Lightning-driven plasma bubbles provide prebiotic synthesis and biogeochemical cycling
Dingwei
Gan
 a,
Longfei
Hong
a,
Xiaofan
Guo
b,
Haoxuan
Jiang
a,
Santu
Luo
a,
Tianqi
Zhang
*c,
Jianxi
Ying
a,
Longfei
Hong
a,
Xiaofan
Guo
b,
Haoxuan
Jiang
a,
Santu
Luo
a,
Tianqi
Zhang
*c,
Jianxi
Ying
 *b,
Rusen
Zhou
a,
Dingxin
Liu
a,
H. James
Cleaves
d,
Yufen
Zhao
*b,
Rusen
Zhou
a,
Dingxin
Liu
a,
H. James
Cleaves
d,
Yufen
Zhao
 b and
Renwu
Zhou
b and
Renwu
Zhou
 *a
*a
aState Key Laboratory of Electrical Insulation and Power Equipment, Centre for Plasma Biomedicine, Xi’an Jiaotong University, Xi'an 710049, China. E-mail: renwu.zhou@xjtu.edu.cn
bQian Xuesen Collaborative Research Center of Astrochemistry and Space Life Sciences, Ningbo University, Ningbo 315211, China. E-mail: yingjianxi@nbu.edu.cn
cSchool of Chemical and Biomolecular Engineering, The University of Sydney, Sydney, New South Wales 2006, Australia. E-mail: Tianqi.zhang@sydney.edu.au
dEarth and Planets Laboratory, Carnegie Institution for Science, Washington, DC 20015, USA
First published on 13th December 2025
Abstract
Lightning–sea interactions generate plasma bubbles that act as electrochemical reactors, producing amino acids, nucleobases, and peptides with yields 100–300% higher than spark discharges. This process accelerates carbon-nitrogen coupling and enhances biogeochemical cycling, linking CO2 and NH3 to bioavailable organic compounds.
The origin of life and the evolution of its key biochemical components are deeply intertwined with Earth's early atmospheric conditions and biogeochemical cycles. Since Miller's landmark contribution in 1953,5 extensive research has explored the origin of prebiotic building blocks under diverse conditions.6–10 Currently, most studies focus on synthesizing individual prebiotic molecules, while one-pot synthesis of multiple components remains a major challenge in understanding life's emergence on early Earth.
Lightning, a key driver of prebiotic chemistry on early Earth, likely provided energy for atmospheric reactions, promoting the formation of amino acids and other organic compounds, as recent studies suggest.11–15 Furthermore, a significant portion of early Earth's surface was covered by liquid environments, where lightning strikes likely generated bubbles. This gas–liquid plasma environment could have provided a highly reactive microenvironment, promoting the synthesis of complex organic molecules.16,17
Based on this, plasma bubbles from lightning–sea interactions may have played a key role in prebiotic chemistry, creating a high-reactivity environment that accelerates organic molecule synthesis, similar to early Earth processes. Our previous work18,19 demonstrated that CO2 plasma bubbles can produce organic acids and long-chain hydrocarbons at high yields, further supporting the idea of plasma bubbles as a potential “cradle” for prebiotic molecular formation. Recent advances in electrified and plasma-assisted gas–liquid interfaces have shown that non-equilibrium microenvironments can dramatically enhance C–N coupling and molecular complexity, in both material synthesis and sustainable chemical production.20–22 Our lightning-driven plasma bubbles represent a prebiotic analogue of such systems, where similar interface effects are harnessed to generate amino acids and nucleobases from CO2/NH3 mixtures.
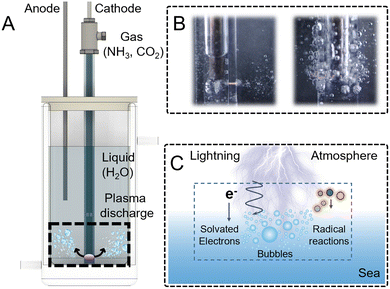
Here we developed a plasma bubble discharge system to simulate lightning strikes on the ocean (Fig. 1A and Fig. S1, S2). By applying negative DC voltage and introducing NH3 and CO2, we replicated early Earth conditions. Micropores at the reactor base promote plasma bubble formation (Fig. 1B), creating an environment for prebiotic biomolecule synthesis via gas–liquid plasma chemistry (Fig. 1C).
J. David Felix's estimates of substantial NH3/NH4+ fluxes from Archean-like hydrothermal systems23 and the experimental demonstration by Gao et al. that Fe-rich rock–water interactions can efficiently generate NH3 from nitrate together suggest that ammonia could have been locally abundant on the early Earth,24 which motivated our use of an NH3/CO2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 gas mixture to simulate a nitrogen-rich prebiotic atmosphere–ocean interface.
1 gas mixture to simulate a nitrogen-rich prebiotic atmosphere–ocean interface.
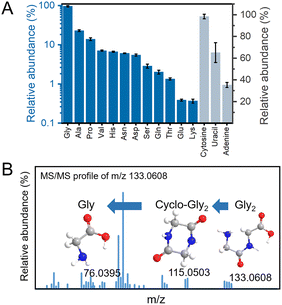
LC-MS analysis identified 12 amino acids and 3 canonical nucleobases (Fig. 2A and Fig. S3–S16, S38, Table S1). MS/MS analysis identified Gly–Gly, with the m/z of [M + H]+ 133.0608 and Gly diketopiperazine (DKP), with the m/z of [M + H]+ 115.0503 (Fig. 2B), suggesting that under early Earth conditions, plasma bubbles could facilitate the formation of amino acids, peptides, and cyclic peptides. This one-pot reaction, producing 12 amino acids, peptides, DKP, and three nucleobases, is unprecedented. To evaluate how early Earth conditions affected prebiotic synthesis, we systematically analysed the impact of lightning discharge intensity and gas flow rate on biomolecule production (Table S2). Notably, the gas flow rate governs the size of bubbles in the discharge reactor (Fig. S17). Smaller micro- and nano-bubbles provide a greater interfacial area and longer lifetime, enhancing the generation of reactive species. Recent studies on electrospark-induced bubble rupture confirm that discharge fragmentation produces micro/nano-bubbles that act as highly efficient electrochemical micro-reactors.25 The promoting effect is therefore most pronounced for bubbles ∼100 µm in diameter. With quantitative analysis performed using standard concentration curves (Fig. S18–S21), the observed concentration gradient (Gly, 0.4 nM > Ala, 0.15 nM > Asn, 0. 04 nM > Glu, 0.02 nM) suggests preferential formation of smaller, less polar amino acids under the simulated early Earth conditions. We also assessed the effect of varying voltages (3–5 kV) on product formation, finding minimal impact on the synthesis of the same product (Table S2). And it was found that the discharge temperature at different voltages increased proportionally with time (Fig. S22). Further research has shown that the yield of Gly decreased continuously as the concentration of NH3 decreased, revealing that the NH3-rich condition is important for amino acid formation (Fig. S23). This might be because NH3 can provide a reducing environment, which is of great significance for the chemical synthesis of life molecules. The emergence of life likely involved the interaction and symbiosis of various molecular species, rather than the evolution of a single substance, the simultaneous synthesis of multiple amino acids and nucleobases under these conditions provides a key insight into the molecular interactions that may have driven the co-evolution of life's building blocks.26–29
To highlight the superiority of plasma bubbles in synthesizing life-building blocks, we compared them with needle plasma discharge environments, both plausible in lightning scenarios. Fig. S24 shows that plasma bubbles significantly outperform needle plasma in product formation, with glycine levels more than three times higher in bubble plasma. This enhancement is likely due to spark discharges within gas bubbles, providing a large surface area and promoting efficient NH3–CO2 reactions. Our previous work demonstrates that plasma bubbles create an optimal gas–liquid interface, enhancing the generation and transfer of active particles.30,31
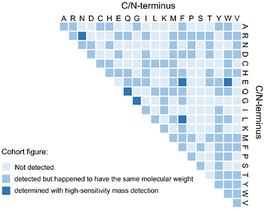
The emergence of biomolecules from non-living matter has long been a research focus. However, the direct transition from inorganic gases to functional peptides or cyclic peptides (e.g., cyclic-Gly2) remains rare. Here we demonstrate the prebiotic synthesis of dipeptides and cyclic dipeptides from inorganic gases and explore the reactivity of the 20 proteinogenic amino acids in a plasma bubble environment, proposing a novel pathway for amino acid conversion to polypeptides on early Earth. Furthermore, we explored the reactivity of amino acids in the plasma bubble system. As shown in Fig. 3, 106 dipeptides were detected, with amino acids such as Gly, Leu, Val, Gln, and Ile—likely present on early Earth—forming the corresponding dipeptides. Higher-order peptides, including a hexamer (Gly6) (Fig. S25), were also observed. These results highlight the plasma bubble system's role in the study of life's molecular origins, particularly in polypeptide formation.
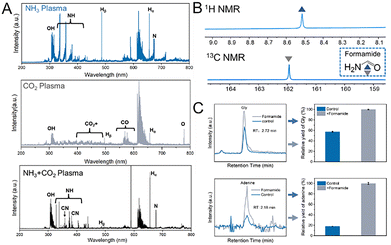
To further explore the reaction mechanism, we first utilized optical emission spectroscopy (OES) to detect the reactive species during the underwater discharge process of (CO2/NH3) gas bubbles. The results are presented in Fig. 4A. The results showed that excited hydroxyl (OH) radicals, atomic oxygen (O), and H radicals were generated in CO2/NH3 plasmas. The formation of OH emissions from the transition of A2Σ+ → X2Π (Δν = 0) at 309 nm and Hα emissions at 656 nm mainly comes from the electron impact dissociations (H2O + e− → H + OH + e−) at the plasma–water interface.32 The O emission at 777 nm, corresponding to the oxygen atom transition of O (2s22p33s–2s2sp33p), was visible due to the energetic collisions of electrons with H2O molecules (H2O + e− → 2H + O + e−).33 In addition, it shows different bands indicating the various reactions and excitation substances produced during the decomposition of CO2 and NH3 in the plasma environment. For CO2 plasma discharge, the emission spectra show significant electronic transitions of the CO2+ and CO Angstrom band, which are key intermediates in plasma-induced CO2 decomposition reactions.34 Among them, CO may play an important role in the subsequent formation of reactive molecules in the reaction process. For NH3 plasma discharge, the characteristic emission peaks of the NH group were observed between 330 and 400 nm, indicating that NH3 underwent dissociation, which is related to the electronic excitation and recombination of the nitrogen–hydrogen bonds.35 Notably, the CN radical was observed at 359 nm and 388 nm, indicating the coupling of CO2 and NH3. The CN species could play an important role in the subsequent plasma synthesis of nucleobases.36
Formamide formation was confirmed via NMR and GC-MS (Fig. 4B and Fig. S26), suggesting its role as a key precursor for amino acid and nucleobase synthesis, as proposed by Ferus, Bada, and Saitta et al. To investigate the chemical pathways of formamide synthesis and its role in amino acid and nucleobase formation, DFT calculations and chemical kinetic simulations were combined with experimental results. Product synthesis was most efficient at the gas–liquid interface (discharge at the bubble–water interface), as compared to needle plasma discharge (Fig. 2C). We propose the following reaction pathways: (i) CO2 dissociation forms CO and O (Fig. 4A); (ii) CO reacts with OH and H from H2O dissociation to form formic acid (HCOOH) (Fig. S27); (iii) HCOOH then reacts with NH2 radicals to form formamide (HCONH2) (Fig. 4B); (iv) Amino acids and canonical nucleobases are subsequently generated from formamide. DFT calculations show that CO reacts with H2O, overcoming a 30 kcal mol−1 energy barrier, while HCOOH formed from CO and H2O reacts with NH3 to overcome a 59 kcal mol−1 barrier, predicting fast kinetics under plasma bubble discharge conditions (Fig. S28).
Based on these findings, we propose a chemical mechanism for converting inorganic gases into prebiotic building blocks in a plasma bubble environment (Fig. 5). The reaction kinetics in the NH3–CO2 plasma bubble system reveal formamide (HCONH2) as a key prebiotic intermediate, with its formation occurring in two phases. During the rapid formation phase (0–0.4 s), high fluxes of radicals and small molecules drive formamide synthesis through CO–NH3 coupling and a HCOOH-derived pathway. Formamide concentration peaks at ∼10−8–10−12 cm−3 before declining due to hydrolysis and competition from nitrogenous by-products. Formamide's transient accumulation highlights its potential as a precursor for nucleobases and amino acids (Fig. 5C and D).37,38 For example, amino acids via Strecker synthesis, consistent with the amino nitrile species observed in our mass spectrometry analysis (Fig. S29–S34), additional experiment (Fig. S35) and previous reports,39–41 further confirmed the reaction pathway, supporting lightning-induced reactive environments as plausible drivers of early biomolecular synthesis. These amino acids can then further polymerize to form peptides, as demonstrated in this study. Regarding nucleobases, formamide dissociates to generate CN, which subsequently undergoes a series of reactions to form nucleobases, with uracil being derived from cytosine.42 Taken together with previous studies,43–45 our results highlight the key role of formamide in prebiotic chemistry: in the plasma-bubble system, it serves both as a direct precursor to nucleobases and other organics and as a transient reservoir of C–N species in a lightning-activated CO2/NH3 atmosphere–ocean setting. All of the above underscores the role of non-equilibrium plasma dynamics, where transient radical fluxes and competing pathways govern the synthesis and stability of life's building blocks.
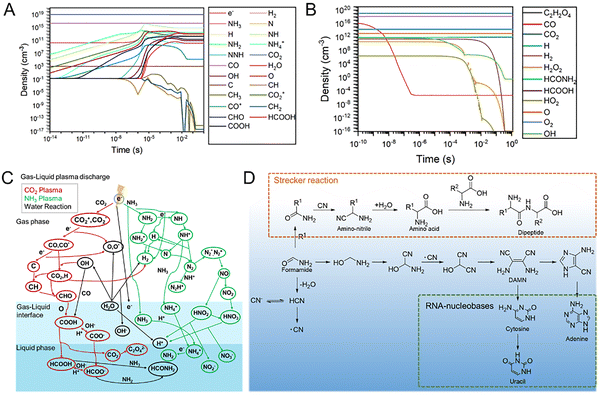 | ||
| Fig. 5 The mechanism of prebiotic chemistry in the plasma bubble. Density profiles of important (A) gas phase and (B) aqueous phase species, showed that the three key species (C2H2O4, HCOOH and HCONH2) exhibit a decreasing trend. (C) Plasma discharge chemistry under the gas–liquid conditions, from NH3 and CO2 to the formamide. (D) The reactions from formamide to prebiotic building blocks; the pathways shown in the figure are either confirmed in this study or are based on previous literature reports.1–4 | ||
In summary, the abiotic origin of life's molecular precursors on early Earth necessitates efficient pathways for biomolecule synthesis and polymerization. This study shows that lightning-induced plasma bubbles serve as high-energy geochemical reactors, enabling rapid synthesis of amino acids (12 types), RNA nucleobases (3 types), urea, biuret (Fig. S36 and S37) and oligopeptides at gas–liquid interfaces. Simulated plasma discharges in NH3/CO2 atmospheres generate reactive nitrogen and carbon species (e.g., CN, NH2, CO), driving formamide-mediated Strecker synthesis and HCN oligomerization. These processes produce biomolecules within minutes, surpassing conventional methods (Table S3). By bypassing thermodynamic barriers via non-equilibrium plasma excitation, the system concurrently synthesizes nucleobases and amino acids. This plasma-mediated pathway completes a critical biogeochemical cycle, converting atmospheric CO2 and NH3 into bioavailable organic compounds. Whilst such high abundances of NH3 may not have been uniformly present in primordial Earth's atmosphere, geochemical and photochemical flux studies indicate that substantial local or episodic NH3 inputs were plausible, thereby supporting the relevance of investigating NH3/CO2 mixtures. In this context, our 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NH3/CO2 mixture should be interpreted as a model system to probe fundamental plasma-driven prebiotic chemistry. Moreover, ammonia-rich settings have been widely proposed for several icy bodies in the outer solar system—including Enceladus, Titan, Triton, and Pluto—where NH3–H2O or NH3–CO2–H2O ices and vapour phases are thought to have co-existed. Observational, experimental, and thermochemical modelling studies provide substantial evidence for the presence and chemical importance of ammonia in these environments.46–48 In this broader astrochemical context, our 1
1 NH3/CO2 mixture should be interpreted as a model system to probe fundamental plasma-driven prebiotic chemistry. Moreover, ammonia-rich settings have been widely proposed for several icy bodies in the outer solar system—including Enceladus, Titan, Triton, and Pluto—where NH3–H2O or NH3–CO2–H2O ices and vapour phases are thought to have co-existed. Observational, experimental, and thermochemical modelling studies provide substantial evidence for the presence and chemical importance of ammonia in these environments.46–48 In this broader astrochemical context, our 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NH3/CO2 mixture is therefore best interpreted as a model system designed to probe fundamental plasma-driven nitrogen–carbon coupling chemistry. The identified mechanism positions atmospheric electrical discharges as a key driver in early Earth's surface–atmosphere exchanges, complementing existing prebiotic synthesis paradigms.
1 NH3/CO2 mixture is therefore best interpreted as a model system designed to probe fundamental plasma-driven nitrogen–carbon coupling chemistry. The identified mechanism positions atmospheric electrical discharges as a key driver in early Earth's surface–atmosphere exchanges, complementing existing prebiotic synthesis paradigms.
Conflicts of interest
There are no conflicts to declare.Data availability
All data of this study are included in this published article and its supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5cc04325e.Acknowledgements
This work is supported by the National Natural Science Foundation of China (524B2109, 52377160, GYKP010, and 42388101) and the Shaanxi Provincial Natural Science Program (2023-JC-YB-425).References
- S. Miyakawa, K. Kobayashi and A. B. Sawaoka, Adv. Space Res., 1999, 24, 465–468 CrossRef CAS PubMed.
- A. Pastorek, J. Hrnčířová, L. Jankovič, L. Nejdl, S. Civiš, O. Ivanek, V. Shestivska, A. Knížek, P. Kubelík, J. Šponer, L. Petera, A. Křivková, G. Cassone, M. Vaculovičová, J. E. Šponer and M. Ferus, Chem. Commun., 2019, 55, 10563–10566 Search PubMed.
- M. Yadav, R. Kumar and R. Krishnamurthy, Chem. Rev., 2020, 120, 4766–4805 Search PubMed.
- M. Ferus, F. Pietrucci, A. M. Saitta, O. Ivanek, A. Knizek, P. Kubelík, M. Krus, L. Juha, R. Dudzak, J. Dostál, A. Pastorek, L. Petera, J. Hrncirova, H. Saeidfirozeh, V. Shestivská, J. Sponer, J. E. Sponer, P. Rimmer, S. Civiš and G. J. A. Cassone, Astron. Astrophys., 2019, 626, A52 CrossRef CAS.
- S. L. Miller, Science, 1953, 117, 528–529 Search PubMed.
- H. James CleavesII, A. M. Scott, F. C. Hill, J. Leszczynski, N. Sahai and R. Hazen, Chem. Soc. Rev., 2012, 41, 5502–5525 RSC.
- D. W. Gan, Y. T. Guo, X. M. Lei, M. Zhang, S. S. Fu, J. X. Ying and Y. F. Zhao, Earth Planet. Sci. Lett., 2023, 607, 118072 CrossRef CAS.
- L. Zhang, M. Zhang, X. F. Guo, D. W. Gan, Y. Ye, Y. F. Zhao and J. X. Ying, Chem. Commun., 2024, 60, 2748–2751 Search PubMed.
- Z. R. Todd, R. Szabla, J. W. Szostak and D. D. Sasselov, Chem. Commun., 2019, 55, 10388–10391 RSC.
- A. Pastorek, J. Hrnčířová, L. Jankovič, L. Nejdl, S. Civiš, O. Ivanek, V. Shestivska, A. Knížek, P. Kubelík, J. Šponer, L. Petera, A. Křivková, G. Cassone, M. Vaculovičová, J. E. Šponer and M. Ferus, Chem. Commun., 2019, 55, 10563–10566 Search PubMed.
- B. L. Hess, S. Piazolo and J. Harvey, Nat. Commun., 2021, 12, 1535 CrossRef CAS PubMed.
- M. Ferus, F. Pietrucci, A. M. Saitta, O. Ivanek, A. Knizek, P. Kubelík, M. Krus, L. Juha, R. Dudzak and J. Dostál, Astron. Astrophys., 2019, 626, A52 CrossRef CAS.
- K. Kobayashi and P. Cyril, Origins Life Evol. Biospheres, 1985, 16, 57–67 CrossRef CAS PubMed.
- X. F. Xiang, L. Guo, X. Wu, X. X. Ma and Y. S. Xia, Environ. Chem. Lett., 2012, 10, 295–300 CrossRef CAS.
- C. R. Stark, C. Helling, D. A. Diver and P. B. Rimmer, Int. J. Astrobiol., 2014, 13, 165–172 CrossRef CAS.
- E. Tekin, A. Salditt, P. Schwintek, S. Wunnava, J. Langlais, J. Saenz, D. Tang, P. Schwille, C. Mast and D. Braun, ChemBioChem, 2022, 23, e202200423 CrossRef CAS PubMed.
- N. Ben-Amots and M. Anbar, Ultrason. Sonochem., 2007, 14, 672–675 CrossRef CAS PubMed.
- T. Q. Zhang, J. Knezevic, M. Y. Zhu, J. M. Hong, R. S. Zhou, Q. Song, L. Y. Ding, J. Sun, D. X. Liu, K. K. Ostrikov, R. W. Zhou and P. J. Cullen, J. Am. Chem. Soc., 2023, 145, 28233–28239 CrossRef CAS PubMed.
- J. Knezevic, T. Q. Zhang, R. W. Zhou, J. M. Hong, R. S. Zhou, C. Barnett, Q. Song, Y. T. Gao, W. P. Xu, D. X. Liu, N. Proschogo, B. Mohanty, J. Strachan, B. Soltani, F. Li, T. Maschmeyer, E. C. Lovell and P. J. Cullen, J. Am. Chem. Soc., 2024, 146, 12601–12608 Search PubMed.
- D. Li, Y. Zhao, Y. Miao, C. Zhou, L. P. Zhang, L. Z. Wu and T. Zhang, Adv. Mater., 2022, 34, e2207793 Search PubMed.
- Y. Zhao, Y. Zhao, G. I. N. Waterhouse, L. Zheng, X. Cao, F. Teng, L. Z. Wu, C. H. Tung, D. O’Hare and T. Zhang, Adv. Mater., 2017, 29, 1703828 CrossRef PubMed.
- Y. Wang, Y. Liu, J. Gan, L. Hong, J. Shen, Z. Zhang, H. Zheng and X. Wang, ACS Sustainable Chem. Eng., 2024, 12, 14505–14513 CrossRef CAS.
- J. D. Felix, Sci. Rep., 2024, 14, 1544 CrossRef CAS PubMed.
- Y. Gao, M. Lei, B. Sravan Kumar, H. B. Smith, S. H. Han, L. Sangabattula, J. Li and I. I. Abate, Joule, 2025, 9, 101805 CrossRef CAS.
- M. Zhu, M. Zhang, X. Wang, C. Liu, R. Zhou, J. Sun, R. Zhou and D. Liu, J. Phys. Chem. Lett., 2025, 16, 11101–11108 CrossRef CAS PubMed.
- M. Frenkel-Pinter, M. Samanta, G. Ashkenasy and L. J. Leman, Chem. Rev., 2020, 120, 4707–4765 Search PubMed.
- C. J. Butch, M. Meringer, J.-S. Gagnon and H. J. Cleaves, Commun. Chem., 2021, 4, 11 CrossRef PubMed.
- S. Tagami, J. Attwater and P. Holliger, Nat. Chem., 2017, 9, 325–332 CrossRef CAS PubMed.
- S. Chatterjee, Phys. Chem. Chem. Phys., 2016, 18, 20033–20046 RSC.
- D. W. Gan, J. W. Huang, L. F. Hong, H. X. Jiang, X. R. Wang, R. S. Zhou, J. Sun and R. W. Zhou, Green Chem., 2025, 27, 8811–8817 RSC.
- D. W. Gan, L. F. Hong, S. Yuan, M. Y. Zhu, Y. T. Gao, T. Q. Zhang, T. Y. Li, B. H. Chen, A. Dzimitrowicz, P. Jamroz, P. J. Cullen and R. Zhou, Green Chem., 2025, 27, 3715–3726 RSC.
- S. Wang, D.-Z. Yang, R. S. Zhou, R. W. Zhou, Z. Fang, W. C. Wang and K. Ostrikov, Plasma Processes Polym., 2020, 17, 1900146 CrossRef CAS.
- X. Lu, G. V. Naidis, M. Laroussi, S. Reuter, D. B. Graves and K. Ostrikov, Phys. Rep., 2016, 630, 1–84 CrossRef CAS.
- Y. J. Du, K. Tamura, S. Moore, Z. M. Peng, T. Nozaki and P. J. Bruggeman, Plasma Chem. Plasma Process., 2017, 37, 29–41 CrossRef CAS.
- U. Müller and G. Schulz, J. Chem. Phys., 1992, 96, 5924–5937 CrossRef.
- M. Ferus, D. Nesvorný, J. Šponer, P. Kubelík, R. Michalčíková, V. Shestivská, J. E. Šponer and S. Civiš, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 657–662 CrossRef CAS PubMed.
- D. Deamer, J. Mol. Evol., 2024, 92, 530–538 Search PubMed.
- A. S. Burton, J. C. Stern, J. E. Elsila, D. P. Glavin and J. P. Dworkin, Chem. Soc. Rev., 2012, 41, 5459–5472 RSC.
- N. J. Green, D. A. Russell, S. H. Tanner and J. D. Sutherland, J. Am. Chem. Soc., 2023, 145, 10533–10541 CrossRef CAS PubMed.
- K. K. Farnsworth, H. L. McLain, A. Chung and M. G. Trainer, ACS Earth Space Chem., 2024, 8, 2380–2392 CrossRef CAS PubMed.
- K. Ashe, C. Fernández-García, M. K. Corpinot, A. J. Coggins, D.-K. Bučar and M. W. Powner, Commun. Chem., 2019, 2, 23 Search PubMed.
- M. Yadav, R. Kumar and R. Krishnamurthy, Chem. Rev., 2020, 120, 4766–4805 CrossRef CAS PubMed.
- R. Saladino, E. Di Mauro and J. M. García-Ruiz, Chemistry, 2019, 25, 3181–3189 CrossRef CAS PubMed.
- R. Saladino, C. Crestini, F. Ciciriello, G. Costanzo and E. Di Mauro, Chem. Biodiversity, 2007, 4, 694–720 CrossRef CAS PubMed.
- R. Saladino, C. Crestini, S. Pino, G. Costanzo and E. Di Mauro, Phys. Life Rev., 2012, 9, 84–104 CrossRef PubMed.
- M. Nasralla, H. Laurent, O. L. G. Alderman and L. Dougan, Commun. Chem., 2025, 8, 227 CrossRef CAS PubMed.
- G. M. Marion, J. S. Kargel, D. C. Catling and J. I. Lunine, Icarus, 2012, 220, 932–946 CrossRef CAS.
- W. Zheng, D. Jewitt and R. I. Kaiser, Astrophys. J., Suppl. Ser., 2009, 181, 53 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |