Synthesis of high-density jet fuel from plastics via catalytically integral processes†
Xuesong Zhang and
Hanwu Lei*
Bioproducts, Sciences and Engineering Laboratory, Department of Biological Systems Engineering, Washington State University, Richland, WA 99354-1671, USA. E-mail: hlei@wsu.edu; Fax: +1 509 372 7690; Tel: +1 509 372 7628
First published on 8th January 2016
Abstract
The present study was aimed at synthesizing JP-5 navy fuel from plastics through a novel pathway. The consecutive processes for manufacturing JP-5 navy fuel principally included the catalytic microwave-induced degradation of low-density polyethylene (a model compound of waste plastics) and the hydrotreatment of obtained liquid organics. The catalytic microwave degradation was conducted at the catalytic temperature of 375 °C and catalyst to feed ratio of 0.1. The carbon yield of the liquid organics from the catalytic microwave degradation was 66.18%, mainly consisting of a mixture of aromatic hydrocarbons and aliphatic olefins. Several variables, such as initial pressure and catalyst to reactant ratio, were employed to determine the optimal condition for the production of alternative jet fuels in the hydrotreating process. We observed that the aromatic hydrocarbons and aliphatic olefins as the precursors of jet fuels could be converted into jet fuel range aliphatic alkanes and cycloalkanes. The hydrotreated organics from the experiment conducted at the reaction temperature of 250 °C for 2 h included 31.23% selectivity towards aliphatic alkanes, 53.06% selectivity towards cycloalkanes, and 15% selectivity towards remaining aromatic hydrocarbons, which were consistent with the specifications of JP-5 navy fuel. In this regard, the catalytic microwave degradation of plastics and the hydrotreatment of obtained liquid organics can be regarded as a clear breakthrough to producing alternative jet fuels. From a commercial point of view, the catalytically integrated processes could be the most feasible for synthesizing advanced jet fuels (e.g. JP-5 navy fuel).
1. Introduction
The consumption of virgin plastics has increased exponentially over the past decades, since they are acting as an indispensable ingredient of utmost importance in daily life. Global plastic consumption is predicted to continuously rise by 4% in the forthcoming years,1 leading in parallel to growing waste plastics generated. Most waste plastics disposed in landfills cause a serious danger towards the environment owing to plastics degradation and subsequent contaminant generation.2 Plastic that pollutes oceans and waterways has severe impacts on the environment and economy. A new study in Science indicated the horrifying numbers: in 2010, the study found between 4.8 and 12.7 million metric tons (that was about 10.5 billion to 28 billion pounds) of plastic entered the oceans. Plastic pollution has an incalculably lethal effect on everything from plankton to whales.3 The current fate of these waste plastics indicates approximately 50% were disposed as they cannot be recovered.4 Landfills and incineration for disposal of waste plastics are commonly utilized among the conventional existing methods.5,6 Energy recovery by means of energetic valorization (incineration) stimulates the release of harmful compounds such as acid gases, dioxins, and furans into the atmosphere together with heavy metals causing damage towards the environment and human health.7These inherent issues of plastics recycling impel us to seek alternative valorization technologies for the production of high value-added chemicals or fuels from waste plastics. Hence conversion of waste plastics into valuable chemicals and fuels has attracted crucial interest worldwide. The state of the art on tertiary recycling technologies like depolymerization offers a promising alternative to plastic recycling.6,8 Waste plastics valorization approached by thermal degradation give rise to a heterogeneous hydrocarbon mixture of paraffins and olefins over a wide range of molecular weights.9 It is noted that a reactively broad spectrum of products is generated from the thermal degradation of macromolecules to small molecules complicating their utilization on an industrial scale at present.10 Therefore, the preliminary products have to undergo downstream catalytic upgrading prior to being used as transportation fuels.
Obtaining highly valuable fuels from waste plastics by means of simple technologies is extremely tough because of strict quality standard for fuel substitutes with regard to the presence of unsaturated and aromatic compounds.11 Catalytic cracking of waste plastics towards transportation fuels (e.g. gasoline and diesel) are more feasible.12 The application of a catalyst in a thermal degradation process presents remarkable merits because products in the desired range of carbon atom number can be enhanced and lower operating temperatures are attained.13 Nonetheless the in situ catalysis, which means the direct contact between the catalysts and waste plastics, noticeably resulted in poor conversion rates and fast catalyst deactivation.14 Compared to conventional waste management strategies, the ex situ catalysis (combination of sequential pyrolysis and catalytic reforming) for waste plastics valorization has evidenced to entail sound energy and environmental benefits.15 A downstream packed-bed reactor introduced in the studies was conducive to an upward tendency in contents of aromatic hydrocarbons and light olefins.16,17 Suitable catalysts and reactors can in principle control the product yield, product distribution as well as reduce the reaction temperature.1 In light of these premises, the conversion of waste plastics into fuels or valuable chemicals by means of catalytic degradation is regarded as the sustainable way to valorize the waste plastics.
In the context of increasing price of crude oils, the demand for diesel and jet fuels in the United States is expected to continue increasing by 27% in the following years as opposed to gasoline;18 therefore it is essential to shift alternative fuels towards distillate-range liquid alkanes in the future. The current jet fuels originated from fossil resources are principally comprised of aliphatic alkanes (paraffins) and cycloalkanes (naphthenes).19,20 These C8–C16 alkanes are hydrogen saturated, clean burning, and chemically stable. Nevertheless, linear-chain alkanes and branched-chain alkanes own lower densities (∼0.76–0.78 g mL−1), which cannot satisfy the specifications of jet fuels.21 To overcome the shortage of aliphatic alkanes, jet fuel range cycloalkanes or aromatic hydrocarbons should be synthesized and added into commercial jet fuels (e.g. Jet A and JP-8).20,22 Most efforts to produce military jet fuels have concentrated on increasing the cycloalkanes content; for example, JP-5 navy fuel contains 52.8% of cycloalkanes, 30.8% of aliphatic alkanes, and 15.9% of aromatic hydrocarbons.23 Notwithstanding, waste plastics can be implemented to produce advanced products via thermal degradation and catalytic reforming, the combined processes from waste plastics to high valuable jet fuels have not paved a feasible route.
In our previous work, Lei and his co-workers have found that microwave-induced degradation could enhance the selectivity of aromatic hydrocarbons.17,24,25 It was also obtained that the microwave-induced degradation of low-density polyethylene (a model of waste plastics) generated significant amounts of aromatic and aliphatic hydrocarbons.17 These unsaturated hydrocarbons belonged to the jet fuel range compounds, which provide an indication to manufacture jet fuel range alkanes from these preliminary products. From an industrial implementation point of view, the catalysts introduced in a process condition favor the economy of the process commercialized in a continuous operating plant.6 As most of waste plastics like polyethylene and polypropylene possess approximately 14 wt% hydrogen content (H/Ceff = 2), these hydrogen-efficient feedstock could reduce or even eliminate the coke formation in the catalytic degradation process.26 The formation of coke deposited on the ZSM-5 catalyst used in the aforementioned study was as low as 0.1 wt%, which could prolong the life time of the catalyst and contribute to lowering the catalyst cost.
In addition, it was noticed that jet fuel range aromatic hydrocarbons (C8–C16) are prone to be hydrogenated into cycloalkanes under mild conditions.27,28 Toward this end, the plastics (low-density polyethylene) were converted into jet fuel range aromatic and aliphatic hydrocarbons over well-modified ZSM-5 catalyst, according to the optimal condition for maximizing the carbon yield of aromatic hydrocarbons. The unsaturated hydrocarbons were stepwise hydrogenated into C8–C16 hydrocarbons (including cycloalkanes, aliphatic alkanes and aromatic hydrocarbons) by using RANEY® nickel as the catalysts, which meet basic specifications of conventional jet fuels.
2. Experimental
2.1 Materials
Low density polyethylene (LDPE) (CAS number 9002-88-4) in the form of pellets was purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA). The density and melting point of LDPE are 0.925 g cm−3 at ambient temperature and 116 °C, respectively. Parent ZSM-5 (SiO2/Al2O3 mole ratio: 50) was purchased from Zeolyst International, USA. RANEY® Ni 4200 (slurry in water) in an activated form was supplied by Sigma-Aldrich Corporation (St. Louis, MO, USA). Toluene (99.7%), p-xylene (99%), ethylbenzene (99%), n-octane (98%), n-propylbenzene (98%), indane (95%), n-nonane (99%), naphthalene (99.6%), 1,2,3,4-tetrahydronaphthalene (97%), decahydronaphthalene (98%), n-decane (99%), 1-methylnaphthalene (96%), 2-methylnaphthalene (97%), n-undecane (99%), n-dodecane (99%), anthracene (99%), n-tridecane (98%), n-tetradecane (99%), n-pentadecane (99%), and n-hexadecane (99%) were used as purchased from Alfa Aesar (Ward Hill, MA, USA). 1,2-Dimethylcyclohexane (99%), 1,3-dimethylcyclohexane (99%), 1,4-dimethylcyclohexane (99%), ethylcyclohexane (99%), 1,2,4-trimethylbenzene (98%), 1,2,4-trimethylcyclohexane (97%), propylcyclohexane (99%), and hexahydroindan (99%) were supplied by Sigma-Aldrich Corporation (St. Louis, MO, USA).2.2 Catalyst preparation
The activity of parent ZSM-5 was improved by suffering both hydrothermal and calcined treatments. Under the gentle stirring, parent ZSM-5 powder was added into deionized water (mass ratio = 1) at 60 °C. After addition, the mixture was kept on stirring for 2 h under this condition. The slurry was then dried at 105 °C till constant weight. The sequential process was the catalyst calcination: hydrothermally treated ZSM-5 was calcined at 550 °C for 5 h in a muffle furnace. The catalysts were pelletized and sieved to 20–40 mesh. The main characteristics of the catalyst were reported in our previous study.17,25RANEY® nickel is notorious for its pyrophoricity, and it may ignite spontaneously when dried in air. The RANEY® nickel 4200 (slurry in water) was thus dried at 60 °C till constant weight in the atmosphere of nitrogen to avoid contact with air, prior to the subsequent catalytic test. The textural properties of as-received RANEY® Ni 4200 catalyst and the SEM image are outlined in Table 1 and Fig. 1, respectively.
| SBET (m2 g−1) | Vpore (cm3 g−1) | Spore (m2 g−1) | dpore nm | |
|---|---|---|---|---|
| a SBET: BET surface area; Vpore: pore volume; Spore: pore surface area; dpore: average pore size. | ||||
| RANEY®-Ni catalyst | 38.1 | 0.112 | 43.0 | 10.4 |
2.3 Catalytic microwave-assisted degradation of low-density polyethylene (LDPE)
The schematic diagram of the microwave-assisted degradation system integrated with the catalysis process is shown in Fig. S1.† Detailed experimental setting was described in our previous studies.17,29 Fixed loading of low-density polyethylene pellets (100 g) for each run were placed in a 500 mL quartz flask inside the microwave oven (Shanghai, China) by a constant microwave power setting (700 W). 0.5 g of activated carbon powder was used as the absorber for the microwave-assisted degradation. All reactions of microwave degradation were conducted at the temperature of 350 °C for 20 min until all LDPE pellets were completely vaporized. The pyrolytic volatile vapors from the flask passed through a packed bed catalysis reactor which was filled with catalyst. As previous work reported, the optimal condition to maximize the liquid yield was set at 375 °C.17 In this regard, the catalytic temperature was hold at 375 °C and catalyst (10 g) to feed ratio was kept constantly at 0.1. The catalytic microwave degradation was duplicated for four times in order to gain abundant liquid organics which were thereafter combined together for the hydrogenated process.2.4 Hydrotreatment of liquid organics derived from catalytic microwave degradation
To saturate the liquid organics evolved from catalytic microwave degradation, a closed reaction system with a stirred stainless batch reactor of the 4592 micro stirred reactor (with a 50 mL vessel) and a 4848 reactor controller from Parr Instrument Company (Moline, IL, USA) was used (Fig. S2†). The liquid organics (8 g) and the catalysts with intended ratio were loaded into the reactor. Then the reactor was sealed and vented for five times with hydrogen to get rid of the air present in the vessel. Hydrogen was subsequently adjusted to reach the set pressure. The automatic controller was employed to control the temperature and the revolution of stirrer (100 rpm). The pressure inside the reactor was recorded and the reactions proceeded at a set temperature for the intended time. After the experiment finished, stirring was stopped and the reactor was rapidly cooled to ambient temperature. Then, the gas was collected for analysis and the reactor was depressurized. Consequently the liquid product was filtered to remove catalyst particles.2.5 Analytical techniques
Elemental analysis of LDPE pellets, liquid samples, char, and coke deposited on spent catalysts was conducted using a 2400 Series II CHN/O Elemental Analyzer (PerkinElmer, USA).The chemical composition of the bio-oils was characterized and qualified by Agilent 7890A GC-MS (GC-MS; GC, Agilent 7890A; MS, Agilent 5975C) with a DB-5 capillary column. The GC was first programmed to heat to 45 °C for 3 min followed by heating to 300 °C at a rate of 10 °C min−1. The injection sample size was 1 μL. The flow rate of the carrier gas (helium) was 0.6 mL min−1. The ion source temperature was 230 °C for the mass selective detector. Compounds were identified by comparing the spectral data with that in the NIST Mass Spectral library. The area percent of changed concentrations of model compounds obtained from GC/MS results was utilized to predict product concentration in bio-oils.
The gaseous product was collected in a 1 L Tedlar gas bag and then offline analyzed by an INFICON 3000 Micro-GC (INFICON Inc., Santa Clara, CA, USA) system with a thermal conductivity detector (TCD). A standard gas mixture consisting of H2, N2, CH4, CO, CO2, C2H4, C2H6, and C3H6 was used to calibrate the yield of non-condensable gas. Alkanes and olefins (>C4) in gas samples were either not detected or negligible in this research.
2.6 Experimental methods and data evaluation
A central composite experimental design (CCD) was employed to optimize the process conditions and the yield of jet fuel range alkanes. The initial pressure of hydrogen (X1, psi) and catalyst to reactant ratio (X2) were chosen as independent variables as illustrated in Table 2. In these experiments, the mass of catalyst varied from 0.08 to 0.72 g (with respect to the ratio from 0.01 to 0.09), while the initial pressure ranged from 76 to 924 psi.| Entry | Initial pressure (psi) | Catalyst to reactant ratio | Yieldb (area in %) | ||||
|---|---|---|---|---|---|---|---|
| AA | CA | HAH | AH | Others | |||
| a Reaction conditions: reaction temperature, 150 °C; reaction time, 1 h.b AA: aliphatic alkanes; CA: cyclic alkanes; HAH: hydro-aromatic hydrocarbons; AH: aromatic hydrocarbons. | |||||||
| H-1 | 200 | 0.02 | 17.13 | 2.61 | 12.05 | 67.05 | 1.16 |
| H-2 | 200 | 0.08 | 18.00 | 3.05 | 11.17 | 67.05 | 0.73 |
| H-3 | 800 | 0.02 | 23.19 | 12.70 | 16.80 | 45.80 | 1.51 |
| H-4 | 800 | 0.08 | 23.09 | 27.23 | 11.76 | 36.74 | 1.18 |
| H-5 | 76 | 0.05 | 18.01 | 3.76 | 10.96 | 66.48 | 0.79 |
| H-6 | 924 | 0.05 | 21.68 | 16.57 | 9.56 | 50.56 | 1.63 |
| H-7 | 500 | 0.05 | 18.58 | 6.94 | 13.81 | 59.37 | 1.30 |
| H-8 | 500 | 0.05 | 18.69 | 7.81 | 14.37 | 58.12 | 1.01 |
| H-9 | 500 | 0.05 | 19.11 | 7.23 | 14.18 | 58.51 | 0.97 |
| H-10 | 500 | 0.05 | 18.99 | 7.15 | 14.47 | 58.46 | 0.93 |
| H-11 | 500 | 0.05 | 19.02 | 7.03 | 14.22 | 58.65 | 1.08 |
| H-12 | 500 | 0.01 | 18.10 | 5.78 | 14.09 | 60.20 | 1.83 |
| H-13 | 500 | 0.09 | 19.56 | 9.91 | 13.94 | 55.01 | 1.58 |
The coke mass was determined by the difference before and after catalytic degradation. The weight of non-condensable gas was calculated using the following equation:
| Gas mass = LDPE mass − liquid mass − char mass − coke mass | (1) |
Overall carbon yields of the liquid, gas, and solid products and carbon selectivity of a specific product were calculated based on the following equations.
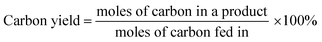 | (2) |
 | (3) |
3. Results and discussion
3.1 Catalytic transformation of LDPE into liquid organics
In order to manufacture advanced jet fuels, the transformation of LDPE into the precursors of jet fuels in the carbon number range from 8 to 16 is primarily required. Based on our previous study for catalytic microwave degradation of LDPE,17 optimal catalytic temperature set at 375 °C could maximize the yield of liquid organics. Henceforth, the LDPE was subjected to the optimized catalytic temperature. It was found that the liquid organics from catalytic microwave degradation of LDPE was 64.41 ± 5.20 wt%. There have never been any results could achieve such high yield organics.4,12 The mild catalytic temperature and sufficient catalyst loading could favor the conversion rate of waxes from direct thermal degradation into the lumps of liquid organics through catalytic cracking, cyclization, and oligomerization reactions. The overall carbon yield distribution from catalytic microwave degradation of LDPE is shown in Fig. 2(A). It is noteworthy that the share of liquid organics accounted for the dominant composition, occupying 66.18%; while both the minor carbon yield of coke and char were approximately 1%, which is as low as our aforementioned result.17 It is also noted that the low carbon yield (32.01%) of non-condensable gas was calculated by difference. The gas consisted of ethylene, ethane, hydrogen, and methane, originating from the large extent of cracking and oligomerization reactions. Comparing with the feedstock using lignocellulosic biomasses in the system of catalytic pyrolysis,29,30 the carbon yield of non-condensable gas derived from the feedstock of plastics was relatively low.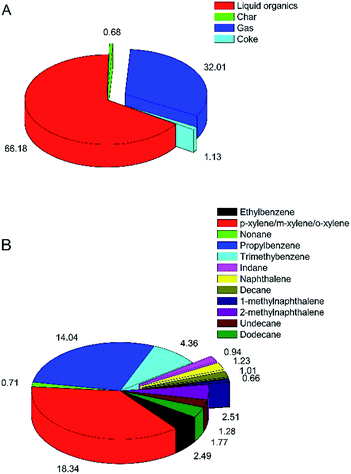 | ||
| Fig. 2 Overall carbon yield distribution (C mol%), (A) and carbon selectivity of main chemical compounds (C mol%), (B) in the liquid organics from catalytic microwave degradation of LDPE. | ||
The carbon selectivity of main chemical compounds in the liquid organics from catalytic microwave degradation of LDPE is depicted in Fig. 2(B). In general, the typical compositions have been categorized into aromatic hydrocarbons, olefins, alkanes; and partial chemicals in the organics were quantified. Carbon selectivity towards xylenes (C8H10) was 18.34%, which was the dominant part in the liquid organics. Likewise, the large amount of propylbenzene (C9H12) was also obtained, displaying remarkable carbon selectivity (14.04%). Consequently, mono-cyclic aromatic hydrocarbons in the jet fuel range were preferentially formed over the well-promoted ZSM-5 catalyst with regard to aliphatic hydrocarbons, which can be ascribed to the fast diffusion of the intermediates toward the active sites inside the micropores. A small amount of double-cyclic aromatic hydrocarbons including naphthalene and its derivatives was evolved from the oligomerization and polymerization reactions of mono-cyclic aromatic hydrocarbons.31 Catalytic cracking of waxes from thermal degradation of LDPE also gave rise to small amounts of aliphatic hydrocarbons. The results imply that the products gained after catalytic microwave degradation of LDPE at 375 °C is mostly made up of hydrocarbons in the jet fuel range (C8–C16).
3.2 Hydrotreatment of liquid organics derived from catalytic microwave degradation
The experimental design and product yield distribution of hydrotreated liquid organics are summarized as a function of initial pressure and catalyst to reactant ratio in Table 2. It was observed that remaining aromatic hydrocarbons were in the range from 36.74 to 67.05% depending on alterations of reaction conditions. The high amounts of aromatic hydrocarbons retained are due in part to the mild reaction conditions, such as low reaction temperature and reaction time. These results also indicate that the initial pressure and catalyst to reactant ratio had a slight influence in the total amounts of aliphatic alkanes; while the enhancement in the two variables led toward a parallel increase in the yields of cycloalkanes. The optimal condition for maximum yields of saturated hydrocarbons was found to be the initial pressure of 800 psi and the catalyst to reactant ratio of 0.08. The corresponding amount of cycloalkanes reached at the highest selectivity (27.23%), whereas the yield of aromatic hydrocarbon dropped to 36.74%. Thus, these augments of cycloalkanes took place mostly at the expense of the aromatic hydrocarbon because its share decreased. As such, increasing the initial pressure and catalyst to reactant ratio contributed to improving the yield of hydro-aromatic hydrocarbons. These results are consistent with hydrogenation reactions predominantly occurring under low-severity conditions.32 Since these high amounts of aromatic hydrocarbon remained in the hydrotreated organics cannot be straightforward utilized as jet fuels, further efforts for converting aromatic hydrocarbon into saturated hydrocarbons containing aliphatic and cyclic alkanes should be made.
| Temperature (°C) | |||
|---|---|---|---|
| 150 | 175 | 200 | |
| a Reaction condition: initial pressure, 500 psi; RANEY® Ni catalyst, 5 wt% with respect to reactant mass. | |||
| Overall selectivity (% in area) | |||
| Aliphatic alkanes | 18.88 | 22.91 | 25.12 |
| Cycloalkanes | 7.23 | 24.70 | 29.37 |
| Hydro-aromatic hydrocarbons | 14.21 | 11.05 | 11.42 |
| Aromatic hydrocarbons | 58.62 | 39.66 | 32.71 |
| Others | 1.06 | 1.68 | 1.38 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Alkanes selectivity (C mol%) | |||
| 1,4-Dimethylcyclohexane | — | 4.16 | 6.63 |
| 1,3-Dimethylcyclohexane | — | 1.63 | 2.05 |
| 1,2-Dimethylcyclohexane | — | 1.33 | 2.12 |
| Ethylcyclohexane | 2.78 | 1.83 | 5.78 |
| Octane | 0.91 | 1.22 | 1.58 |
| 1,2,4-Trimethylcyclohexane | 0.20 | 0.69 | 0.35 |
| Propylcyclohexane | 1.24 | 5.73 | 4.55 |
| Nonane | 0.89 | 1.41 | 1.98 |
| Hexahydroindan | 0.54 | 2.00 | 2.18 |
| Decalin | 0.11 | 0.53 | 0.55 |
| Decane | 1.12 | 2.06 | 2.31 |
| Undecane | 1.45 | 2.30 | 2.04 |
| Dodecane | 1.72 | 2.34 | 3.12 |
| Tridecane | 1.26 | 1.43 | 1.58 |
| Tetradecane | 1.28 | 1.47 | 1.73 |
| Pentadecane | 1.17 | 1.40 | 1.64 |
| Hexadecane | 1.32 | 1.58 | 1.71 |
On the other hand, the total amounts of aromatic hydrocarbons experienced a gradually declined tendency as the reaction temperature went up to 200 °C. The reduction of aromatic hydrocarbons was hydrogenated into cycloalkanes and aliphatic alkanes via hydrogenation and hydrocracking reactions. It is noted that the share of hydro-aromatic hydrocarbons was not significantly impacted by the reaction temperature. Although some aromatic hydrocarbons were hydrogenated into hydro-aromatic hydrocarbon, these hydro-aromatic hydrocarbons as the intermediates were simultaneously converted into saturated hydrocarbons. In the gas fraction, unreacted hydrogen was detected at the end of reaction accompanied with minor volume of small hydrocarbons (such as methane and ethane), implying that the experiments were not carried out under hydrogen starved conditions. These gaseous hydrocarbons detected could be produced from the hydrocracking of liquid hydrocarbons. These results suggest that not only hydrogenation reactions but also hydrocracking reactions could take place at high reaction temperature.
Reaction temperatures also had a significant effect on the carbon selectivity of specific alkanes including aliphatic and cyclic alkanes. It was noteworthy that the carbon selectivity of mono-cyclic alkanes significantly increased as the reaction temperature increased, especially from 150 to 175 °C. Meanwhile, 1,4-dimethylcyclohexane (C8H16) and propylcyclohexane (C9H18) gradually increased to 4.16% and 5.73%, which indicates that the reaction temperature above 175 °C presumably accelerated the hydrogenation rate of the corresponding aromatic hydrocarbons. For the carbon selectivity of ethylcyclohexane (C8H16), the enhancement of reaction temperature from 175 to 200 °C led toward a remarkable increase. The carbon selectivity of polycyclic alkanes (e.g. decalin) was slightly impacted by the increment of reaction temperature. There were small upward tendencies towards carbon selectivity of hexahydroindan (C9H16) and decalin (C10H18) in the range from 150 to 200 °C. This fact was possibly ascribed to the low amounts of double-ring aromatic hydrocarbon in the liquid organic from catalytic microwave degradation; and it is very tough for the double-ring aromatic hydrocarbons to be hydrogenated into saturated hydrocarbon below 200 °C. Likewise, the carbon selectivity of aliphatic alkanes was also affected by the reaction temperature. The carbon selectivity of aliphatic alkanes, such as octane (C8H18) and nonane (C9H20), showed a steady increment as the reaction temperature grew. These outcomes imply that the increment of aliphatic alkanes was attributed to hydrogenation of aliphatic olefins and hydrocracking of cycloalkanes simultaneously occurring at the high temperature range.12
Nevertheless, the optimal result (over 30% of aromatic hydrocarbons plus approximately 10% hydro-aromatic hydrocarbons remained in the hydrotreated organics) cannot meet the specifications of conventional jet fuels, especially for advanced jet fuels. Other variable (e.g. reaction time) should be investigated to obtain higher valuable fuels that can directly be used as conventional jet fuels or drop-in fuels. Apart from the aforementioned variables, reaction time was another crucial factor that influenced product distribution and chemicals' carbon selectivity.17 Table 4 summarizes products distribution and partial alkanes' carbon selectivity with regard to reaction temperature at the reaction time of 2 h. In particular, the production distribution prominently shifted towards the cycloalkanes (31.23–53.06%) with increasing reaction temperature to 250 °C, suggesting that hydrogenation reactions were enhanced because of the longer reaction duration and higher reaction temperature. The amount of cycloalkanes in the hydrotreated organics at 250 °C was equal to that in JP-5. It was noticeable that the total amount of aliphatic alkanes appeared to generally increase to 31.23% at 250 °C, whose amount was similar with the content (30.8%) in conventional JP-5.23 Unlike the saturated hydrocarbons, the total amounts of aromatic hydrocarbon and hydro-aromatic hydrocarbons distantly declined, particularly from 200 to 250 °C. The total amounts of hydro-aromatic hydrocarbons and aromatic hydrocarbons were below 15% at 250 °C, which satisfies the limited ceiling of aromatic hydrocarbons in Jet-A and JP-8. It is noteworthy to point out that this remarkable drop in the amount of aromatics is a positive effect for the quality of JP-5 fraction provided that the legislation limit (15.9%) is not surpassed. These results indicate that the obtained liquid products at 250 °C for 2 h can be directly used as alternatives for the formulation of JP-5 navy fuel.
| Temperature (°C) | |||
|---|---|---|---|
| 150 | 200 | 250 | |
| a Reaction condition: initial pressure, 500 psi; RANEY® Ni catalyst, 5 wt% with respect to reactant mass. | |||
| Overall selectivity (% in area) | |||
| Aliphatic alkanes | 21.12 | 26.37 | 31.23 |
| Cycloalkanes | 31.13 | 40.18 | 53.06 |
| Hydro-aromatic hydrocarbons | 9.81 | 10.84 | 7.22 |
| Aromatic hydrocarbons | 36.19 | 21.12 | 7.78 |
| Others | 1.76 | 1.49 | 0.71 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Alkanes selectivity (C mol%) | |||
| 1,4-Dimethylcyclohexane | 8.40 | 8.18 | 9.45 |
| 1,3-Dimethylcyclohexane | 1.79 | 2.02 | 3.04 |
| 1,2-Dimethylcyclohexane | 3.77 | 2.98 | 4.21 |
| Ethylcyclohexane | 2.34 | 1.62 | 3.88 |
| Octane | 1.01 | 0.93 | 1.84 |
| 1,2,4-Trimethylcyclohexane | 0.25 | 1.05 | 2.92 |
| Propylcyclohexane | 5.75 | 9.80 | 10.21 |
| Nonane | 1.22 | 0.98 | 1.45 |
| Hexahydroindan | 1.81 | 1.34 | 2.02 |
| Decalin | 0.66 | 0.76 | 0.87 |
| Decane | 1.54 | 2.00 | 2.77 |
| Undecane | 2.43 | 3.45 | 4.21 |
| Dodecane | 2.21 | 3.80 | 4.56 |
| Tridecane | 1.45 | 2.08 | 2.79 |
| Tetradecane | 1.47 | 2.08 | 2.92 |
| Pentadecane | 1.44 | 2.04 | 3.01 |
| Hexadecane | 1.51 | 2.22 | 3.33 |
The carbon selectivity of mono-cyclic alkanes significantly increased at the reaction time of 2 h, especially for propylcyclohexane, as the reaction temperature was elevated; while the carbon selectivity for hexahydroindan and decalin slightly went up in the range of 150–250 °C. Even if naphthalenes can be converted into corresponding cycloalkanes under this condition,27 the low amounts of naphthalenes could make the slight variation of double-cyclic alkanes. Meanwhile, the high reaction temperature (250 °C) could allow the double-cyclic alkanes to be hydrocracked and oligomerized, forming monocyclic alkanes or their derivatives. As expected, the carbon selectivity of main linear alkanes also increased with the augment of reaction temperature. From the optimal result at 250 °C, up to 26.88% of the total carbon selectivity towards jet fuel range linear alkanes (C8–C16) was achieved. The high purity of the linear alkanes provides an excellent performance of the hydrotreated organics as the replacement of jet fuels. In terms of gaseous results at 250 °C, a trace volume of small-chain hydrocarbons were found, suggesting that the hydrocracking and oligomerization reaction have taken place in the process. Wherefore, prolonging the reaction time can principally enhance the hydrogenation reactions; hydrocracking and oligomerization reactions might also occur jointly at the high reaction temperature.
3.3 Reaction pathway for the conversion of plastics (LDPE) into JP-5 navy fuel
These observations are the key point to propose the reaction pathway for the conversion of plastics (LDPE) into JP-5 navy fuel. Based on the quantified products distribution in this study, and related results from catalytic microwave-induce degradation of LDPE as a function of several variables,17 the overall reactions network (including catalytic microwave degradation and hydrotreating process) is shown in Fig. 5. Thermal degradation of polyethylene has been reported to take place through two mechanisms: (1) random scission to yield long-chain hydrocarbons; and (2) chain-end scission of the oligomers to yield low-molecular weight products.33,34 The two above mentioned mechanisms took place simultaneously, giving rise to free radicals along with the carbon chains; thereafter the cleavage of the molecule caused the formation of a molecule with an unsaturated end and the other with a terminal free radical.35 Consequently the radical fragments were converted into straight chain dienes, alkenes and alkanes through hydrogen transfer reactions.35The volatiles subsequently underwent catalytic cracking over ZSM-5 through two carbocationic mechanisms.33,36 Once these carbocations were generated, various acid-catalyzed reaction could take place over the acid sites, including aromatization, cyclization, cracking, isomerization, and oligemerization.12 Aromatization and cyclization reactions proceed by the way of hydrogen transfer reactions, whilst cracking reactions usually occurs by means of β-scission reactions. Herein, lager intermediate olefins derived from the thermal degradation could not enter ZSM-5 micropores where the majority of the active acid sites were located. The macromolecules were degraded on the active sites on the external surface of the zeolite crystallites; small fractions relatively diffused into the zeolite micropores and further reacted on the internal sites.37 Moreover, the intermediate olefins stepwise underwent oligomerization, cyclization and hydrogen transfer reactions, contributing to forming aromatic hydrocarbons.38 Eventually the liquid organic with high quality of aromatics and aliphatic olefins were obtained when the suitable loading of well-promoted ZSM-5 catalyst was introduced.
It was observed that different reactions took place in the hydroreforming experiments of liquid organics from the LDPE thermal degradation, such as hydrogenation, hydrocracking, and hydroisomerization.4 Since the liquid organics resulting from the catalytic microwave degradation step in mostly produced by a mixture of aromatic hydrocarbons, aliphatic olefins, and alkanes, hydrogenation of the alkenes is the one of the first step to take place.4 When the much higher-severity conditions were employed, the aromatic hydrocarbons were partially hydrogenated into cycloalkanes or hydro-aromatic hydrocarbons. Likewise, the total amounts of aliphatic alkanes were enhanced because the formed cycloalkanes could further undergo the hydrocracking to generate the aliphatic alkanes. According to the gaseous fraction, hydrocracking of liquid hydrocarbons over the metal sites, leading toward methane and ethane, should occur. However, the gaseous small hydrocarbons in all reactions carried out were practically negligible.
4. Conclusions
This study illustrated that the microwave-induced degradation followed by hydrotreating process is a profound approach for the production of jet fuels from plastics (LDPE). Diverse variables (initial pressure, catalyst to reactant ratio, reaction temperature, and reaction time) were taken consideration to manufacture advanced jet fuels. In the first step of microwave-induced degradation of LDPE over well-promoted ZSM-5 catalyst, the overall carbon yield of liquid organics was 66.18% at the catalytic temperature of 375 °C with the catalyst to reactant ratio of 0.1; while the carbon yields of both coke and char were approximately 1%. Such high carbon yield of liquid organics could improve the feasibility of the process by using waste plastics as the feedstock in an industrial scale. Furthermore, the trace yield of coke deposited on the catalyst assisted in the promotion of the catalyst lifetime (results not shown), which could significantly reduce the operating cost of the catalyst in a biorefinery.After the hydrotreating process by using RANEY® nickel as the catalyst, the recovery of the hydrotreated organics could reach more than 95 wt% in all experiments. It was observed that the optimal reaction for the production of advanced jet fuels was conducted at the reaction temperature of 250 °C for 2 h. The total amounts of aliphatic alkanes (31.23%), cycloalkanes (53.06%), and aromatic hydrocarbons (15.00%) in the jet fuel range were obtained under the mild reaction condition. The outcomes indicate that the specifications of hydrotreated organics are equal to those of JP-5 navy fuel, which can be directly used as the alternatives or additives for JP-5 navy fuel. From this perspective, the catalytically integral processes of waste plastics by using inexpensive catalysts deliver a novel and feasible pathway in a biorefinery, specifically targeting JP-5 navy fuel.
Acknowledgements
This study was supported partially by the Joint Center for Aerospace and Technology Innovation (JCATI), The Agriculture and Food Research Initiative of National Institute of Food and Agriculture, United States Department of Agriculture (Award Number: 2015-67021-22911; Grant Number: 2015-11888712) and Chinese Scholarship Council. We are grateful to Dr Aftab Ahamed and Dr Xin Li for helping us run GCMS measurements and Dr Valerie Lynch-Holm from Franceschi Microscopy & Imaging Center (FMIC), Washington State University for the help of SEM training.Notes and references
- A. S. Burange, M. B. Gawande, F. L. Y. Lam, R. V. Jayaram and R. Luque, Green Chem., 2015, 17, 146–156 RSC.
- G. S. Miguel, D. P. Serrano and J. Aguado, Ind. Eng. Chem. Res., 2009, 48, 8697–8703 CrossRef.
- J. R. Jambeck, R. Geyer, C. Wilcox, T. R. Siegler, M. Perryman, A. Andrady, R. Narayan and K. L. Law, Science, 2015, 347, 768–771 CrossRef CAS PubMed.
- J. M. Escola, J. Aguado, D. P. Serrano, L. Briones, J. L. Díaz de Tuesta, R. Calvo and E. Fernandez, Energy Fuels, 2012, 26, 3187–3195 CrossRef CAS.
- M.-H. Cho, S.-H. Jung and J.-S. Kim, Energy Fuels, 2010, 24, 1389–1395 CrossRef CAS.
- A. López, I. de Marco, B. M. Caballero, M. F. Laresgoiti, A. Adrados and A. Aranzabal, Appl. Catal., B, 2011, 104, 211–219 CrossRef.
- C. Dorado, C. A. Mullen and A. A. Boateng, ACS Sustainable Chem. Eng., 2014, 2, 301–311 CrossRef CAS.
- M. Artetxe, G. Lopez, M. Amutio, G. Elordi, J. Bilbao and M. Olazar, Ind. Eng. Chem. Res., 2013, 52, 10637–10645 CrossRef CAS.
- G. S. Miguel, D. P. Serrano and J. Aguado, Ind. Eng. Chem. Res., 2009, 48, 8697–8703 CrossRef.
- M. d. R. Hernández, Ä. N. García, A. Gómez, J. Agulló and A. Marcilla, Ind. Eng. Chem. Res., 2006, 45, 8770–8778 CrossRef.
- I. Baraniec-Mazurek and A. Mianowski, Chem. Eng. J., 2010, 163, 284–292 CrossRef CAS.
- D. P. Serrano, J. Aguado and J. M. Escola, ACS Catal., 2012, 2, 1924–1941 CrossRef CAS.
- J. M. Escola, J. Aguado, D. P. Serrano and L. Briones, Waste Manage., 2014, 34, 2176–2184 CrossRef CAS PubMed.
- A. Marcilla, M. I. Beltrán, F. Hernández and R. Navarro, Appl. Catal., A, 2004, 278, 37–43 CrossRef CAS.
- D. Iribarren, J. Dufour and D. P. Serrano, J. Mater. Cycles Waste Manage., 2012, 14, 301–307 CrossRef CAS.
- R. Bagri and P. T. Williams, J. Anal. Appl. Pyrolysis, 2002, 63, 29–41 CrossRef CAS.
- X. Zhang, H. Lei, G. Yadavalli, L. Zhu, Y. Wei and Y. Liu, Fuel, 2015, 144, 33–42 CrossRef CAS.
- J. Q. Bond, A. A. Upadhye, H. Olcay, G. A. Tompsett, J. Jae, R. Xing, D. M. Alonso, D. Wang, T. Zhang, R. Kumar, A. Foster, S. M. Sen, C. T. Maravelias, R. Malina, S. R. H. Barrett, R. Lobo, C. E. Wyman, J. A. Dumesic and G. W. Huber, Energy Environ. Sci., 2014, 7, 1500–1523 CAS.
- R. Xing, A. V. Subrahmanyam, H. Olcay, W. Qi, G. P. van Walsum, H. Pendse and G. W. Huber, Green Chem., 2010, 12, 1933 RSC.
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044–4098 CrossRef CAS PubMed.
- H. A. Meylemans, L. C. Baldwin and B. G. Harvey, Energy Fuels, 2013, 27, 883–888 CrossRef CAS.
- C. Zhao, Y. Kou, A. A. Lemonidou, X. Li and J. A. Lercher, Angew. Chem., 2009, 48, 3987–3990 CrossRef CAS PubMed.
- D. E. Keil, D. A. Warren, M. J. Jenny, J. G. EuDaly, J. Smythe and M. M. Peden-Adams, Toxicol. Sci., 2003, 76, 347–356 CrossRef CAS PubMed.
- L. Wang, H. Lei, S. Ren, Q. Bu, J. Liang, Y. Wei, Y. Liu, G.-S. J. Lee, S. Chen, J. Tang, Q. Zhang and R. Ruan, J. Anal. Appl. Pyrolysis, 2012, 98, 194–200 CrossRef CAS.
- X. Zhang, H. Lei, L. Wang, L. Zhu, Y. Wei, Y. Liu, G. Yadavalli and D. Yan, Green Chem., 2015, 17, 4029–4036 RSC.
- X. Li, H. Zhang, J. Li, L. Su, J. Zuo, S. Komarneni and Y. Wang, Appl. Catal., A, 2013, 455, 114–121 CrossRef CAS.
- X. Zhang, H. Lei, L. Zhu, J. Wu and S. Chen, Green Chem., 2015, 17, 4736–4747 RSC.
- X. Zhang, H. Lei, L. Zhu, Y. Wei, Y. Liu, G. Yadavalli, D. Yan, J. Wu and S. Chen, Fuel, 2015, 160, 375–385 CrossRef CAS.
- L. Wang, H. Lei, J. Lee, S. Chen, J. Tang and B. Ahring, RSC Adv., 2013, 3, 14609–14615 RSC.
- H. Lei, S. Ren, L. Wang, Q. Bu, J. Julson, J. Holladay and R. Ruan, Bioresour. Technol., 2011, 102, 6208–6213 CrossRef CAS PubMed.
- T. R. Carlson, J. Jae, Y.-C. Lin, G. A. Tompsett and G. W. Huber, J. Catal., 2010, 270, 110–124 CrossRef CAS.
- R. Feiner, N. Schwaiger, H. Pucher, L. Ellmaier, M. Derntl, P. Pucher and M. Siebenhofer, RSC Adv., 2014, 4, 34955 RSC.
- M. Artetxe, G. Lopez, M. Amutio, G. Elordi, J. Bilbao and M. Olazar, Chem. Eng. J., 2012, 207–208, 27–34 CrossRef CAS.
- M. Artetxe, G. Lopez, G. Elordi, M. Amutio, J. Bilbao and M. Olazar, Ind. Eng. Chem. Res., 2012, 51, 13915–13923 CrossRef CAS.
- D. P. Serrano, J. Aguado, J. M. Escola, J. M. Rodríguez and G. San Miguel, J. Anal. Appl. Pyrolysis, 2005, 74, 370–378 CrossRef CAS.
- A. Lopez-Urionabarrenechea, I. de Marco, B. M. Caballero, M. F. Laresgoiti and A. Adrados, J. Anal. Appl. Pyrolysis, 2012, 96, 54–62 CrossRef CAS.
- G. Manos, A. Garforth and J. Dwyer, Ind. Eng. Chem. Res., 2000, 39, 1198–1202 CrossRef CAS.
- A. Marcilla, M. I. Beltrán and R. Navarro, Energy Fuels, 2008, 22, 2917–2924 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra25327f |
| This journal is © The Royal Society of Chemistry 2016 |




