DOI:
10.1039/C5RA12910A
(Paper)
RSC Adv., 2015,
5, 69251-69258
A naked-eye pH-modulated ratiometric photoluminescence sensor based on dual-emission quantum dot@silica nanoparticles for Zn2+ and IO3−†
Received
3rd July 2015
, Accepted 25th July 2015
First published on 29th July 2015
Abstract
In this study, we report development of a ratiometric photoluminescence (PL) sensor comprising dual-emission quantum dots (QDs)@silica nanoparticles for the detection of Zn2+ and IO3−. The PL signal of the red-emitting QDs in the silica nanoparticles core worked as a reference, while the PL signal of the green-emitting QDs covalently linked onto the silica surface could be selectively quenched or restored by the analyte. After PL quenching of the green-emitting QDs by phenanthroline (Phen), Zn2+ can recover the PL under alkaline conditions via the formation of a Zn–Phen complex in the solution. Besides, IO3− can interact with the green-emitting QDs through oxidation–reduction reactions under acidic conditions, leading to further PL quenching. Under the optimized conditions, linear relationships between the PL intensity ratio of the ratiometric system and ion concentration were obtained from 5 to 100 μM for Zn2+ and from 5 to 150 μM for IO3−. The limits of detection (LOD) for Zn2+ and IO3− were 1.15 and 1.76 μM, respectively. The proposed method was sensitive, highly selective and intuitional. It has been successfully applied towards the determination of Zn2+ and IO3− in serum samples and table salt with satisfactory results.
1. Introduction
To date, a series of functional nanoparticles has been utilized to establish various types of nanosensor systems such as QDs, Au nanoparticles, Fe3O4 nanoparticles, and carbon dots.1–3 Among them, QDs possess distinct optical properties, including broad excitation spectra, narrow emission peaks, high quantum yields and resistance to photobleaching.4 QDs with different sizes can emit different bands, affording the application of incorporating different-sized QDs in one nanosensor or nanoprobe. In addition, changes in the polychrome color provide convenience for detection by the naked eye. QD-based fluorescence methods have been widely applied to fluorescent labeling, biological imaging and sensing systems due to their simplicity, sensitivity, easy operation and low cost features. However, there are still two key questions in the development of QD-based PL sensors: the reliability and scope of their applications. On the one hand, most of the reported QD-based sensors are based on the quenching or enhancement of a single PL signal. These single lumophore-based sensors tend to be compromised by some other factors such as the instrumental efficiency and environmental fluctuations. On the other hand, the QD-based PL sensing systems modified using different chemical methods have been extensively exploited in the quantitative determination of cations, especially transition metal ions. However, there are few reports on their use for anion detection.5 Therefore, designing a variety of more sensitive and reliable QD-based PL sensors suitable for cations and anions is of great significance.
To improve the sensing reliability, ratiometric sensors have been proposed. Ratiometric PL sensors comprising two signal reporters have attracted increasing attention in recent years owing to their remarkable advantages.6,7 In the ratiometric PL sensor systems, one signal transducer works as the analyte-sensor, whereas the other signal reporter maintains a stable signal as a reference. Two signal reporters provide a better measured response to the analyte by reducing the experimental fluctuations. Thus, the ratiometric PL sensors greatly improve the sensitivity and reliability at trace quantity levels via self-calibration of two or more different PL signals.8–10 For example, a silica nanobead-based ratiometric Cu2+ sensor was reported.11 RBITC (response dye) was covalently linked on the surface of silica nanoparticles, whereas FITC (reference dye) was doped in the interior. The RBITC emission band decreased through quenching in the presence of Cu2+, while the reference FITC was unaffected. In addition, we have designed a direct naked-eye colorimetric analysis of Hg2+ based on the bi-color QDs multilayer films.12
Zinc exists in the human body in the form of Zn2+ and is the second most abundant transition metal in the body.13 Zn2+ serves as a catalytic and structural co-factor, affecting gene expression, neural related signal transmission, and facilitates enzyme regulation. Disorder of Zn2+ metabolism can result in various diseases, such as Alzheimer's disease, epilepsy and infantile diarrhea.14,15 Iodine is a necessary trace element during the growth of the human body. The lack of iodine can lead to thyromegaly, and can impact people's mental development at the same time. Long-term excessive iodine intake can also cause diseases and can affect the health of the human body.16–18 Introducing potassium iodate in table salt is the most simple and effective method to prevent iodine deficiency. To date, many PL sensors have been designed for Zn2+ or IO3− detection based on proteins, nanoparticles, rhodamine, fluorescein, and quinoline derivatives.19–22 In addition, a number of Zn2+ ratiometric sensors have been proposed such as polyethylenimine conjugated [Ru(bpy)3]2+@SiNPs ratiometric fluorescent nanoprobe, squaraine based ratiometric PL probe, tetrazolylpyridine-based ratiometric fluorescent chemosensor, and phenylbenzimidazole derivative-based ratiometric PL probe.23–26 Nevertheless, the ratiometric PL sensors based on QDs for Zn2+ and IO3− in one system have been rarely reported.
In this study, we have developed a novel type of dual-emission QD@silica nanoparticles-based ratiometric PL sensor (QSR) for the detection of Zn2+ and IO3−. As shown in Scheme 1, the QSR consisted of two different CdTe QDs, namely, the red-emitting QDs were embedded in the core of silica nanoparticles and the green-emitting QDs were covalently linked onto the silica surface. The red-emitting QDs in the silica nanoparticles emitted stable red PL, avoiding the interference of the analyte, and their relatively constant PL intensity served as an internal reference to eliminate the instrument and surrounding environmental interference. In the presence of Phen, the PL intensity of the green-emitting QDs was quenched via the electronic interaction between the QDs and Phen, and the PL intensity ratio of the QSR was decreased accordingly. Under alkaline pH conditions, the PL intensity of the green-emitting QDs can be selectively recovered upon the addition of Zn2+. On the contrary, IO3− can continue to quench the PL intensity of the green-emitting QDs under acidic pH conditions due to the oxidation–reduction reactions between IO3− and CdTe QDs. Therefore, QSR can detect Zn2+ and IO3− with high sensitivity and selectivity via the modulation of the pH.
 |
| | Scheme 1 The schematic illustration of the QSR structure and the detection principle for Zn2+ and IO3−. | |
2. Experimental section
2.1 Materials and apparatus
All the chemical reagents were of analytical reagent grade and were used without further purification. Tellurium powder, CdCl2, NaBH4 and tetraethoxysilane (TEOS) were purchased from Aldrich Chemical Co. Mercaptosuccinic acid (MPA) was purchased from J&K Chemical Co. 1-Ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), 3-mercaptopropyltrimethoxysilane (MPS) and 3-aminopropyltriethoxysilane (APTS) were purchased from Sigma-Aldrich Corporation. Phen, ammonium hydroxide, Zn(NO3)2·6H2O, KIO3, NaCl and other chemicals used were obtained from Beijing Dingguo Biotechnology Co. Ltd. 10 mM phosphate buffer solutions (PBS) of various pH values were prepared. The water used in all the experiments had a resistivity higher than 18 MΩ cm.
The fluorescence experiments were performed on a RF-5301 PC spectrofluorophotometer (Shimadzu Co., Kyoto, Japan) equipped with a xenon lamp at right-angle geometry. A 1 cm path-length quartz cuvette was used during the measurements of the PL spectrum. ICP (OPTIMA 3300DV) was used as a reference method for measuring serum zinc. All the pH measurements were made with a PHS-3C pH meter (Hangzhou, China).
2.2 Synthesis of CdTe QDs
CdTe QDs were synthesized by refluxing routes described in a previous method.27 Briefly, the precursor sodium hydrogen telluride (NaHTe) was produced as an aqueous solution by the reaction of sodium borohydride (NaBH4) with Te powder at a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Then, 1.25 mM CdCl2 solution at pH 11.4 and the stabilizing agent MPA were placed into a 250 mL three-necked flask under an N2 atmosphere. The molar ratio of Cd2+/MPA/HTe− was 1
1. Then, 1.25 mM CdCl2 solution at pH 11.4 and the stabilizing agent MPA were placed into a 250 mL three-necked flask under an N2 atmosphere. The molar ratio of Cd2+/MPA/HTe− was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4
2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5. A fresh precursor solution was injected into the CdCl2 solution, and the solution was heated at reflux (100 °C) with stirring under a condenser. Stable, water-compatible MPA-capped CdTe QDs were obtained. The PL emission wavelengths of the green and red emissive CdTe QDs used in the present experiments were 555 nm and 654 nm, respectively. The concentrations of both QDs were 1 μM.
0.5. A fresh precursor solution was injected into the CdCl2 solution, and the solution was heated at reflux (100 °C) with stirring under a condenser. Stable, water-compatible MPA-capped CdTe QDs were obtained. The PL emission wavelengths of the green and red emissive CdTe QDs used in the present experiments were 555 nm and 654 nm, respectively. The concentrations of both QDs were 1 μM.
2.3 Preparation of QD@silica nanoparticles and surface modification
QD@silica nanoparticles were synthesized using a modified process based on the Stöber method.28,29 In a typical process, 15 mL ultrapure water, 40 mL ethanol, and 7 mL red-emitting CdTe QDs solution were mixed with stirring in a 100 mL flask for 10 min. The flask was coated with an aluminum foil, 20 μL of MPS was added, and the mixed solution was stirred for 12 h. Then, 0.5 mL of TEOS and ammonium hydroxide were added into the solution, and the solution was stirred for another 12 h. Then, 100 μL of APTS was introduced into the above mentioned mixture to modify the silica surface with amino groups and stirred for another 12 h. After the reaction, the resulting silica nanoparticles embedded with red-emitting QDs were obtained by centrifugation and washed with ethanol and ultrapure water for several times to remove the unreacted chemicals. Ultimately, the amino-modified QD@silica nanoparticles were redispersed in 10 mL ultrapure water and preserved for further experiments.
2.4 Preparation of dual-emission QD@silica nanoparticles-based ratiometric PL sensor (QSR)
0.5 mL of the green-emitting QDs solution was dispersed in 1 mL H2O in a flask. 2 mL of EDC/NHS (2 mg mL−1) was added and the solution was stirred for 15 min. Then, 3 mL of the amino-modified QD@silica nanoparticles was introduced, and the mixture was stirred for 4 h in the dark. The resulting dual-emission silica nanoparticles were centrifuged and washed three times with ultrapure water. The final product QSR was dispersed in 10 mL ultrapure water for further use.
2.5 Photoluminescence assays
In the experiments, all PL spectral measurements were performed under the same conditions: the slit widths of the excitation and emission were 5 nm and 10 nm, respectively, and the excitation wavelength was set at 400 nm. The emission was scanned from 420 to 780 nm. In the PL “turn off” step, 20 μL of QSR and different concentrations of Phen solution were placed into a 2.0 mL calibrated test tube. PL spectrum was obtained and the corresponding PL intensity ratio was calculated. In the procedure for detecting Zn2+ and IO3−, different concentrations of Zn2+ and IO3− solution were added into the above mentioned Phen-QSR solution under alkaline or acidic conditions and the corresponding PL spectra were recorded. The relationships between Zn2+ or IO3− concentration and the PL intensity ratio of the QSR were plotted and the calibration curves were established.
2.6 Detection of Zn2+and IO3− in real samples
Fresh human blood samples were supplied from China-Japan Union Hospital, a hospital in Changchun. All the blood samples were mixed with acetonitrile (acetonitrile/blood was 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and centrifuged at 10
1) and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The supernatant serum samples were obtained by eliminating the large molecules and proteins. The serum samples were diluted by a factor of 2 with PBS buffer before analysis and different concentrations of Zn2+ were added to prepare the spiked samples. These serum samples were analyzed via the PL measurements under the optimal conditions. Table salt samples were purchased from local markets and dried at 120 °C for 6 h. Subsequently, a sample solution was prepared by dissolving 10 g of table salt in 50 mL of ultrapure water. The table salt samples were diluted by a factor of 2 with PBS buffer, and different concentrations of IO3− were added to prepare spiked samples.
000 rpm for 10 min. The supernatant serum samples were obtained by eliminating the large molecules and proteins. The serum samples were diluted by a factor of 2 with PBS buffer before analysis and different concentrations of Zn2+ were added to prepare the spiked samples. These serum samples were analyzed via the PL measurements under the optimal conditions. Table salt samples were purchased from local markets and dried at 120 °C for 6 h. Subsequently, a sample solution was prepared by dissolving 10 g of table salt in 50 mL of ultrapure water. The table salt samples were diluted by a factor of 2 with PBS buffer, and different concentrations of IO3− were added to prepare spiked samples.
3. Results and discussion
3.1 Characteristics of the QSR
In this study, ratiometric PL sensor QSR was prepared via the formation of red-emitting QD@silica nanoparticles and subsequent covalent linking of the green-emitting QDs on the silica surface, as shown in Scheme 1. The PL emission spectra of the green-emitting QDs, red-emitting QDs and the QSR are shown in Fig. 1. The green-emitting and red-emitting QDs have an emission maximum at 555 nm and 654 nm, respectively. The QSR exhibited a stable dual-emission PL spectrum (562 and 651 nm) under single wavelength excitation. The PL emission peak of the green-emitting QDs had a slight red shift from 555 nm to 562 nm after being linked on the silica surface. This indicated the surface state of the QDs was changed when chemically bonded on the silica surface. Moreover, the larger volume and surface area of the silica nanoparticles also led to a red shift of the PL emission peak.30,31
 |
| | Fig. 1 The PL spectra of green-emitting QDs (a), red-emitting QDs (b) and QSR (c). | |
3.2 PL recovery and quenching of Phen-QSR system with Zn2+ and IO3−
The QSR exhibited a stable dual-emission PL peak in PBS buffer, as shown in Fig. 2 curve a. When Phen was introduced into the QSR system, there was a gradual attachment of Phen onto the QSR surface due to the affinity of the two nitrogen atoms of Phen with Cd atoms on the surface of the QDs. As a result, the PL intensity of the green-emitting QDs was quenched, while the red PL from the QDs in the silica nanoparticles remained stable. Phen quenched the PL of the green-emitting QDs primarily by an electronic interaction rather than a “surface chemistry” interaction. Upon excitation, the photogenerated holes on the QDs were preferentially transferred to the Phen ligands and trapped, preventing effective hole/electron recombination.32 The relatively constant red PL served as an internal reference to improve the sensitivity and reliability of the sensor. Fig. 2 curve b shows that the PL intensity ratio (I562/I651) of the QSR system decreased upon the addition of Phen. Accordingly, the PL colors of the QSR solution changed from yellow to pale red under a UV lamp, which can be seen with the naked eye. This reveals that the QSR gradually emitted red PL of the inner QDs in the silica nanoparticles with the quenching of green-emitting QDs. Upon the addition of Zn2+, the PL intensity ratio (I562/I651) of the Phen-QSR system increased (curve c in Fig. 2) and the PL color of the ratiometric solution turned orange to the naked eye under a UV lamp. This was due to the fact that Phen was dissociated from the surface of green-emitting QDs by Zn2+ and a soluble Zn–Phen chelate was formed in the solution. The electronic interaction between Phen and QDs was terminated, leading to the restoration of the PL intensity of the QDs. When the molar ratio of the added Zn2+ and Phen was higher than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the PL intensity of the green-emitting QDs cannot recover any more, suggesting that the chelating stoichiometric ratio of Zn2+ to Phen was 1
2, the PL intensity of the green-emitting QDs cannot recover any more, suggesting that the chelating stoichiometric ratio of Zn2+ to Phen was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. To verify the occurrence of the chelating reaction rather than the influence of Zn2+ itself on the system, the PL intensity of the QSR was recorded in the presence of Zn2+. As shown in Fig. S1,† Zn2+ itself did not affect the PL intensity ratio of QSR system in a wide range of concentrations, which demonstrated that the chelating reaction between Zn2+ and Phen indeed occurred. When IO3− was added to the Phen-QSR system under acidic conditions, IO3− reacted with the superficial green-emitting CdTe QDs, resulting in further quenching of its PL intensity. The PL intensity ratio of QSR system subsequently decreased, as shown in Fig. 2 curve d. In addition, the QSR solution changed from pale red to dark red under a UV lamp. The reaction mechanism is expressed by the following equations:33
2. To verify the occurrence of the chelating reaction rather than the influence of Zn2+ itself on the system, the PL intensity of the QSR was recorded in the presence of Zn2+. As shown in Fig. S1,† Zn2+ itself did not affect the PL intensity ratio of QSR system in a wide range of concentrations, which demonstrated that the chelating reaction between Zn2+ and Phen indeed occurred. When IO3− was added to the Phen-QSR system under acidic conditions, IO3− reacted with the superficial green-emitting CdTe QDs, resulting in further quenching of its PL intensity. The PL intensity ratio of QSR system subsequently decreased, as shown in Fig. 2 curve d. In addition, the QSR solution changed from pale red to dark red under a UV lamp. The reaction mechanism is expressed by the following equations:33| | |
IO3− + 3CdTe QDs + 6H+ → I− + 3Te + 3Cd2+ + 3H2O
| (1) |
| | |
5I− + IO3− + 6H+ → 3I2 + 3H2O
| (2) |
 |
| | Fig. 2 The PL spectra of the QSR (a), QSR-200 μM Phen (b), QSR-200 μM Phen-100 μM Zn2+ (c), and QSR-200 μM Phen-40 μM IO3− (d). | |
3.3 Optimum conditions for the detection of Zn2+ and IO3−
A series of experiments were performed to choose the optimal conditions for the detection of Zn2+ and IO3−. Fig. 3 shows the effect of Phen concentration on the PL intensity ratio of the QSR. Upon the addition of Phen, the PL intensity at 562 nm of the green-emitting QDs in the QSR system was gradually decreased with increasing Phen concentration. Correspondingly, the PL intensity ratio of the QSR decreased gradually. When the Phen concentration was increased to 200 μM, the PL intensity ratio (I562/I651) reached its minimum and remained stable. Therefore, 200 μM of Phen was used in the subsequent experiments.
 |
| | Fig. 3 The effect of Phen concentration on the PL intensity ratio I562/I651 of the QSR. (a) 0 μM, (b) 10 μM, (c) 20 μM, (d) 50 μM, (e) 100 μM, (f) 200 μM, (g) 300 μM and (h) 400 μM. Inset: the plot of the PL intensity ratio I562/I651 of the ratiometric sensor toward Phen. | |
The pH value was of significant importance for the sensitive detection of Zn2+ and IO3−. Thus, we investigated the effect of pH on PL quenching and recovery of the QSR system in the pH range of 4–9. The QSR system was stable under weakly acidic or alkaline conditions. At low pH values (less than pH 4), the QSR was etched by the strong acid and the stability of the sensor was greatly reduced. The results in Fig. 4A show the PL intensity ratio of the Phen-QSR system with Zn2+ at different pH values. It can be seen that the maximum PL intensity ratio (I562/I651 = 3.11) of the ratiometric system was obtained at pH 8. This indicates that the chelation between Zn2+ and Phen can easily occur in a weak alkaline solution, and the PL intensity of the green-emitting QDs was recovered. However, a strong basic environment can lead to the hydrolysis of Zn2+, which affects the efficiency of the chelation reaction. Thus, we chose PBS buffer at pH 8 for the detection of Zn2+. The effect of pH on the PL intensity ratio of the Phen-QSR system with IO3− is displayed in Fig. 4B. It can be seen that under alkaline conditions (greater than pH 7.5), IO3− barely quenched the PL intensity of the green-emitting QDs on the surface of the silica nanoparticles. In addition, the quenching efficiency gradually increased with increasing acidity in the range of pH 5.0–7.5, which indicated that IO3− had a strong oxidizability under acidic conditions and the oxidation–reduction reaction between IO3− and CdTe QDs can be performed.34 Therefore, pH 5 was chosen in the subsequent experiments for IO3− detection. In a word, based on the modulation of pH, the QSR system can effectively determine Zn2+ and IO3− under alkaline or acidic conditions, respectively.
 |
| | Fig. 4 (A) The effect of pH on the PL intensity ratios I562/I651 for the QSR upon addition of 200 μM Phen and 100 μM Zn2+ and (B) the effect of pH on the PL intensity ratios I562/I651 for the QSR upon addition of 200 μM Phen and 120 μM IO3−. | |
The effect of reaction time on the QSR sensing system was also studied. For this purpose, QSR and Phen were mixed according to the procedure described above for different time intervals. As shown in Fig. 5, the PL intensity ratio (I562/I651) rapidly decreased with an increase in the reaction time and reached a minimum at 7 min. This indicated that the PL quenching of the green-emitting QDs was completed. When Zn2+ was added to the above mentioned solution, the PL intensity ratio of the ratiometric system increased with increasing reaction time and then remained constant over 6 min. Therefore, 7 min and 6 min were chosen as the optimal reaction time for Phen quenching and Zn2+ detection. Fig. 6 shows the PL intensity ratio of the Phen-QSR system in the presence of IO3− as a function of incubation time. It can be seen that the PL of the green-emitting QDs continued to quench with increasing reaction time until 6 min, suggesting that the oxidation–reduction reactions between IO3− and the CdTe QDs was complete within 6 min. Based on the above mentioned results, we chose 6 min as the optimal reaction time for IO3− detection.
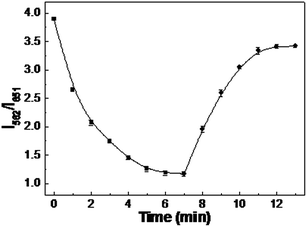 |
| | Fig. 5 The effect of incubation time on the PL intensity ratio I562/I651 for the QSR upon addition of 200 μM Phen (■) and subsequent 100 μM Zn2+ (●). | |
 |
| | Fig. 6 The effect of incubation time on the PL intensity ratio I562/I651 for the QSR upon addition of 200 μM Phen (■) and subsequent 120 μM IO3− (●). | |
3.4 Detection of Zn2+and IO3−
Under the optimized experimental conditions, the PL intensity ratio of the Phen-QSR system was closely related to the concentration of Zn2+ and IO3−. Fig. 7A shows that the PL intensity of the QSR at 562 nm increased gradually with increasing concentration of Zn2+. In Fig. 7B, it can be noticed that the PL intensity ratio (I562/I651) exhibited a linear response to the Zn2+ concentration. The linear range was from 5 to 100 μM and a good linear correlation (R2 = 0.998) was obtained. The linear regression equation was I562/I651 = 1.1526 + 0.0215 × C (Zn2+) (μM). According to the IUPAC 3s criterion, the limit of detection (LOD = 3σ/s) of Zn2+ was calculated to be 1.15 μM. Clearly, the distinguishable color changes of the QSR solution from pale red to pale yellow under a UV lamp were observed (Fig. 7C). As shown in Fig. 8, there was a linear relationship between the PL intensity ratio (I562/I651) of the Phen-QSR system and IO3− concentration in the range of 5–150 μM. The calibration curve can be expressed as I562/I651 = 1.0242 − 0.0076 × C (IO3−), (μM) and I562/I651 = 0.7431 − 0.0018 × C (IO3−), (μM). The correlation coefficients (R2) were 0.997 and 0.998, respectively. The limit of detection (LOD = 3σ/s) for IO3− was 1.76 μM. As shown in Fig. 8C, the color of the probe solution changed from pale red to red under a UV lamp. The reaction could be clearly distinguished by the naked eye. A comparison of the present method and other methods in the linear range and the limits of detection are shown in Table S1.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35–41 Compared with other sensors, our QSR system for Zn2+ and IO3− detection offered a comparable limit of detection and dynamic range.
35–41 Compared with other sensors, our QSR system for Zn2+ and IO3− detection offered a comparable limit of detection and dynamic range.
 |
| | Fig. 7 (A) The PL spectra of Phen-QSR with different Zn2+ concentrations from 0 to 100 μM. (a) 0 μM, (b) 5 μM, (c) 10 μM, (d) 20 μM, (e) 40 μM, (f) 60 μM and (g) 100 μM. (B) The linear plot of the PL intensity ratio I562/I651 for the QSR toward Zn2+. (C) The photographs of the mixed solution under a UV lamp. | |
 |
| | Fig. 8 (A) The PL spectra of Phen-QSR with different IO3− concentrations from 0 to 150 μM. (a) 0 μM, (b) 5 μM, (c) 10 μM, (d) 20 μM, (e) 30 μM, (f) 50 μM, (g) 80 μM, (h) 100 μM, (i) 130 μM and (j) 150 μM. (B) The linear plot of the PL intensity ratio I562/I651 of the ratiometric sensor toward IO3−. (C) The photographs of the mixed solution under a UV lamp. | |
3.5 Interference study
To evaluate the selectivity of the nanosensor for Zn2+ and IO3−, PL changes in the QSR system in the presence of various ions were investigated. Tolerable concentrations, the concentrations of foreign species causing less than ±5% relative error, were examined. As shown in Table S2,† the tolerable concentration ratios of interference substances for Zn2+ detection was 500 fold for Na+, K+, Cl−, and NO3−, 200 fold for Mg2+, SO42−, and Mn2+, 50 fold for Ni2+ and Ba2+, and 10 fold for Fe3+, Cd2+, and Cu2+. Most of the interfering substances had little effect on the detection of Zn2+. It can be seen from Table S3† that the tolerable concentration ratios of common interference ions and oxidant ions for IO3− detection was 500 fold for Na+, K+, and Cl−, 200 fold for Mg2+, Mn2+, NH4+, NO3−, and SO42−, 100 fold for CO32−, HCO3−, CH3COO−, F−, and I−, and 5 fold for SO32−, NO2−, and O2−. This indicates that these interfering ions have little interference on IO3− detection. Thus, the proposed ratiometric PL sensor used for the detection of Zn2+ and IO3− has higher selectivity.
3.6 Detection of Zn2+ and IO3− in real samples
To evaluate the practicability of the QSR system, we detected Zn2+ and IO3− in human serum samples and table salt, respectively. All the samples were prepared by spiking with Zn2+ and IO3− at different concentration levels, respectively. During the determination of IO3− in table salt samples, to eliminate the influence of NaCl, 1 mL of 3.4 M NaCl solution was added to obtain the work curve. The analytical results obtained by the standard addition method are listed in Table 1 and 2. To evaluate the accuracy of the proposed method, ICP and UV spectrophotometry methods were applied as the reference standard methods for the detection of Zn2+ and IO3−, respectively.42,43 It can be seen that the concentrations observed with the proposed method were in good agreement with those obtained by the reference standard methods. The recoveries of the samples were in the range of 97%–103%. The relative standard deviation (RSD) for three repeated measurements was less than 5%. The iodine content of the salt samples was in accordance with a standard level (iodine content label of the sample is 2250 μg/100 g, and it is 17.7 μM before measurement). This indicated our QSR is feasible and reliable for real sample detection.
Table 1 Determination of Zn2+ in human serum samples. A certain amount of human serum sample with different Zn2+ concentrations: (1) 20 μM, (2) 40 μM and (3) 60 μM
| Sample |
Original (μM) |
Added (μM) |
Found (μM) |
Recovery (%) |
RSD (%, n = 3) |
| The proposed method |
ICP |
| 1 |
6.82 |
20.00 |
26.40 |
26.21 |
97.90 |
3.73 |
| 2 |
7.35 |
40.00 |
47.81 |
48.42 |
101.15 |
2.28 |
| 3 |
7.54 |
60.00 |
67.16 |
67.59 |
99.37 |
1.65 |
Table 2 Determination of IO3− in table salt. A certain amount of table salt sample solution with different IO3− concentrations: (1) 20 μM, (2) 40 μM and (3) 60 μM
| Sample |
Original (μM) |
Added (μM) |
Found (μM) |
Recovery (%) |
RSD (%, n = 3) |
| The proposed method |
UV spectrophotometry method |
| 1 |
18.52 |
20.00 |
39.05 |
38.46 |
102.65 |
2.85 |
| 2 |
22.37 |
40.00 |
61.41 |
62.81 |
97.60 |
2.71 |
| 3 |
20.83 |
60.00 |
81.39 |
81.95 |
100.93 |
1.69 |
4. Conclusions
In conclusion, a novel dual-emission QD@silica nanoparticles-based ratiometric PL sensor for the detection of Zn2+ and IO3− has been developed. The red-emitting QDs encapsulated in the silica nanoparticles are inert to the analyte and the green-emitting QDs attached to the surface of the silica nanoparticles are specifically sensitive to the analyte. Phen quenched the PL intensity of the green-emitting QDs. Then, Zn2+ can react with the Phen under alkaline conditions, resulting in the PL recovery of the green-emitting QDs. IO3− can continue to quench the PL of the green-emitting QDs under acidic conditions owing to the oxidation–reduction reaction between IO3− and the QDs. Under the optimized conditions, the pH-modulated sensor shows a limit of detection of 1.15 μM for Zn2+ and 1.76 μM for IO3−. The proposed QSR system exhibits high sensitivity and selectivity to Zn2+ and IO3−, and has been successfully used in the real sample analysis with a simple, convenient, and facile procedure.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Nos 21275063 and 21005029) and the Youth Science Fund of Jilin Province (20140520081JH).
References
- F. Wang, Z. Xie, H. Zhang, C. Y. Liu and Y. G. Zhang, Adv. Funct. Mater., 2011, 21, 1027–1031 CrossRef CAS PubMed.
- Y. Li, Q. Ma, Z. P. Liu, X. Y. Wang and X. G. Su, Anal. Chim. Acta, 2014, 840, 68–74 CrossRef CAS PubMed.
- Q. Gan, X. Y. Lu, Y. Yuan, J. C. Qian and H. J. Zhou, Biomaterials, 2011, 32, 1932–1942 CrossRef CAS PubMed.
- L. H. Jing, C. H. Yang and R. R. Qiao, Chem. Mater., 2010, 22, 420–427 CrossRef CAS.
- M. Xue, X. Wang, L. L. Duan, W. Gao, L. F. Ji and B. Tang, Biosens. Bioelectron., 2012, 36, 168–173 CrossRef CAS PubMed.
- Y. J. Gong, X. B. Zhang, C. C. Zhang, A. L. Luo, T. Fu, W. H. Tan, G. L. Shen and R. Q. Yu, Anal. Chem., 2012, 84, 10777–10784 CrossRef CAS PubMed.
- Z. X. Han, X. B. Zhang, L. Zhuo, Y. J. Gong, X. Y. Wu, J. Zhen and C. M. He, Anal. Chem., 2010, 82, 3108–3113 CrossRef CAS PubMed.
- C. F. Wu, B. Bull, K. Christensen and J. McNeill, Angew. Chem., Int. Ed., 2009, 48, 2741–2745 CrossRef CAS PubMed.
- D. W. Domaille, L. Zeng and C. J. Chang, J. Am. Chem. Soc., 2010, 132, 1194–1195 CrossRef CAS PubMed.
- J. Zhou, C. Fang, T. Chang, X. Liu and D. Shangguan, J. Mater. Chem. B, 2013, 1, 661–667 RSC.
- C. Zong, K. Ai, G. Zhang, H. Li and L. Lu, Anal. Chem., 2011, 83, 3126–3132 CrossRef CAS PubMed.
- F. P. Yang, Q. Ma, Y. Wei and X. G. Su, Talanta, 2011, 84, 411–415 CrossRef CAS PubMed.
- P. J. Fraker and L. E. King, Annu. Rev. Nutr., 2004, 24, 277–298 CrossRef CAS PubMed.
- W. C. Fischer and R. E. Black, Annu. Rev. Nutr., 2004, 24, 255–275 CrossRef PubMed.
- P. A. Adlard and A. I. Bush, J. Alzheimer's Dis., 2006, 10, 145–163 Search PubMed.
- A. M. H. Shabani, P. S. Ellis and I. D. McKelvie, Food Chem., 2011, 129, 704–707 CrossRef CAS PubMed.
- Y. X. Hou, L. P. Liu and Z. X. Du, Phys. Test. Chem. Anal., 2011, 47, 1262–3111 CAS.
- T. L. Wang, S. Z. Zhao, C. H. Shen, J. Tang and D. Wang, Food Chem., 2009, 112, 215–220 CrossRef CAS PubMed.
- D. E. Benson, M. S. Wisz and H. W. Hellinga, Curr. Opin. Biotechnol., 1998, 9, 370–376 CrossRef CAS.
- M. J. Ruedas-Rama and E. A. H. Hall, Anal. Chem., 2008, 80, 8260–8268 CrossRef CAS PubMed.
- G. Crivat, K. Kikuchi, T. Nagano, T. Priel, M. Hershfinkel, I. Sekler, N. Rosenzweig and Z. Rosenzweig, Anal. Chem., 2006, 78, 5799–5804 CrossRef CAS PubMed.
- Y. Chen, K. Y. Han and Y. Liu, Bioorg. Med. Chem., 2007, 15, 4537–4542 CrossRef CAS PubMed.
- Y. P. Shi, Z. H. Chen, X. Cheng, Y. Pan and C. Q. Yi, Biosens.
Bioelectron., 2014, 61, 397–403 CrossRef CAS PubMed.
- P. Kaleeswaran, I. A. Azath, V. Tharmaraj and K. Pitchumani, ChemPlusChem, 2014, 79, 1361–1366 CrossRef CAS PubMed.
- Y. W. Huang, Q. Lin, J. M. Wu and N. Y. Fu, Dyes Pigm., 2013, 99, 699–704 CrossRef CAS PubMed.
- L. J. Tang, M. J. Cai, P. Zhou, J. Zhao and Y. J. Bian, J. Lumin., 2014, 147, 179–183 CrossRef CAS PubMed.
- Q. Ma, E. Ha, F. Yang and X. G. Su, Anal. Chim. Acta, 2011, 701, 60–65 CrossRef CAS PubMed.
- K. Zhang, Q. S. Mei, G. J. Guan, B. H. Liu, S. H. Wang and Z. P. Zhang, Anal. Chem., 2010, 82, 9579–9586 CrossRef CAS PubMed.
- J. L. Yao, K. Zhang, H. J. Zhu, F. Ma, M. T. Sun and H. Yu, Anal. Chem., 2013, 85, 6461–6468 CrossRef CAS PubMed.
- Y. Q. Wang, Y. Y. Zhang, F. Zhang and W. Y. Li, J. Mater. Chem., 2011, 21, 6556–6562 RSC.
- F. Vollmer, S. Arnold, D. Braun and I. Teraoka, Biophys. J., 2003, 85, 1974–1979 CrossRef CAS.
- S. Banerjee, S. Kar and S. Santra, Chem. Commun., 2008, 3037–3039 RSC.
- M. Fan, L. Zhang and Q. L. Liu, J. Anal. Sci., 2014, 30, 16–20 CAS.
- C. R. Tang, Z. H. Su, B. G. Lin, H. W. Huang and Y. L. Zeng, Anal. Chim. Acta, 2010, 678, 203–207 CrossRef CAS PubMed.
- Z. P. Liu, G. Y. Li, Q. Ma and X. G. Su, Microchim. Acta, 2014, 181, 1385–1391 CrossRef CAS.
- Q. Ma, Z. H. Lin, N. Yang, Y. Li and X. G. Su, Acta Biomater., 2013, 10, 868–874 CrossRef PubMed.
- H. Xu, Z. P. Wang, Y. Li, S. J. Ma and P. Y. Hu, Analyst, 2013, 138, 2181–2191 RSC.
- B. Haghighi, H. Hamidi and L. Gorton, Electrochim. Acta, 2010, 55, 4750–4757 CrossRef CAS PubMed.
- S. Abdollah, M. K. Hussein, H. Rahman and Z. Shiva, Electrochim. Acta, 2007, 52, 6097–6105 CrossRef PubMed.
- M. A. Tabrizi and L. Ebrahimi, J. Electroanal. Chem., 2014, 724, 8–14 CrossRef PubMed.
- J. Jakmunee and K. Grudpan, Anal. Chim. Acta, 2001, 438, 299–304 CrossRef CAS.
- Y. J. Li, W. T. Ma, J. H. Liu and E. R. Ma, Chin. J. Prev. Med., 1997, 31, 112–113 Search PubMed.
- G. N. P. Silva, A. Oliveira and E. A. Neves, J. Braz. Chem. Soc., 1998, 9, 171–174 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra12910a |
| ‡ These authors contributed equally to this work. |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Then, 1.25 mM CdCl2 solution at pH 11.4 and the stabilizing agent MPA were placed into a 250 mL three-necked flask under an N2 atmosphere. The molar ratio of Cd2+/MPA/HTe− was 1
1. Then, 1.25 mM CdCl2 solution at pH 11.4 and the stabilizing agent MPA were placed into a 250 mL three-necked flask under an N2 atmosphere. The molar ratio of Cd2+/MPA/HTe− was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4
2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5. A fresh precursor solution was injected into the CdCl2 solution, and the solution was heated at reflux (100 °C) with stirring under a condenser. Stable, water-compatible MPA-capped CdTe QDs were obtained. The PL emission wavelengths of the green and red emissive CdTe QDs used in the present experiments were 555 nm and 654 nm, respectively. The concentrations of both QDs were 1 μM.
0.5. A fresh precursor solution was injected into the CdCl2 solution, and the solution was heated at reflux (100 °C) with stirring under a condenser. Stable, water-compatible MPA-capped CdTe QDs were obtained. The PL emission wavelengths of the green and red emissive CdTe QDs used in the present experiments were 555 nm and 654 nm, respectively. The concentrations of both QDs were 1 μM.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and centrifuged at 10
1) and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The supernatant serum samples were obtained by eliminating the large molecules and proteins. The serum samples were diluted by a factor of 2 with PBS buffer before analysis and different concentrations of Zn2+ were added to prepare the spiked samples. These serum samples were analyzed via the PL measurements under the optimal conditions. Table salt samples were purchased from local markets and dried at 120 °C for 6 h. Subsequently, a sample solution was prepared by dissolving 10 g of table salt in 50 mL of ultrapure water. The table salt samples were diluted by a factor of 2 with PBS buffer, and different concentrations of IO3− were added to prepare spiked samples.
000 rpm for 10 min. The supernatant serum samples were obtained by eliminating the large molecules and proteins. The serum samples were diluted by a factor of 2 with PBS buffer before analysis and different concentrations of Zn2+ were added to prepare the spiked samples. These serum samples were analyzed via the PL measurements under the optimal conditions. Table salt samples were purchased from local markets and dried at 120 °C for 6 h. Subsequently, a sample solution was prepared by dissolving 10 g of table salt in 50 mL of ultrapure water. The table salt samples were diluted by a factor of 2 with PBS buffer, and different concentrations of IO3− were added to prepare spiked samples.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the PL intensity of the green-emitting QDs cannot recover any more, suggesting that the chelating stoichiometric ratio of Zn2+ to Phen was 1
2, the PL intensity of the green-emitting QDs cannot recover any more, suggesting that the chelating stoichiometric ratio of Zn2+ to Phen was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. To verify the occurrence of the chelating reaction rather than the influence of Zn2+ itself on the system, the PL intensity of the QSR was recorded in the presence of Zn2+. As shown in Fig. S1,† Zn2+ itself did not affect the PL intensity ratio of QSR system in a wide range of concentrations, which demonstrated that the chelating reaction between Zn2+ and Phen indeed occurred. When IO3− was added to the Phen-QSR system under acidic conditions, IO3− reacted with the superficial green-emitting CdTe QDs, resulting in further quenching of its PL intensity. The PL intensity ratio of QSR system subsequently decreased, as shown in Fig. 2 curve d. In addition, the QSR solution changed from pale red to dark red under a UV lamp. The reaction mechanism is expressed by the following equations:33
2. To verify the occurrence of the chelating reaction rather than the influence of Zn2+ itself on the system, the PL intensity of the QSR was recorded in the presence of Zn2+. As shown in Fig. S1,† Zn2+ itself did not affect the PL intensity ratio of QSR system in a wide range of concentrations, which demonstrated that the chelating reaction between Zn2+ and Phen indeed occurred. When IO3− was added to the Phen-QSR system under acidic conditions, IO3− reacted with the superficial green-emitting CdTe QDs, resulting in further quenching of its PL intensity. The PL intensity ratio of QSR system subsequently decreased, as shown in Fig. 2 curve d. In addition, the QSR solution changed from pale red to dark red under a UV lamp. The reaction mechanism is expressed by the following equations:33


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35–41 Compared with other sensors, our QSR system for Zn2+ and IO3− detection offered a comparable limit of detection and dynamic range.
35–41 Compared with other sensors, our QSR system for Zn2+ and IO3− detection offered a comparable limit of detection and dynamic range.





