH generation ability of Ru1–Ru4 was validated using MB degradation in the presence of tert-butanol, a ˙OH scavenger.14 In the presence of tert-butanol, Ru1–Ru4 + light showed no change in absorbance of MB, confirming that the MB degradation in Ru1–Ru4 + light is specifically due to ˙OH generation (Fig. S23, SI). NADH plays a crucial role as an electron donor in cellular redox processes in bacteria, producing ATP for survival.15 Its oxidation to NAD+ disturbs the bacterial redox equilibrium and metabolic activity, impairing energy generation.15 Therefore, the NADH oxidation ability of Ru2 in the presence of light was investigated using UV-Vis. spectroscopy by monitoring characteristic bands of NADH and NAD+ in a PBS-DMSO (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution.9Ru2 (2 µM) did not change the absorption spectra of NADH (180 µM) in the dark (Fig. S24, SI). However, under light, Ru2 gave a turnover frequency (TOF) of 40.5 ± 4.8 h−1 for NADH photo-oxidation (Fig. 2b). This photo-oxidation of NADH by Ru2 might contribute to its aPDT activity via in-cell NADH oxidation.2 Furthermore, upon light irradiation, Ru2 generated H2O2 in the presence of NADH (Fig. 2b), promoting ROS generation via the type I pathway. This observation matches well with previous reports.2,13 Thus, this series of Ru(II) complexes may have prospects in aPDT, through the dual mode of ROS generation, i.e., type I and type II pathways.
1, v/v) solution.9Ru2 (2 µM) did not change the absorption spectra of NADH (180 µM) in the dark (Fig. S24, SI). However, under light, Ru2 gave a turnover frequency (TOF) of 40.5 ± 4.8 h−1 for NADH photo-oxidation (Fig. 2b). This photo-oxidation of NADH by Ru2 might contribute to its aPDT activity via in-cell NADH oxidation.2 Furthermore, upon light irradiation, Ru2 generated H2O2 in the presence of NADH (Fig. 2b), promoting ROS generation via the type I pathway. This observation matches well with previous reports.2,13 Thus, this series of Ru(II) complexes may have prospects in aPDT, through the dual mode of ROS generation, i.e., type I and type II pathways.
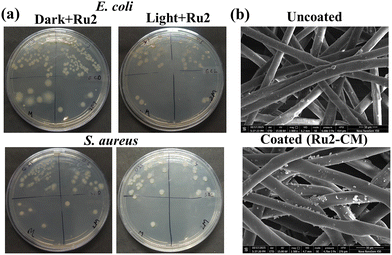
Ru1–Ru4 demonstrated significant antibacterial activity against both Gram-positive (S. aureus) and Gram-negative (E. coli) bacteria under dark and light conditions (Figs. S25, S26; Table S8, SI). The ZOI increased with concentration, confirming dose-dependent activity. Under white light, Ru3 (0.5 µg mL−1) showed the highest ZOI against S. aureus (0.92 cm), while Ru2 (0.5 µg mL−1) was most effective against E. coli (0.60 cm). Additionally, assays performed under green light irradiation showed lower ZOI (Fig. S27 and Table S9, SI). Furthermore, minimum inhibitory concentrations (MICs) of Ru1–Ru4 were evaluated against both E. coli and S. aureus in the presence of light and dark (Fig. S28, S29, and Table S10, SI). The MIC in light conditions is lower compared to dark conditions, reflecting strong antimicrobial potency even at low doses in the presence of light. The MIC further showed a similar response for Ru1–Ru4, ranging from 0.05 to 0.09 µg mL−1. Ru1 and Ru2 exhibited the lowest MIC of 0.05 µg mL−1 for S. aureus and 0.06 µg mL−1 for E. coli, respectively (Table S10, SI). MIC under green light conditions was higher than the white light irradiation, with the lowest MIC for G(+) being 0.07 µg mL−1 for Ru1 and Ru2, whereas the lowest MIC for G(−) was 0.07 µg mL−1 for Ru2 and Ru4 (Fig. S30 and Table S11, SI). To further confirm the antibacterial activity and its mechanism, a bacterial membrane lysis assay was performed. The membrane lysis and nucleic acid release by the microbes in the presence of Ru1–Ru4 were measured by monitoring the absorbance at 260 nm under white light conditions and green light (Fig. 2c and Fig. S31 and S32, SI). The most prominent effect was observed for Ru2 and Ru3, where the OD reached approximately 1.3 after 120 min at 0.5 µg mL−1, indicating extensive cell lysis. This effect was both dose and time-dependent, indicating that photoactivated Ru1–Ru4 generated ROS, which were capable of compromising membrane integrity, leading to bacterial cell death.8–10Ru1–Ru4 showed slightly higher sensitivity against G(+) (S. aureus) compared to G(−) (E. coli), due to differences in cell wall composition and permeability of the two groups.16 Moreover, the biofilm inhibition assay of Ru2 was studied against S. aureus in both light and dark conditions. This result demonstrated a dose-dependent inhibition of the bacterial biofilm in the presence of light where at 0.25 MIC, inhibition reached ca. 58%, which is only ca. 5% in the dark, and the effect intensified at higher concentration (98–100%) at MIC and 2MIC under light, while samples not treated with light showed inhibition at ca. 42–50% (Fig. 2d). Overall, these results demonstrate that Ru(II)-complexes, particularly Ru2, exhibit strong light-activated antibacterial activity.9,17 Therefore, coating Ru2 on surgical masks could offer localized, on-demand antimicrobial protection using light-triggered ROS generation, providing a promising strategy for next-generation infection control. The Ru2 was sprayed on the mask matrix to form a photoactive coating (Ru2-CM). The antimicrobial properties of the Ru2-CM showed significant reduction of bacterial load for both G(−) and G(+) bacteria under light (Fig. 3a) compared to dark conditions, as evidenced by the reduced CFU/mL of 1.2 × 106 (E. coli) and 4 × 105 (S. aureus) under light illumination, which was 1.6 × 106 for S. aureus and 3.2 × 106 for E.coli under dark conditions at MIC concentration. The reduction of CFU/mL is noticeable at concentrations below and above the MIC (Table S12, SI). This enhanced antibacterial property is attributed to the immobilization of photo-responsive Ru2, as also observed in the SEM images of Ru2-CM. The surface morphology of Ru2-CM, as examined using SEM, showed that it retains the pristine fibrous nature of the mask while exhibiting a uniform deposition of the Ru2 on the fibre, confirming effective interaction (Fig. 3b). The photo-responsive nature of Ru2 is examined by thermal imaging of Ru2-CM under light irradiation, showing a rapid rise in temperature to ∼44.8 °C within 5 min, demonstrating efficient photothermal effect (Fig. 4a, b and Fig. S33, SI), where the Ru(II) complex absorbs incident light energy through metal-to-ligand charge transfer (MLCT) transition and non-radiatively dissipates the absorbed energy as localized heat. The reversible temperature fluctuations across multiple light-on/off cycles indicate dynamic and stable photoactivation behaviour, even at low concentration of coating (0.25 MIC). The minimal loading of Ru2 reduces the risk of toxicity and allows targeted clinical application while maintaining the therapeutic impact. Furthermore, contact angle measurements revealed a light-dependent alteration in surface wettability exhibiting hydrophobic characteristics. Ru2-CM displayed a higher contact angle (131.5 ± 0.1°) under light illumination, whereas the contact angle is lower under dark conditions (∼122.8 ± 0.3°) at the MIC concentration (Fig. 4c). The variation of contact angle with time and light illumination was evaluated for the MIC- and 2MIC-coated Ru2-CM (Fig. S34, SI). The initial contact angle was 123.6 ± 0.2° (MIC) and 131.3 ± 0.3° (2 MIC), which gradually decreased over repeated measurements due to water absorption. After four cycles in the dark, the contact angle decreased 116.2 ± 0.5° (MIC) and 113 ± 0.6° (2 MIC), while under illumination, a higher contact angle of 117.5 ± 0.4° (MIC) and 124.5 ± 0.3° (2 MIC) (Fig. S35, SI) was retained, confirming light-induced modulation of the surface wettability. The hydrophobic coating prevents the penetration and accumulation of pathogen droplets. Additionally, the self-cleaning property allows droplets to roll off the mask surface, removing contaminants and ensuring surface hygiene. The incorporation of Ru2 into Ru2-CM imparts synergistic photothermal and photodynamic properties, enabling light-triggered antimicrobial activity, attributed to the combined effects of localized photothermal heating, dynamic modulation of surface energy, and surface hydrophobicity, which together minimize bacterial adhesion while sustaining efficient photoactive disinfection. The enhanced bactericidal efficacy is further attributed to intracellular NADH photo-oxidation-mediated ROS generation by Ru2.
In summary, a series of novel Ru(II)-polypyridyl-based photo-antibiotics (Ru1–Ru4) were synthesized and characterized, and their antibacterial efficacy was investigated under visible light exposure against both G(+) and G(−) bacteria. The UV-Vis. spectra of Ru1–Ru4 suggested their ability to absorb light in the visible region. Among these, Ru2 showed better activity in the presence of light due to high ROS generation (via type I & type II pathways) and efficient catalytic NADH photo-oxidation. In vitro studies revealed that Ru2 + light showed antibacterial efficacy against E. coli and S. aureus. The Ru2 was further coated onto a surgical mask (Ru2-CM) to create a photo-responsive antibacterial interface capable of self-sterilization under visible light. The Ru2-CM mask exhibited strong antibacterial properties through ROS-mediated membrane disruption and oxidative damage, effectively preventing bacterial adhesion while maintaining the mask's breathability and durability. The antibacterial coating of Ru(II)-based PSs on face masks could be a promising light-assisted antibacterial approach for reducing bacterial infections. Further in-depth studies are still needed to coat these photosensitive agents for health protection applications in the future.
This work was supported by the SERB (now ANRF), India (SRG/2022/000030) and (BT/HRD/35/02/2006) DBT, India. A. A. M. and A. M. thank the GOI for the PMRF. S. K. thank the Ministry of Education, India for its provision of financial support. P. D. thanks DBT, GOI for the Ramalingaswami and IYBA fellowship. P. D. acknowledges the research grant received from DST and Ministry of Textiles, GOI.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: syntheses and characterization, NMR spectra, crystallographic details including ORTEP representations, UV-Vis details, and details of the DFT calculations, including the XYZ-files. See DOI: https://doi.org/10.1039/d5cc06235g.CCDC 2499129 contains the supplementary crystallographic data for this paper.18
References
- World Health Organization, WHO warns of widespread resistance to common antibiotics worldwide, 2025, https://www.who.int/news/item/13-10-2025-who-warns-of-widespread-resistance-to-common-antibiotics-worldwide.
- World Health Organization, Antimicrobial resistance, 2023, https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
- X. Zhang, F. Chen, Y. Jiang, Y. Yan, L. Yang, L. Yang, X. Wang, C. Yu, L. Hu, Y. Dai and Q. Wang, ACS Appl. Nano Mater., 2022, 5, 16332–16343 CrossRef CAS.
- D. K. Chu, et al. , Lancet, 2020, 395, 1973–1987 CrossRef CAS PubMed.
- M. Sharma, G. Randhawa, F. Xu, E. Cudmore and T. Hoare, Polymer, 2025, 328, 128428 CrossRef CAS.
- F. Saliu, M. Veronelli, C. Raguso, D. Barana, P. Galli and M. Lasagni, Environ. Adv., 2021, 4, 100042 CrossRef CAS.
- S. Kumar, M. Karmacharya, S. R. Joshi, O. Gulenko, J. Park, G.-H. Kim and Y.-K. Cho, Nano Lett., 2021, 21, 337–343 CrossRef CAS PubMed.
- I. Singh, A. Upadhyay, A. A. Mandal, S. Saha, P. Pragya, L. Pradhan, M. Nayak, A. Dutta, A. K. Agrawal, S. Mukherjee and S. Banerjee, J. Med. Chem., 2025, 68, 4453–4465 CrossRef CAS PubMed.
- A. A. Mandal, A. Upadhyay, A. Mandal, M. Nayak, K. M. Sabeel, S. Mukherjee and S. Banerjee, ACS Appl. Mater. Interfaces, 2024, 16, 28118–28133 CrossRef CAS PubMed.
- R. Kushwaha, S. Kumari, A. Mishra, A. Upadhyay, A. Rai, M. Nayak, S. Mukherjee and S. Banerjee, Chem. Commun., 2025, 61, 10307–10310 RSC.
- T. Ganguly, S. Das, D. Maity and S. Baitalik, Inorg. Chem., 2024, 63, 6883 CrossRef CAS PubMed.
- S. Noimark, E. Salvadori, R. G. Bombarelli, A. J. MacRobert, I. P. Parkin and C. W. M. Kay, Phys. Chem. Chem. Phys., 2016, 18, 28101 RSC.
- H. Huang, S. Banerjee, K. Qiu, P. Zhang, O. Blacque, T. Malcomson, M. J. Paterson, G. J. Clarkson, M. Staniforth, V. G. Stavros, G. Gasser, H. Chao and P. J. Sadler, Nat. Chem., 2019, 11, 1041–1048 CrossRef CAS PubMed.
- J. Jiang, H. Li and L. Zhang, Chem. – Eur. J., 2012, 18, 6360 CrossRef CAS PubMed.
- K. Zhou, L. Du, R. Ding, L. Xu, S. Shi, S. Wang, Z. Wang, G. Zhang, G. He, Z. Zhao and B. Z. Tang, Nat. Commun., 2024, 15, 10551 CrossRef CAS PubMed.
- D. D. Bannerman, M. J. Paape, J.-W. Lee, X. Zhao, J. C. Hope and P. Rainard, Clin. Diagn. Lab. Immunol., 2004, 11, 463–472 Search PubMed.
- D. Monneret, F. Mestari, G. Atlan, C. Corlouer, Z. Ramani, J. Jaffre, S. Dever, V. Fressart, R. Alkouri, F. Lamari, C. Devilliers, F. I.-Bismut and D. B.-Rousselot, Scand. J. Clin. Lab. Invest., 2015, 75, 162–169 CrossRef CAS PubMed.
- CCDC 2499129: Experimental Crystal Structure Determination, 2025 DOI:10.5517/ccdc.csd.cc2pwk27.
Footnote |
| † Equal contribution. |
| This journal is © The Royal Society of Chemistry 2026 |