Intermolecular interactions in dictating the self-assembly of halogen derivatives of bis-(N-substituted oxamato)palladate(II) complexes†‡
Francisco Ramón Fortea-Péreza,
Nadia Marinob,
Giovanni de Munnob,
Donatella Armentano*b,
Miguel Julvea and
Salah-Eddine Stiriba*ac
aInstituto de Ciencia Molecular (ICMol), Universitat de València, C/ Catedrático José Beltrán 2, 46980 Paterna, València, Spain. E-mail: salah.stiriba@uv.es
bDipartimento di Chimica e Tecnologie Chimiche, Università della Calabria, 87036 Rende, Cosenza, Italy. E-mail: donatella.armentano@unical.it
cEquipe de Chimie Moléculaire, Matériaux et Modélisation-C3M, Faculté Polydisciplinaire de Safi, Université Cadi Ayyad, Safi, Morocco
First published on 7th January 2016
Abstract
Three palladium(II) complexes of formula (n-Bu4N)2[Pd(4-Fpma)2] (1), (n-Bu4N)2[Pd(4-Clpma)2]·4H2O (2) and (n-Bu4N)2[Pd(4-Brpma)2]·4H2O (3) [n-Bu4N+ = tetra-n-butylammonium cation, 4-Fpma = N-4-fluorophenyloxamate, 4-Clpma = N-4-chlorophenyloxamate and 4-Brpma = N-4-bromophenyloxamate] have been prepared and their structures determined by single crystal X-ray diffraction. Each palladium(II) ion in 1–3 is four-coordinate with two oxygen and two nitrogen atoms from two fully deprotonated oxamate ligands building PdO2N2 square planar surroundings, the oxamate ligands exhibiting trans (1 and 2) and cis (3) dispositions. The fluoro substituent and the organic counterion in 1 are involved in C–H⋯F type interactions whereas the anionic complex assembly is driven by C–H⋯Ooxamate interactions. The structures of 2 and 3 shed light on the effects of the different chemical nature of chloro and bromo substituents on the C–H⋯X interactions joined to hydrogen bonds involving oxamate oxygen atoms and crystallization water molecules. The complex anions in 2 are interlinked by water chains to afford a supramolecular two-dimensional network where Ow⋯Ow, Ow⋯Ooxamate and Ow⋯Cl hydrogen bonds are involved. The reduction of the polarity of C–Br bonds with respect to C–X (X = F, Cl) is very likely to be one of the key reasons allowing for the cis-arrangement of the two 4-Brpma ligands around each palladium(II) ion of the anionic complex in 3 in water. These units are interconnected by hydrogen bonds between the peripheral oxamato-oxygens and a four-membered ring of water molecules leading to supramolecular cationic chains extending along the crystallographic a axis.
Introduction
Since the late 1990s, it was evident that other intermolecular forces than hydrogen bonds, namely halogen atom interactions, could be used as robust design elements in crystal engineering.1–8 Although the understanding of the competitiveness and/or cooperativeness between such intermolecular forces to assemble molecules in both chemistry and biology is still not well-understood, a structural competition between halogen and hydrogen bonds9,10 has recently been detected, pointing out the great difficulty in defining the hierarchy of these two intermolecular interactions whose strength and directionality are comparable.2 Besides creating fundamental advances in understanding such forces in driving the formation of complex architectures, the main interest in such studies relies on the evidence that the properties of a given material deeply depend on the arrangement of either organic or inorganic entities in the solid state.11Supramolecular motifs that can be achieved by the arrangement of mononuclear complexes as building units via intermolecular forces (hydrogen bonds and stacking interactions, for instance) have experienced a rapid growth during the last decades. This increasing interest has been driven by the large variety of supramolecular assemblies and molecular architectures with desired properties that can be achieved based on such directing intermolecular forces, contributing to the development of the crystal engineering.12–25 A careful choice of the ligands, metal centres, spacers and reaction conditions are required in order to gain specific control over the topology of the resulting frameworks.18,19
Herein, we report on the supramolecular features on three non-isostructural compounds of formula (n-Bu4N)2[Pd(4-Fpma)2] (1), (n-Bu4N)2[Pd(4-Clpma)2]·4H2O (2) and (n-Bu4N)2[Pd(4-Brpma)2]·4H2O (3) (n-Bu4N+ = tetra-n-butylammonium cation), containing N-4-Xphenyloxamate ligands (4-Xpma with X = F, Cl and Br), where the two fragments (oxamate and Xphenyl) can be involved in hydrogen-bonding or supramolecular halogen⋯halogen interactions. The charge balance is ensured by bulky tetra-n-butylammonium cation for which it is reasonable to assume that electrostatic forces dominate conventional intermolecular interactions of the C–H⋯O and/or C–H⋯X type.26
The reported results are related to our current studies on oxamato-containing metal complexes envisaging the rational design of molecule-based multifunctional materials.27–29 Very recently, we have isolated novel palladium(II) complexes with N-substituted oxamate ligands and proved their activity in the sustainable palladium(II)-catalysed carbon–carbon coupling reactions, namely Suzuki and Heck reactions.30,31 Their easy and cheap preparation, environmentally benign character and, furthermore, cytotoxic activity against leukemia cells32 make them very appealing systems. The reaction of aqueous [PdCl4]2− with N-4-Xphenyloxamate ligands afforded compounds 1–3, exhibiting intriguing different three-dimensional assemblies, well driven by hydrogen bonds and/or weak C–H⋯O and C–H⋯X type interactions.33–37 The structural study of 1 reveals that C–H⋯F interactions occur between the organic counterion and the fluoro substituent from the phenyl fragment, but the assembly of the complex anions is driven by C–H⋯Ooxamate type interactions.26–28 The structures of 2 and 3 shed light on the effects of the different chemical nature of Cl and Br substituents on the C–H⋯X interactions joined to hydrogen bonds involving oxamate oxygen atoms and crystallization water molecules. In fact, this work focus on the structural characterization of compounds 1–3, special attention being paid to the supramolecular interactions that dictate and drive their crystal packing.
Experimental section
Preparation of the complexes
Compounds 1–3 were prepared as described elsewhere.38 The recrystallization in water of powder samples of 1–3 afforded X-ray quality yellow prisms for all three compounds whose stoichiometry was determined by X-ray diffraction methods (see below).X-ray data collection and structure determination
Single crystal X-ray diffraction data for 1–3 were collected at 296 K on a Bruker-Nonius X8-APEXII CCD area detector system using graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å), and processed through the SAINT39 reduction and SADABS40 absorption software. All the structures were solved by direct methods and subsequently completed by Fourier recycling using the SHELXTL-2013 (ref. 41) software packages, then refined by the full-matrix least-squares refinements based on F2 with all observed reflections. All structures, especially 2, show large thermal motion on chains of n-tetrabutylammonium cations as often found in other complexes containing this entity. All non-hydrogen atoms were refined anisotropically, except C38 and C48 in 2. The hydrogen atoms on the phenyl-substituted oxamate ligand and on the tetra-n-butylammonium cations were included at geometrically calculated positions and refined using a riding model. The hydrogen atoms on the water molecules in 2 and 3 were neither found nor calculated. Crystal data and refinements conditions for 1–3 are summarized in Table S1,‡ whereas selected bond lengths and angles and hydrogen bonds are listed in Tables S2 and S3,‡ respectively. The final graphical manipulations were carried out with the Crystal Maker programs.42Results and discussion
Description of the structures
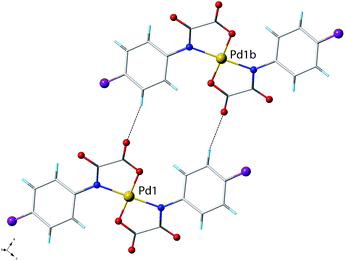
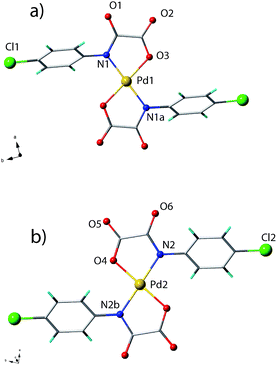
Compounds 1 and 3 crystallize in the monoclinic space groups P21/c and P2/c, respectively, with half a molecule in the asymmetric unit, the second half being generated by the crystallographic inversion centre. Compound 2 crystallizes in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group with two half a molecule in the asymmetric unit. Each structure consists of [Pd(4-Xpma)2]2− anions and n-Bu4N+ counterions (1–3) plus crystallization water molecules (2–3) (see Fig. 1–3). The complex anions in 1 are grouped into pairs through weak C–H⋯O type interactions (Fig. 4), these units being well separated from each other by the bulky organic cations which in turn are involved in weak C–H⋯F contacts (Fig. 5). On the contrary, the bis(oxamate)palladate(II) species in 2 are assembled into a pretty supramolecular three-dimensional structure by means of an extended network of hydrogen bonds and C–H⋯Cl interactions (Fig. 6–9). In the case of 3, the complex anions are inter-connected through hydrogen bonds involving four membered rings of water molecules and leading to supramolecular anionic chains which grow along the crystallographic a axis (Fig. 10 and 11).
space group with two half a molecule in the asymmetric unit. Each structure consists of [Pd(4-Xpma)2]2− anions and n-Bu4N+ counterions (1–3) plus crystallization water molecules (2–3) (see Fig. 1–3). The complex anions in 1 are grouped into pairs through weak C–H⋯O type interactions (Fig. 4), these units being well separated from each other by the bulky organic cations which in turn are involved in weak C–H⋯F contacts (Fig. 5). On the contrary, the bis(oxamate)palladate(II) species in 2 are assembled into a pretty supramolecular three-dimensional structure by means of an extended network of hydrogen bonds and C–H⋯Cl interactions (Fig. 6–9). In the case of 3, the complex anions are inter-connected through hydrogen bonds involving four membered rings of water molecules and leading to supramolecular anionic chains which grow along the crystallographic a axis (Fig. 10 and 11).
 | ||
| Fig. 1 Top (a) and side (b) views of trans-[Pd(4-Fpma)2]2− complex in 1 [symmetry code: (a) = −x + 1, −y + 1, −z]. | ||
 | ||
| Fig. 2 Perspective view of the two crystallographically independent trans-[Pd(4-Clpma)2]2− units in 2 [symmetry code: (a) = −x + 2, −y, −z + 1; (b) = −x + 2, −y, −z + 2]. | ||
 | ||
| Fig. 3 Top (a) and side (b) views of the cis-[Pd(4-Brpma)2]2− complex in 3 [symmetry code: (a) = −x + 1, y, −z + 1/2]. | ||
 | ||
| Fig. 7 Details of weak C–H⋯Cl type interactions [C20⋯Cl2 and H20A⋯Cl2 3.502(1) and 2.955(1) Å, respectively] in 2. | ||
The structures of 1 and 2 have in common the presence of the trans-[Pd(4-Xpma)2]2− anions [X = F (1) or Cl (2)] where the palladium(II) ion is four-coordinate with two amidate-nitrogen [N1 and N1a] and two carboxylate-oxygen atoms [O1 and O1a] building a slightly distorted square-planar surrounding. The reduced bite angle of the chelating oxamate [81.67(6) (1) and 81.0(1)° (2)] accounts for the deviations from the ideal geometry. The Pd(II) ion and the atoms building its surrounding lie exactly on a plane for symmetry reasons. The Pd–N/Pd–O bond lengths are 2.041(2)/1.999(2) (1) and 2.013(3)/2.008(2) Å (2), values which compare well with those found in other oxamato-containing palladium(II) complexes.30–32,38,43–45
The plane at the Pd(II) ion and the mean plane of the oxamate group are almost coplanar [the values of the dihedral angle between these two planes (φ) are 4.70(4) (1) and 4.0(2) and 0.5(2)° (2) for Pd1 and Pd2, respectively]. Focusing on the phenyloxamate ligand, the dihedral angle between the 4-substituted phenyl ring and the mean plane of the oxamate group are 36.71(3) (1) and 62.3(3) and 46.9(2)° (2), values which are not so close to orthogonality as those observed in {[Na(H2O)]2trans-[PdII(2,6-Me2pma)2]}n [φ = 75.79(4)] (2,6-Me2pma = N-2,6-dimethylphenyloxamate),31 but they compare well with those found in (n-Bu4N)2trans-[Pd(2-Mepma)2]·4H2O and (n-Bu4N)2trans-[Pd(4-Mepma)2]·4H2O [φ = 51.1(1) and 52.2(1)°, respectively] (2-Mepma = N-2-methylphenyloxamate and 4-Mepma = N-4-methylphenyloxamate).30
The [Pd(4-Brpma)]2− mononuclear units in the structure of 3 exhibit a cis conformation. However, the Pd(II) environment is practically the same of 1 and 2 being four-coordinate with two amidate nitrogen and two carboxylate-oxygen atoms from the two cis-oxamate ligands and building a slightly distorted square-planar [NON′O′] surrounding.
Again, the reduced bite of the chelating oxamate in 3 [81.5(1)°] accounts for the deviations from the ideal geometry. The Pd(II) ion belongs practically to its square plane. The Pd–N and Pd–O bond lengths [2.021(2)/2.008(2) Å] compare well with those found in 1 and 2 and in the already mentioned literature species.30–32,38,43,44 The square plane at the Pd(II) and the mean plane of the chelating oxamate group in the cis-[PdII(4-Brpma)2]2− units are very close to coplanarity as observed in 1 and 2 [the value of the dihedral angle between these two planes (φ) is 0.4(1)° while the dihedral angle between the 4-substituted phenyl ring and the mean plane of the oxamate group is 57.00(2)°]. Intramolecular face-to-face stacking interactions are clearly established in 3 between the phenyl rings on each chelating ligand of the cis-[PdII(4-Brpma)2]2− unit, the two 4-substituted phenyl ring forming a dihedral angle of 14.2(1)°. The interactions together with the reduction of the polarity of C–Br bonds respect to C–X (X = F, Cl) are likely to be one of the key reasons allowing for the cis-arrangement of the two 4-Brpma ligands around each palladium(II) ion. Furthermore a very weak (since the two Br atoms are at a distance 0.5 Å greater than the sum of van der Waals radii) intramolecular bromo⋯bromo interaction [4.27 Å for Br(1)⋯Br(1a)] would support the cis-conformation of the two 4-Brpma ligands observed in 3.
A detailed description of the different crystal packings of 1–3 is given in the following section.
Crystal packing of (n-Bu4N)2trans-[Pd(4-F-pma)2] (1)
The study of supramolecular structure of 1 shows the concomitant presence of either C–H⋯O or C–H⋯F interactions which are the driving forces towards the association of ions in the solid state.In fact, the bis(oxamate)palladium(II) units are well separated from each other due to the presence of bulky (n-Bu4N)+ cations. Nevertheless, there are noticeable Cph–H⋯Oox interactions [2.488(1) and 2.975(1) Å for H4⋯O1 and C4⋯O1 respectively, and 2.722(1) and 3.485(1) Å for H5⋯O2 and C5⋯O2] one of being related to the shortest Pd(1)⋯Pd(1b) separation of 9.645(3) Å [symmetry code: (b) = −x, y, z] (Fig. 4 and 5a) and even slightly shorter Ccat–H⋯Oox interactions where the H⋯O distance is 2.432(1) Å [C17⋯H17A separation 3.338(1) Å] (Fig. 5b). Furthermore, weak C–H⋯F interactions involving the organic n-Bu4N+ cations are present [shortest C–H⋯F and H⋯F separations of 3.381(2) and 2.468(2) Å, respectively] (Fig. 5b).
Crystal packing of (n-Bu4N)2trans-[Pd(4-Cl-pma)2]·4H2O (2)
Compound 2 shows a fascinating crystal packing due to the cooperation of different H-bonds involving also the co-crystallized water molecules and building together an elaborated supramolecular net. One of the two independent complex anions [Pd1] is engaged in such H-bond interactions between the terminal oxygen atoms of the oxamate ligand and the lattice water molecules [Ooxamate⋯Ow distances varying in the range 2.847(3)–2.908(3) Å; see Table S5‡] (Fig. 6).Additional H-bonds between water molecules [Ow⋯Ow distances in the range 2.664(1)–2.801(1) Å] give rise to a supramolecular 2D motif developing in the crystallographic ab plane, yielding a noteworthy intercalation of water chains, which are trapped in well-defined water ribbons (Fig. 6).
The supramolecular three-dimensional packing is reached by means of weak C–H⋯Cl type interactions [C20⋯Cl2 and H20A⋯Cl2 distances of 3.502(1) and 2.955(1) Å, respectively] involving more isolated trans-[Pd(4-Clpma)2]2− moieties (Fig. 7). These interactions are most likely responsible of the grasping of the two non-equivalent complex anions in 2 (Fig. 7 and 8).
In fact, one of the two independent complex anions [Pd2] is involved in H-bond interactions only with one of the terminal oxygen atoms of the ligand and a water lattice molecule [Ooxamate⋯Ow distance equal to 2.785(3) Å] (Fig. 7). As shown in Fig. 8, the whole packing is made up of layers with water ribbons [Pd1] staggered by other independent anions [Pd2]. The remaining voids are filled by the bulky organic countercations.
Crystal packing of (n-Bu4N)2cis-[Pd(4-Br-pma)2]·4H2O (3)
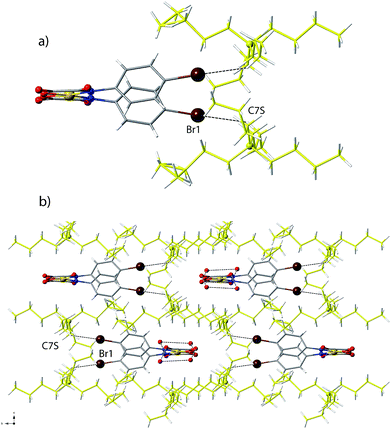
Compound 3 it is the only one containing complex anions in a cis conformation, namely the cis-[PdII(4-Brpma)2]2− units. As expected for such a conformation, intramolecular face-to-face stacking interactions are clearly established between the phenyl rings on each chelating ligand of the cis-[PdII(4-Br-pma)2]2− unit (Fig. 3). The value of the centroid–centroid distance (h) between the two facing benzene rings is 3.401(1) Å, while the value of the off-set angle (φ) between the centroid–centroid vector and the normal to the plane of the benzene rings is 19.9(1)°. As mentioned above, the reduction of the polarity of C–Br bonds respect to C–X (X = F, Cl) most likely would account for the cis-conformation of the two 4-Brpma ligands in 3. The steric effects produced by the adopted conformation are likely responsible for the non-zero twist angle [τ = 4.2(2)°] observed in 3 and in related compounds with cis stereochemistry.31 As in 1 and 2, C–H⋯X interactions involving counterions are present also in 3 [the values of C7S⋯Br1 and H7s1⋯Br1 being 3.656(1) and 2.821(1) Å, respectively] (Fig. 9). These forces together with H-bonds, involving both the terminal oxygen atoms of the ligand and the lattice water molecules [Ooxamate⋯Ow and Ow⋯Ow distances of 2.817(3)–2.840(3) and 2.860(3)–2.892(3) Å, respectively; see Table S6‡] give rise to a 1D supramolecular motif (Fig. 10 and 11) developing along the crystallographic a axis. The shortest Pd⋯Pd separation of 8.489(1) Å corresponds to an interchain distance while the shortest intrachain separation (through the network of H-bonded water molecules) is 14.827(1) Å (Fig. 11).Adjacent supramolecular 1D motifs are disposed into a zig-zag arrangement leaving pseudo-hydrophobic voids to be filled by the bulky n-Bu4N+ cations (Fig. 9b).
It has been found that both the trans and cis isomers of the [PdII(4-Brpma)2]2− complex anion can be isolated in the solid state even if from different solvents.38 The trans isomer of formula (n-Bu4N)2[Pd(4-Brpma)2]·H2O crystallized as a monohydrated species38 by slow vapor diffusion of ether into an acetonitrile solution of the complex. As mentioned in the Experimental section, suitable crystals for X-ray diffraction of 3 were obtained by recrystallization in water of the powder samples. On the contrary, we have no evidence of the formation of the cis isomer when 4-Fpma4− and 4-Clpma4− ligands were used. This result induces us to speculate on the C–X (X = F, Cl, Br) polarity as main reason for such experimental finding. The polarity of C–Br bonds is most likely to be considerably smaller than that of C–F and C–Cl bonds. Thus, while corresponding bond dipole moments in the trans isomers (X = F, Cl) have opposite orientations and are likely to combine favorably in an individual mononuclear entity, leading to a low energy structure, this is much less true for the cis isomer (X = Br). This reduction of the bond polarity in the case of the Br derivative (3), may well reduce or invert the energy difference between the cis and trans isomers, and hence be one of the key reasons allowing the formation of the cis isomer with water and of the trans isomer in the presence of less polar solvents. In any case, being aware that different plausible reasons exist for the occurrence of the cis along with the trans isomer only in the case of the Br derivative. Appropriate calculations which might give more insights on that matter will be further performed.
Conclusions
In summary, concerted supramolecular motifs in fluoro-, chloro- and bromo-derivatives of the mononuclear bis-oxamatopalladate(II) complexes have been investigated. The different nature and size of the halogen atoms show a clear putative role in the supramolecular assemblies of the similar anionic complexes. These intriguing different assemblies described are undoubtedly driven by C–H⋯X and C–H⋯O type interactions together with ionic forces, (1–3), or combined H-bonds and C–H⋯X interactions (2–3) and/or π⋯π stacking and very weak interactions Br⋯Br (3) merely depending on the substitution on the ligand used. This study is a uncommon report on structural modularity of the supramolecular assemblies in complexes as ligands for which a modulation of supramolecular features can be achieved with a judicious choice of slightly modified ligands.The supramolecular driving forces in the field of crystal engineering may be so far not all explored for the prediction of the crystal structures and then for the design of new functional molecule-based materials that exhibit intriguing physical–chemical properties.
Acknowledgements
Financial support from the Spanish Ministerio de Economía y Competitividad [Projects CTQ2013-448449 and MDM-2015-0538 (Unidades de Excelencia Ramiro de Maetzu)], the Generalitat Valenciana (ISIC/2012/002) and the Italian Ministero dell'Istruzione dell'Università e della Ricerca Scientifica (MiUR) is acknowledged. F. R. F.-P. thanks the MCIIN for a predoctoral FPU grant and N. M. also thanks the European Commission, FSE (Fondo Sociale Europeo) and Calabria Region for a post-doctoral fellowship.Notes and references
- (a) G. R. Desiraju, The Crystal as a Supramolecular Entity, John Wiley & Sons, New York, 1996 Search PubMed; (b) D. S. Reddy, K. Panneerselvam, T. Pilati and G. R. Desiraju, J. Chem. Soc., Chem. Commun., 1993, 661–662 RSC.
- P. Metrangolo, H. Neukirch, T. Pilati and G. Resnati, Acc. Chem. Res., 2005, 38, 386–395 CrossRef CAS PubMed.
- G. R. Desiraju and R. L. Harlow, J. Am. Chem. Soc., 1989, 111, 6757–6764 CrossRef CAS.
- D. S. Reddy, D. C. Craig and G. R. Desiraju, J. Am. Chem. Soc., 1996, 118, 4090–4093 CrossRef CAS.
- P. K. Thallapally, K. Chakraborty, A. K. Katz, H. L. Carrell, S. Kotha and G. R. Desiraju, CrystEngComm, 2001, 3, 134–136 RSC.
- P. Metrangolo and G. Resnati, Chem.–Eur. J., 2001, 7, 2511–2519 CrossRef CAS.
- V. R. Thalladi, B. S. Goud, V. J. Hoy, F. H. Allen, J. A. K. Howard and G. R. Desiraju, Chem. Commun., 1996, 401–402 RSC.
- V. K. Singh, S. K. Verma, R. Kadu and S. M. Mobin, RSC Adv., 2015, 5, 43669–43686 RSC.
- C. B. Aakeröy, M. Fasulo, N. Schultheiss, J. Desper and C. Moore, J. Am. Chem. Soc., 2007, 129, 13772–13773 CrossRef PubMed.
- K. Bouchmella, B. Boury, S. G. Dutremez and A. van der Lee, Chem.–Eur. J., 2007, 13, 6130–6138 CrossRef CAS PubMed.
- (a) G. R. Desiraju, Crystal Engineering. The Design of Organic Solids, Elsevier, Amsterdam, 1989 Search PubMed; (b) G. R. Desiraju, Curr. Opin. Solid State Mater. Sci., 1997, 2, 451–454 CrossRef CAS.
- (a) J.-M. Lehn, Supramolecular Chemistry: Concepts and Perspectives, Wiley-VCH, Wenheim, Germany, 1995 Search PubMed; (b) J. W. Steed and J. L. Atwood, Supramolecular Chemistry, Wiley, Chichester, 2000 Search PubMed.
- M. T. Albelda, J. C. Frías, E. García-España and H.-J. Schneider, Chem. Soc. Rev., 2012, 41, 3859–3877 RSC.
- P. Starha, Z. Trávnícek and I. Popa, J. Inorg. Biochem., 2009, 103, 978–988 CrossRef CAS PubMed.
- P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. S. Morris and C. Serre, Chem. Rev., 2012, 112, 1232–1268 CrossRef CAS PubMed.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS PubMed.
- H. Amouri and M. Gruselle, Chirality in Transition Metal Chemistry, Molecules, Supramolecular Assemblies and Materials, John Wiley & Sons, Chichester United Kingdom, 2008 Search PubMed.
- C. Train, M. Gruselle and M. Verdaguer, Chem. Soc. Rev., 2011, 40, 3297 RSC.
- S. Horike, D. Umeyama and S. Kitagawa, Acc. Chem. Res., 2013, 46, 2376–2384 CrossRef CAS PubMed.
- Multifunctional Molecular Materials, ed. L. Ouahab, Pan Stanford Publishing, Singapore, 2013, ch. 1, 2, 5 and 7 Search PubMed.
- M. Andruh, Chem. Commun., 2007, 2565–2577 RSC.
- M. Andruh, Chem. Commun., 2011, 47, 3025–3042 RSC.
- R. W. Gable, B. F. Hoskins and R. Robson, J. Chem. Soc., Chem. Commun., 1990, 1677–1678 RSC.
- B. F. Hoskins and R. Robson, J. Am. Chem. Soc., 1990, 112, 1546–1554 CrossRef CAS.
- C. A. Hunter, Angew. Chem., Int. Ed., 2004, 43, 5310–5324 CrossRef CAS PubMed.
- A. Mukherjee, S. Tothadi and G. R. Desiraju, Acc. Chem. Res., 2014, 47, 2514–2524 CrossRef CAS PubMed.
- M.-C. Dul, E. Pardo, R. Lescouëzec, Y. Jounaux, J. Ferrando-Soria, R. Ruiz-García, J. Cano, M. Julve, F. Lloret, D. Cangussu, L. M. Pereira, H. O. Stumpf, J. Pasán and C. Ruiz-Pérez, Coord. Chem. Rev., 2010, 254, 2281–2296 CrossRef CAS.
- (a) T. Grancha, J. Ferrando-Soria, M. Castellano, M. Julve, J. Pasán, D. Armentano and E. Pardo, Chem. Commun., 2014, 50, 7569–7585 RSC; (b) E. Pardo, R. Ruiz-García, J. Cano, X. Ottenwaelder, R. Lescouëzec, Y. Journaux, F. Lloret and M. Julve, Dalton Trans., 2008, 2780–2805 RSC.
- M. Castellano, R. Ruiz-García, J. Cano, J. Ferrando-Soria, E. Pardo, F. Fortéa-Pérez, S.-E. Stiriba, M. Julve and F. Lloret, Acc. Chem. Res., 2015, 48, 510–520 CrossRef CAS PubMed.
- F. R. Fortea-Pérez, I. Schlegel, M. Julve, D. Armentano, G. de Munno and S.-E. Stiriba, J. Organomet. Chem., 2013, 743, 102–108 CrossRef.
- F. R. Fortea-Pérez, N. Marino, D. Armentano, G. de Munno, M. Julve and S.-E. Stiriba, CrystEngComm, 2014, 16, 6971–6988 RSC.
- W. X. C. Oliveira, M. M. da Costa, A. P. S. Fontes, C. B. Pinheiro, F. C. S. de Paula, E. H. L. Jaimes, E. F. Pedroso, P. P. de Souza, E. C. Pereira-Maia and C. L. M. Pereira, Polyhedron, 2014, 76, 16–21 CrossRef CAS.
- K. Rissanen, CrystEngComm, 2008, 10, 1107–1113 RSC.
- Y. Nie, J. Miao, H. Pritzkow, H. Wadepohl and W. Siebert, J. Organomet. Chem., 2013, 747, 174–177 CrossRef CAS.
- V. R. Hathwar, S. M. Roopan, R. Subashini, F. N. Khan and T. N. Gururow, J. Chem. Sci., 2010, 122, 677–685 CrossRef CAS.
- S. Yamada, N. Sako, M. Okuda and A. Hozumi, CrystEngComm, 2013, 15, 199–205 RSC.
- C. B. Aakeröy, N. Schultheiss, A. Rajbanshi, J. Desper and C. Moore, Cryst. Growth Des., 2009, 9, 432–441 Search PubMed.
- F. R. Fortea-Pérez, B. L. Rothenpieler, N. Marino, D. Armentano, G. de Munno, M. Julve and S.-E. Stiriba, Inorg. Chem. Front., 2015, 2, 1029–1039 RSC.
- SAINT, Version 6.45, Bruker Analytical X-ray Systems Inc., Madison, WI, USA, 2003 Search PubMed.
- SADABS, Version 2.03, Bruker AXS Inc., Madison, WI, USA, 2000 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- D. Palmer, CRYSTAL MAKER, Cambridge University Technical Services, Cambridge, 1996 Search PubMed.
- F. R. Fortea-Pérez, D. Armentano, M. Julve, G. de Munno and S.-E. Stiriba, J. Coord. Chem., 2014, 67, 4003–4015 CrossRef.
- W. X. C. Oliveira, M. A. Ribeiro, C. B. Pinheiroda, M. M. Costa, A. P. S. Fontes, W. C. Nunes, D. Cangussu, M. Julve, H. O. Stumpf and C. L. M. Pereira, Cryst. Growth Des., 2015, 15, 1325–1335 CAS.
- W. X. C. Oliveira, M. A. Ribeiro, C. B. Pinheiro, W. C. Nunes, M. Julve, Y. Journaux, H. O. Stumpf and C. L. M. Pereira, Eur. J. Inorg. Chem., 2012, 34, 5685–5693 CrossRef.
Footnotes |
| † Dedicated to our friend Prof. Juan Faus for his outstanding contribution to the progress of the coordination chemistry field both as teacher and researcher. |
| ‡ Electronic supplementary information (ESI) available: Crystallographic data including tables of H-bonds are provided (Tables S1–S6). CCDC 1408631–1408633. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra25136b |
| This journal is © The Royal Society of Chemistry 2016 |