Facile synthesis of nitrogen-doped carbon derived from polydopamine-coated Li3V2(PO4)3 as cathode material for lithium-ion batteries†
Cunliang Zhangab,
Hongshen Lia,
Nie Pinga,
Gang Panga,
Guiyin Xua and
Xiaogang Zhang*a
aCollege of Material Science and Engineering and Key Laboratory for Intelligent Nano-Materials and Devices, Ministry of Education, Nanjing University of Aeronautics and Astronautics, Nanjing, 210016, PR China. E-mail: azhangxg@163.com
bShangqiu Polytechnic, Shangqiu, 476000, PR China
First published on 6th August 2014
Abstract
Nitrogen-doped, carbon-coated Li3V2(PO4)3 cathode materials were prepared by the oxidative self-polymerization of dopamine on the Li3V2(PO4)3 surface and subsequent carbonization of polydopamine. Field emission scanning electron microscopy (FESEM), scanning transmission electron microscopy-energy dispersive X-ray spectroscopy (STEM-EDS) and X-ray photoelectron spectroscopy (XPS) were performed to characterize morphologies and structures. The uniform nitrogen-doped, carbon coating provided a continuous electronic conducting network. Furthermore, the existence of nitrogen in the composite can improve electronic conductivity. As cathode materials for lithium-ion batteries, the nitrogen-doped, carbon-coated Li3V2(PO4)3 composites display superior rate performance and excellent cycle performance over the pristine Li3V2(PO4)3 and carbon-coated Li3V2(PO4)3. The electrode delivers high specific capacity of 74 mA h g−1 at 10 C, and still remains at 95.4% of initial capacity after 100 cycles at 1 C.
Introduction
With increasing concerns about environmental issues caused by conventional energy, there is an urgent demand to develop renewable and clean energy sources.1,2 Recently, lithium-ion batteries (LIBs) have been widely used in portable electronic devices, transportation, and storage of renewable energy for smart grids due to their high energy density. However, LIBs suffer from deficiencies, including low power density, poor cycling performance and poor safety that hinder large-scale applications.3–5 Therefore, the search for efficient electrode materials, especially with enhanced lithium storage properties, is underway.6–9Cathode materials effectively determine energy density, safety and life cycle of lithium-ion batteries.10 Up to now, layered transition metal oxides, spinel-type oxides and polyanion cathode materials have been investigated intensively as potential cathode materials. Layered transition metal oxides have several advantages: fully developed synthetic routes, high capacity and facile processing. Nevertheless, they all have a drawback, namely oxygen evolution at high charging potential, which could cause serious safety issues in practical applications. Spinel-type oxides such as LiMn2O4 have the advantages of thermal stability, low cost and environmental friendliness. However, they suffer fast capacity fading during cycling, especially at elevated temperatures. Polyanion materials have attracted tremendous attention as cathodes for LIBs, owing to their robust structure, low cost and high voltage plateau.6,7,11 In particular, monoclinic lithium vanadium phosphate, Li3V2(PO4)3, one of the phosphate compounds with a sodium super-ionic conductor (NASICON) framework, has been considered a promising cathode material due to its high operating voltage, large theoretical specific capacity, structural stability and abundant reserves.12–14 However, the poor electronic conductivity (2.4 × 10−7 S cm−1) of Li3V2(PO4)3 due to its separated VO6 octahedral arrangement hinders practical applications. Carbon coating is an effective way to improve electrochemical performance, because it improves the surface electronic conductivity and the electric contact between particles and conducting agents.13,14 Recently, a Li3V2(PO4)3/C nanosphere cathode with a three-dimensional conductive network was synthesized, where the 3D structure effectively enhanced the electron transport and the electrochemical performance.17 Duan and co-workers reported a hydrothermal-assisted sol–gel method to prepare the Li3V2(PO4)3@C core–shell nanocomposite, which exhibited remarkably high rate capability and long cyclability.18 The introduction of nitrogen into a carbon structure can tune the carbon properties, because it fabricates more active defects that show higher Li+ diffusion efficiency compared with conventional carbon coating.19–22
Dopamine (2-(3,4-dihydroxyphenyl)ethylamine), a nitrogenous nature-inspired biomimetic material, which contains catechol and amine functional groups, can self-polymerize and deposit on virtually any surface.23–27 Thus, polydopamine has opened a new route to modify electrode materials and has stimulated extensive research.28–34 The polyethylene separator properties were dramatically improved by simple immersion into the dopamine solution.28 Polydopamine was also used as a binder for the silicon anode to substantially improve electrochemical properties, due to its unique wetness-resistant adhesion.29 Nitrogen-doped carbon-coated Li4Ti5O12 microspheres were used as anode materials, which displayed a superior rate performance and excellent capacity retention.30 Anatase TiO2 nanoparticles with the carbon coating derived from polydopamine were reported.32 Kong et al. reported the enhanced electrochemical properties of SnO2-based anodes prepared by in situ polymerization of dopamine.33
In this paper, a facile method is proposed for the synthesis of Li3V2(PO4)3 composite with a carbon-coating structure by using dopamine as a nitrogen-doped carbon precursor. The nitrogen-doped carbon shell as a conductive network is beneficial for lithium ion transport and electric conductivity across the interface between the active materials and the electrolyte. The LVP/NC exhibits excellent high-rate performance as well as superior cyclability compared with pristine Li3V2(PO4)3 and Li3V2(PO4)3/C.
Experimental
Synthesis of pristine Li3V2(PO4)3 (LVP)
All of the reactants and solvents were analytical grade and used without further purification. The LVP was prepared via a sol–gel route reported by Zhou's group, with minor modifications.35 In a typical procedure, pristine LVP was synthesized using Li2CO3, V2O5, NH4H2PO4 and oxalic acid as starting materials, with a Li![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V
V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxalic acid molar ratio of 3
oxalic acid molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Stoichiometric V2O5 and oxalic acid were dissolved in deionized water at 70 °C to form a blue transparent aqueous solution. Then NH4H2PO4 and Li2CO3 in a stoichiometric ratio were added to the solution with continuous stirring until a gel formed. Finally, the gel was decomposed at 350 °C in a nitrogen atmosphere for 4 h, followed by sintering at 750 °C for 4 h.
3. Stoichiometric V2O5 and oxalic acid were dissolved in deionized water at 70 °C to form a blue transparent aqueous solution. Then NH4H2PO4 and Li2CO3 in a stoichiometric ratio were added to the solution with continuous stirring until a gel formed. Finally, the gel was decomposed at 350 °C in a nitrogen atmosphere for 4 h, followed by sintering at 750 °C for 4 h.
Synthesis of nitrogen-doped, carbon-coated Li3V2(PO4)3 (LVP/NC)
Typically, 200 mg LVP was dispersed in Tris-buffer (50 mL, pH = 8.5) by ultrasonication for 30 min to form a suspension. Subsequently, specific amounts of dopamine were added to the suspension and constantly stirred for 6 h at room temperature. Then the precipitate was collected by centrifugation, washed several times with deionized water, and then dried at 60 °C in an electric oven for 12 h. Finally the powder was annealed in a tube under N2 atmosphere at 750 °C for 4 h. To accurately indicate the modification effects of the nitrogen-doped carbon coating, a comparative experiment of carbon-coated Li3V2(PO4)3(LVP/C) was carried out (see ESI†).36Material characterization
The crystal structure of the obtained samples was characterized by X-ray diffraction (XRD) (Bruker D8 Advance) with Cu-Kα radiation. Elemental analysis was carried out using the Heraeus CHNO-Rapid. The X-ray photoelectron spectroscopy (XPS) analysis was performed on a Perkin-Elmer PHI 550 spectrometer with Al-Kα (1486.6 eV) as the X-ray source. The microstructure properties were observed by scanning transmission electron microscopy-energy dispersive X-ray spectroscopy (STEM-EDS, JEM-2100, JEOL), and field emission scanning electron microscopy (FESEM, JEOL, JSM-7000).Electrochemical measurements
Electrochemical characterization was carried out by galvanostatic cycling in CR2016-type coin cells that were assembled in an argon-filled glove box. The working electrodes were formed by mixing 80 wt% active materials, 10 wt% carbon black and 10 wt% polyvinylidene fluoride (PVDF) dissolved in N-methyl pyrrolidinone (NMP) and uniformly pasting the mixture on an aluminum-foil current collector. Finally, the electrodes were dried at 110 °C for 12 h in a vacuum oven. Lithium metal was used as both the counter and reference electrode, and the polypropylene membrane (Celgard 2400) was served as separator. The electrolyte was a 1 M solution of LiPF6 dissolved in ethylene carbonate (EC)–dimethyl carbonate (DMC) with a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Galvanostatic charge–discharge experiments were performed at different current densities between 3 and 4.3 V (vs. Li/Li+) by using a CT2001A cell test instrument (LAND Electronic Co.). Electrochemical impedance spectra (EIS) were measured on an electrochemical workstation (CHI750D). EIS measurements were performed over a frequency range of 100 kHz to 0.01 Hz with an amplitude of 5 mV. All the measurements were carried out at room temperature.
1. Galvanostatic charge–discharge experiments were performed at different current densities between 3 and 4.3 V (vs. Li/Li+) by using a CT2001A cell test instrument (LAND Electronic Co.). Electrochemical impedance spectra (EIS) were measured on an electrochemical workstation (CHI750D). EIS measurements were performed over a frequency range of 100 kHz to 0.01 Hz with an amplitude of 5 mV. All the measurements were carried out at room temperature.
Results and discussion
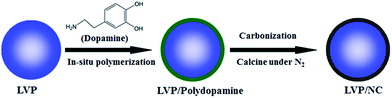
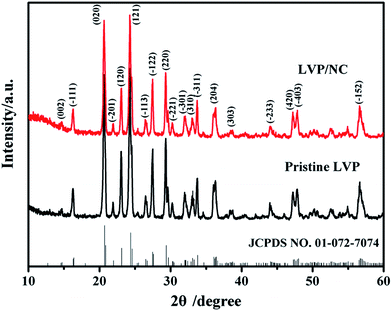
The schematic of the LVP/NC synthesis is illustrated in Fig. 1. First, the LVP was prepared by a typical sol–gel route. The as-synthesized LVP, mixed with dopamine, was dispersed in the Tris-buffer (pH = 8.5). Through the spontaneous oxidative polymerization of dopamine on the surface of the LVP, the LVP/polydopamine was achieved. Finally, LVP/NC was obtained via carbonization of polydopamine by calcination under an inert atmosphere.The X-ray diffraction (XRD) patterns of the as-synthesized pristine LVP and LVP/NC are presented in Fig. 2. The major identified peaks at 2θ = 16.3, 20.6, 23.0, 24.3, 27.5, 29.3, 36.2, 44.0, 47.2, 47.8 and 56.6° correspond to the (−111), (020), (120), (121), (−122), (220), (204), (−233), (420), (−403) and (−152) planes of a monoclinic lithium vanadium phosphate phase (space group: P21/n, JCPDS no. 01-072-7074) without any discernable impurities such as Li3PO4, and V2O3, conforming well to the previously reported literature.7,15–18 The characteristic peak of the graphitized carbon is not detected in the pattern of LVP/NC, indicating that carbon obtained from polydopamine is amorphous. Furthermore, the carbon and nitrogen contents in the composite were 2.61% and 0.18%, respectively, according to elemental analysis, whereas the carbon content in the LVP/C was 2.7%, according to thermogravimetric (TG) analysis (Fig. S1†).
Fig. 3a shows the scanning electron microscopy (SEM) image of the as-synthesized pristine LVP sample, which consists of nearly spherical particles with a diameter of approximately 400 nm. Compared with the other synthesized LVP via solid-state reaction or sol–gel method,37,38 the particles display better homogeneity, which is beneficial for contact between particles and electrolyte. Transmission electron microscopy (TEM) examination reveals that the surface of pristine LVP is very smooth (Fig. 3c). As can be seen from the SEM (Fig. 3b) and TEM (Fig. 3d) images, the LVP/NC composite has the same overall morphology as pristine LVP, except for the uniform and continuous carbon coating. Fig. 3d shows that LVP is uniformly coated with a ∼7 nm thin carbon layer, displaying a typical core–shell nanostructure, which is beneficial for enhancing electronic conductivity and facilitating electrochemical performance of the composite electrode.39
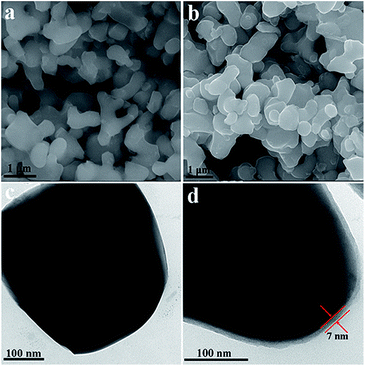 | ||
| Fig. 3 SEM images of the pristine LVP (a) and LVP/NC (b), and TEM images of the pristine LVP (c) and LVP/NC (d). | ||
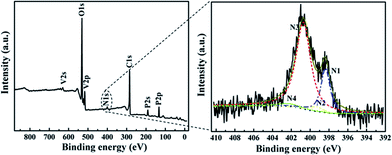
The XPS survey spectra of LVP/NC are shown in Fig. 4a and b. The peak at around 399 eV can be ascribed to N 1s in the LVP/NC survey spectra, suggesting the presence of N in LVP/NC samples.40 In the high-resolution XPS spectrum of N 1s for LVP/NC, the N 1s region exhibited three main contributions: the peak at 398.4 eV can be ascribed to pyridinic nitrogen (N1), the peak at 399.4 eV to pyrrolic nitrogen (N2), and the peak at 400.7 eV to graphite nitrogen (N3). The minor N 1s peak at 402.5 eV (N4) indicated presence of the N-oxide in pyridinic nitrogen. The XPS survey spectrum indicates that polydopamine was transformed into nitrogen-doped carbon through carbonization, which is beneficial for Li+ transport in the interface due to defects in the carbon caused by nitrogen doping.41 The nitrogen content measured by XPS was 2 atom%.
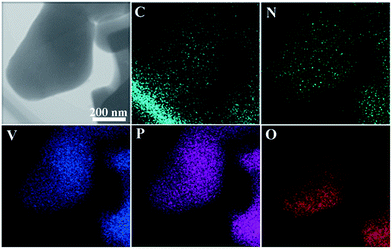
To further investigate element distribution in the LVP/NC composite, energy dispersive X-ray (EDX) mapping was carried out, as shown in Fig. 5. It can be seen that the images were overlapped with each other, which indicates that C and N distributed uniformly through the LVP sample. Both XPS and EDX analysis confirmed the existence of nitrogen in the composite, which can improve the electronic conductivity due to hybridization of nitrogen lone-pair electrons with the p electrons in carbon.42 Furthermore, nitrogen-doped carbon has more defects in the graphite structure.20,39,43 Hence, lithium ions can easily diffuse from the carbon shell to the LVP core.
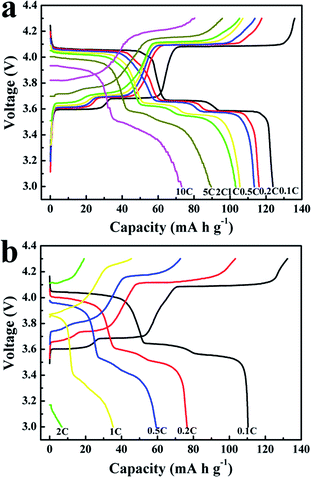
The typical discharge–charge voltage profiles of the LVP/NC electrode at different current rates over a potential window of 3–4.3 V are shown in Fig. 6a. At the initial lower rate of 0.1 C, during the charge process, the sample showed three charge plateaus around 3.6, 3.7 and 4.1 V, which corresponded to a sequence of phase transition processes of Li3V2(PO4)3 → Li2.5V2(PO4)3 → Li2V2(PO4)3 → LiV2(PO4)3. Accordingly, three flat discharge plateaus could be discerned at 4.0, 3.7 and 3.6 V, which were identified as the two-phase transition processes during the electrochemical reactions. At the initial lower rate of 0.1 C, the LVP/NC samples gave a discharge capacity of 124 mA h g−1, which was close to its theoretical capacity of 133 mA h g−1. Moreover, with the increase of discharge–charge rate, the discharge plateau voltages decreased slightly and the electrochemical plateaus became undistinguishable, due to the increase of cell polarization at high current rates.44 In Fig. 6b and S2,† both LVP and LVP/C samples show similar plateaus, but deliver lower discharge capacity of 111 mA h g−1 and 122 mA h g−1 at 0.1 C, respectively. Furthermore, the electrochemical plateau voltages of LVP and LVP/C at various current rates were also lower. These results imply that LVP/NC has lower polarization and fast reaction kinetics during charge–discharge.45
Rate performance of the LVP/NC, LVP and LVP/C electrodes were also evaluated (Fig. 7 and S3†). The LVP/NC electrode showed the smaller capacity decay, compared to LVP and LVP/C, with the increase of rate. It was noteworthy that the capacity (74 mA h g−1) obtained by LVP/NC at a rate of 10 C is higher than that at a rate of 0.5 C for the pristine LVP. At any discharge rate, the discharge capacity of LVP/NC was always higher than that of LVP or LVP/C. Even when the rate was turned back to 0.1 C, a discharge capacity of 113 mA h g−1 (as high as 91% of initial capacity for LVP/NC) could be retained, much higher than that of 68 mA h g−1 for LVP and 109 mA h g−1 for LVP/C. These results indicate that the LVP/NC electrode has a remarkable rate capability.
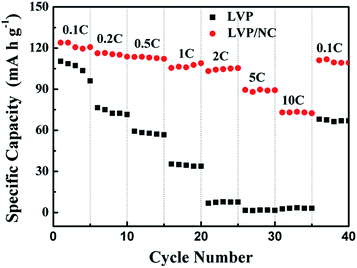 | ||
| Fig. 7 Rate performances of LVP/NC and LVP samples at different current rates over a potential window of 3–4.3 V. | ||
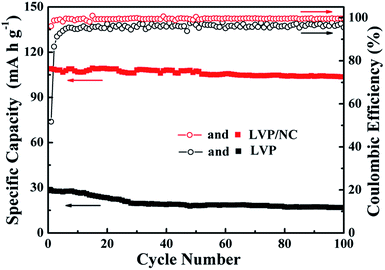
The LVP/NC electrode also revealed excellent cycling performance. Fig. 8 and S4† show the cycling performances of the LVP/NC, LVP and LVP/C at 1 C between the voltage limits of 3.0 and 4.3 V. For the LVP/NC electrode, the initial discharge capacity was 109 mA h g−1 at the rate of 1 C, after 100 cycles with 4.6% capacity loss, but for LVP and LVP/C, the corresponding values were 16 mA h g−1 and 42.9%, and 100 mA h g−1 and 7.6%. The Coulombic efficiency of the LVP/NC remained approximately 100%, which indicates that the electrochemical Li+ insertion–extraction process is reversible even at high rates. These results further confirm the advantage of nitrogen-doped carbon as a shell for LVP electrodes.
In order to gain insight into the remarkable rate performance of LVP/NC, we carried out AC impedance spectra measurements. As demonstrated in Fig. 9 and S4,† Nyquist plots spectra of electrodes exhibit a semicircle in the high-frequency range and an inclined line in the low-frequency range. The high-frequency region featuring the semicircle is attributed to the electrochemical reaction resistance and the double layer capacity of the electrode, while the low-frequency region of the straight line corresponds to the diffusion of lithium ions into the bulk of the cathode material, the so-called Warburg diffusion. The fitting results were obtained by ZView software, which was set using an equivalent circuit. As seen from the equivalent circuit, Rs and Rct are the uncompensated bulk resistance (total resistance of the electrolyte, separator and electrical contact) and charge-transfer resistance, respectively. CPE is the constant phase angle element, involving double layer capacitance, and Zw represents the Warburg impedance related to the diffusion of lithium ions into the bulk electrodes.46 As shown in Table 1 and S1,† the value of the charge-transfer resistance (Rct) is 172 Ω for LVP/NC, which is significantly lower than those for LVP (521 Ω) and LVP/C (266 Ω), indicating that nitrogen-doped carbon coating substantially enhances the electrochemical activity of LVP/NC.17,29
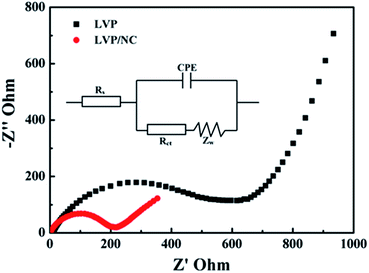 | ||
| Fig. 9 EIS spectra for LVP and LVP/NC with frequency range of 100 kHz to 0.01 Hz after five full cycles at 0.1 C. | ||
| Samples | Rs (Ω) | Rct (Ω) |
|---|---|---|
| LVP | 6.88 | 521 |
| LVP/NC | 1.85 | 172 |
Conclusions
In summary, a facile approach has been developed to synthesize LVP/NC composites. The amorphous carbon layer derived from polydopamine formed a continuous electronic conducting network that can effectively improve electronic conductivity of the materials. Moreover, the defects in the nitrogen-doped carbon coating also provided Li+ diffusion paths. Consequently, the resultant LVP/NC exhibits improved lithium storage properties, including high rate capability and excellent cycle stability. At a current rate of 10 C, LVP/NC delivered a discharge capacity of 74 mA h g−1. Furthermore, the capacity retention was up to 95.4% of its initial capacity, with negligible capacity fading after 100 cycles at 1.0 C. In addition to the LVP/NC application, we anticipate that this versatile synthetic approach can be extended to modify other electrode materials for high-performance energy storage devices.Acknowledgements
This work was supported by China's National Basic Research Program (973 Program) (2014CB239701), National Natural Science Foundation (21173120, 51372116), Natural Science Foundation of Jiangsu Province (BK2011030), and Fundamental Research Funds for Central Universities (NP2014403).Notes and references
- J. B. Goodenough and K. S. Park, J. Am. Chem. Soc., 2013, 135, 1167 CrossRef CAS PubMed.
- B. Dunn, H. Kamath and J. M. Tarascon, Science, 2011, 334, 928 CrossRef CAS PubMed.
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652 CrossRef CAS PubMed.
- K. S. Park, A. Benayad, D. J. Kang and S. G. Doo, J. Am. Chem. Soc., 2008, 130, 14930 CrossRef CAS PubMed.
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930 CrossRef CAS PubMed.
- C. Masquelier and L. Croguennec, Chem. Rev., 2013, 113, 6552 CrossRef CAS PubMed.
- Q. L. Wei, Q. Y. An, D. D. Chen, L. Q. Mai, S. Y. Chen, Y. L. Zhao, K. M. Hercule, L. Xu, A. Minhas-khan and Q. J. Zhang, Nano Lett., 2014, 14, 1042 CrossRef CAS PubMed.
- L. F. Shen, H. S. Li, E. Uchaker, X. G. Zhang and G. Z. Cao, Nano Lett., 2012, 12, 5673 CrossRef CAS PubMed.
- L. F. Shen, H. S. Li, E. Uchaker, X. G. Zhang and G. Z. Cao, Adv. Mater., 2012, 24, 6502 CrossRef CAS PubMed.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359 CrossRef CAS PubMed.
- Z. L. Gong and Y. Yang, Energy Environ. Sci., 2011, 4, 3223 CAS.
- J. Kim, J. K. Yoo, Y. S. Jung and K. Kang, Adv. Energy Mater., 2013, 3, 1004 CrossRef CAS PubMed.
- H. Huang, S. C. Yin, T. Kerr, N. Taylor and L. F. Nazar, Adv. Mater., 2002, 14, 1525 CrossRef CAS.
- S. C. Yin, H. Grondey, P. Strobel, M. Anne and L. Nazar, J. Am. Chem. Soc., 2003, 125, 10402 CrossRef CAS PubMed.
- Z. Y. Chen, H. Z. Jin, C. S. Dai, G. Wu, M. Nelson and Y. F. Chen, Int. J. Electrochem. Sci., 2013, 8, 8153 CAS.
- Y. Z. Li, Z. Zhou, X. P. Gao and J. Yan, Electrochim. Acta, 2007, 52, 4922 CrossRef CAS PubMed.
- L. Q. Mai, S. Li, Y. F. Dong, Y. L. Zhao, Y. Z. Luo and H. M. Xu, Nanoscale, 2013, 5, 4864 RSC.
- W. C. Duan, Z. Hu, K. Zhang, F. Y. Cheng and Z. L. Tao, Nanoscale, 2013, 5, 6485 RSC.
- W. H. Shin, H. M. Jelong, B. G. Kim, J. K. Kang and J. W. Choi, Nano Lett., 2012, 12, 2283 CrossRef CAS PubMed.
- Z. J. Ding, L. Zhao, L. M. Suo, Y. Jiao, S. Meng, Y. S. Hu, Z. X. Wang and L. Q. Chen, Phys. Chem. Chem. Phys., 2011, 13, 15127 RSC.
- C. Wang, W. Shen and H. M. Liu, New J. Chem., 2014, 38, 430 RSC.
- J. P. Paraknowitsch, A. Thomas and M. Antonietti, J. Mater. Chem., 2010, 20, 6746 RSC.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426 CrossRef CAS PubMed.
- R. Liu, S. M. Mahurin, C. Li, R. Unocic, J. C. Idrobo, H. Gao, S. J. Pennycook and S. Dai, Angew. Chem., Int. Ed., 2011, 50, 6799 CrossRef CAS PubMed.
- A. Postma, Y. Yan, Y. Wang, A. N. Zelikin, E. Tjipto and F. Caruso, Chem. Mater., 2009, 21, 3042 CrossRef CAS.
- J. H. Jiang, L. P. Zhu, L. J. Zhu, B. K. Zhu and Y. Y. Xu, Langmuir, 2011, 27, 14180 CrossRef CAS PubMed.
- Y. L. Liu, K. L. Ai and L. H. Lu, Chem. Rev., 2014, 114, 5057 CrossRef CAS PubMed.
- M. H. Ryou, Y. M. Lee, J. K. Park and J. W. Choi, Adv. Mater., 2011, 23, 3066 CrossRef CAS PubMed.
- M. H. Ryou, J. Kim, I. Lee, S. Kim, Y. K. Jeong, S. Hong, J. H. Ryu, T. S. Kim, J. K. Park, H. Lee and J. W. Choi, Adv. Mater., 2013, 25, 1571 CrossRef CAS PubMed.
- H. S. Li, L. F. Shen, K. B. Yin, J. Ji, J. Wang, X. Y. Wang and X. G. Zhang, J. Mater. Chem. A, 2013, 1, 7270 CAS.
- Z. X. Chi, W. Zhang, F. Q. Cheng, J. T. Chen, A. M. Cao and L. J. Wan, RSC Adv., 2014, 4, 7795 RSC.
- L. Tan, P. Lei, C. Y. Cao, B. F. Wang and L. Li, J. Power Sources, 2014, 253, 193 CrossRef CAS PubMed.
- J. H. Kong, W. A. Yee, L. P. Yang, Y. F. Wei, S. L. Phua, H. G. Ong, J. M. Ang, X. Li and X. H. Lu, Chem. Commun., 2012, 48, 10316 RSC.
- C. Lei, F. Han, D. Li, W. C. Li, Q. Sun, X. Q. Zhang and A. H. Lu, Nanoscale, 2013, 5, 1168 RSC.
- M. M. Ren, Z. Zhou, X. P. Gao, W. X. Peng and J. P. Wei, J. Phys. Chem. C, 2008, 14, 5689 Search PubMed.
- X. M. Sun and Y. D. Li, Angew. Chem., Int. Ed., 2004, 43, 597 CrossRef PubMed.
- C. S. Dai, Z. Y. Chen, H. Z. Jin and X. G. Hu, J. Power Sources, 2010, 195, 5775 CrossRef CAS PubMed.
- Y. Z. Li, Z. Zhou, M. M. Ren, X. P. Gao and J. Yan, Electrochim. Acta, 2006, 51, 6498 CrossRef CAS PubMed.
- C. X. Wang, G. J. Shao, Z. P. Ma, S. Liu, W. Song and J. J. Song, Electrochim. Acta, 2014, 130, 679 CrossRef CAS PubMed.
- H. S. Li, L. F. Shen, J. Wang, B. Ding, P. Nie, G. Y. Xu, X. Y. Wang and X. G. Zhang, ChemPlusChem, 2014, 79, 128 CrossRef CAS PubMed.
- Z. J. Ding, L. Zhao, L. M. Suo, Y. Jiao, S. Meng, Y. S. Hu, Z. X. Wang and L. Q. Chen, Phys. Chem. Chem. Phys., 2011, 13, 15127 RSC.
- N. Y. Wang, C. H. Shih, P. T. Chiueh and Y. F. Huang, Energies, 2013, 6, 871 CrossRef CAS PubMed.
- C. C. Ma, X. H. Shao and D. P. Cao, J. Mater. Chem., 2012, 22, 8911 RSC.
- D. L. Li, M. Tian, R. Xie, Q. Li, X. Y. Fan, P. Zhao, S. L. Ma, Y. X. Shi and H. T. H. Yong, Naonoscale, 2014, 6, 3302 RSC.
- H. S. Li, L. F. Shen, X. G. Zhang, J. Wang, P. Nie, Q. Che and B. Ding, J. Power Sources, 2013, 221, 122 CrossRef CAS PubMed.
- S. B. Yang, X. L. Feng and K. Müllen, Adv. Mater., 2011, 23, 3575 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra05089d |
| This journal is © The Royal Society of Chemistry 2014 |