Supported palladium nanoparticles-catalyzed decarboxylative coupling approaches to aryl alkynes, indoles and pyrrolines synthesis†
C. Bal Reddyab,
Richa Bharti‡
ab,
Sandeep Kumarab and
Pralay Das*ab
aNatural Product Chemistry and Process Development Division, CSIR-Institute of Himalayan Bioresource Technology, Palampur-176061, H. P., India. E-mail: pdas@ihbt.res.in; pdas_nbu@yahoo.com; Fax: +91-1894-230-433
bAcademy of Scientific and Innovative Research (AcSIR), New Delhi, India
First published on 11th July 2016
Abstract
The polystyrene supported palladium (Pd@PS) nanoparticles (NPs) catalyzed decarboxylative coupling (DC) of arylhalides and alkynyl carboxylic acids was developed for the synthesis of diaryl alkynes. Indole and 3-pyrroline heterocycles were also synthesized from 2-iodo anilines/amino benzocycloheptene bromide and alkynyl carboxylic acids, following a domino decarboxylative coupling-cyclization (DCC) approach under the same catalytic conditions. The combined anchoring and catalytic behaviour of Pd@PS makes the process favourable for the product formation.
Introduction
The decarboxylative couplings of alkynyl carboxylic acids have been identified as a viable tool for C–C and C-heteroatom bond formation processes.1 Alkynyl carboxylic acids have emerged as coupling partners in Sonogashira type reactions for the synthesis of pharmaceutically potent2 diaryl motifs, rather than terminal alkynes, due to easy handling, high boiling points, and high stability.3 Since the development of the first Pd2dba3/dppf4a catalytic system for the decarboxylative coupling of alkynyl carboxylic acids with aryl halides, a number of protocols using palladium complexes4b–4i (Pd2dba3/dppb, Pd2dba3CHCl3/PPh3 or Xantphos, Pd(OAc)2/XPhos, Pd(PPh3)2Cl2/dppb, and palladacycle/XPhos) and copper5 (CuI/1,10-Phen, CuI/PPh3 and CuI/Fe(acac)3) catalysts have been developed. The only heterogeneous catalyst reported for decarboxylative Sonogashira coupling is Pd-CNT (carbon nanotubes).4g Although decarboxylative Sonogashira coupling reactions under homogeneous catalytic conditions are well documented, less attention has been paid to applying the decarboxylative coupling strategy to the synthesis of functionalized indoles and 3-pyrrolines, an important scaffold for natural and pharmaceutical products.6 Reported protocols to access 2-substituted indoles include (i) cycloisomerization of 2-alkynyl anilines using transition metal catalysts, Lewis acids, and strong bases (Scheme 1, method A),7 and (ii) domino coupling-cyclization of protected 2-haloanilines and terminal alkynes under copper- and palladium-catalyzed conditions (Scheme 1, method B).8 Moreover, 3-pyrrolines can be afforded by 5-endo cyclization of α-amino allenes, prompted by transition metal catalysts and base.9 In addition, few multi-component reactions were also demonstrated by using copper- and organo-catalytic conditions.9e,9f Most of the reported methods for the synthesis of indoles and 3-pyrrolines were either performed in homogeneous catalytic conditions, or required additional cyclizing agents. Recently, the domino decarboxylative coupling-cyclization was reported for the synthesis of substituted indoles, performed in a CuBr/L-proline catalytic system, which is only applicable for 2-iodotrifluoro acetanilide substrates.10Therefore, the development of a heterogeneous palladium catalyzed, novel and reliable decarboxylative coupling strategy for the synthesis of di-aryl alkynes and five member nitrogen heterocycles under ligand free conditions is highly desirable.
As a part of our continuous research on the development of supported transition metal nanoparticles as catalysts, and their applications in various organic transformations,11 herein we present Pd@PS NPs catalyzed decarboxylative coupling for the synthesis of di-aryl alkynes and domino DCC to synthesize functionalized indoles. Further, structurally similar antidepressant molecules,12 amino benzocycloheptene vinyl bromides were also converted to the corresponding new class of 3-pyrrolines by the domino decarboxylative coupling 5-exo cyclization reaction with phenylpropiolic acid under the same catalytic conditions.
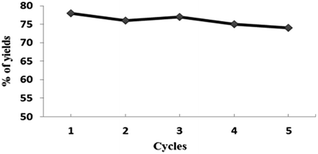
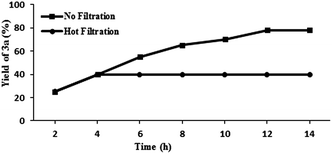
The Pd@PS NPs were prepared by following our earlier reported reduction deposition method (ESI†). The developed Pd@PS catalyst was further analyzed for its morphology and crystalline structure by scanning electron microscopy (SEM), transmission electron microscopy (TEM) and selected area electron diffraction (SAED) studies. The particles at the surface were analyzed by SEM and SEM-EDS (energy dispersive spectra), which revealed the presence of palladium NPs at the surface of PS (Fig. 1a and b). The low field TEM image of Pd@PS further confirmed the impregnation of palladium NPs of size ranging from 1–5 nm, with the largest average number in the range 1–3 nm (Fig. 1c–e). The high resolution TEM (HRTEM) image of Pd@PS showed an interplanar distance of 0.22 nm, corresponding to the (111) plane, and 0.19 nm corresponding to the (200) planes of the face centered cubic arrangement of palladium (Fig. 1f). The heterogeneity of the Pd@PS catalyst was further confirmed by Hg(0) poisoning and hot filtration tests (Fig. 3), which revealed that the catalysis took place in a truly heterogeneous manner, as the negligible leaching of palladium was detected by ICP-AES analysis after the fifth reaction cycle (Fig. 2).
We started our investigation using 4-iodo anisole and phenyl propiolic acid as model substrates. Among the different bases (Table 1, entries 1–6) and solvents (Table 1, entries 7–13) screened, DBU as base and DMF as solvent gave the best results. Gratifyingly, the Pd@PS catalyst was found to be more active than the commercially available Pd/C (Table 1, entries 14). The optimum condition was found to be Pd@PS (3 mol%), DBU (3 equiv.) in DMF at 110 °C for 12 h, giving 78% of the desired product. Under the optimized conditions, phenyl acetylene was found to be less active for the coupling reaction (Table 1, entry 15); therefore, the oxidative adduct (Ar–Pd-halide) may be responsible for the decarboxylation of palladium carboxylate to form the aryl alkynyl palladium, and further reductive elimination gave the desired product.
| Entry | Catalyst (mol%) | Base | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.42 mmol), 2a (0.51 mmol), base (3 equiv.), catalyst (3 mol%) and DMF (2 mL) at 110 °C.b GC-MS yield, the isolated yield is given in parentheses.c At 90 °C.d 2a = phenyl acetylene. | ||||
| 1 | Pd@PS (3) | K2CO3 | DMF | 22 |
| 2 | Pd@PS (3) | Et3N | DMF | nr |
| 3 | Pd@PS (3) | K3PO4 | DMF | 14 |
| 4 | Pd@PS (3) | DABCO | DMF | 16 |
| 5 | Pd@PS (3) | KOtBu | DMF | 37 |
| 6 | Pd@PS (2) | DBU | DMF | 65 |
| 7 | Pd@PS (3) | DBU | DMF | 89 (78) |
| 8c | Pd@PS (3) | DBU | DMF | 45 |
| 9 | Pd@PS (3) | DBU | 1,4-dioxane | 6 |
| 10 | Pd@PS (3) | DBU | Toluene | nr |
| 11 | Pd@PS (3) | DBU | CH3CN | 10 |
| 12 | Pd@PS (3) | DBU | DMA | 20 |
| 13 | Pd@PS (3) | DBU | DMSO | 56 |
| 14 | Pd/C (3) | DBU | DMF | 10 |
| 15d | Pd@PS (3) | DBU | DMF | 34 |
The scope of the DC reaction was investigated by using different aryl iodides and aryl bromides with alkynyl carboxylic acids under the optimized reaction conditions (Scheme 2). Electron-rich and neutral aryl iodides successfully coupled with phenylpropiolic acid in good yields (70 and 66%) of 3b and 3c, respectively. Moreover, base sensitive and electron withdrawing groups (e.g., CO2CH3, COCH3, CHO, CN, CF3 and Cl) were well tolerated under the reaction conditions, and furnished the corresponding internal alkynes 3d–3i in 58–96% yields. In addition, ortho substituted aryl iodides, such as 2-iodoaniline and 2-iodo acetanilide smoothly yielded desired products 3j and 3k in 74% and 76% yield, respectively. Alcohol substituted aryl halide, such as 3-iodobenzylalcohol was allowed to react with phenylpropiolic acid under the optimized reaction conditions, di-aryl alkyne 3l was formed in 86% yield. Heterocyclic halide, such as 2-iodothiophene was also found to be compatible with the employed reaction conditions, producing 3m in 76% yield. Poly substituted aromatic iodide also reacted smoothly and produced 3n in 82% yield. It is gratifying to say that the reaction conditions were compatible with a variety of propiolic acids bearing electron donating and withdrawing functionalities in the coupling reaction with different aryl iodides, and gave the coupled products 3o–p and 3s in high yields. To our surprise, the reaction of 4-iodoanisole with 2-butynoic acid gave the corresponding internal alkyne 3r in 58% yield. In addition, the decarboxylative coupling of arylbromides were also studied under the same catalytic conditions and the corresponding products 3d, 3e, 3t and 3u were attained in moderate yields. Finally, the decarboxylative coupling of aryl chlorides with phenyl propiolic acid were also attained, to get the coupled products 3e and 3t in lower isolated yields and the major quantity of starting material was recovered.
The reactions of diiodobenzenes with phenylpropiolic acid were also carried out under the aforementioned reaction conditions (Scheme 3). Intriguingly, the reaction of 1,2-diiodobenzene with phenylpropiolic acid gave the corresponding bis-coupled product 5a in 94% yield. Similarly, the coupling reaction of 1,3- and 1,4-diiodobenzenes with phenylpropiolic acid afforded the desired bis coupled products 5b and 5c in 77% and 72% yields, respectively, which indicates that the present protocol may have broad potential for constructing extended π-electron systems.
We next attempted the Pd@PS catalyzed decarboxylative coupling of 2-iodo-N-tosylanilines with various alkynyl carboxylic acids (Scheme 4). We were delighted to observe the synthesis of N-tosylated indoles without any need for an external cyclizing agent. The mechanistic investigation reveals that palladium catalyst, as well as DBU, assisted the cyclization reaction (ESI†). The various substituents on 2-iodo-N-tosylanilines had no effect, as all reacted smoothly with phenylpropiolic acid to give approximately the same yield of corresponding products 7a–7c. Similarly, 3-(4-chlorophenyl)propiolic acid and but-2-ynoic acid were also found to be effective coupling partners, yielding the desired products 7d–7f in good yields. Surprisingly, N-(2-iodophenyl)methane sulfonamide, 2-iodotriflouroacetanilide, 2,2,2-trifluoro-1-(2-iodo-4-methylphenyl)ethanone reacted with phenylpropiolic acid to give indole 7g and 7h in one pot coupling cyclization and deprotection approaches.
The methodology was further extended to the synthesis of bioactive 3-pyrroline derivatives by tandem decarboxylative coupling 5-exo cyclization of amino benzocycloheptene bromides with phenylpropiolic acid (Scheme 5).12 Different benzyl substituted amino benzocycloheptene bromides were easily coupled and cyclized to furnish a new class of 3-pyrroline compounds 9a–9d in 52–76% yields. Naphthyl-methyl substituted amino benzocycloheptenebromide also coupled to get the desired pyrroline 9e in 73%. Interestingly, aryl substituted amino benzocycloheptene bromide also participated in the similar reaction with phenyl propiolic acid to produce the corresponding 3-pyrroline product 9f in considerably good yield of 60%. Alkyl amine substituted amino benzocycloheptene bromides were also found to be reactive and gave the corresponding products 9g and 9h in good yields.
Recyclability experiments
The recyclability of the Pd@PS catalyst was investigated in the decarboxylative coupling of 4-iodoanisole with phenylpropiolic acid under standard reaction conditions. After completion of the reaction, the catalyst was recovered by simple filtration and washed with water and acetone, and dried under reduced pressure and reused. It was found that the catalyst was recycled up to 5 times without significant loss of activity (Fig. 2). ICP-AES analysis was used to detect the palladium leaching into the resulting reaction solution and only <1 ppm of palladium was detected (ESI†).Mercury test
The mercury drop test was carried out to evaluate the heterogeneity of the catalyst, as well as the catalytically active species, under the reaction conditions. The addition of Hg(0) to the heterogeneously catalysed reactions is known to inhibit the activity of the catalyst, due to amalgam formation on the catalytic surface. In contrast, Hg(0) does not inhibit the catalytic activity of homogeneous palladium catalysts. When a drop of mercury was added to the reaction mixture of 4-iodoanisole and phenylpropiolic acid under optimized reaction conditions, traces of product were observed, whereas, the mercury free experiment give the highest yield of the desired product, indicating that the mercury leads to the amalgamation of the catalytic surface of the heterogeneous catalyst (Pd@PS), which further confirms that the catalytic active species under the reaction conditions are Pd(0) and the reaction occurred in a truly heterogeneous manner.Hot filtration test
The reaction of 4-iodoanisole with phenylpropiolic acid was carried out under the standard reaction conditions; after 2 h (the yield of the product was 30%) the solid catalyst was filtered out and the reaction continued for 12 h. No further increase in the yield of the product was observed, indicating the absence of palladium(II) species in the solution, and that the decarboxylation reaction was truly heterogeneous (Fig. 3).Conclusions
In conclusion, Pd@PS NPs was found to be a highly active catalyst for the decarboxylative coupling of arylhalides and alkynyl carboxylic acids to produce diaryl alkynes. The ability of the catalyst has also been exploited in the domino decarboxylative coupling-cyclization (DCC) reaction for indoles and new classes of pyrroline synthesis. The benefits of this process, such as simple operation, recyclability, vast selectivity profile and milder reaction conditions, produce an alternative decarboxylative coupling strategy to the existing procedures.Acknowledgements
Authors are grateful to the Director, CSIR-IHBT for providing necessary facilities during the course of the work. We thank Dr G. Saini, AIRF, JNU-New Delhi, India for the TEM and Biotechnology Division, CSIR-IHBT, for SEM and EDS analysis; R. B. thanks CSIR, New Delhi, for financial support as part of XII Five Year Plan programme under the title ORIGIN (CSC-0108). C. B. R., S. K. thank UGC, New Delhi for awarding fellowship.Notes and references
- (a) K. Park and S. Lee, RSC Adv., 2013, 3, 14165 RSC; (b) G. Rong, J. Mao, H. Yan, Y. Zheng and G. Zhang, J. Org. Chem., 2015, 80, 7652 CrossRef CAS PubMed.
- (a) J. Boukouvalas, S. Cote and B. Ndzi, Tetrahedron Lett., 2007, 48, 105 CrossRef CAS; (b) D. Falcone, J. Li, A. Kale and G. B. Jones, Bioorg. Med. Chem. Lett., 2008, 18, 934 CrossRef CAS PubMed.
- (a) K. Park, T. Palani, A. Pyo and S. Lee, Tetrahedron Lett., 2012, 53, 733 CrossRef CAS; (b) K. Park, J.-M. You, S. Jeon and S. Lee, Eur. J. Org. Chem., 2013, 1973 CrossRef CAS.
- (a) J. Moon, M. Jeong, H. Nam, J. Ju, J. H. Moon, H. M. Jung and S. Lee, Org. Lett., 2008, 10, 945 CrossRef CAS PubMed; (b) J. Moon, M. Jang and S. Lee, J. Org. Chem., 2009, 74, 1403 CrossRef CAS PubMed; (c) H. Kim and P. H. Lee, Adv. Synth. Catal., 2009, 351, 2827 CrossRef CAS; (d) W.-W. Zhang, X.-G. Zhang and J.-H. Li, J. Org. Chem., 2010, 75, 5259 CrossRef CAS PubMed; (e) K. Park, G. Bae, J. Moon, J. Choe, K. H. Song and S. Lee, J. Org. Chem., 2010, 75, 6244 CrossRef CAS PubMed; (f) S. Tartaggia, O. D. Lucchi and L. J. Goossen, Eur. J. Org. Chem., 2012, 1431 CrossRef CAS; (g) A. Pyo, J. D. Kim, H. C. Choi and S. Lee, J. Organomet. Chem., 2013, 724, 271 CrossRef CAS; (h) P. V. Reddy, P. Srinivas, M. Annapurna, S. Bhargava, J. Wagler, N. Mirzadeh and M. L. Kantam, Adv. Synth. Catal., 2013, 355, 705 CrossRef CAS; (i) X. Li, F. Yang and Y. Wu, J. Org. Chem., 2013, 78, 4543 CrossRef CAS PubMed.
- (a) J. Mao, M. Wu, G. Xie and S. Ji, Adv. Synth. Catal., 2009, 351, 2101 CrossRef CAS; (b) X. Qu, T. Li, P. Sun, Y. Zhu, H. Yang and J. Mao, Org. Biomol. Chem., 2011, 9, 6938 RSC; (c) D. Zhao, C. Gao, X. Su, Y. He, J. You and Y. Xue, Chem. Commun., 2010, 46, 9049 RSC; (d) T. Li, P. Sun, H. Yang, Y. Zhu, H. Yan, L. Lu and J. Mao, Tetrahedron Lett., 2012, 68, 6413 CrossRef.
- (a) S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873 CrossRef CAS PubMed; (b) M. Somei and F. Yamada, Nat. Prod. Rep., 2004, 21, 278 RSC; (c) W. Shi, S. L. Marcus and T. L. Lowary, Bioorg. Med. Chem., 2011, 19, 603 CrossRef CAS PubMed; (d) D. O'Hagen, Nat. Prod. Rep., 2000, 17, 435 RSC; (e) W. K. Anderson and A. S. Milowsky, J. Med. Chem., 1986, 29, 2241 CrossRef CAS PubMed; (f) G. R. Petti, Y. Kamano, C. Dufresne, R. L. Cerny, C. L. Herald and J. M. Schmidt, J. Org. Chem., 1989, 54, 6005 CrossRef.
- (a) K. Hiroya, S. Itoh and T. Sakamoto, J. Org. Chem., 2004, 69, 1126 CrossRef CAS PubMed; (b) Y. Yin, W. Ma, Z. Chai and G. Zhao, J. Org. Chem., 2007, 72, 5731 CrossRef CAS PubMed; (c) N. Sakai, K. Annaka, A. Fujita, A. Sato and T. Konakahra, J. Org. Chem., 2008, 73, 4160 CrossRef CAS PubMed; (d) X. Li, A. R. Chianese, T. Vogel and R. H. Crabtree, Org. Lett., 2005, 7, 5437 CrossRef CAS PubMed; (e) M. Nakamura, L. Ilies, S. Otsubo and E. Nakamura, Org. Lett., 2006, 8, 2803 CrossRef CAS PubMed; (f) J. McNulty and K. Keskar, Eur. J. Org. Chem., 2014, 1622 CrossRef CAS; (g) R. Sanz, V. Cuilarte and M. P. Castroviejo, Synlett, 2008, 3006 CrossRef CAS , and references cited therein..
- (a) F. Liu and D. Ma, J. Org. Chem., 2007, 72, 4844 CrossRef CAS PubMed; (b) R. Wang, S. Mo, Y. Lu and Z. Shen, Adv. Synth. Catal., 2011, 353, 713 CrossRef CAS; (c) L. Djakovitch, V. Dufaud and R. Zaidi, Adv. Synth. Catal., 2006, 248, 715 CrossRef.
- (a) R. K. Dieter and H. Yu, Org. Lett., 2001, 3, 3855 CrossRef CAS PubMed; (b) N. Morita and N. Krause, Org. Lett., 2004, 6, 4121 CrossRef CAS PubMed; (c) M. Sai and S. Matsubara, Org. Lett., 2011, 13, 4676 CrossRef CAS PubMed; (d) H. Ohno, Y. Kodoh, N. Fujii and T. Tanaka, Org. Lett., 2006, 8, 947 CrossRef CAS PubMed; (e) S. L. Cui, J. Wang and Y. G. Wang, Org. Lett., 2007, 9, 5023 CrossRef CAS PubMed; (f) A. Desmarchelier, V. Coeffard, X. Moreau and C. Greck, Chem.–Eur. J., 2012, 18, 13222 CrossRef CAS PubMed.
- T. Ponpandian and S. Muthusubramanian, Tetrahedron Lett., 2012, 53, 4248 CrossRef CAS.
- (a) P. Das, D. Sharma, A. K. Shil and A. Kumari, Tetrahedron Lett., 2011, 52, 1176 CrossRef CAS; (b) N. R. Guha, C. Bal Reddy, N. Aggarwal, D. Sharma, A. K. Shil, Bandna and P. Das, Adv. Synth. Catal., 2012, 354, 2911 CrossRef CAS; (c) A. K. Shil and P. Das, Green Chem., 2013, 15, 3421 RSC; (d) N. R. Guha, D. Bhattacharjee and P. Das, Catal. Sci. Technol., 2015, 5, 2575 RSC; (e) A. K. Shil, S. Kumar, C. Bal Reddy, S. Dadwal, V. Thakur and P. Das, Org. Lett., 2015, 17, 5352 CrossRef CAS PubMed; (f) N. R. Guha, S. Sharma, D. Bhattacherjee, V. Thakur, R. Bharti, C. Bal Reddy and P. Das, Green Chem., 2016, 18, 1206 RSC.
- (a) A. Chaudhary, P. Das, A. Mishra, P. Kaur, B. Singh and R. K. Goel, Mol. Diversity, 2012, 16, 357 CrossRef CAS PubMed; (b) A. Chaudhary and P. Das, Curr. Org. Chem., 2015, 19, 179 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and spectral data. See DOI: 10.1039/c6ra12046f |
| ‡ Scheme 5 work contributed by R. B. |
| This journal is © The Royal Society of Chemistry 2016 |