The metabolism and analysis of isoflavones and other dietary polyphenols in foods and biological systems
Stephen
Barnes
*ab,
Jeevan
Prasain
ab,
Tracy
D'Alessandro
ab,
Ali
Arabshahi
b,
Nigel
Botting
c,
Mary Ann
Lila
bd,
George
Jackson
b,
Elsa M.
Janle
b and
Connie M.
Weaver
b
aDepartment of Pharmacology & Toxicology, MCLM 452, University of Alabama at Birmingham, 1918 University Boulevard, Birmingham, AL 35294, USA. E-mail: sbarnes@uab.edu; Fax: (+205) 934-6944; Tel: (+205) 934-7117
bThe Purdue University-University of Alabama at Birmingham Botanicals Research Center, Birmingham, AL 35294, USA
cDepartment of Chemistry, University of St. Andrews, Fife, Scotland
dThe Plants for Human Health Institute, North Carolina State University, Kannapolis, NC 28081, USA
First published on 9th May 2011
Abstract
Polyphenols in dietary and botanical matrices are usually present as simple and complex O-glycosides. In fermented dietary materials, the glycosidic moiety is removed and accompanied in some cases by more complex changes to the polyphenol. As for most xenobiotics, polyphenols undergo phase II conjugation in the intestinal wall during their absorption from the gut. In contrast, a few polyphenols, such as puerarin in the kudzu vine, are C-glycosides and are stable in the gut and during absorption, distribution and excretion. Large bowel bacteria reduce polyphenol aglycones, causing opening of the heterocyclic B-ring and ring cleavage. The products are mostly absorbed and enter the bloodstream. Phase I and II metabolism events occur in the intestine and the liver – most polyphenols predominantly circulate as β-glucuronides and sulfate esters with very little as the aglycones, the presumed active forms. In addition, metabolism can occur in non-hepatic tissues and cells including breast tumor cells that have variable amounts of cytochrome P450s, sulfatase and sulfotransferase activities. Inflammatory cells produce chemical oxidants (HOCl, HOBr, ONO2−) that will react with polyphenols. The isoflavones daidzein and genistein and the flavonol quercetin form mono- and dichlorinated products in reaction with HOCl. Genistein is converted to 3′-nitrogenistein in the lung tissue of lipopolysaccharide-treated rats. Whereas polyphenols that can be converted to quinones or epoxides react with glutathione (GSH) to form adducts, chlorinated isoflavones do not react with GSH; instead, they are converted to β-glucuronides and are excreted in bile. Analysis of polyphenols and their metabolites is routinely carried out with great sensitivity, specificity and quantification by LC-tandem mass spectrometry. Critical questions about the absorption and tissue uptake of complex polyphenols such as the proanthocyanins can be answered by labeling these polyphenols with 14C-sucrose in plant cell culture and then purifying them for use in animal experiments. The 14C signature is quantified using accelerator mass spectrometry, a technique capable of detecting one 14C atom in 1015carbon atoms. This permits the study of the penetration of the polyphenols into the interstitial fluid, the fluid that is actually in contact with non-vascular cells.
 Stephen Barnes | Stephen Barnes received his PhD in Biochemistry in 1972 from Imperial College, University of London. He is a Professor of Pharmacology and Toxicology at the University of Alabama at Birmingham. He has a broad interest in the study of dietary polyphenols. He and Connie Weaver have co-directed a Botanicals Center for Age-related Disease since 2000 with a particular focus on mechanisms of action of these compounds. |
 Jeevan Prasain | Jeevan K. Prasain received his PhD in 1998 from Toyama Medical & Pharmaceutical University, Japan. Currently, he is an Assistant Professor in University of Alabama at Birmingham and his research interests include the use of tandem mass spectrometry in the study of the metabolomics, bioavailability and pharmacokinetics of dietary natural products. |
 Tracy D'Alessandro | Dr Tracy D'Alessandro received a PhD in Pharmacology and Toxicology from the University of Alabama at Birmingham in 2008. She did a postdoctoral fellowship in Dr Candace Floyd's lab and is expert in the application of surgical models for research on polyphenol metabolism. She currently is a staff scientist at the Alabama Department of Forensic Science. |
 Mary Ann Lila | Mary Ann Lila is Director of the Plants for Human Health Institute, North Carolina State University. She is the endowed David H. Murdock Chair, and is a Professor in the Department of Food, Bioprocessing, and Nutrition Sciences. Her research focuses on both wild and domesticated berries and their wide-ranging health and unique human health benefits, alleviating the symptoms of diabetes and metabolic syndrome. |
 Elsa Janle | Dr Elsa Janle, PhD, a graduate of Purdue University in Physiology, is an Associate Research Professor in the Department of Foods and Nutrition and a member of the Purdue-UAB Botanicals Research Center for Age Related diseases. Her research interests include the effects of polyphenols on glucose metabolism in diabetes, and the brain bioavailability of grape polyphenols in models of neurodegenerative diseases. |
 Connie M. Weaver | Connie M. Weaver, Ph.D., is Distinguished Professor and Head of the Department of Foods & Nutrition at Purdue University in Indiana and is a member of the Institute of Medicine. From 2000 to 2010, she was Director of the NIH Purdue-UAB Botanical Research Center to study polyphenolics in age-related diseases. Her research interests include mineral bioavailability, calcium metabolism, and bone health. |
Introduction
Research on the effects of dietary polyphenols on human health is seriously influenced by the gap between what is in the food and how individual polyphenols are delivered and operate at the level of the cell. While numerous investigators have demonstrated interesting biochemical, biological and mechanistic effects of polyphenols in cell culture, the concentrations required (in the micromolar range) far exceed those that can be delivered from the diet. As for xenobiotics developed by the pharmaceutical industry for therapeutic interventions, the questions of the chemical and physical forms of polyphenols and their oral bioavailability and metabolism are huge issues to consider. This summary of the metabolism and distribution of polyphenols takes into account the changing chemistry that occurs before the dietary source is consumed as well as the more traditional metabolic events within the human or animal model used. With the advent of personalized medicine we can expect that diet may need to be personalized to take into account the variable metabolism of foods and their components (also xenobiotics) in individuals. This will represent a considerable challenge for the food industry to develop suitable products that match human tastes and needs and can be economically viable to produce for consumption.Polyphenols
These encompass a large group of plant-derived compounds containing more than one phenolic group. These include the bioflavonoids (anthocyanins, flavanols, flavonols, flavones, flavanones, isoflavones and proanthocyanins), coumestanes, lignans, and stilbenoids (Fig. 1). Consideration should also be given to their intermediates formed during synthesis as well as their metabolic products since it should not be assumed a priori that the bioactive form of a dietary source of polyphenols is any of the recognized polyphenols in the food.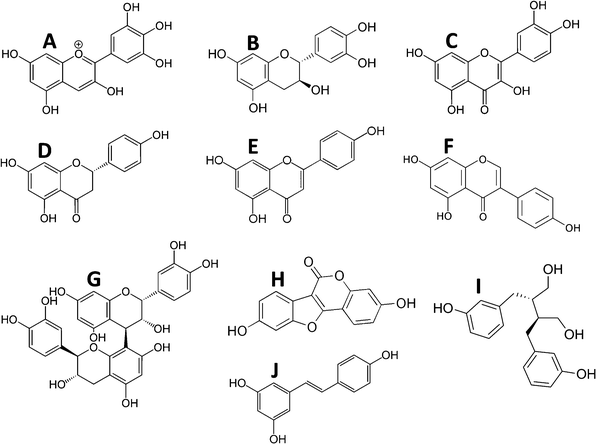 | ||
| Fig. 1 Examples of polyphenol structures by class. A, Anthocyanins (delphinidin); B, flavanols (epi-catechin); C, flavonols (quercetin); D, flavanones (naringenin); E, flavones (apigenin); F, isoflavones (genistein); G, proanthocyanidins (proanthocyanidin B1); H, coumestanes (coumesterol); I, lignans (enterodiol); and J, stilbenoids (resveratrol). | ||
In the plants, polyphenols are mostly found in complex chemical forms as β-glycosides (mono- and diglycosides of hexoses and pentoses), often with the sugar moiety further esterified. In the cotyledon and hypocotyl of the soybean the isoflavone genistein (5,7,4′-trihydroxyisoflavone) is present as its 6′′-O-malonyl-7-O-β-D-glucoside1,2 (Fig. 2A). In the tuber of Apios americana there is a 7-O-β-D-glucosylglucoside of genistein3 (Fig. 2C). Besides the addition of hydrophilic sugars, in some plants the polyphenols are more hydrophobic because of prenylation.4,5 For example, the bioactive in Horny Goat Weed (a Chinese botanical preparation from Epimedium grandiflorum) is icariin, a 3,7-diglycoside of the polyphenol kaempferol with a 8-prenyl substituent (Fig. 3). It is believed to have a role in increasing the bioavailability of nitric oxide.5 In pharmacology, many lead compounds are modified to introduce hydrophobic groups to increase their residence time at a receptor or target binding site. Investigators should therefore be aware of the possible role of small amounts of these modified polyphenols since their bioactivity may be otherwise underestimated. One should not assume that the largest peak in a high performance liquid chromatographic (HPLC) or liquid chromatography-mass spectrometric (LC-MS) chromatogram is automatically the one with the most bioactivity.
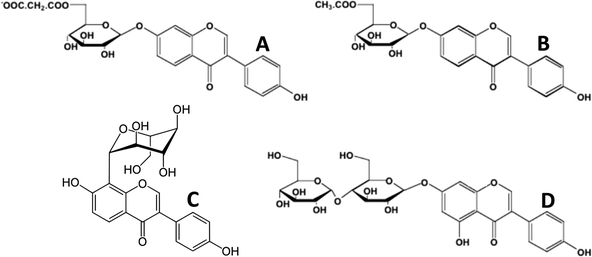 | ||
| Fig. 2 Glycoside esters of isoflavones. A, 6′′-O-malonyl-7-O-β-D-glucoside of daidzein (soy); B, 6′′-O-acetyl-7-O-β-D-glucoside of daidzein (soy); C, 8-C-glycoside of daidzein (Kudzu root, Puerariae lobata); D, 7-O-β-D-glucosylglucoside of genistein (Apios americana). | ||
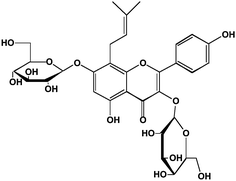 | ||
| Fig. 3 Structure of the prenylated flavonoid, icariin, from horny goat weed. Icariin is 8-prenyl kaempferol 3,7-diglucoside. | ||
Impact of food processing on polyphenol presentation
Interest in polyphenols and health has largely come about from epidemiological studies designed to explain world-wide differences in the incidence of chronic diseases such as cancer and atherosclerosis. These epidemiological data are dependent on what was the polyphenols content of the foods eaten in different parts of the world. This creates a problem in translating the dietary practices of one country to another – since taste is usually established early in life, successful translation involves consideration of taste as well as other dietary norms. The peoples from the countries of Southeast Asia have a noticeably lower incidence of and mortality from breast6 and prostate cancer7 which has been attributed to their intake of soy and its isoflavones.8,9 Many of the Southeast Asian foods are fermented (miso in Japan, tempeh in Indonesia and soy paste in Korea). Fermentation typically converts the polyphenol glycosides to their aglycone forms. This is significant from the standpoint of intestinal uptake since in most cases the aglycones are more readily absorbed from the small intestine than their glycosidic counterparts. Prolonged fermentation also leads to additional modifications to the polyphenols. For instance, fermented soy sauces contain 6- and 8-hydroxylated isoflavones10 (Fig. 4A and 4B). These modifications increase the antioxidant potential of the isoflavone.11Fermentation also leads to formation of tartaric acid ethers of isoflavones (Fig. 4C).12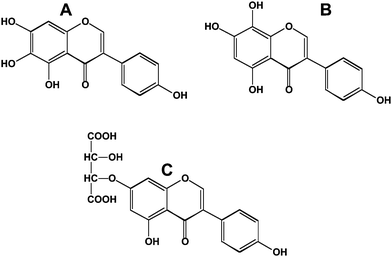 | ||
| Fig. 4 Modified isoflavones in fermented soy sauces. A; 6-hydroxygenistein; B, 8-hydroxygenistein; C, genistein-7-tartaric acid ether. | ||
Soy is consumed in Southeast Asia principally in the form of soymilk and tofu (a coagulated, largely proteinaceous product derived from soymilk). Due to the need to inactivate the protease inhibitors in soybeans, soymilk is treated at super-heated steam temperatures (121 °C). This causes the hydrolysis of the 6′′-O-malonoyl ester moiety yielding simple β-glycosides.13 Many of the latter are easily hydrolyzed to the aglycones by the small intestinal enzyme lactose phlorizin hydrolase (LPH).14 Therefore, both fermentation and soymilk preparation lead to foods in which the isoflavones are in forms that are quickly taken up from the small intestine. In contrast, soy foods prepared in the USA and Western Europe are focused on protein and low-fat forms. They are mostly manufactured from soy flour, a material derived from hexane extraction of soybean flakes. This retains the isoflavones as their 6′′-O-malonyl-7-O-β-D-glucosides1,2 (Fig. 2A). The soy flour is converted into soy protein concentrates (70% protein w/w) and soy protein isolates (90+% protein w/w). In these processes, the isoflavones may be essentially lost (because of an aqueous alcohol wash step) or (partially) converted by dry heat from the 6′′-O-malonyl-7-O-β-D-glucosides into their 6′′-O-acetyl-7-O-β-D-glucosides by decarboxylation (Fig. 2B). These forms of the isoflavones are not effective substrates of small intestinal LPH and therefore proceed to the large intestine where they are hydrolyzed by bacterial enzymes. The latter also cause the conversion of the isoflavone aglycones into several metabolites prior to first-pass uptake.
Uptake and bioavailability of polyphenols
There has been an excellent set of reviews of differences in the uptake and bioavailability of polyphenols and the reader is encouraged to refer to them.15–17 In theory, polyphenols ought to be substrates for the small intestine sodium-dependent glucose transporters. While this is true for polyphenols such as phlorizin (which is well known to inhibit glucose transport) and C-glucosides such as puerarin), β-glucosidase activity in the intestinal lumen or LPH in the enterocyte causes hydrolysis of polyphenolO-glycosides. In essence, the challenge is the same for all xenobiotics; in the absence of a specific transporter for polyphenols, their uptake is dependent on their hydrophobicity and aqueous solubility since they utilize passive diffusion to cross the intestinal cell membrane. Hydrophobic polyphenols are more easily transported; however, their aqueous solubility is lower and may limit how they approach the intestinal wall from the luminal side. Once inside the intestinal cell, polyphenols undergo metabolism to their β-D-glucuronide and sulfonate esters. As a result, very little of the aglycone diffuses through the intestinal cell to be excreted on the basolateral side into the bloodstream. The capacity of the intestinal cell to carry out metabolism is extensive and therefore orally administered polyphenols for the most part are converted to phase II metabolites. This is in contradistinction to polyphenols administered parenterally, intramuscularly or intravenously. In each of these latter routes of administration, there will be substantial amounts of unconjugated polyphenols in the blood.18 The biological response will therefore be quite different. An analogy is the different effects of orally and intravenously (i.v.) administered estrogens. 17β-Estradiol has to be chemically converted to its 17α-ethinyl or 3-methyl ether forms to be used pharmacologically since orally administered 17β-estradiol is 500 times less estrogenic than when it's administered intravenously.An exception to the intestinal metabolic barrier to polyphenols is the class of polyphenols containing C-linked glycosides. A prominent member of this class is puerarin, the 8-C-glucoside of daidzein (Fig. 2C), which is present in large amounts in kudzu root of a vine (Pueraria lobata) endemic to Southeast Asia. It was presented as a gift from the Japanese government to the USA in recognition of their 100 years of Independence. It was successfully used as a ground cover to protect from soil erosion during the Great Depression in the 1930s; however, it has grown without opposition from predators and is draped over many of the trees in the Southeastern USA. The C-glycosides are not substrates for either the luminal hydrolases or the bacterial hydrolases that hydrolyze the O-glycosides. As a consequence, they are substrates for the sodium-dependent glucose transporter and enter the blood stream rapidly and in an unmetabolized form.19 Peak blood concentrations are achieved in less than 60 min after oral intake and puerarin is largely excreted into the urine without further metabolism.20,21 Small amounts of puerarin are observed in bile as its β-D-glucuronide.21 It is likely that puerarin can be taken up by other cells containing glucose transporters. Indeed, puerarin has been detected in the eye and brain.21
Importance of bacterial transformation of polyphenols
As noted earlier, most dietary polyphenols are in a latent chemical form and depend in part on bacterial hydrolysis for their availability. As a consequence, the nature of the bacterial populations and the intestinal transit time are important aspects of what is actually absorbed into the blood. Rapid large bowel transit limits the secondary stages of metabolism that cause reduction, ring opening and ring cleavage of polyphenols. For an isoflavone such as daidzein (7,4′-dihydroxyisoflavone), the Δ2–3group is reduced to make dihydrodaidzein and subsequently S-(−)-equol, or the heterocyclic C-ring is cleaved to make O-desmethylangolensin (Fig. 5). As shown by Setchell et al.,22,23 only the S-(−) enantiomer of equol (Fig. 5) is present in man and rodents. He also showed that S-(−)-equol is about 30 times more potent as an estrogen than its R-(+)-enantiomer (Fig. 5).22 Interestingly, R-(+)equol is chemopreventive in a rat model of breast cancer, whereas S-(−)equol is not.24 This result suggests that chemoprevention in this animal model is not via effects on estrogen receptor signaling. It should be noted that there is a chiral center on the C3 carbon atom in dihydrodaidzein and O-desmethylangolensin; whether they exhibit the marked differences in their properties as observed for equol awaits further investigation.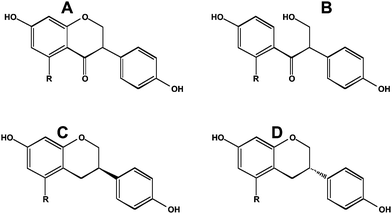 | ||
| Fig. 5 Reduced metabolites of daidzein and genistein. A, dihydrodaidzein; B, O-desmethylangolensin; C, R-(+)equol; D, S-(−)equol. For equol, R = H. | ||
Delayed transit through the large bowel results in opportunities for further degradation of polyphenols. An inverse relationship between large bowel transit time and peak isoflavone blood concentrations has been reported.25 The non-isoflavone metabolites arise from cleavage of the heterocyclic ring to yield p-ethylphenol (Fig. 6A), 2-(4-hydroxyphenyl)-propionic acid (Fig. 6B) and 4-hydroxyphenylacetic acid (Fig. 6C) among other metabolites.26 The flavonoids undergo similar reactions; however, the 2-position of the B-phenolic ring results in their conversion to 3-(4-hydroxyphenyl)-propionic acid (Fig. 6D).27 Interestingly, these hydroxyphenylacetic and hydroxyphenylpropionic acids can undergo reactions with oxidants such as peroxynitrite to make nitro-derivatives.28Benzoic acid derivatives from the A-ring of the bioflavonoids can be converted to glycine conjugates (the hippuric acids). p-Ethylphenol is converted to its β-glucuronide and sulfonate.29
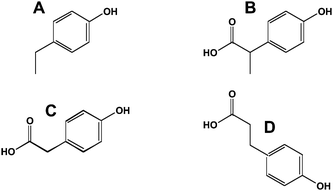 | ||
| Fig. 6 Ring opened metabolites of flavonoids and isoflavonoids. A, p-ethylphenol; B, 2-(4-hydroxyphenyl)-propionic acid; C, phenylacetic acid; D, 3-(4-hydroxyphenyl)-propionic acid. | ||
Consequently, the bacterial flora of the gut represents an important aspect of polyphenols metabolism. In the case of equol production, only about 30% of the population are equol-producers.30,31 In some studies, equol producers appear to have a health advantage over the non-equol producers.32 However, a randomized controlled clinical trial revealed no association of equol production and vascular function in postmenopausal women.33 Fermented soy germ products containing S-(−)-equol have become available and it will be interesting to test the equol hypothesis by administering them to equol non-producers.34
Another aspect to consider is to what extent polyphenols regulate the composition of the bacterial flora. This may be particularly important for the poorly absorbable proanthocyanidins, oligomers (n = 2–10) of flavanols. Potentially, these complex polyphenols could alter the bacterial populations as well as change the composition of other quite unrelated bacterial metabolites produced from dietary components that are absorbed into the bloodstream. In this scenario, the effect of the polyphenols could be entirely indirect and not require any absorption of the polyphenols or its metabolites.
Peripheral metabolism of polyphenols
Polyphenols that enter the blood stream via the gut are transported to the liver where further phase I (cytochrome P450-mediated) or phase II reactions can occur. The latter, besides β-glucuronidation and sulfonation (Fig. 7A and 7B), include methylation and glycine conjugation (for metabolites where a carboxylic acid group has been previously introduced). In cases where epoxides are generated because of vicinal hydroxyl groups on the polyphenol, the polyphenol can form glutathione conjugates (Fig. 7C) and eventually mercapturic acids.35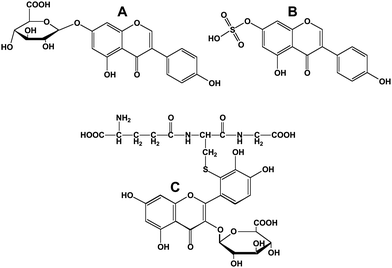 | ||
| Fig. 7 Conjugated flavonoids and isoflavonoids. A; genistein-7-O-β-D-glucuronide; B, genistein-7-sulfonate; C, 3′-glutathionyl quercetin 3-D-glucuronide. | ||
Polyphenols have been long described as anti-oxidants. An important site for the production of oxidants is during activation of neutrophils, macrophages and other inflammatory cells. The oxidative bursts to produce superoxide and hydrogen peroxide to kill perceived foreign intruders also result in the formation of hypohalous acids and peroxynitrite. Neutrophil myeloperoxidase catalyzes the formation of hypochlorite (HOCl) from hydrogen peroxide and chloride ions.36 In eosinophils, eosinophil peroxidase carries out a similar reaction but 1000 times more specifically with bromide ions to form hypobromite (HOBr) in the lung where alveolar macrophages reside.37HOBr is also a product of myeloperoxidase.38 In each of these cells, the generation of superoxide (O2−˙) leads to radical-radical reaction with nitric oxide (NO˙) to form peroxynitrite (ONO2−).39 In addition to reacting with polyphenols, these oxidants react with tyrosine residues on proteins. Urines of patients with atherosclerosis contain elevated levels of 3′-bromo-, 3′-chloro- and 3′-nitrotyrosine.40,41 The oxidants in a similar way react with polyphenols to generate 3′-bromo-, 3′-chloro- and 3-nitro-derivatives,42–44 as well as oxidation of the phenyl B-ring.45 Unlike tyrosine, chlorination of polyphenols can also occur on the 6- and 8-positions in the A-ring.46,47 Those modified polyphenols have been shown to have altered biochemical properties.48,49 This includes a 2–3 fold increase in their anti-oxidation properties in an LDL oxidation assay. Interestingly, in a pharmacophore analysis of the epidermal growth factortyrosine kinase, 3′-chloro-5,7-dihydroxyisoflavone was identified as a 10-fold better inhibitor than genistein with an IC50 of 98 nM.50
HOCl not only reacts with genistein, but also its β-glucoside, genistin (Boersma, B., Kirk, M. and Barnes, S., unpublished data). This suggests that HOCl should also react with isoflavone β-D-glucuronides if they are in the same biological compartment. However, attempts to identify chlorinated isoflavones in the blood and urine of rats treated with lipopolysaccharide have not been successful. However, 3′-nitrogenistein was discovered in the lungs of these animals (D'Alessandro, T., Wang, C.-C., Kirk, M. and Barnes, S., unpublished observations).
If chlorinated isoflavones are formed in vivo, they would be expected to be handled similarly to other isoflavones and excreted in bile. Synthetic chloroisoflavones were infused into the portal and femoral veins of anesthesized rats with an indwelling cannula in the bile duct. 3′-chlorodaidzein rapidly appeared in the bile as its β-D-glucuronide when infused into either vein (D'Alessandro, T; Moore, D. R., Barnes, S., unpublished observations). This argues that either chlorinated isoflavones are not formed in vivo or that they are converted to other metabolites under conditions of oxidative stress. Since all of the administered dose was not recovered after infusion of the chlorodaidzeins, it was hypothesized that chlorodaidzeins could form glutathione conjugates. However, attempts to demonstrate the formation of glutathione conjugates of chlorinated isoflavones have not been successful; this may reflect the specific substrate requirements of the glutathione S-transferases. It is possible that isoflavone metabolites with vicinal hydroxyl groups such as orobol (3′-hydroxygenistein) may form glutathione conjugates and hence mercapturic acids. Another factor was the plasma binding of these compounds. Other polychlorinated compounds such as the pesticide dichloro-diphenyl-trichloroethane (DDT) are known to be highly bound to plasma proteins,51 as was observed with dichlorodaidzeins. At 10 μM, less than 30% of dichlorodaidzein was not bound to proteins in the plasma fraction (D'Alessandro, T; Moore, D. R., Barnes, S., unpublished observations). This adds another level of complexity when considering the efficacy of dietary supplements.
Another site of polyphenol metabolism is in specific cell types used in tissue culture experiments. Breast cancer cells have highly variable amounts of phenol sulfotransferases. In the estrogen-responsive ZR-75-1 breast cancer cells, genistein added to the medium is completely converted to its 7-sulfate ester within 24 h of its addition to the cell culture medium.52 Therefore, in these cells, genistein has no estrogenic effect despite their sensitivity to estradiol-stimulated cell proliferation. In other cells, this sulfating capacity is either lower or totally absent52,53 and this may result in very different responsiveness of individual cell types to polyphenols and hence conclusions as to likely events in vivo.
Distribution of polyphenols
Polyphenols are distributed in widely different concentrations throughout the body (Fig. 8). The highest concentrations of polyphenols are present in the lumen of the gastrointestinal tract. If polyphenols and/or their metabolites cross the gastrointestinal barrier, they enter the bloodstream and to a lesser extent the lymphatics. Polyphenols transported into the bile are present in high concentrations. Urine concentrations of polyphenols and their metabolites are also high (typically 30 times or higher than in the blood) because of (1) their effective filtration at the glomerulus and (2) the efficient recovery of the water from the glomerular filtrate. In addition, there is evidence that active proximal tubular anion excretion of polyphenols occurs. In rats, this appears to account for the large proportion of equol in blood since its renal clearance is much lower than its parent isoflavone, daidzein and other isoflavones.54 Tissue distribution of polyphenols has been described in a few cases. One interesting extrahepatic site where significant accumulation of isoflavones occurs is in prostatic fluid55,56 and in the prostate.57 In prostatic fluid, concentrations of daidzein and its metabolites (but not genistein) are 20–50 times higher than in the blood.56 A rationale for this observation has yet to be established. Possible other extrahepatic sites of accumulation are suggested by the high volumes of distribution derived from analysis of plasma disappearance curves of i.v. administered isoflavones.58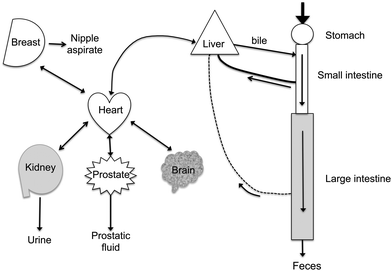 | ||
| Fig. 8 Distribution of polyphenols in the body. Ingested polyphenols are mostly taken up after hydrolysis by passive diffusion from the small and large intestines. Bacterial metabolites are formed in the colon. The mesenteric blood supply takes them back to the liver. Hepatic extraction is efficient and conjugated polyphenols are secreted into the bile. Polyphenols and their metabolites circulate at sub-μM concentrations in the blood that perfuses the organs. Small amounts pass the blood-brain barrier. Concentrations in the μM range are found in nipple aspirate and prostatic fluid. The kidney readily filters polyphenols and their metabolites and active secretion may also occur. Urine concentrations are often >20 μM for the bioavailable polyphenols (isoflavones), but are much lower for the complex polyphenols (proanthocyanidins) which are poorly absorbed. | ||
An interesting microcompartment not usually studied is the exosomal fraction. Exosomes are double-layered lipid particles secreted by many cells and represent a pathway for maintaining lipid balance in a cell. They are derived from lysosomal processing. On exit from the cell, they carry proteins whose composition may be regulated by polyphenols. Exosomes fuse with other cells in the vascular space and transfer their protein baggage to these cells. They therefore have endocrine-like properties. Breast tumor cells secrete exosomes that inactivate the response of natural killer cells to interleukin-2 and thereby establish immune tolerance.59Curcumin (diferuloylmethane) inhibits this inactivation effect of exosomes at low nM concentrations and appears to do so via its effects on ubiquitination of the proteins contained in the exosomes.60 The ubiquitinated proteins would be processed by the natural killer cells. Interestingly, curcumin administered in exosomes has a substantially higher ability to prevent lipopolysaccharide-induced inflammation.61 This has implications for the delivery of bioactive compounds in foods by exploiting food processing methods that micronize these compounds.
Identifying and quantifying polyphenol metabolites
Many of the earlier studies of polyphenols metabolism were carried out using gas-liquid (GC) chromatography. We have reviewed the relative merits of the different methods of polyphenol analysis.62 More recently, high-pressure liquid chromatography (HPLC or LC) combined with electrospray ionization63 or atmospheric pressure chemical ionization64 has overcome the problem of getting complex polyphenols into the gas phase for analysis by mass spectrometry. We have described in detail the various mass spectrometry methods for polyphenol analysis.65,66While mass spectrometry of the molecular ions of polyphenols ([M + H]+ or [M − H]−) can be easily carried out, the presence of several isomers of the same molecular weight means that it is critical to have sufficient chromatographic separation of isobaric isomers. An alternative is to isolate the molecular ion and fragment it by collision-induced dissociation in a triple quadrupole or ion trap mass spectrometer.66 The pattern of fragment ions reveals the different structures of the isomers. For instance, daidzin and puerarin, the O- and C-glycosides of daidzein, which have identical molecular weights, are readily differentiated because daidzin loses the entire glucose moiety ([M-162]) upon fragmentation, whereas puerarin retains the link to the sugar but undergoes multiple losses of water.67 Quantitative assays can be built on combining the parent and specific fragment ions, thereby allowing individual polyphenols and their metabolites to be detected in very complex milieu.68 This can be carried out on 11–15 compounds at a time for samples with concentrations in the low nanomolar range.69 For the best approach to quantitative analysis, heavy, isotopically labeled internal standards are used. Both d6- and 13C3-labeled isoflavones have been used in this type of application.70,71
Challenges in polyphenol metabolism
Quantifying the amount of polyphenols in specific tissues, particularly the brain, is fraught with difficulties, largely associated with effective extraction and sensitivity. An approach being taken in the Purdue University-University of Alabama at Birmingham Botanicals Center for Age-Related Disease is the use of plant cell synthesis of 14C-labeled polyphenols in combination with accelerator mass spectrometry (AMS).72 A cartoon summarizing this process is illustrated in Fig. 9. Fig. 9, step A shows the use of the plant cell to carry out the specific synthesis of radiolabeled polyphenols which overcomes problems associated with the complexity of polyphenols structures and, where they exist, of chiral centers which could not be produced selectively by chemical methods. Details of duration and amount of 14C exposure in labeling experiments vary, but generally the amount of 14C needed is quite low.73–77 The challenge is separating the radioactive polyphenols, but assuming suitable chromatographic methods overcome this, then very small amounts (typically ∼50 nCi or 105 μrem if the dose is in the form of an ingested organic compound) of the purified 14C-labeled polyphenols can be used in rats or human studies (step B of Fig. 9).78 By way of comparison, this is one third of the hourly radiation dose received by a passenger on a Paris-Buenos Aires flight in 1991.79 Besides radiological safety, the smallness of the dose also means that in many cases physiologically relevant oral doses are possible. Returning to our example, if the polyphenol is labeled at 10 μCi/μmol, then the 50 nCi dose represents 1.1 × 105dpm, 5 nmol, or ∼1.2–1.5 μg. If only 0.1% of the polyphenol was absorbed by the brain, then this would be 1.1 × 102dpm, 5 pmol or 1.2–1.5 ng. An investigator examining the brain of a rat (3 g) could take 3 mg of wet tissue for analysis. This would contain 0.11 dpm, 5 fmol or 1.2–1.5 pg. To measure this by GC-MS, LC-MS and most other techniques would be a struggle or, in the case of radioactive disintegrations, impossible. However, by converting the 3 mg of tissue to elemental carbon, accelerator mass spectrometry can measure the 14C content as low as 10 attomoles or 1 14C atom in 1015 total carbon atoms.73 The conversion to elemental carbon is accomplished by combusting the sample to form CO2 and then reducing it to form graphite.80 The small vials shown in Fig. 9, step C contain the graphite. The final step in the process (step D) is to take the graphite and pound it into a small, cylindrical piece of metal that has a hole in it for acceptance of the sample. The cylindrical piece of metal is called a cathode because this is the electrical role it plays in the AMS ion source. This step is called loading. After loading the sample is inserted into the AMS ion source and assayed. For the, dose, recovery, and sample characteristics mentioned earlier the signal-to-noise for this measurement would be approximately 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
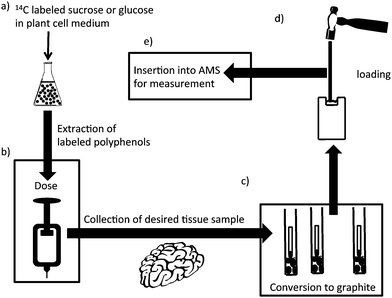 | ||
| Fig. 9 Design of an experiment using accelerator mass spectrometry. (A) Plant cells are incubated with 14C-labeled glucose or sucrose and the polyphenols extracted and chromatographically purified; (B) a small dose of 14C-polyphenol is administered to the animal or clinical subject; (C) the biological fluid or tissue is recovered and converted to graphite; (D) the graphitic material is converted to a plug; and (E) the plug is inserted into the AMS for analysis. | ||
The low detection limit of AMS is made possible by variety of techniques; such as, chemically preparing the sample so that it yields high current for the element of interest in the AMS ion source whilst suppressing interfering ions; the destruction of interfering molecular ions in the accelerator; multiple levels of traditional mass spectrometric electric and magnetic separation; and use of a detector that can differentiate between differing nuclear charges.81 The major steps in an AMS analysis are summarized in Fig. 10. Step a) shows the cesium sputter ion source. Positively charged cesium ions are formed on the surface of an ionizer which is typically a slice of a sphere. The positively formed cesium ions are accelerated onto the sample and negatively charged ions of the sample of interest are accelerated away from the after impact from the cesium ions. Next (step B), there is low-energy mass analysis. This is usually done with a large magnet that allows only ions with the mass-to-charge ratio of interest to pass. Essentially, the source and the magnet on the low-energy side comprise a magnet sector mass spectrometer. Next, the beam is injected into an accelerator (typically 1–10 MeV) and accelerated through a gas, metal foil, or both. At this point multiple electrons are stripped from all the ions and interfering molecules become unbound. On the high-energy side, a multiply charged ion (for carbon this is typically +3 or +4) of the element of interest is selected by another magnet and subjected to energy analysis (i.e., an electrostatic analyzer) and injected into a gas ionization detector (step E). This type of detector can differentiate between different nuclear charges. This is important for differentiating between 14C and the small amount of 14N that makes it down to the detector.
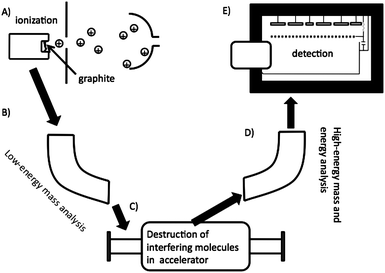 | ||
| Fig. 10 A cartoon depicting a typical AMS experiment. Ionization of the sample in ion source (A), is followed by mass separation (B), destruction of interfering molecules (C), more mass and energy analysis (D), and finally detection in a gas-ionization detector (E). | ||
The ultra sensitivity of AMS allows for unique science. For example, studies can be carried out for the lifetime of a human subject with one small dose,82 over multiple generations in an animal model as the tracer is passed to offspring in mother's milk,83 and for physiologically relevant doses to be explored in small mammals.84 It should be noted that traditional AMS instruments are very large due to the high energies required. The AMS available at Purdue University is roughly 50 meters from source to detector. However, many routine carbon analyses can be conducted at lower energy and smaller AMS instruments are becoming more common. Even though they have a higher detection limit than the larger instruments, most biological samples are high enough above background that this is not a consideration for most studies.
For our purposes, this level of sensitivity permits the investigation of the kinetics of how polyphenols' metabolites enter the interstitial fluid space. For non-vascular cells, it is the interstitial fluid (ISF) that bathes cells rather than the blood and it is therefore more important to understand the polyphenols and their metabolites composition and concentration in this fluid rather than blood. By implanting membrane probes in the tissue of interest one can follow the concentrations of 14C labeled compounds into the tissue of interest with a high degree of precision85 (Fig. 11). Ultrafiltrate probes remove ISF under a negative pressure gradient. With microdialysis probes, an iso-osmotic fluid is pumped through the semi-permeable membrane implanted in the tissue and labeled compounds diffuse into the probe along a concentration gradient. The microdialysis probes combined with AMS are especially useful for studying brain chemistry. Because of the blood brain barrier concentrations in brain ISF are often orders of magnitude less than plasma concentrations. However, with the sensitivity of the AMS it is still possible to determine pharmacokinetics and bioavailability in brain with high precision.
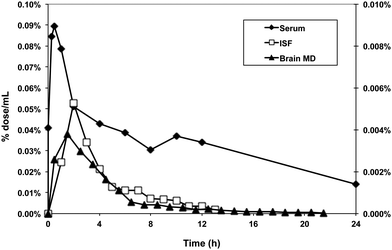 | ||
| Fig. 11 Use of accelerator mass spectrometry to track the distribution of 14C-labeled polyphenols in tissues. A 14C-preparation of proanthocyanidins (50 nCi) was administered to adult rats by gavage. Blood, interstitial fluid and brain microdialysate were collected from unanesthetized, free-living rats implanted with in-dwelling cannulas to recover these fluids. | ||
Acknowledgements
The work discussed in this summary includes research funded by a grant to the Purdue University-University of Alabama at Birmingham Botanicals Center for Age-Related Disease from the National Center for Complementary and Alternative Medicine and the National Institutes of Health Office of Dietary Supplements (P50 AT00477, C. Weaver, PI). Tracy D'Alessandro was a recipient of a NRSA pre-doctoral fellowship from the National Heart Lung and Blood Institute Training Program in Cardiovascular Pathophysiology (5T32HL007918, V. Darley-Usmar, PI).References
- S. Kudou, Y. Fleury, D. Welti, D. Magnolato, T. Uchida, K. Kitamura and K. Okubo, Agric. Biol. Chem., 1991, 55, 2227–2233 CAS.
- S. Barnes, M. Kirk and L. Coward, J. Agric. Food Chem., 1994, 42, 2466–2474 CrossRef CAS.
- S. Barnes, C.-C. Wang, M. Kirk, M. Smith-Johnson, L. Coward, N. C. Barnes, G. Vance and B. Boersma, HPLC-Mass Spectrometry of Isoflavonoids in Soy and the American Groundnut, Apios Americana, in, Flavonoids in Cell Function, ed. B. S. Buslig, J. A. Manthey, Kluwer Academic/Plenum Publishers, New York, NY, 2002, pp.77–88 Search PubMed.
- K. Yazaki, K. Sasaki and Y. Tsurumaru, Phytochemistry, 2009, 70, 1739–1745 CrossRef CAS.
- A. W. Shindel, Z. C. Xin, G. Lin, T. M. Fandel, Y. C. Huang, L. Banie, B. N. Breyer, M. M. Garcia, C. S. Lin and T. F. Lue, J. Sex. Med., 2010, 7, 1518–1528 CrossRef CAS.
- F. Bray, P. McCarron and D. M. Parkin, Breast Cancer Res., 2004, 6, 229–239 CrossRef.
- M. Quinn and P. Babb, BJU Int., 2002, 90, 162–173 CrossRef CAS.
- A. H. Wu, R. G. Ziegler, P. L. Horn-Ross, A. M. Nomura, D. W. West, L. N. Kolonel, J. F. Rosenthal, R. N. Hoover and M. C. Pike, Cancer Epidemiol Biomarkers Prev., 1996, 5, 901–906 CAS.
- N. Kurahashi, M. Iwasaki, S. Sasazuki, T. Otani, M. Inoue and S. Tsugane, Cancer Epidemiol. Biomarkers Prev., 2007, 16, 538–545 CrossRef CAS.
- H. Esaki, S. Kawakishi, Y. Morimitsu and T. Osawa, Biosci., Biotechnol., Biochem., 1999, 63, 1637–1639 CrossRef CAS.
- H. Esaki, T. Shirasaki, K. Yamashita, Y. Nakamura, S. Kawakishi and T. Osawa, J. Nutr. Sci. Vitaminol., (Tokyo)., 2005, 51, 80–86 Search PubMed.
- E. Kinoshita, S. Murakami and T. Aishima, J. Agric. Food Chem., 2000, 48, 2149–2154 CrossRef CAS.
- S. Barnes, Lymphat. Res. Biol., 2010, 8, 89–98 CrossRef CAS.
- A. J. Day, F. J. Cañada, J. C. Díaz, P. A. Kroon, R. Mclauchlan, C. B. Faulds, G. W. Plumb, M. R. Morgan and G. Williamson, FEBS Lett., 2000, 468, 166–170 CrossRef CAS.
- N. G. Coldham, A. Q. Zhang, P. Key and M. J. Sauer, Eur. J. Drug Metab. Pharmacokinet., 2002, 27, 249–258 Search PubMed.
- C. Manach, G. Williamson, C. Morand, A. Scalbert and C. Rémésy, Am. J. Clin. Nutr., 2005, 81, 230S–242S CAS.
- G. Williamson and C. Manach, Am. J. Clin. Nutr., 2005, 81, 243S–255S CAS.
- A. Crozier, I. B. Jaganath and M. N. Clifford, Nat. Prod. Rep., 2009, 26, 1001–1043 RSC.
- J. K. Prasain, K. Jones, N. Brissie, D. R. Moore II, J. M. Wyss and S. Barnes, J. Agric. Food Chem., 2004, 52, 3708–3712 CrossRef CAS.
- J. K. Prasain, N. Peng, E. Acosta, D. R. Moore II, A. Arabshahi, E. Meezan, S. Barnes and J. M. Wyss, Biomed. Chromatogr., 2007, 21, 410–414 CrossRef CAS.
- J. K. Prasain, N. Peng, D. R. Moore II, A. Arabshahi, S. Barnes and J. M. Wyss, Phytomedicine, 2009, 16, 65–71 CrossRef CAS.
- K. D. Setchell, C. Clerici, E. D. Lephart, S. J. Cole, C. Heenan, D. Castellani, B. E. Wolfe, L. Nechemias-Zimmer, N. M. Brown, T. D. Lund, R. J. Handa and J. E. Heubi, Am. J. Clin. Nutr., 2005, 81, 1072–1079 CAS.
- K. D. Setchell, X. Zhao, P. Jha, J. E. Heubi and N. M. Brown, Am. J. Clin. Nutr., 2009, 90, 1029–1037 CrossRef CAS.
- N. M. Brown, C. A. Belles, S. L. Lindley, L. Zimmer-Nechemias, X. Zhao, D. P. Witte, M. O. Kim and K. D. Setchell, Carcinogenesis, 2010, 31, 886–893 CrossRef CAS.
- A. L. Simons, M. Renouf, P. A. Murphy and S. Hendrich, J. Agric. Food Chem., 2010, 58, 141–147 CrossRef CAS.
- L. Schoefer, R. Mohan, A. Braune, M. Birringer and M. Blaut, FEMS Microbiol. Lett., 2002, 208, 197–202 CrossRef CAS.
- A. R. Rechner, M. A. Smith, G. Kuhnle, G. R. Gibson, E. S. Debnam, S. K. Srai, K. P. Moore and C. A. Rice-Evans, Free Radic. Biol. Med., 2004, 36, 212–225 CrossRef CAS.
- K. M. Miranda, K. Yamada, M. G. Espey, D. D. Thomas, W. DeGraff, J. B. Mitchell, M. C. Krishna, C. A. Colton and D. A. Wink, Arch. Biochem. Biophys., 2002, 401, 134–144 CrossRef CAS.
- T. Nanbo, Biol. Pharm. Bull., 1993, 16, 518–520 CAS.
- M. Axelson, J. Sjövall, B. E. Gustafsson and K. D. Setchell, J. Endocrinol., 1984, 102, 49–56 Search PubMed.
- C. Atkinson, K. M. Newton, E. J. Bowles, M. Yong and J. W. Lampe, Am. J. Clin. Nutr., 2008, 87, 679–687 CAS.
- K. D. Setchell, N. M. Brown and E. Lydeking-Olsen, J. Nutr., 2002, 132, 3577–3584 CAS.
- S. Kreijkamp-Kaspers, L. Kok, M. L. Bots, D. E. Grobbee, J. W. Lampe and Y. T. van der Schouw, Am. J. Clin. Nutr., 2005, 81, 189–195 CAS.
- S. Barnes and H. Kim, J. Nutr., 2010, 140, 1390S–1394S CrossRef CAS.
- Y. J. Hong and A. E. Mitchell, Chem. Res. Toxicol., 2006, 19, 1525–1532 CrossRef CAS.
- J. E. Harrison and J. Schultz, J. Biol. Chem., 1976, 251, 1371–1374 CAS.
- A. N. Mayeno, A. J. Curran, R. L. Roberts and C. S. Foote, J. Biol. Chem., 1989, 264, 5660–5668 CAS.
- R. Senthilmohan and A. J. Kettle, Arch. Biochem. Biophys., 2006, 445, 235–244 CrossRef CAS.
- H. Ischiropoulos, L. Zhu and J. S. Beckman, Arch. Biochem. Biophys., 1992, 298, 446–451 CrossRef.
- H. J. Chen and W. L. Chiu, Toxicol. Lett., 2008, 181, 31–39 CrossRef CAS.
- M. Schwemmer, B. Fink, R. Köckerbauer and E. Bassenge, Clin. Chim. Acta, 2000, 297, 207–216 CrossRef CAS.
- B. J. Boersma, R. P. Patel, M. Kirk, P. L. Jackson, D. Muccio, V. M. Darley-Usmar and S. Barnes, Arch. Biochem. Biophys., 1999, 368, 265–275 CrossRef CAS.
- B. J. Boersma, T. D'Alessandro, M. R. Benton, M. Kirk, L. S. Wilson, J. Prasain, N. P. Botting, S. Barnes, V. M. Darley-Usmar and R. P. Patel, Free Radical Biol. Med., 2003, 35, 1417–1430 CrossRef CAS.
- T. D'Alessandro, J. Prasain, M. R. Benton, N. P. Botting, D. R. Moore II, V. M. Darley-Usmar, R. P. Patel and S. Barnes, J. Nutr., 2003, 133, 3773S–3777S CAS.
- S. E. Pollard, G. G. Kuhnle, D. Vauzour, K. Vafeiadou, X. Tzounis, M. Whiteman, C. Rice-Evans and J. P. Spencer, Biochem. Biophys. Res. Commun., 2006, 350, 960–968 CrossRef CAS.
- J. K. Prasain, R. Patel, M. Kirk, L. Wilson, N. Botting, V. M. Darley-Usmar and S. Barnes, J. Mass Spectrom., 2003, 38, 764–771 CrossRef CAS.
- R. Binsack, B. J. Boersma, R. P. Patel, M. Kirk, C. R. White, V. Darley-Usmar, S. Barnes, F. Zhou and D. A. Parks, Alcohol.: Clin. Exp. Res., 2001, 25, 434–443 CrossRef CAS.
- B. K. Chacko, R. T. Chandler, T. L. D'Alessandro, A. Mundhekar, N. K. Khoo, N. P. Botting, S. Barnes and R. P. Patel, J. Nutr., 2007, 137, 351–356 CAS.
- W. S. Xiang, J. Zhang, J. D. Wang, L. Jiang, B. Jiang, Z. D. Xiang and X. J. Wang, J. Agric. Food Chem., 2010, 58, 1933–1938 CrossRef CAS.
- P. Traxler, J. Green, H. Mett, U. Séquin and P. Furet, J. Med. Chem., 1999, 42, 1018–1026 CrossRef CAS.
- M. Gülden, S. Mörchel, S. Tahan and H. Seibert, Toxicology, 2002, 175, 201–213 CrossRef CAS.
- T. G. Peterson, L. Coward, M. Kirk, C. N. Falany and S. Barnes, Carcinogenesis, 1996, 17, 1861–1869 CrossRef CAS.
- T. G. Peterson, G.-P. Ji, M. Kirk, L. Coward, C. N. Falany and S. Barnes, Am. J. Clin. Nutr., 1998, 68, 1505–1511.
- S. Barnes, C. Grubbs, M. Smith, M. Kirk and R. Lubet, J. Nutr., 2002, 132, 619S.
- M. S. Morton, P. S. Chan, C. Cheng, N. Blacklock, A. Matos-Ferreira, L. Abranches-Monteiro, R. Correia, S. Lloyd and K. Griffiths, Prostate, 1997, 32, 122–128 CrossRef CAS.
- T. E. Hedlund, P. D. Maroni, P. G. Ferucci, R. Dayton, S. Barnes, K. Jones, D. R. Moore II, L. G. Ogden, K. Wähälä, H. M. Sackett and K. J. Gray, J. Nutr., 2005, 135, 1400–1406 CAS.
- C. D. Gardner, B. Oelrich, J. P. Liu, D. Feldman, A. A. Franke and J. D. Brooks, Prostate, 2009, 69, 719–726 CrossRef CAS.
- K. D. Setchell, M. S. Faughnan, T. Avades, L. Zimmer-Nechemias, N. M. Brown, B. E. Wolfe, W. T. Brashear, P. Desai, M. F. Oldfield, N. P. Botting and A. Cassidy, Am. J. Clin. Nutr., 2003, 77, 411–419 CAS.
- C. Liu, S. Yu, K. Zinn, J. Wang, L. Zhang, Y. Jia, J. C. Kappes, S. Barnes, R. P. Kimberly, W. E. Grizzle and H. G. Zhang, J. Immunol., 2006, 176, 1375–1385 CAS.
- H. G. Zhang, H. Kim, C. Liu, S. Yu, J. Wang, W. E. Grizzle, R. P. Kimberly and S. Barnes, Biochim. Biophys. Acta, 2007, 1773, 1116–1123 CAS.
- D. Sun, X. Zhuang, X. Xiang, Y. Liu, S. Zhang, C. Liu, S. Barnes, W. E. Grizzle, D. Miller and H. G. Zhang, Mol. Ther., 2010, 18, 1606–1614 CrossRef CAS.
- C.-C. Wang, J. K. Prasain and S. Barnes, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2002, 777, 3–28 CrossRef CAS.
- J. B. Fenn, M. Mann, C. K. Meng, S. F. Wong and C. M. Whitehouse, Science, 1989, 246, 64–71 CrossRef CAS.
- T. R. Covey, B. A. Thomson and B. B. Schneider, Mass Spectrom. Rev., 2009, 28, 870–879 CrossRef.
- J. Prasain, C.-C. Wang and S. Barnes, Mass spectrometry in the analysis of phytoestrogens in biological samples, in, Phytoestrogens and Health, ed. G. S. Gilani, J. J. Anderson, AOCS Press, Champaign, IL, 2002, pp 147–177 Search PubMed.
- J. K. Prasain, C.-C. Wang and S. Barnes, Free Radical Biol. Med., 2004, 37, 1324–1350 CrossRef CAS.
- J. K. Prasain, K. Jones, M. Kirk, L. Wilson, M. Smith-Johnson, C. M. Weaver and S. Barnes, J. Agric. Food Chem., 2003, 51, 4213–4218 CrossRef CAS.
- L. Coward, M. Kirk, N. Albin and S. Barnes, Clin. Chim. Acta, 1996, 247, 121–142 CrossRef CAS.
- J. K. Prasain, A. Arabshahi, D. R. Moore II, G. A. Greendale, J. M. Wyss and S. Barnes, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2010, 878, 994–1002 CrossRef CAS.
- K. Wähälä, T. Hase and H. Adlercreutz, Proc. Soc. Exp. Biol. Med, 1995, 208, 27–32 Search PubMed.
- D. B. Clarke, A. S. Lloyd, N. P. Botting, M. F. Oldfield, P. W. Needs and H. Wiseman, Anal. Biochem., 2002, 309, 158–172 CrossRef CAS.
- C. M. Weaver, S. Barnes, J. M. Wyss, H. Kim, D. M. Morré, D. J. Morré, J. E. Simon, M. A. Lila, E. M. Janle and M. G. Ferruzzi, Am. J. Clin. Nutr., 2008, 87, 493S–497S CAS.
- B. A. Buchholz, A. Arjomand, S. R. Dueker, P. D. Schneider, A. J. Clifford and J. S. Vogel, Anal. Biochem., 1999, 269, 348–352 CrossRef CAS.
- A. J. Clifford, S. R. Dueker, E. E. Fabbro, Y. M. Lin, N. Hong, M. W. Lame, H. J. Segall, B. A. Buchholz and J. S. Vogel, in: Proceedings of the 3rd International Conference on Isotopes: Isotope Production and Applications in the 21st Century; Stevenson, N. R., Ed., 2000, pp 377–379 Search PubMed.
- S. R. Dueker and B. A. Buchholz, Adv. Mass Spectrom., 2004, 16, 95–122 Search PubMed.
- A. Reppert, G. G. Yousef, R. B. Rogers and M. A. Lila, J. Agric. Food Chem., 2008, 56, 7860–7865 CrossRef CAS.
- G. G. Yousef, D. S. Seigler, M. A. Grusak, R. B. Rogers, C. T. Knight, T. F. Kraft, J. W. Erdman and M. A. Lila, J. Agric. Food Chem., 2004, 52, 1138–1145.
- J. Mun, M. Grannan, P. Lachcik, A. Reppert, G. G. Yousef, R. B. Rogers, E. M. Janle, C. M. Weaver and M. A. Lila, Br. J. Nutr., 2009, 102, 1523–1530 CrossRef CAS.
- J. F. Bottollier-Depois, Q. Chau, P. Bouisset, G. Kerlau, L. Plawinski and L. Lebaron-Jacobs, Radiat. Res., 2000, 153, 526–532 CrossRef CAS.
- J. S. Vogel, Radiocarbon, 1992, 34, 344–350.
- C. Tuniz, J. R. Bird, D. Fink and G. F. Herzog, Accelerator Mass Spectrometry: Ultrasensitive Analysis for Global Science. Accelerator Mass Spectrometry: Ultrasensitive Analysis for Global Science. CRC Press, Boca Raton, Fl. 1998 Search PubMed.
- J. M. K. Cheong, B. R. Martin, G. S. Jackson, D. Elmore, G. P. McCabe, J. R. Nolan, S. Barnes, M. Peacock and C. M. Weaver, J. Clin. Endocrinol. Metab., 2007, 92, 577–582 CAS.
- E. Janle, J. Sojka, G. S. Jackson, P. Lachcik, J. A. Einstein and C. R. Santerre, Nucl. Instrum. Methods Phys. Res., Sect. B, 2007, 259, 758–762 CrossRef CAS.
- K. H. Dingley, S. P. H. T. Freeman, D. O. Nelson, C. R. Garner and K. W. Turteltaub, Drug Metab Dispos, 1998, 26, 825–828 CAS.
- E. M. Janle, M. A. Lila, M. Grannan, L. Wood, A. Higgins, G. G. Yousef, R. B. Rogers, H. Kim, G. S. Jackson and C. M. Weaver, Nucl. Instrum. Methods Phys. Res., Sect. B, 2010, 268, 1313–1316 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
