Effective tuning of the electronic and photophysical properties of tetrathiafulvalene pyrroles via aromatic heterocycle annulation†
Jung Su Park*a,
Jihae Kima,
Yong-Hoon Kim*b and
Jonathan L. Sessler*c
aDepartment of Chemistry, Sookmyung Womens's University, Cheongpa-ro 47-gil, Yongsan-gu, Seoul, South Korea. E-mail: jspark@sookmyung.ac.kr
bDepartment of Agricultural Engineering, National Academy of Agricultural Science, Rural Development Administration, 310 Nongsaengmyeng-ro, Wansan-gu, Jeonju, Korea. E-mail: yhkim0420@korea.kr
cDepartment of Chemistry, The University of Texas at Austin, 105 East 24th Street-Stop A5300, Austin, Texas 78712-1224, USA. E-mail: sessler@cm.utexas.edu
First published on 5th August 2015
Abstract
We report the synthesis and characterization of a series of fused molecular triads, consisting of tetrathiafulvalene-pyrrole derivatives annulated with pyrrole, benzene, thiophene, furan, pyrazine and thiadiazole. Unique and readily tunable optical and electrochemical features are displayed by these new systems.
Since the discovery of the tetrathiafulvalene (TTF) based organic conductors,1 the oxidized radical forms of TTF [TTF1+Cl−], and the charge transfer complex (TTF-TCNQ),2 in the early 1970s, investigations into the chemical, physical and functional properties of TTF and its higher chalcogen analogues have continued at an accelerating pace.3 This research has gone beyond the initial emphasis on electrically conducting materials and now encompasses various fields, such as functional material chemistry, nonlinear optics, photovoltaics, molecular electronics, redox active molecular receptors, molecular switches, logic gates, and artificial machines to name but a few.3–8
Among the various fields of research related to the synthetic chemistry of TTF and its analogues, we are particularly interested in the preparation and study of new functional TTF building blocks bearing fused heterocyclic aromatic rings, especially pyrroles. Pyrroles are versatile synthetic building blocks in organic synthesis and have proved to be very important intermediates in the construction of a wide range of natural products9 and functional supramolecular architectures.10 In this context, Becher and coworkers have first synthesized α-free TTF-pyrrole derivatives and applied these precursors for the preparation of several types of pyrrole-based supramolecular materials.11–13 Since then, several groups, including our own, have prepared pyrrole-annulated TTF derivatives and used them to prepare a range of functional supramolecular materials, including porphyrins, expanded porphyrins, calix[n]pyrroles, and rotaxanes.13–27 To date, it has been found that incorporation of TTFs into a pyrrole-based supramolecular frameworks endows the resulting system with a capability to recognize and sense a particular guest through a combination of electron donor–acceptor interactions and receptor-specific binding.14–25 In certain cases, the TTF subunits have been used as signalling units to monitor host–guest interactions via changes in the electrochemical or optical properties of the supramolecular systems, depending on nature of features induced upon substrate binding.13–25 In the particular case of TTF-calix[4]pyrrole (TTF-C4Ps) receptors and nitroaromatic explosives, it was shown that the binding affinities could be enhanced (by up to three orders of magnitude) through electronic modulation of the parent TTF-pyrrole used to construct the calixpyrrole receptor. This enhancement was achieved through annulation of aromatic moieties (benzene or thiophene) onto the TTF-pyrrole.25 We believe that further benefits would accrue as the result of annulating other aromatic subunits onto the TTF-pyrrole core. In particular we suggest that the resulting systems would act as molecular triads, showing unique photophysical and electrochemical features as the result of annulation. Reported here is a test of this hypothesis.
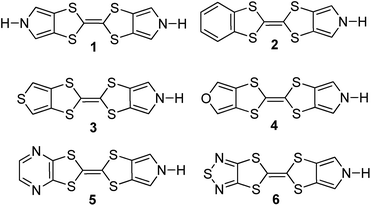
Annulation of aromatic moieties onto a TTF-pyrrole core is expected to modulate a variety of factors affecting the strength of various putative host–guest interactions, including the nature of the π-surfaces, the redox potentials, as well as the dipole moment (orientation and magnitude), flexibility, and the hydrogen bonding donor ability of the pyrrolic NH protons. With such consideration in mind, three new aromatic heterocycles, namely the furan 4, pyrazine 5, and thiadiazole 6 annulated TTF-pyrroles, were targeted. These compounds were synthesized, and fully characterized by 1H- and 13C-NMR, and High Resolution-Mass Spectra (HR-MS) (ESI†). The known aromatic annulated TTF-pyrrole derivatives 1–3 were also synthesized to allow for systematic comparison in terms of inter alia the electrochemical, chemical, and optical properties (Chart 1). The particular interest in the furan, pyrazine, and thiadiazole derivatives stems from their electron-deficient nature as compared to the pyrrole, benzene, and thiophene congeners.
All six TTF-pyrrole derivatives (i.e., 1–6) were prepared by a phosphite-mediated cross coupling reaction between 5-tosyl-5H-[1,3]dithiolo[4,5-c]pyrrol-2-one and the corresponding heterocyclic 1,3-dithiole-2-thione derivatives, followed by the detosylation reactions (ESI†).11,12 All systems gave spectral data consistent with their proposed structures.
The molecular structure of the compound 5 was determined from a single crystal X-ray diffraction analysis.† The resulting molecular structure and its packing arrangements of 5 are displayed in Fig. 2. As shown in Fig. 2A, the complex 5 adopts nearly planar configuration. The N–H of pyrrole in 5 is engaged in hydrogen bonding interactions with one of the N atoms of a neighboring pyrazine ring, as inferred from an N–H⋯N bond distance of 2.18 Å and a corresponding angle of 173°. These presumed hydrogen bonding interactions lead to a head-to-tail one dimensional zigzag chainlike structure being observed in the solid state (cf. Fig. 1B). An alternating regular π–π stacking arrangement between the pyrazine moiety and either the pyrrole or TTF-core moiety is also seen orthogonal to the principle direction of the hydrogen bonding interactions. This stacking between the pyrazine and pyrrole likely results from donor–acceptor interactions involving the electron deficient pyrazine and electron rich TTF-pyrrole subunits within 5.
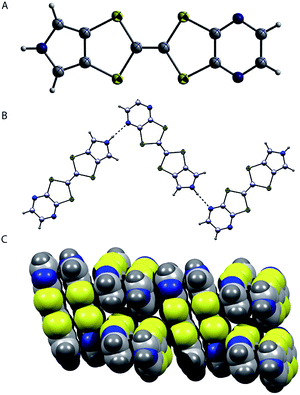 | ||
| Fig. 1 (A) Molecular structure of 5. (B) The intermolecular hydrogen-bonding motif. (C) The π–π stacking pattern. | ||
In order to investigate the effect of heterocycle annulation on the electrochemical properties of the TTF-pyrrole moiety, cyclic voltammetry (CV) analyses of compounds 1–6 were carried out in acetonitrile solution. The first and second half wave oxidation potentials resulting from these analyses are summarized in Table 1. Under identical conditions of study these aromatic annulated derivatives display two consecutive redox waves that are well-resolved and reversible, as seen in the case of TTF.3 The pyrrole annulated TTF-pyrrole 1 exhibits the lowest oxidation potential. Replacing the pyrrole ring with another aromatic ring results in an increase in the first and second oxidation potentials in the following order: pyrrole 1 < benzene 2 < thiophene 3 < furan 4 < pyrazine 5 < thiadiazole 6. Particularly large cathodic shifts in the first oxidation potential with respect to 1 were observed for 5 (by 0.401 V) and 6 (by 0.464 V), a finding considered to reflect the electron-deficient nature of the pyrazine and thiadiazole rings.
| No | Eox11/2 (V) | Eox21/2 (V) | HOMO (eV) | LUMO (eV) | Dipole (debye) |
|---|---|---|---|---|---|
| 1 | 0.356 | 0.701 | −4.221 | −0.590 | 0 |
| 2 | 0.479 | 0.810 | −4.429 | −0.858 | 2.293 |
| 3 | 0.523 | 0.854 | −4.535 | −0.879 | 3.320 |
| 4 | 0.566 | 0.908 | −4.579 | −0.872 | 3.600 |
| 5 | 0.756 | 1.111 | −4.724 | −1.731 | 3.721 |
| 6 | 0.819 | 1.312 | −4.943 | −1.860 | 6.236 |
The HOMO/LUMO energies of these compounds were calculated using the DFT B3LYP/6-21G(d) method.28 The results were found to reflect the major trends inferred from the experimental first oxidation potentials (cf. Fig. 2). As importantly, these calculations support the notion that the electron donating ability of TTF-pyrrole can be effectively tuned over a wide range by the present heterocyclic annulation strategy.
It was also expected that the choice of aromatic heterocycle used for annulation would result in significant changes in the dipole moment amplitude relative for the TTF-pyrrole moiety relative to the symmetric bis-pyrrolo-TTF system 1. Based on the downfield shifts seen for the pyrrolic N–H resonance, within this series the dipole moment shifts as follows: 1 < 2 < 3 < 4 < 5 < 6 (see Table S1, ESI†).
To study the optical properties of 1–6, the UV-vis absorption and fluorescence emission spectra were recorded in dichloromethane at room temperature. The known compounds 1–3 display an intense broad absorption band in the 250–350 nm spectral region (ESI†). The furan annulated TTF-pyrrole 4 also shows a similar absorption spectrum. On the other hand, the electronic absorption spectral features of compounds 5 and 6 are significantly different from those of 1–4. They exhibit an additional red-shifted absorption band centered at 425 nm for 5 and 372 nm for 6. These new spectral features reflect an internal charge-transfer band from the electron rich TTF-pyrrole core to the electron-deficient pyrazine or thiadiazole moieties. The presence of this band is rationalized by the DFT calculations, which reveal localized HOMO and LUMO orbitals on the TTF-pyrrole moiety and the pyrazine or thiadiazole subunits, respectively (Fig. 3). This type of internal charge transfer band is also observed in the case of several donor–acceptor fused TTF derivatives.29
 | ||
| Fig. 3 Electronic absorption and emission spectra of compounds 4–6 (25 μM) in dichloromethane (room temperature). | ||
The emission behavior of the heterocyclic annulated TTF pyrroles 1–6 was also investigated. Dichloromethane was again used as the solvent. The derivatives 1–4 did give rise to an appreciable fluorescence in the visible region of the spectrum. In contrast, compounds 5 and 6 when excited at their corresponding ICT bands, exhibited broad, largely red-shifted emission bands centred at 602 and 557 nm, respectively (Fig. 3).
Compounds 5 and 6 both contain a weakly acidic pyrrolic N–H, as well as a basic nitrogen center (in the pyrazine or thiadiazole moiety) within the same molecular entity. In principle, these systems are capable of either donating or accepting a proton depending on the conditions. The affect of treating with trifluoroacetic acid (TFA), tetrabutylammonium fluoride (TBAF), and tetrabutylammonium hydroxide (TBAOH) on the photophysical properties of compounds 5 and 6 were thus explored (Fig. 4 and ESI†). As can be seen from an inspection of Fig. 4a and c, the gradual addition of TFA to a dichloromethane solution of 5 or 6 leads to a decrease in the initial fluorescence spectral intensity. However, a corresponding gradual addition of TBAOH to dichloromethane solutions of 5 or 6 results in a largely blue-shift in the fluorescence emission bands with significantly enhanced intensities (Fig. 4b and d). A larger blue-shift was observed for compound 6 (Δλmax = 96 nm) than for compound 5 (Δλmax = 62 nm). Upon treatment with TBAF, similar phenomena were observed as with TBAOH; this was true for both 5 and 6, a result we interpret in terms of deprotonation by the fluoride anion (ESI†). In the event, there exists striking differences in the optical emission features of 5 and 6 induced by protonation vs. deprotonation. This responsiveness, which is not fully mirrored in the chemistry of 1–4, leads us to suggest that these particular annulated systems may have a role to play as environmentally responsive materials.
In summary, we report here three newly prepared aromatic heterocycle annulated (furan-, pyrazine-, and thiadiazole-) TTF-pyrrole derivatives. These TTF-pyrrole derivatives were systematically compared in terms of their electrochemical and optical properties. The resulting analysis revealed that the electronic features and the magnitude of the dipole moments within a series of homologous TTF-pyrrole derivatives can be readily tuned via the present heterocycle annulations strategy. The functional changes are dramatic, as evidenced by both CV measurements and DFT calculations. Two of the new derivatives, namely 5 and 6, act as a donor–donor–acceptor system and, in contrast to 1–4, exhibit ICT-based absorption and emission bands. We anticipate that the α-free pyrrole moiety present in these new derivatives can be exploited to create a variety of advanced functional supramolecular frameworks, while the systems themselves may be exploited as environmentally responsive switching elements. These possibilities are currently being explored in our laboratories.
Acknowledgements
This work was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ010506)” from the Rural Development Administration, Republic of Korea. The work in Austin was supported by the U.S. National Science Foundation (Grant CHE-1402004 to J. L. S.).Notes and references
- F. Wudl, D. Wobschall and E. Hufnagel, J. Am. Chem. Soc., 1972, 94, 670–672 CrossRef CAS.
- F. John, D. O. Cowan, V. Walatka Jr and J. H. Perlstein, J. Am. Chem. Soc., 1973, 3, 948–949 Search PubMed.
- J.-I. Yamada and T. Sugimoto, TTF Chemistry: Fundamentals and Application of Tetrathiafulvalene, Springer, 2004 Search PubMed.
- S. S. Park, E. R. Hontz, L. Sun, C. H. Hendon, A. Walsh, T. van Voorhis and M. Dincă, J. Am. Chem. Soc., 2015, 137, 1774–1777 CrossRef CAS PubMed.
- M. Shatruk and L. Ray, Dalton Trans., 2010, 11105–11121 RSC.
- P. Frere and P. J. Skabara, Chem. Soc. Rev., 2005, 34, 69–98 RSC.
- C.-G. Liu, Mol. Phys., 2014, 112, 199–205 CrossRef CAS PubMed.
- J. J. Bergkamp, S. Decurtins and S.-X. Liu, Chem. Soc. Rev., 2015, 44, 863–874 RSC.
- I. S. Young, P. D. Thornton and A. Thompson, Nat. Prod. Rep., 2010, 27, 1801–1839 RSC.
- A. L. Jerry and S. W. Jonathan, Encyclopedia of Supramolecular Chemistry, 2004, pp 1139–1185 Search PubMed.
- J. Jeppesen, K. Takimiya, F. Jensen, T. Brimert, K. Nielsen, N. Thorup and J. Becher, J. Org. Chem., 2000, 65, 5794–5805 CrossRef CAS PubMed.
- J. O. Jeppesen, K. Takimiya, F. Jensen and J. Becher, Org. Lett., 1999, 1, 7903–7904 CrossRef.
- J. O. Jeppesen, M. B. Nielsen and J. Becher, Chem. Soc. Rev., 2004, 104, 5115–5131 CrossRef CAS PubMed.
- K. A. Nielsen, E. Levillain, V. M. Lynch, J. L. Sessler and J. O. Jeppesen, Chem.–Eur. J., 2009, 15, 506–516 CrossRef CAS PubMed.
- A. Jana, M. Ishida, K. Cho, S. K. Ghosh, K. Kwak, K. Ohkubo, Y. M. Sung, C. M. Davis, V. M. Lynch, D. Lee, S. Fukuzumi, D. Kim and J. L. Sessler, Chem. Commun., 2013, 49, 8937–8939 RSC.
- C. M. Davis, J. M. Lim, K. R. Larsen, D. S. Kim, Y. M. Sung, D. M. Lyons, V. M. Lynch, K. A. Nielsen, J. O. Jeppesen, D. Kim, J. S. Park and J. L. Sessler, J. Am. Chem. Soc., 2014, 136, 10410–10417 CrossRef CAS PubMed.
- J. S. Park, E. Karnas, K. Ohkubo, P. Chen, K. M. Kadish, S. Fukuzumi, C. W. Bielawski, T. W. Hudnall, V. M. Lynch and J. L. Sessler, Science, 2010, 329, 1324–1327 CrossRef CAS PubMed.
- J. S. Park, K. Y. Yoon, D. S. Kim, V. M. Lynch, C. W. Bielawski, K. P. Johnston and J. L. Sessler, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 20913–20917 CrossRef CAS PubMed.
- C. M. Davis, J. M. Lim, K. R. Larsen, D. S. Kim, Y. M. Sung, D. M. Lyons, V. M. Lynch, K. A. Nielsen, J. O. Jeppesen, D. Kim, J. S. Park and J. L. Sessler, J. Am. Chem. Soc., 2014, 136, 10410–10417 CrossRef CAS PubMed.
- C. M. Davis, Y. Kawashima, K. Ohkubo, J. M. Lim, D. Kim, S. Fukuzumi and J. L. Sessler, J. Phys. Chem. C, 2014, 118, 13503–13513 CAS.
- D. S. Kim, V. M. Lynch, J. S. Park and J. L. Sessler, J. Am. Chem. Soc., 2013, 135, 14889–14894 CrossRef CAS PubMed.
- S. Fukuzumi, K. Ohkubo, Y. Kawashima, D. S. Kim, J. S. Park, A. Jana, V. M. Lynch, D. Kim and J. L. Sessler, J. Am. Chem. Soc., 2011, 133, 15938–15941 CrossRef CAS PubMed.
- D. S. Kim, V. M. Lynch, J. S. Park and J. L. Sessler, J. Am. Chem. Soc., 2013, 135, 14889–14894 CrossRef CAS PubMed.
- J. S. Park, C. Bejger, K. R. Larsen, K. A. Nielsen, A. Jana, V. M. Lynch, J. O. Jeppesen, D. Kim and J. L. Sessler, Chem. Sci., 2012, 3, 2685–2689 RSC.
- J. S. Park, F. Le Derf, C. M. Bejger, V. M. Lynch, J. L. Sessler, K. A. Nielsen, C. Johnsen and J. O. Jeppesen, Chem.–Eur. J., 2010, 16, 848–854 CrossRef CAS PubMed.
- A. Coskun, J. M. Spruell, G. Barin, A. C. Fahrenbach, R. S. Forgan, M. T. Colvin, R. Carmieli, D. Benítez, E. Tkatchouk, D. C. Friedman, A. A. Sarjeant, M. R. Wasielewski, W. A. Goddard and J. F. Stoddart, J. Am. Chem. Soc., 2011, 133, 4538–4547 CrossRef CAS PubMed.
- S. Bivaud, J. Balandier, M. Chas, M. Allain, S. Goeb and M. Salle, J. Am. Chem. Soc., 2012, 134, 11968–11970 CrossRef CAS PubMed.
- M. Gonza, L. Segura, C. Seoane, N. Martı, J. Garı, R. Alcala and J. T. Lo, J. Org. Chem., 2001, 66, 8872–8882 CrossRef PubMed.
- J. J. Bergkamp, S. Decurtins and S.-X. Liu, Chem. Soc. Rev., 2015, 44, 863–874 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of all the new compounds 4, 5, and 6, absorption and emission spectra, CV curves, and DFT calculations of 1–6. Single crystal X-ray structure of 5. CCDC 1404846. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra14811a |
| This journal is © The Royal Society of Chemistry 2015 |