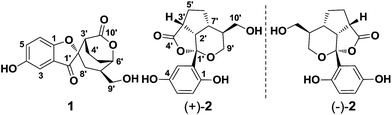
Applanatumols A and B, meroterpenoids with unprecedented skeletons from Ganoderma applanatum†
Qi Luoab,
Lei Dia,
Xiao-Hua Yangac and
Yong-Xian Cheng*a
aState Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650204, People's Republic of China. E-mail: yxcheng@mail.kib.ac.cn; Fax: +86-871-65223048; Tel: +86-871-65223048
bUniversity of Chinese Academy of Sciences, Yuquan Road 19, Beijing 100049, People's Republic of China
cGuangdong Pharmaceutical University, Guangzhou 510006, People's Republic of China
First published on 26th April 2016
Abstract
Applanatumols A (1) and B [(±)-2], two unique meroterpenoids with a novel spiro[benzofuran-2,2′-bicyclo[3.2.2]nonane] ring system and a naturally unusual dioxacyclopenta[cd]inden motif, respectively, were isolated from Ganoderma applanatum. Their structures were determined using spectroscopic data. In particular, the absolute configurations of 1 and 2 were assigned by X-ray diffraction analysis using the Flack parameter and ECD calculations, respectively. A plausible biosynthetic pathway for 1 and 2 is proposed. The biological activities of 1 and 2 against renal fibrosis were accessed in rat proximal tubular epithelial cells.
1. Introduction
Chronic kidney disease (CKD) has become a global public health problem that affects more than 10% of the population in the developed countries.1 Renal fibrosis is known to be the inevitable consequence of CKD, which could in turn lead to a progressive and irreversible loss of renal function, a process known as end-stage renal disease (ESRD) that requires dialysis or organ transplantation. Thus, finding new approaches aimed at attenuating renal fibrosis is of great importance. Despite multiple-drug therapy leading to a slowing of renal fibrosis progression towards ESRD over the years, there are still hundreds of people who develop ESRD every year with a big economic burden. Renal fibrosis is characteristic of abnormalities of extracellular matrix (ECM) components and therefore targeting ECM balance might be an important strategy to avoid renal failure.2In recent years, the search for renoprotective substances from natural sources has become our research focus. Inspired by the traditional uses of Chinese medicine, we have conducted an extensive investigation of Ganoderma fungi, which led to the isolation of several novel meroterpenoids with profound kidney protection activities. For examples, lingzhiol was identified as a potent Smad3 phosphorylation inhibitor and Nrf2 activator from G. lucidum,3 and lingzhilactone B from G. lingzhi was found to be able to ameliorate adriamycin-induced nephropathy in mice via scavenging reactive oxygen species and activating Nrf2.4 These findings not only add new facets to Ganoderma chemistry but also prompt us to further explore the structural diversity of meroterpenoids in this genus and their biological potential to fight kidney diseases. G. applanatum is a medicinal mushroom and has been used to treat various chronic diseases in Chinese folk medicine. We have characterized applanatumin A from this species, which exhibits potent antifibrotic activity.5 In our follow-up study on this mushroom, two structurally novel compounds applanatumols A (1) and B (2) were isolated and their biological activities towards renal fibrosis were evaluated. Interestingly, 1 is a spiro compound with a novel 6/5/7/6 ring system, and 2 bears an unusual dioxacyclopenta[cd]inden motif making it look like a naturally hemisphere-shaped structure. A plausible biosynthetic pathway further confirmed the structural rationality of 1 and 2 (Fig. 1).
2. Results and discussion
2.1. Structure elucidation
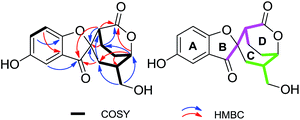
The EtOH extract of G. applanatum was suspended in H2O and partitioned with EtOAc. A combination of chromatography on an EtOAc soluble fraction afforded 1 and 2.Applanatumol A (1) was obtained as an optically active yellow gum. Its molecular formula, C16H16O6, was determined by means of HRESIMS, 13C NMR, and DEPT spectra, having 9 degrees of unsaturation. The 1H NMR spectrum (Table 1) of 1 contains an ABX spin system [δH 6.98 (1H, d, J = 2.7 Hz, H-3), 7.29 (1H, dd, J = 8.9, 2.7 Hz, H-5), 7.06 (1H, d, J = 8.9 Hz, H-6)], suggesting the presence of a 1,2,4-trisubstituted benzene ring. The 13C NMR and DEPT spectra show 16 carbons ascribed to 4 methylene (one oxygenated), 6 methine (three sp2 and three sp3), and 6 quaternary carbons (one ketone, one carbonyl, three olefinic, including two oxygenated, and one aliphatic). The 1H-1H COSY spectrum (Fig. 2) of 1 shows the presence of H-5/H-6, H-3′/H-4′/H-5′/H-6′/H-7′/H-8′ and H-7′/H-9′ fragments. The planar structure of 1 was determined mainly through 2D NMR experiments. The HMBC correlations of H-3, H-3′, H-8′/C-1′ (δC 202.5), H-3′, H-4′, H-8′/C-2′ (δC 87.6), and H-8′/C-3′ (δC 44.9) and in consideration of their downfield shifts indicate that C-1 and C-2′ are linked via an oxygen bridge. Moreover, this suggests that rings B and C (Fig. 2) are present as part of the novel spiro[benzofuran-2,2′-bicyclo[3.2.2]nonane] ring system. In addition, except for rings A-C, one ketone, and one carbonyl, the remaining one degree of unsaturation is attributed to an additional ring in 1. HMBC correlations of H-3′, H-4′, H-6′ (δH 4.79)/C-10′ (δC 170.3) suggest that C-10′ and C-6′ are linked by formation of an ester bond (ring D). These findings also indicate the presence of two isopentyl moieties (Fig. 2, pink line and green line).
| 1 | 2 | ||||
|---|---|---|---|---|---|
| No. | δH | δC | No. | δH | δC |
| a In acetone-d6.b In DMSO-d6 (600 MHz). | |||||
| 1 | 165.7 | 1 | 148.5 | ||
| 2 | 119.9 | 2 | 129.7 | ||
| 3 | 6.98 (d, 2.7) | 108.3 | 3 | 6.87 (d, 2.8) | 114.0 |
| 4 | 153.7 | 4 | 150.8 | ||
| 5 | 7.29 (dd, 8.9, 2.7) | 128.4 | 5 | 6.64 (d, 8.6, 2.8) | 117.6 |
| 6 | 7.06 (d, 8.9) | 115.2 | 6 | 6.67 (d, 8.6) | 118.5 |
| 1′ | 202.5 | 1′ | 108.9 | ||
| 2′ | 87.6 | 2′ | 3.14 (dd, 10.4, 8.6) | 41.9 | |
| 3′ | 2.76 (dd, 6.0, 2.6) | 44.9 | 3′ | 3.28 (t-like, 8.1) | 50.6 |
| 4′a | 2.56 (m) | 17.9 | 4′ | 181.3 | |
| 4′b | 1.92 (m) | 5′a | 2.18 (m) | 29.9 | |
| 5′ | 2.15 (m) | 20.1 | 5′b | 1.73 (m) | |
| 6′ | 4.79 (t-like, 5.9) | 78.0 | 6′a | 1.90 (m) | 32.5 |
| 7′ | 2.45 (m) | 43.9 | 6′b | 1.51 (m) | |
| 8′a | 2.07 (overlap) | 30.1 | 7′ | 2.28 (m) | 37.9 |
| 8′b | 1.66 (dd, 15.0, 4.1) | 8′ | 1.69 (m) | 38.5 | |
| 9′a | 3.58 (dd, 11.0, 5.2) | 64.0 | 9′a | 3.98 (dd, 12.1, 2.4) | 59.0 |
| 9′b | 3.48 (dd, 11.0, 8.0) | 9′b | 3.91 (dd, 12.1, 3.5) | ||
| 10′ | 170.3 | 10′a | 4.02 (dd, 10.7, 3.9) | 64.1 | |
| 4-OHa | 8.67 (s) | 10′b | 3.77 (dd, 10.7, 2.9) | ||
| 9′-OHa | 4.04 (s) | 1-OHb | 9.17 (s) | ||
| 4-OHb | 8.80 (s) | ||||
| 10′-OHb | 4.68 (s) | ||||
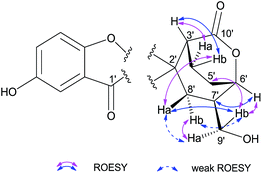
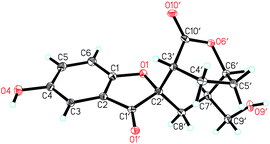
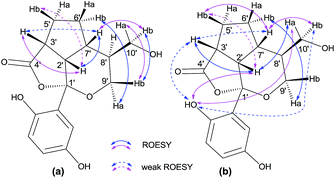
There are four stereogenic centers in 1 and the conformationally immobile bicyclo[3.2.2]nonane ring system in 1 requires H-3′ and H-6′ to be at the same orientation. ROESY correlations of Hb-4′ (δH 1.92)/Ha-8′, Ha-8′/H-9′, H-9′/H-5′ (Fig. 3) show that all these protons are spatially adjacent, which naturally assign the relative configuration at C-7′. This conclusion was further confirmed by the observed ROESY correlation of H-6′/H-7′. Unfortunately, it is impossible to assign the relative configuration at the spiro center using ROESY observations. Finally, the absolute configuration of 1 was unambiguously assigned as 2′S,3′R,6′S,7′R by utilizing single-crystal X-ray diffraction data and anomalous scattering of CuKα radiation. As shown in Fig. 4, the formation of rings A–D made the structure of 1 cage-like.
(±)-Applanatumol B (2), obtained as a yellow gum, has a molecular formula C16H18O6 (8 degrees of unsaturation), based on its HREIMS, 13C NMR and DEPT spectra. The 13C NMR and DEPT spectra show that this substance contains 16 carbons, including four methylene (two oxygenated), seven methine (three olefinic and four aliphatic), and five quaternary carbons (one carbonyl, three olefinic including two oxygenated, and one oxygenated aliphatic). The 1H NMR spectrum (Table 1) of 2 contains a typical ABX spin system [δH 6.87 (1H, d, J = 2.8 Hz, H-3), 6.64 (1H, dd, J = 8.6, 2.8 Hz, H-5), 6.67 (1H, d, J = 8.6 Hz, H-6)]. The architecture of 2 was constructed mainly based on the 1H-1H COSY and HMBC spectra (Fig. 5). The 1H-1H COSY spectrum shows correlations of H-5/H-6, H-2′/H-3′/H-5′/H-6′/H-7′/H-8′/H-9′, H-2′/H-7′, and H-8′/H-10′. Moreover, the HMBC spectrum presents correlations between H-3′, H-7′, H-9′/C-1′, H-2′, H-3′, H-5′/C-4′. Considering the chemical shift of C-1′ (δC 108.9) and C-4′ (δC 181.3) and the degree of unsaturation of 2, the data indicates the existence of a naturally unusual dioxacyclopenta[cd]inden motif comprising rings A–C, which makes the architecture of 2 hemisphere-shaped. In addition, HMBC correlations of H-3, H-6/C-1′, H-2′/C-2 (δC 129.7) suggest that one phenyl unit (ring D) in 2 is linked to C-1′ through C-2. With this, the planar structure of 2 was deduced as shown.
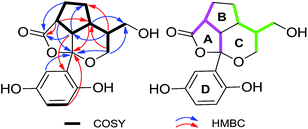 | ||
| Fig. 5 Key COSY and HMBC correlations of 2; pink and green in 2 represent two independent isopentyl moieties. | ||
The relative configuration of 2 was assigned by ROESY correlations (Fig. 6). In the ROESY spectrum, correlations between Hb-9′/Ha-10′, Hb-10′, Ha-9′/Hb-6′, and H-7′/Hb-10′ were observed, indicating the relative configurations at C-7′ and C-8′. Due to the ring tension, the formation of rings A and B naturally requires H-2′ and H-7′ to be at the same orientation. This was further confirmed by ROESY correlations of H-2′/H-7′ and H-2′/Ha-10′ (in DMSO-d6). In addition, ROESY correlation of H-2′/H-3′ along with weak cross peak between H-3′ and H-7′ suggests that these protons are spatially adjacent, allowing the stereochemistry at C-3′ to be assigned. As far as the relative configuration at C-1′, we measured ROESY correlations in DMSO-d6, in which interactions between 1-OH/H-2′, Ha-10′, H-3′ (weak), Hb-10′ (weak) were observed, and clearly indicate the orientation of the phenyl group. With this, the relative configuration of 2 was assigned. It can be noted that compound 2 was isolated as a racemic mixture and further purification by chiral-phase HPLC yielded (+)-2 and (−)-2 in a ratio of 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Computational methods were relied on to determine the absolute configuration of (+)-2 (ESI†). As shown in Fig. 7, the calculated ECD spectrum of (+)-2 agrees well with those of the experimental one, suggesting 1′S,2′S,3′R,7′R,8′S-configuration for (+)-2.
1. Computational methods were relied on to determine the absolute configuration of (+)-2 (ESI†). As shown in Fig. 7, the calculated ECD spectrum of (+)-2 agrees well with those of the experimental one, suggesting 1′S,2′S,3′R,7′R,8′S-configuration for (+)-2.
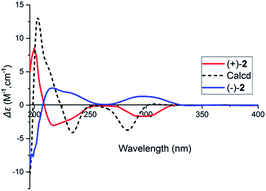 | ||
| Fig. 7 Comparison of calculated ECD spectrum for (1′S,2′S,3′R,7′R,8′S) with the experimental spectra of (+)-2 and (−)-2 in MeOH. σ = 0.27 eV; shift = +15 nm. | ||
Meroterpenoids in the genus Ganoderma feature a hydroquinone and a terpenoidal side chain. To date, several structurally diverse meroterpenoids have been characterized by us,4,6,7 providing structure targets for synthetic chemists. Lingzhiol from G. lucidum is such an example that has been successfully synthesized by Yang and co authors,8 demonstrating that Ganoderma could be a potential source for searching molecules of interest. The present finding of exceptional structures of 1 and 2 makes a convincing footnote for our abovementioned hypothesis. In general, the ketone group at the benzylic location is rather stable. However, the ketalation reaction occurred in 2 and the formation of C-2′-C-7′ led to the construction of rings A–C, representing, to the best of our knowledge, the first example of a new carbon skeleton meroterpenoid with a rare dioxacyclopenta[cd]inden group. Moreover, that rings A–C of 2 sharing a common carbon (C-2′) makes it a hemisphere-like structure, which might be a challenge for synthetic chemists to finish the total synthesis of 2.
One more thing that needs to be mentioned is that 2 was isolated as a racemic mixture. Similar phenomena have also been found in the other meroterpenoids from Ganoderma.4,6,7 We have tried to check whether the previously isolated racemic compounds are artificial products, but our efforts gave negative feedback for this question.7 The reasons for the racemic nature of enzyme-catalyzed natural products in nature remain unknown to date. One possibility is that these racemic meroterpenoids might only act as intermediates for fungi to synthesize their end products. However, this needs further exploration.
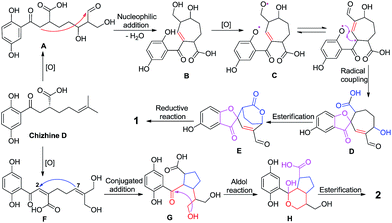
Analyses of the structures of 1 and 2 indicate that they are derived from the combination of the shikimic acid pathway and the mevalonic acid pathway (Scheme 1).9 First, chizhine D,10 a precursor, which has been isolated from G. lucidum, is produced from geranyl diphosphate and 4-hydroxybenzonic acid under geranyltransferase.9 Two intermediates, A and F, are generated from chizhine D by oxidation reaction. The monomer A could be cyclized via nucleophilic addition to generate B, which could be transformed to C by further oxidation. An interesting feature in the biosynthesis of 1 is the formation of a spiro structure by a stereospecific phenol radical coupling.5 Second, esterification and reduction of monomer D would yield 1. Finally, monomer G can be cyclized by conjugated addition of F and ring A in 2 is formed via esterification (Scheme 1).
2.2. Biological evaluation
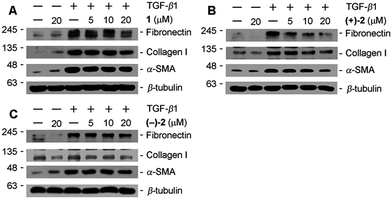
The antifibrotic properties of compounds 1 and 2 were examined by Western blotting in TGF-β1 induced rat proximal tubular epithelial cells in the condition that no cytotoxicity was observed for 1 and 2 at the indicated concentrations (ESI†). The results show that 1 and (+)-2 could inhibit ECM components (fibronectin and collagen I) and α-SMA in a dose-dependent manner. In contrast to (+)-2, the potency of (−)-2 is weaker (Fig. 8), indicating the influence of optical activity on biological properties.3. Experimental section
3.1. General procedure
Optical rotations were recorded on a Horiba SEPA-300 polarimeter. UV spectra were obtained on a Shimadzu UV-2401PC spectrometer. CD spectra were obtained on a Chirascan instrument. NMR spectra were obtained on a Bruker AV-400 spectrometer, with TMS as an internal standard. ESIMS, HRESIMS, and HREIMS were measured on an API QSTAR Pulsar 1 spectrometer. C-18 silica gel (40−60 μm; Daiso Co., Japan), MCI gel CHP 20P (75−150 μm, Mitsubishi Chemical Industries, Tokyo, Japan) and Sephadex LH-20 (Amersham Pharmacia, Sweden) were used for column chromatography. Semi-preparative HPLC was carried out using an Agilent 1200 liquid chromatograph, the columns used were a 250 mm × 10 mm, i.d., 5 μm, Daicel Chiralpak (IC), flow rate: 2.5 mL min−1, and a 250 mm × 9.4 mm, i.d., 5 μm, Zorbax SB-C18, flow rate: 2 mL min−1.3.2. Fungal material
The fruiting bodies of G. applanatum were obtained from Tongkang Pharmaceutical Co. Ltd. in Guangzhou Province, P. R. China, in September 2013. The material was identified by Prof. Zhu-Liang Yang at our institute, and a voucher specimen (CHYX-0590) was deposited at the State Key Laboratory of Photochemistry and Plant Resources in West China of our institute.3.3. Extraction and isolation
Powdered fruiting bodies of G. applanatum (30 kg) were extracted by refluxing with 80% EtOH (3 × 120 L × 2 h) to give a crude extract, which was suspended in water followed by extraction with EtOAc to afford an EtOAc soluble extract (0.9 kg). The EtOAc extract was divided into seven parts (Fr.1–Fr.7) using a MCI gel CHP 20P column eluted with gradient aqueous MeOH (20–100%). Fr.3 (73 g) was separated by MCI gel CHP 20P eluted with gradient aqueous MeOH (30–60%) to yield nine fractions (Fr.3.1–Fr.3.9). Among these, Fr.3.3 (12 g) was purified by Sephadex LH-20 (MeOH) followed by successive RP-18 column (MeOH/H2O, 10–55%) and semi-preparative HPLC (MeOH/H2O, 25%) to yield compounds 1 (3.4 mg) and 2 (2.3 mg). Compound 2 was isolated as a racemate, which was further purified by chiral phase on Daicel Chiralpak IC (n-hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH, 80
EtOH, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, flow rate: 2.5 mL min−1) to afford enantiomers (+)-2 (1.1 mg) and (−)-2 (0.9 mg).
20, flow rate: 2.5 mL min−1) to afford enantiomers (+)-2 (1.1 mg) and (−)-2 (0.9 mg).
3.4. Spectral data of the new compounds
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 370 (3.46), 254 (3.82), 219 (4.15) nm; CD (MeOH) Δε196 −6.98, Δε216 +5.32, Δε233 −3.83, Δε370 +0.87; ESIMS m/z 303 [M − H]−, HRESIMS m/z 303.0874 [M − H]− (calcd for C16H15O6, 303.0869). 1H and 13C NMR data, see Table 1.
ε) 370 (3.46), 254 (3.82), 219 (4.15) nm; CD (MeOH) Δε196 −6.98, Δε216 +5.32, Δε233 −3.83, Δε370 +0.87; ESIMS m/z 303 [M − H]−, HRESIMS m/z 303.0874 [M − H]− (calcd for C16H15O6, 303.0869). 1H and 13C NMR data, see Table 1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 372 (2.55), 287 (3.58), 202 (4.07) nm; ESIMS m/z 305 [M − H]−, HREIMS m/z 306.1092 [M]+ (calcd for C16H18O6, 306.1103). [α]22.5D +25.6 (c 0.88, MeOH); CD (MeOH) Δε200 +8.55, Δε217 −3.02, Δε301 −1.65; (+)-2; [α]22.4D −26.9 (c 0.72, MeOH); CD (MeOH) Δε199 −7.70, Δε215 +2.98, Δε297 +1.49; (−)-2. 1H and 13C NMR data, see Table 1.
ε) 372 (2.55), 287 (3.58), 202 (4.07) nm; ESIMS m/z 305 [M − H]−, HREIMS m/z 306.1092 [M]+ (calcd for C16H18O6, 306.1103). [α]22.5D +25.6 (c 0.88, MeOH); CD (MeOH) Δε200 +8.55, Δε217 −3.02, Δε301 −1.65; (+)-2; [α]22.4D −26.9 (c 0.72, MeOH); CD (MeOH) Δε199 −7.70, Δε215 +2.98, Δε297 +1.49; (−)-2. 1H and 13C NMR data, see Table 1.3.5. Computational study
Geometry optimizations of compounds 1 and 2 were carried out at the DFT/B3LYP/6-311G (d,p) level by means of a Gaussian 09 program. The ECD spectra were obtained by using Gaussian 09 software employing the TDDFT-B3LYP functional and the 6-311G (d,p) basis sets. The calculated CD spectra in Δε were obtained using overlapping Gaussian functions.113.6. Cell culture and treatment
Rat proximal tubular epithelial cells (NRK-52E; American Type Culture Collection, Rockville, MD) were maintained in DMEM/F-12 containing 10% fetal bovine serum and penicillin/streptomycin (all obtained from Gibco BRL, Grand Island, NY). Cells were grown to 80–90% confluence and starved in a serum-free DMEM/F-12 overnight before stimulation with 5 ng mL−1 TGF-β1 (100-21, PeproTech, NJ, USA). For some studies, NRK-52E cells were pre-incubated with compounds of 1, (+)-2 and (−)-2 at 37 °C for 1 h. Whole-cell lysates were prepared and subjected to Western blot analyses.3.7. MTT assay
MTT assay was used to detect cell viability. NRK-52E cells in log phase were growing by 6000 cells per well of 96-well flat-bottomed microtiter plates. Cells were incubated with different concentrations of the compounds. After 24 h of incubation, 20 μL of 5 mg mL−1 MTT were introduced to each well and incubated for 4 h of exposure. The plates were centrifuged and medium was decanted. The cells were subsequently dissolved in 150 μL DMSO with gentle shaking for 30 min at room temperature followed by measurement of absorbance at 492 nm.3.8. Western blot analysis
Protein expression was analyzed by Western blot analysis as described previously.12 Proteins were transferred onto polyvinylidene difluoride membranes (Millipore, Schwalbach, Germay) and probed with the primary antibodies as follows: rabbit polyclonal anti-fibronectin (F3648; Sigma, St. Louis, MO), mouse anti-alpha-smooth muscle antibody (A2547, Sigma, St. Louis, MO), rabbit anti-collagen I (BA0325, BOSTER, Wuhan, P. R. China) and rabbit anti-beta-tubulin (ab179513; Abcam, MA, USA). Horseradish peroxidase-conjugated goat anti-rabbit antibody were from Boster (BA1054, Boster, Wuhan, P. R. China).4. Conclusions
In conclusion, two structurally unusual meroterpenoids were isolated from the fungus G. applanatum. Biological evaluation showed that 1 and (−)-2 are potent ECM inhibitors in TGF-β1 induced rat proximal tubular epithelial cells, suggesting that these metabolites could be used as novel structure templates for synthesizing more potent agents, which are beneficial for CKD.Acknowledgements
This study was supported by the National Natural Science Foundation of China (21472199), the NSFC-Joint Foundation of Yunnan Province (U1202222), the National Science Fund for Distinguished Young Scholars (81525026) and a project from State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany (P2016-ZZ02).Notes and references
- M. M. F. Jose, G. N. Maria, M. S. Carlos and M. L. N. Jose, Pharmacol. Ther., 2015, 156, 44 CrossRef PubMed.
- Y. Liu, Kidney Int., 2006, 69, 213 CrossRef CAS PubMed.
- Y. M. Yan, J. Ai, Y. N. L. L. Zhou, A. C. K. Chung, R. Li, J. Nie, P. Fang, X. L. Wang, J. Luo, Q. Hu, F. F. Hou and Y. X. Cheng, Org. Lett., 2013, 15, 548 Search PubMed.
- Y. M. Yan, X. L. Wang, L. L. Zhou, F. J. Zhou, R. Li, Y. Tian, Z. L. Zuo, P. Fang, A. C. K. Chung, F. F. Hou and Y. X. Cheng, J. Ethnopharmacol., 2015, 176, 385 CrossRef CAS PubMed.
- Q. Luo, L. Di, W. F. Dai, Q. Lu, Y. M. Yan, Z. L. Yang, R. T. Li and Y. X. Cheng, Org. Lett., 2015, 17, 1110 CrossRef CAS PubMed.
- M. Dou, L. Di, L. L. Zhou, Y. M. Yan, X. L. Wang, F. J. Zhou, Z. L. Yang, R. T. Li, F. F. Hou and Y. X. Cheng, Org. Lett., 2014, 16, 6064 CrossRef CAS PubMed.
- F. J. Zhou, Y. Nian, Y. M. Yan, Y. Gong, Q. Luo, Y. Zhang, B. Hou, Z. L. Zuo, S. M. Wang, H. H. Jiang, J. Yang and Y. X. Cheng, Org. Lett., 2015, 17, 3082 CrossRef CAS PubMed.
- R. Long, J. Huang, W. Shao, S. Liu, Y. Lan, J. Gong and Z. Yang, Nat. Commun., 2014, 5, 5707 CrossRef CAS PubMed.
- Q. Lou, L. Tian, L. Di, Y. M. Yan, X. Y. Wei, X. F. Wang and Y. X. Cheng, Org. Lett., 2015, 17, 1565 CrossRef PubMed.
- Q. Luo, X. L. Wang, L. Di, Y. M. Yan, Q. Lu, X. H. Yang, D. B. Hu and Y. X. Cheng, Tetrahedron, 2015, 71, 840 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision C.01, Gaussian, Inc., Wallingford, CT: 2010 Search PubMed.
- L. L. Zhou, Y. J. Li, D. Zhou, R. J. Tan and Y. H. Liu, J. Am. Soc. Nephrol., 2013, 24, 771 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1D, 2D NMR, and MS spectra, crystallographic data, MTT assay. CCDC 1439639. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra05148k |
| This journal is © The Royal Society of Chemistry 2016 |