Impact of peripheral alkyl chain length on mesocrystal assemblies of G2 dendrons†
Taesuk
Jun‡
a,
Hyunjun
Park‡
b,
Junsu
Kim
a,
Wooseop
Lee
c,
Hyungju
Ahn
 c,
Woo-Dong
Jang
c,
Woo-Dong
Jang
 *b,
Byeongdu
Lee
*b,
Byeongdu
Lee
 *d and
Du Yeol
Ryu
*d and
Du Yeol
Ryu
 *a
*a
aDepartment of Chemical and Biomolecular Engineering, Yonsei University, 50 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea. E-mail: dyryu@yonsei.ac.kr
bDepartment of Chemistry, Yonsei University, 50 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea. E-mail: wdjang@yonsei.ac.kr
cIndustry Technology Convergence Centre, Pohang Accelerator Laboratory, 80 Jigok-ro, Nam-gu, Pohang 37673, Korea
dAdvanced Photon Source, Argonne National Laboratory, Argonne, IL 60439, USA. E-mail: blee@anl.gov
First published on 1st May 2023
Abstract
Unique sphere-packing mesophases such as Frank–Kasper (FK) phases have emerged from the viable design of intermolecular interactions in supramolecular assemblies. Herein, a series of Cn-G2-CONH2 dendrons possessing an identical core wedge are investigated to elucidate the impact of peripheral alkyl chain lengths (Cn) on the formation of the close-packed structures. The C18 and C14 dendrons, of which the contour lengths of the periphery Lp are longer than the wedge length Lw, assemble into a uniform sphere-packing phase such as body-centred cubic (BCC), whereas the C8 dendron with short (Lp < Lw) corona environment forms the FK A15 phase. Particularly in the intermediate C12 and C10 dendrons (Lp ≈ Lw), cooling the samples from an isotropic state leads to cooling-rate-dependent phase behaviours. The C12 dendron produces two structures of hexagonal columnar and sphere-packing phases (BCC and A15), while the C10 dendron generates the A15 and σ phases by the fast- and slow-cooling processes, respectively. Our results show the impact of peripheral alkyl chain lengths on the formation of mesocrystal phases, where the energy landscape of the dendrons at Lp/Lw ≈ 1 must be more complex and delicate than those with either longer or shorter peripheral alkyl chains.
Introduction
Supramolecular assemblies due to intermolecular interactions are a promising platform for creating mesoscopic lattices or scaffolds, where physicochemical properties such as solubility, catalytic reactivity, and optics vary with their morphologies.1–4 One of the most studied supramolecular assemblies is the sphere-forming structure, where the molecules organize into spherical particles and further form close-packed arrangements.5–7 The observed packing structures of these soft mesospheres are analogous to the intricate atomic configuration in metallic alloys coordinated with large and small atoms.8–10 A class of materials forming metallic alloy-like structures includes dendrons,11–13 surfactants,10,14 block copolymers,15,16 and other soft matters.5,17–20 Recent explorations of these mesoscale assemblies have shown that the architectural design in molecular building components plays a significant role in determining their packing structures.21–23In this regard, the liquid crystal (LC) from dendron assembly has been of interest as it has a simple and versatile framework that offers a wide range of three-dimensional (3D) sphere-packing mesophases including 2D columnar analogs.24,25 These alloy-like structures encompass the Frank–Kasper (FK) phases and quasicrystal approximants, which consist of spherical particles with different sizes, shapes, and coordination numbers.26,27 The thermodynamic origin that determines such a packing structure is a delicate balance between intermolecular interactions to minimize the surface contact area with neighbouring particles and entropic chain stretching associated with filling the interparticle space uniformly.28 In the close-packed structures, a particle with a long corona generates a high curvature between its core and corona, whereas for the limit of a very short corona, its curvature is presumed to be flatter as a result of short-range interactions.29,30 Earlier theoretical investigations have suggested that a chain length ratio (Lp/Lw) between the outer corona (Lp) and wedges (Lw) is a parameter to modulate mesocrystal assemblies.31–33
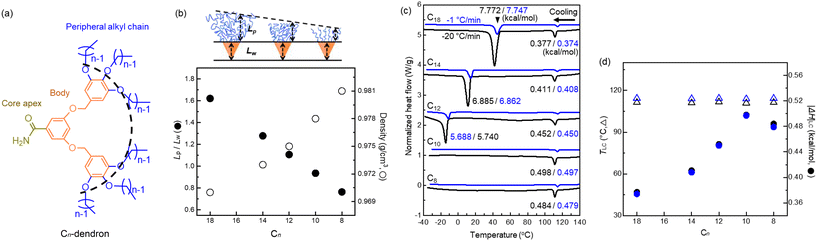
In this study, we fixed the core interaction and Lw of the dendrons and varied the length of peripheral alkyl chains that form the corona. The dendrons (Fig. S1 and S2†) were synthesized by a convergent method using different 1-bromoalkanes and characterised by 1H nuclear magnetic resonance (NMR) and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF). The effect of corona length in mesocrystal assemblies employing a model system Cn-G2-CONH2 (hereafter; Cn dendron) was investigated (Fig. 1a). They all have the same wedge of a benzyl-ether body (orange) terminated with strong hydrogen-bonding CONH2 apex (yellow), but various peripheral alkyl chains (blue) according to the Cn of n = 18, 14, 12, 10, and 8. A contour length ratio of Lp/Lw decreases from 1.62 to 0.76 as the Cn decreases (Fig. 1b), where C–C and C–N bond lengths are 1.21 and 1.13 Å, respectively, considering the bond angle restriction. This trend is inversely proprotional to the density variation of the dendrons, indicating that the Cn dendrons are well-designed to control over the fraction of periphery Lp.
Experimental
A series of Cn-G2-CONH2s were synthesized by a convergent method reported in a previous study using different 1-bromoalkanes with carbon numbers of n = 18, 14, 12, 10, and 8.34 The detailed processes and conditions are provided in the ESI.† Each Cn-G2-CONH2 was characterized by 1H NMR and MALDI-TOF. The densities of the dendrons were evaluated from a density gradient method using water/ethanol solutions. The thermal properties of the samples were examined by a PerkinElmer Diamond differential scanning calorimetry (DSC) with loading amounts of approximately 10–15 mg under nitrogen flow.Small-angle X-ray scattering (SAXS) experiments were conducted at the 4C and 9A beamlines of the Pohang Accelerator Laboratory, Korea, and the 12-ID-B beamline of Advanced Photon Source, U.S. The operating conditions for the 4C and 9A beamlines were set to a wavelength of λ = 0.733–1.127 Å, a sample-to-detector distance (SDD) of 1.0–2.5 m, and an exposure time of 1–15 s; these conditions were similarly set at the 12-ID-B beamline with λ = 0.954 Å, SDD = 2 m, and an exposure time of 1–10 s. The scattered intensities were collected on a 2D detector of Rayonix SX165 and Pilatus2M at the target temperatures ranging from 30 to 130 °C.
Electron density (ED) maps of each morphology were reconstructed with the collected SAXS intensity profiles of dendron assemblies by a similar method to a previous study.34 The peak positions and structure-factor intensities across q = 0.1–5.0 nm−1 were assigned in accordance with the corresponding space-group symmetry and lattice parameter,35,36 then recorded as the input values for SUPERFLIP software.37 The converged results were averaged and visualized using MATLAB software (in 90% contour levels) to construct Voronoi cells for the experimental polyhedrons.
Results and discussion
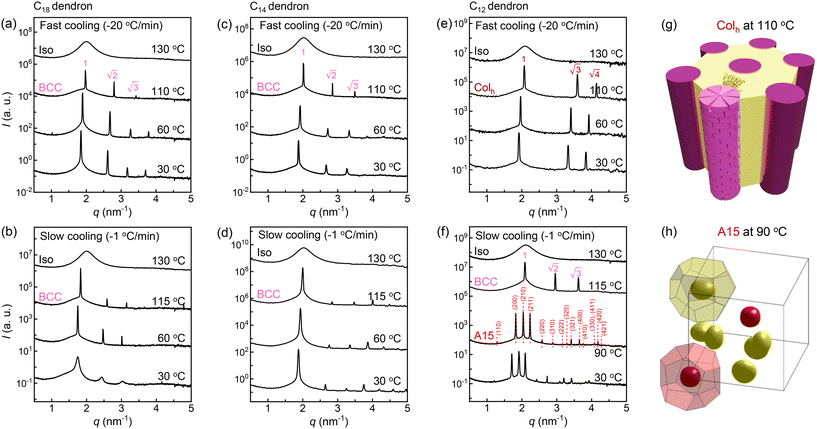
DSC thermograms of the Cn dendrons (Fig. 1c) were measured at cooling rates of −20 and −1 °C min−1 (black and blue colours, respectively) from 140 to −40 °C. The thermograms with two different cooling rates appear to have no difference except for some slight shift of the transition temperatures. While the DSC signals from the slow-cooling (−1 °C min−1) appear weaker, the enthalpic changes (|ΔH|s) of the two cooling rates are nearly identical because the heat flows during each transition are integrated as a function of transitional time. The exotherms at about 110 °C upon cooling represent the liquid crystallization from an isotropic state. The liquid-crystallization temperatures (TLC) are consistent with the isotropization temperatures (Tiso) upon heating (Fig. S3†). The |ΔH|LC slightly increases with decreasing Cn. Large exotherms at lower temperatures, observed in the C18 to C12 dendrons, correspond to the crystallization of peripheral alkyl chains. Both the crystallization temperature Tc and |ΔH|c decrease with decreasing Cn and disappear below −30 °C for the C10 and C8 dendrons. The TLC obtained upon cooling remains consistent at a high temperature of 126 °C for all the Cn dendrons (Fig. 1c and d) because of the identical cohesive interactions from CONH2 apex. Since |ΔH|LC should be proportional to the total number of hydrogen bonding involved in the mesocrystal assemblies, its overall increase with decreasing Cn may be related with an increase in crystallinity on the formation of LC.SAXS intensity profiles of all dendron assemblies were recorded during cooling from the isotropic state (Iso), shown as a function of the scattering vector q = (4π/λ)sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, where 2θ and λ are the scattering angle and wavelength of X-ray beam, respectively. The samples were first heated to 130 °C (Fig. S4†) and additionally annealed for 10 min to erase the thermal history, then subjected to a cooling process. We performed crystallization experiments with two different rates of −20 and −1 °C min−1 to 30 °C, which are hereafter denoted as the fast- and slow-cooling processes, respectively. The fast cooling of the C18 dendron (Fig. 2a) exhibits a primary peak (q*) and higher-order peaks below 110 °C, and the peak intensities increase as the temperature decreases. A set of peaks with the position ratio of
θ, where 2θ and λ are the scattering angle and wavelength of X-ray beam, respectively. The samples were first heated to 130 °C (Fig. S4†) and additionally annealed for 10 min to erase the thermal history, then subjected to a cooling process. We performed crystallization experiments with two different rates of −20 and −1 °C min−1 to 30 °C, which are hereafter denoted as the fast- and slow-cooling processes, respectively. The fast cooling of the C18 dendron (Fig. 2a) exhibits a primary peak (q*) and higher-order peaks below 110 °C, and the peak intensities increase as the temperature decreases. A set of peaks with the position ratio of 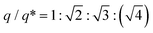 corresponds to a BCC structure composed of single-type particles with equal size. The slow cooling of the C18 dendron (Fig. 2b) generates the same BCC structure from 115 to 60 °C; consistent with the DSC result, this TLC observed in the slow-cooling process is slightly higher than that in the fast-cooling process due to a kinetic effect. Similarly, the C14 dendron presents the same phase behaviour of Iso-BCC (Fig. 2c and d) regardless of the cooling rate.
corresponds to a BCC structure composed of single-type particles with equal size. The slow cooling of the C18 dendron (Fig. 2b) generates the same BCC structure from 115 to 60 °C; consistent with the DSC result, this TLC observed in the slow-cooling process is slightly higher than that in the fast-cooling process due to a kinetic effect. Similarly, the C14 dendron presents the same phase behaviour of Iso-BCC (Fig. 2c and d) regardless of the cooling rate.
In the case of the fast cooling of the C12 dendron, the position ratio of 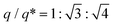 is observed, indicating a hexagonal columnar (Colh) structure (Fig. 2e). A 3D morphology of the stacked Colh (Fig. 2g), of which the 2D ED map was reconstructed from the data at 110 °C, displays the columnar packing assemblies of the flat discotic-shaped dendrons. In contrast, the slow-cooling process causes a completely different phase transition; this C12 dendron forms a BCC structure at 115 °C, which then transforms to the FK A15 structure at 90 °C (Fig. 2f). The A15 phase reconstructed from the data at 90 °C (Fig. 2h) displays two types of polyhedrons with the coordination number (CN) = 14 and 12. With further decreasing temperature, it retains the same A15 phase and no further transition to Colh is observed even for an extended annealing period of 4 months at 30 °C. Interestingly to note, when the Colh phase is heated back, it transitions to A15 and then BCC phases, indicating that the Colh is a low-temperature structure but it cannot be generated once the dendrons are assembled into spherical particles.
is observed, indicating a hexagonal columnar (Colh) structure (Fig. 2e). A 3D morphology of the stacked Colh (Fig. 2g), of which the 2D ED map was reconstructed from the data at 110 °C, displays the columnar packing assemblies of the flat discotic-shaped dendrons. In contrast, the slow-cooling process causes a completely different phase transition; this C12 dendron forms a BCC structure at 115 °C, which then transforms to the FK A15 structure at 90 °C (Fig. 2f). The A15 phase reconstructed from the data at 90 °C (Fig. 2h) displays two types of polyhedrons with the coordination number (CN) = 14 and 12. With further decreasing temperature, it retains the same A15 phase and no further transition to Colh is observed even for an extended annealing period of 4 months at 30 °C. Interestingly to note, when the Colh phase is heated back, it transitions to A15 and then BCC phases, indicating that the Colh is a low-temperature structure but it cannot be generated once the dendrons are assembled into spherical particles.
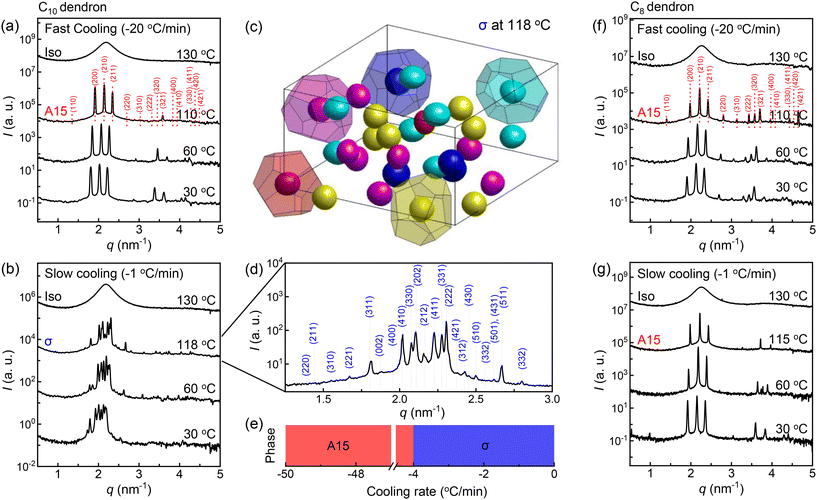
The C10 dendron, in the fast-cooling process (Fig. 3a), forms the A15 structure and no further transition down to 30 °C. In contrast, the slow cooling of the C10 dendron (Fig. 3b) produces new complex peaks of the FK σ structure (Fig. 3d). The ED map (Fig. 3c), which is reconstructed from the data at 118 °C, shows five different types of polyhedrons with CN = 15, 14, 14, 12, and 12, as delineated by red, yellow, magenta, cyan, and blue colours, respectively. We performed the cooling experiments with various cooling rates (Fig. 3e) and found that only two phases of A15 and σ emerge at faster and slower cooling rates than −4 °C min−1, respectively. The C8 dendron (Fig. 3f and g) shows the consistent formation of the A15 structure regardless of the cooling rates we studied. As seen in C18 and C14 samples, the slow-cooling process promotes the structure formation earlier or at higher temperature.
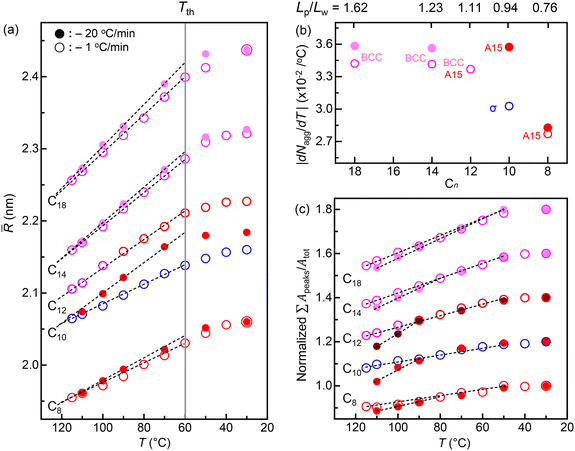
Considering a homologous peripheral region, the phase behaviours are intimately associated with the outer corona environment (or as a function of Lp/Lw). We analyzed the mean radii (![[R with combining macron]](https://www.rsc.org/images/entities/i_char_0052_0304.gif) s) of all the spherical particles as a function of temperature for the fast- and slow-cooling processes (Fig. 4a). As the temperature decreases, all the Cn dendrons reveal the linear increases in R until each threshold temperature (Tth) around 60 °C, followed by a relative plateau value of R. According to our prior study,38 the temperature dependence of R showed that Tth decreases by alleviating the core (or cohesive) interactions from the functional apexes. Hence, the consistent Tths of the Cn dendrons indicate the identical cohesive interactions from CONH2 apex. Note that a curve of the C12 dendron represents only an A15 phase obtained from the slow-cooling process for comparison, not a Colh phase. Upon cooling from the isotropic states, a couple of initial
s) of all the spherical particles as a function of temperature for the fast- and slow-cooling processes (Fig. 4a). As the temperature decreases, all the Cn dendrons reveal the linear increases in R until each threshold temperature (Tth) around 60 °C, followed by a relative plateau value of R. According to our prior study,38 the temperature dependence of R showed that Tth decreases by alleviating the core (or cohesive) interactions from the functional apexes. Hence, the consistent Tths of the Cn dendrons indicate the identical cohesive interactions from CONH2 apex. Note that a curve of the C12 dendron represents only an A15 phase obtained from the slow-cooling process for comparison, not a Colh phase. Upon cooling from the isotropic states, a couple of initial ![[R with combining macron]](https://www.rsc.org/images/entities/i_char_0052_0304.gif) for each Cn dendron are nearly identical at higher temperatures regardless of cooling rates. Except for the C10 dendron, the early-stage slopes gradually decrease as the Cn decreases, and the fast-cooling slopes are somehow greater than the slow-cooling ones. The aggregation number (Nagg) was calculated by Nagg =
for each Cn dendron are nearly identical at higher temperatures regardless of cooling rates. Except for the C10 dendron, the early-stage slopes gradually decrease as the Cn decreases, and the fast-cooling slopes are somehow greater than the slow-cooling ones. The aggregation number (Nagg) was calculated by Nagg = ![[R with combining macron]](https://www.rsc.org/images/entities/i_char_0052_0304.gif) 3NAρ/3Mw and plotted as a function of temperature for the fast- and slow-cooling processes (Fig. S5†), where NA and Mw denote Avogadro's number (6.022 × 1023 mol−1) and the molecular weight, respectively. The density ρ was measured at 30 °C, assuming that its change with temperature is negligible compared to the change of
3NAρ/3Mw and plotted as a function of temperature for the fast- and slow-cooling processes (Fig. S5†), where NA and Mw denote Avogadro's number (6.022 × 1023 mol−1) and the molecular weight, respectively. The density ρ was measured at 30 °C, assuming that its change with temperature is negligible compared to the change of ![[R with combining macron]](https://www.rsc.org/images/entities/i_char_0052_0304.gif) . During cooling from the Iso, the overall values of |dNagg/dT| gently decrease with decreasing Cn (Fig. 4b), and in each Cn dendron, the fast-cooling values run higher than the slow-cooling ones.
. During cooling from the Iso, the overall values of |dNagg/dT| gently decrease with decreasing Cn (Fig. 4b), and in each Cn dendron, the fast-cooling values run higher than the slow-cooling ones.
The SAXS data of all the Cn dendrons upon cooling show sharp but weak peaks at higher temperatures, and the peaks become broader while intensities increase (Fig. 2 and 3), corresponding to the nucleation and growth of the LC structures. At TLC, the mesocrystal domains nucleate and grow fast. As the temperature decreases, new nuclei form in the interstitial region and thus grow into smaller domains. We, however, cannot neglect the possibility that initial larger domains may break into smaller ones as the domains encounter their neighbours. Integrated intensities of the LC scattering peaks were calculated with respect to total scattering intensities (∑Apeaks/Atot), since it should be proportional to crystallinity on the formation of LC.39,40
Assuming each dendron assembly reaches the maximum crystallinity at a low temperature of 30 °C, the temperature-dependent ∑Apeaks/Atot was normalized by the value measured at 30 °C to correlate with the relative crystallinity (Fig. 4c). Even the same BCC phases obtained from C18 and C14 dendrons lead to different crystallinities at lower temperatures, as shown by ∑Apeaks/Atot. A decrease in the slope near TLC with decreasing Cn implies that the crystallinity on the formation of LC is higher for shorter Cn dendrons, agreeing with the DSC result displayed in Fig. 1d, where |ΔH|LC increases with decreasing Cn. Accordingly, the overall decrease in |dNagg/dT| with decreasing Cn (Fig. 4b) presumably attributed to an increase in crystallinity for shorter Cn dendrons, leaving fewer free dendrons available for the particle assemblies as the temperature decreases. In addition, the fast-cooling |dNagg/dT| is greater than the slow-cooling one, indicating lower crystallinity on the formation of LC for the fast-cooling dendrons where more isolated or free dendrons are available to aggregate into the particles. Consistently, in the C10 dendron, a remarkable difference in |dNagg/dT| between the A15 and σ phases from the fast- and slow-cooling processes, respectively, matches a large difference in ∑Apeaks/Atot at higher temperatures near TLC.
Unlike the dendrons with either Lp/Lw > 1 or Lp/Lw < 1, unexpected phases were observed for the C12 and C10 dendrons that have the intermediate corona length of Lp/Lw ≈ 1. Similar phase behaviour has also been observed in the nanoparticle assembly systems by varying the alkane-thiol chain length.41 Particularly, forming σ phase means that the dendrons do not experience a significant energy barrier to generate spherical particles with different sizes, where the formation energies of larger and smaller particles are more advantageous with the intermediate corona length than with either long or short coronas. For the long Cn dendrons, the conformational entropy of peripheral alkyl chains is less likely to allow the formation of spherical particles with different sizes, whereas the short Cn dendron cannot afford to stretch differently. Intriguingly, these C12 and C10 dendrons show cooling-rate-dependent phase behaviours that have been similarly observed in other supramolecular systems42–45 including block copolymers.16 The C12 dendron undergoes structural transitions only between the epitaxially related structures such as the BCC and A15 due to a slight distortion of the lattice without massive interdiffusion of dendrons.46 Once those structures were formed, they didn't transform into the 2D hexagonal Colh or σ structures presumably because it requires a significant rearrangement of dendrons.
Conclusion
In summary, the TLC's (or Tiso's) of all the Cn dendrons remain unchanged, indicating that the phase stability is not associated with the Cn but the cohesive interactions from the core apex. However, the sphere-packing phases achieved from the Cn dendrons turn out to be sensitive to the value of Lp/Lw. Especially at the intermediate corona length of Lp/Lw ≈ 1, the fast-cooling process enables the C12 and C10 dendrons to form low-temperature structures of Colh and A15, respectively, (while the slow-cooling process allows high-temperature structures of BCC-A15 and σ, respectively); these are otherwise inaccessible each other due to the energy barriers. Our results on the crystallization kinetics with the Cn dendrons indicate that the energy landscape of the dendrons at Lp/Lw ≈ 1 must be more complex and delicate than those with either longer or shorter peripheral alkyl chains.Author contributions
Under the instruction of DYR, BL, and WJ constructed the idea of characteristic dendron structures and performed SAXS analysis. TJ evaluated the structural information of sphere-packing phases from dendron assemblies as well as ED map reconstructions. HP synthesized and characterized dendron samples using NMR spectroscopy and MALDI-TOF mass spectrometry. JK assisted with reviewing experimental results. WL and HA provided an assistance with SAXS measurements as well as data analysis advice. DYR, WJ, and BL discussed the results together before writing up their manuscript in collaboration.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We acknowledge the Ministry of Science, ICT & Future Planning in Korea for funding the NRF Grants (2020R1A2C3004520, 2021R1A2C2006588, and 2022R1A4A1020543). SAXS experiments were conducted at the Pohang Accelerator Laboratory in Korea (4C and 9A beamlines) as well as at Advanced Photon Source (APS) within the Argonne National Laboratory in U.S. (12-ID-B beamline). The operation of the APS was supported by the U.S. Department of Energy, Office of Basic Energy Sciences through contract no. DE-AC0206CH11357.References
- M. Schmittel and V. Kalsani, in Functional Molecular Nanostructures, ed. A. D. Schlüter, Springer Berlin Heidelberg, Berlin, Heidelberg, 2005, pp. 1–53, DOI:10.1007/b98165.
- R. Chakrabarty, P. S. Mukherjee and P. J. Stang, Chem. Rev., 2011, 111, 6810–6918 CrossRef CAS PubMed.
- E. R. T. Tiekink, Coord. Chem. Rev., 2017, 345, 209–228 CrossRef CAS.
- Q. Song, Z. Cheng, M. Kariuki, S. C. L. Hall, S. K. Hill, J. Y. Rho and S. Perrier, Chem. Rev., 2021, 121, 13936–13995 CrossRef CAS PubMed.
- M. Huang, C.-H. Hsu, J. Wang, S. Mei, X. Dong, Y. Li, M. Li, H. Liu, W. Zhang, T. Aida, W.-B. Zhang, K. Yue and S. Z. D. Cheng, Science, 2015, 348, 424–428 CrossRef CAS PubMed.
- C. Tschierske, Angew. Chem., Int. Ed., 2013, 52, 8828–8878 CrossRef CAS PubMed.
- S. A. Kim, K.-J. Jeong, A. Yethiraj and M. K. Mahanthappa, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 4072–4077 CrossRef CAS PubMed.
- S. Lee, C. Leighton and F. S. Bates, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 17723–17731 CrossRef CAS PubMed.
- K. Yue, M. Huang, R. L. Marson, J. He, J. Huang, Z. Zhou, J. Wang, C. Liu, X. Yan, K. Wu, Z. Guo, H. Liu, W. Zhang, P. Ni, C. Wesdemiotis, W.-B. Zhang, S. C. Glotzer and S. Z. D. Cheng, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 14195–14200 CrossRef CAS PubMed.
- G. C. Shearman, A. I. I. Tyler, N. J. Brooks, R. H. Templer, O. Ces, R. V. Law and J. M. Seddon, Liq. Cryst., 2010, 37, 679–694 CrossRef CAS.
- V. S. K. Balagurusamy, G. Ungar, V. Percec and G. Johansson, J. Am. Chem. Soc., 1997, 119, 1539–1555 CrossRef CAS.
- G. Ungar, Y. Liu, X. Zeng, V. Percec and W.-D. Cho, Science, 2003, 299, 1208–1211 CrossRef CAS PubMed.
- X. Zeng, G. Ungar, Y. Liu, V. Percec, A. E. Dulcey and J. K. Hobbs, Nature, 2004, 428, 157–160 CrossRef CAS PubMed.
- S. A. Kim, K. J. Jeong, A. Yethiraj and M. K. Mahanthappa, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 4072–4077 CrossRef CAS PubMed.
- S. Lee, M. J. Bluemle and F. S. Bates, Science, 2010, 330, 349–353 CrossRef CAS PubMed.
- K. Kim, M. W. Schulze, A. Arora, R. M. Lewis, M. A. Hillmyer, K. D. Dorfman and F. S. Bates, Science, 2017, 356, 520–523 CrossRef CAS PubMed.
- S. Jeon, T. Jun, S. Jo, H. Ahn, S. Lee, B. Lee and D. Y. Ryu, Macromol. Rapid Commun., 2019, 40, 1900259 CrossRef CAS PubMed.
- K. K. Lachmayr, C. M. Wentz and L. R. Sita, Angew. Chem., Int. Ed., 2020, 59, 1521–1526 CrossRef CAS PubMed.
- J. H. Huang, Z. B. Su, M. J. Huang, R. C. Zhang, J. Wang, X. Y. Feng, R. Zhang, R. M. Zhang, W. P. Shan, X. Y. Yan, Q. Y. Guo, T. Liu, Y. C. Liu, Y. P. Cui, X. P. Li, A. C. Shi and S. Z. D. Cheng, Angew. Chem., Int. Ed., 2020, 59, 18563–18571 CrossRef CAS PubMed.
- X. Y. Feng, G. X. Liu, D. Guo, K. N. Lang, R. M. Zhang, J. H. Huang, Z. B. Su, Y. W. Li, M. J. Huang, T. Li and S. Z. D. Cheng, ACS Macro Lett., 2019, 8, 875–881 CrossRef CAS PubMed.
- T. Kato, N. Mizoshita and K. Kishimoto, Angew. Chem., Int. Ed., 2006, 45, 38–68 CrossRef CAS PubMed.
- P. Rajamalli and E. Prasad, Langmuir, 2013, 29, 1609–1617 CrossRef CAS PubMed.
- R. Fenyves, M. Schmutz, I. J. Horner, F. V. Bright and J. Rzayev, J. Am. Chem. Soc., 2014, 136, 7762–7770 CrossRef CAS PubMed.
- H.-J. Sun, S. Zhang and V. Percec, Chem. Soc. Rev., 2015, 44, 3900–3923 RSC.
- G. Ungar, V. Percec, X. B. Zeng and P. Leowanawat, Isr. J. Chem., 2011, 51, 1206–1215 CrossRef CAS.
- J. Roth and F. Gähler, Eur. Phys. J. B, 1998, 6, 425–445 CrossRef CAS.
- G. Ungar and X. B. Zeng, Soft Matter, 2005, 1, 95–106 RSC.
- C. R. Iacovella, A. S. Keys and S. C. Glotzer, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 20935–20940 CrossRef CAS PubMed.
- G. A. McConnell, A. P. Gast, J. S. Huang and S. D. Smith, Phys. Rev. Lett., 1993, 71, 2102–2105 CrossRef CAS PubMed.
- J. Bang and T. P. Lodge, Macromol. Res., 2008, 16, 51–56 CrossRef CAS.
- M. Daoud and J. P. Cotton, J. Phys., 1982, 43, 531–538 CrossRef CAS.
- Y. Lauw, F. A. M. Leermakers, M. A. Cohen Stuart, O. V. Borisov and E. B. Zhulina, Macromolecules, 2006, 39, 3628–3641 CrossRef CAS.
- A. E. Marras, T. R. Campagna, J. R. Vieregg and M. V. Tirrell, Macromolecules, 2021, 54, 6585–6594 CrossRef CAS.
- T. Jun, H. Park, S. Jeon, S. Jo, H. Ahn, W.-D. Jang, B. Lee and D. Y. Ryu, J. Am. Chem. Soc., 2021, 143, 17548–17556 CrossRef CAS PubMed.
- T. Li, A. J. Senesi and B. Lee, Chem. Rev., 2016, 116, 11128–11180 CrossRef CAS PubMed.
- B. Lee, I. Park, H. Park, C.-T. Lo, T. Chang and R. E. Winans, J. Appl. Crystallogr., 2007, 40, 496–504 CrossRef CAS.
- L. Palatinus and G. Chapuis, J. Appl. Crystallogr., 2007, 40, 786–790 CrossRef CAS.
- T. Jun, H. Park, S. Jeon, H. Ahn, W.-D. Jang, B. Lee and D. Y. Ryu, Nanoscale, 2022, 14, 16936–16943 RSC.
- C. Vonk, J. Appl. Crystallogr., 1973, 6, 148–152 CrossRef CAS.
- D. J. Wilson, C. G. Vonk and A. H. Windle, Polymer, 1993, 34, 227–237 CrossRef CAS.
- B. Pansu, C. Goldmann, D. Constantin, M. Impéror-Clerc and J.-F. Sadoc, Soft Matter, 2021, 17, 6461–6469 RSC.
- B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629–1658 CrossRef CAS.
- F. Tantakitti, J. Boekhoven, X. Wang, R. V. Kazantsev, T. Yu, J. Li, E. Zhuang, R. Zandi, J. H. Ortony, C. J. Newcomb, L. C. Palmer, G. S. Shekhawat, M. O. de la Cruz, G. C. Schatz and S. I. Stupp, Nat. Mater., 2016, 15, 469–476 CrossRef CAS PubMed.
- N. M. Matsumoto, R. P. M. Lafleur, X. Lou, K.-C. Shih, S. P. W. Wijnands, C. Guibert, J. W. A. M. van Rosendaal, I. K. Voets, A. R. A. Palmans, Y. Lin and E. W. Meijer, J. Am. Chem. Soc., 2018, 140, 13308–13316 CrossRef CAS PubMed.
- J. Matern, Y. Dorca, L. Sánchez and G. Fernández, Angew. Chem., Int. Ed., 2019, 58, 16730–16740 CrossRef CAS PubMed.
- P. Xiao, D. Sheppard, J. Rogal and G. Henkelman, J. Chem. Phys., 2014, 140, 174104 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nr01243c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |