Synthesis of Er3+/Yb3+ codoped NaMnF3 nanocubes with single-band red upconversion luminescence†
Zhenhua Baia,
Hui Linb,
Kenji Imakitab,
Reza Montazamia,
Minoru Fujiib and
Nastaran Hashemi*a
aDepartment of Mechanical Engineering, Iowa State University, Ames, IA 50011, USA. E-mail: nastaran@iastate.edu
bDepartment of Electrical and Electronic Engineering, Graduate School of Engineering, Kobe University, Rokkodai, Nada, Kobe 657-8501, Japan
First published on 11th November 2014
Abstract
We have developed a facile low-temperature synthetic method for the preparation of NaMnF3 nanocubes with Er3+ and Yb3+ ions homogeneously incorporated in the host lattice. The effects of the reaction temperature, and the volume ratio between ethanol and DI water on the morphology of NaMnF3 nanocubes are systematically investigated. The NaMnF3 nanocubes can be produced in the low temperature range (25–80 °C), and the higher reaction temperature (80 °C) is favorable for the formation of a smooth surface. The formation of NaMnF3 nanocubes strongly depends on the ethanol solvent. The morphology and single-phase of the obtained samples could be well maintained by controlling the doping concentration (Yb3+ ≤ 20 mol%). Single-band red upconversion emission can be generated in Er3+/Yb3+ codoped NaMnF3 nanocubes due to the energy transfer between host Mn2+ and dopant Er3+ ions. It is revealed that our NaMnF3:Er3+/Yb3+ nanocubes irradiate the brightest red luminescence at the dopant concentrations of Er3+ (2 mol%) and Yb3+ (15 mol%), which is stronger than that of the hexagonal-phase NaYF4:Er3+/Yb3+ phosphor.
1. Introduction
The development of upconversion (UC) phosphors has received considerable attention owing to their applications in solid-state lasers, optical data storage, illumination, color display, and biological labeling.1–3 Especially as a biological labeling material, UC fluorescent labels show very low background light as a result of their unique fluorescence properties and high detection limits compared with their traditional counterparts, such as organic dyes and quantum dots.4,5 The most efficient UC phosphor currently known is based on Er3+ ion in combination with Yb3+ ion as a sensitizer, which exhibits a green emission (∼550 nm) as well as a red emission (∼660 nm).6 The red emission is of technological importance since it is located in the “optical transmission window” of biological tissues, which has the minimum absorption of tissues and the maximum penetration depth.7 On the other hand, the green emission cannot effectively penetrate the deep tissue and may also cause many unwanted side effects that will reduce the sensitivity of the imaging.8 Therefore, avoiding the green emission and achieving strong and single-band red emission from Er3+–Yb3+ couple is eagerly demanded for the development of high-sensitivity and high-specificity probes for bioimaging.Recently, Mn2+-based nanocrystals have been known as ideal host materials to achieve single-band UC emission from Er3+ ions because of the energy transfer between the Er3+ and Mn2+.9–11 Up to now, some approaches have been developed for the preparation of various Mn2+-based nanocrystals including MnF2, KMnF3 and NaMnF3.12–14 It is well established that the shape and size of the material strongly affect the properties and the applications of the material.15–19 Hence, much effort has been dedicated on controlling the size and shape of the particles.10,20 However, in most cases, the geometry of Mn2+-based nanostructures obtained by conventional hydro/solvo-thermal method is spherical, and the synthesis of non-spherical nanostructures still suffers from extra technological difficulties.11,13,14 In addition, the previously reported approaches still suffer from problems including complicated experimental conditions, tedious procedures, and high reaction temperatures (≥160 °C).10,12 Hence, from safety and energy-saving viewpoints, it is highly desirable to develop a novel low-temperature solution-phase synthesis protocol to manipulate the morphology of Mn2+-based nanostructures.
In the present work, we have developed a straightforward wet-chemical approach to fabricate uniform and monodispersed Er3+/Yb3+ codoped NaMnF3 nanocubes. The effects of the reaction temperature, and the volume ratio between ethanol and DI water on the morphology of NaMnF3 nanocubes are systematically investigated. We examine the structural and UC luminescence properties of the NaMnF3:Er3+/Yb3+ nanocubes as a function of dopant concentrations of Er3+/Yb3+ (1–3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5–20 mol%). The UC luminescence properties of as-prepared nanocubes are compared with those of hexagonal-phase NaYF4 with the same dopant concentrations.
5–20 mol%). The UC luminescence properties of as-prepared nanocubes are compared with those of hexagonal-phase NaYF4 with the same dopant concentrations.
2. Experimental
2.1. Sample preparation
NaF (99%), MnCl2·4H2O (99%), YbCl3·6H2O (99.9%), ErCl3·6H2O (99.9%), and absolute ethanol were purchased from Sigma-Aldrich and were used as starting materials without further purification. DI water is used as solvent for the above chemicals to prepare stock solution. The strategy for synthesizing Er3+/Yb3+ codoped NaMnF3 nanocubes is schematically depicted in Scheme 1. In a typical synthesis process, NaMnF3 doped with 2 mol% Er3+ and 20 mol% Yb3+ was synthesized as follows: 3.12 mL of 0.2 M MnCl2·4H2O, 0.8 mL of 0.2 M YbCl3·6H2O and 0.08 mL of 0.2 M ErCl3·6H2O, and 4 mL of 0.6 M NaF were sequentially added to a beaker containing 24 mL of absolute ethanol under vigorous stirring. The reaction temperatures were set to be room temperature (25 °C), 50 °C and 80 °C, according to the experiment requirements. The final products were collected by means of centrifugation, washed with DI water for several times.2.2. Characterization
The crystal structure of prepared products was analyzed by an X-ray powder diffractometer (Rigaku-TTR/S2) using CuKα radiation (λ = 1.54056 Å). The size and morphology of the products were examined by using a field emission scanning electron microscope (FE-SEM, JSM-6700F at an acceleration voltage of 5 kV) equipped with an energy dispersive X-ray spectroscope (EDX, Horiba 7593-H model). The UC luminescence spectra were recorded using a fluorescence spectrophotometer (Horiba Jobin Yvon FluoroLog3) in conjunction with a 980 nm laser as the excitation source. All measurements were performed at room temperature.3. Results and discussion
3.1. Characterizations of structure and morphology
The synthesis of NaMnF3 materials is performed in various methods to study the effects of the experiment parameters such as reaction temperature, solvent and dopant. Fig. 1a presents the XRD patterns of NaMnF3 host materials synthesized at various reaction temperatures. It can be seen that all the diffraction peaks of the samples correspond to the NaMnF3 crystal (JCPDS standard card no. 18-1224). The similar diffraction patterns of all samples reveal that the NaMnF3 crystal can be formed in the temperature range of 25–80 °C. The sharp and strong peaks of NaMnF3 crystals suggest high crystallinity of the obtained samples. The XRD patterns of NaMnF3: 2 mol% Er3+, (10–30) mol% Yb3+ phosphors are also shown in Fig. 1b. It is evidenced that the crystal structure keeps the same until the Yb3+ concentration reaches 20 mol%, indicating that doped elements have been effectively doped into the host lattice. It is notable that an impurity phase is developed for the NaMnF3: 2 mol% Er3+, 30 mol% Yb3+ sample, which can be assigned to the Na5Yb9F32 crystal (JCPDS standard card no. 27-1426). | ||
| Fig. 1 (a) XRD patterns of NaMnF3 particles synthesized at the reaction temperature range of 25–80 °C. (b) XRD patterns of NaMnF3 nanostructures (80 °C) doped with 2 mol% Er3+ and (10–30) Yb3+ ions. | ||
The morphology of NaMnF3 host obtained at different reaction temperatures is characterized by SEM. From the low-resolution SEM images (Fig. 2a–c), uniform and monodispersed nanocubes with an average size around 900 nm can be obtained in the reaction temperature range of 25–80 °C. As revealed by the magnified SEM image (Fig. 2d), the surfaces of nanocubes are very rough, and full of cracks are observed when the reaction is carried out at room temperature. With the increase of reaction temperature to 50 °C, the cracks are gradually disappeared (Fig. 2e), and finally, very smooth surface over the whole particle is obtained at 80 °C (Fig. 2f). The morphology of NaMnF3 host is also investigated by doping various amounts of rare-earth ions. As shown in Fig. 3a and b, the morphologies of Er3+/Yb3+ codoped NaMnF3 nanocrystals are kept well until the Yb3+ doping concentration reaches 20 mol%. However, for the higher Yb3+ doping (30 mol%), besides the nanocubes, the coexistence of nanoparticles with the size of 100 nm is observed (Fig. 3c). It is confirmed that these nanoparticles are responsible for the impurity phase shown in Fig. 1b, which indicates that the excessive Yb3+ ions in solution prefer to react with NaF to form Na5Yb9F32 crystal, rather than doped into NaMnF3 host. Based on the both XRD and SEM results, doping Yb3+ ion lower than 20 mol% is essential to preserve the single-phase and morphology of obtained samples.
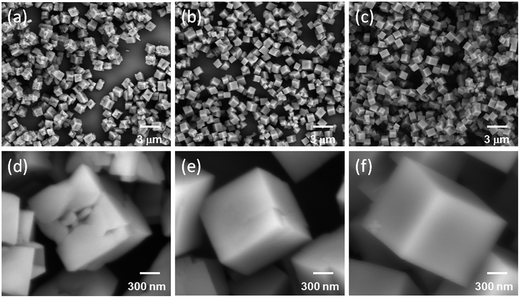 | ||
| Fig. 2 Low-resolution and high-resolution SEM images of NaMnF3 nanocrystals synthesized at various reaction temperatures: (a) and (d) 25 °C; (b) and (e) 50 °C; (c) and (f) 80 °C, respectively. | ||
 | ||
| Fig. 3 SEM images of NaMnF3 nanocubes doped with 2 mol% Er3+ and Yb3+ of (a) 10 mol%, (b) 20 mol% and (c) 30 mol%. | ||
Reaction solvent is another critical factor for the growth of nanocrystals, which can influence the reaction rate of crystal formation and further determine the phase and morphology of the final products.21,22 To evaluate the effect of reaction solvent on the formation of obtained samples, a set of NaMnF3 nanocrystals are fabricated in the mixed solutions of ethanol (ET) and DI water (DW). The sum amount of ethanol and DI water was fixed to 24 mL, and the ET/DW volume ratio was varied to 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 mL, 8
24 mL, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 mL, 16
16 mL, 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 mL, and 24
8 mL, and 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 mL. As shown in Fig. 4a, with the solvent of DI water, the irregularly-shaped and strongly-aggregated large blocks (several micrometers) as well as nanoparticles (∼100 nm) can be produced. With the addition of ethanol (8 mL) into solvent, the formation of aggregated micro-clusters and micro-hexahedrons with large size distributions are confirmed (Fig. 4b). In the solvent with 16 mL ethanol, the micro-clusters are disappeared, and irregular hexahedrons with the size range of 1–2 μm are observed (Fig. 4c). When the reaction is carried out in absolute ethanol, monodispersed nanocubes with a size of about 900 nm can be obtained (Fig. 4d). The results reveal that the introduction of ethanol in reaction system can effectively prevent agglomeration and stimulate the growth into NaMnY3 nanocubic assemblies.
0 mL. As shown in Fig. 4a, with the solvent of DI water, the irregularly-shaped and strongly-aggregated large blocks (several micrometers) as well as nanoparticles (∼100 nm) can be produced. With the addition of ethanol (8 mL) into solvent, the formation of aggregated micro-clusters and micro-hexahedrons with large size distributions are confirmed (Fig. 4b). In the solvent with 16 mL ethanol, the micro-clusters are disappeared, and irregular hexahedrons with the size range of 1–2 μm are observed (Fig. 4c). When the reaction is carried out in absolute ethanol, monodispersed nanocubes with a size of about 900 nm can be obtained (Fig. 4d). The results reveal that the introduction of ethanol in reaction system can effectively prevent agglomeration and stimulate the growth into NaMnY3 nanocubic assemblies.
 | ||
Fig. 4 SEM images of NaMnF3 nanostructures synthesized by various volume ratios of ethanol to water: (a) 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 mL; (b) 8 24 mL; (b) 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 mL; (c) 16 16 mL; (c) 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 mL; (d) 24 8 mL; (d) 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 mL. 0 mL. | ||
The variation of ethanol content has a great influence on the morphology of various nanomaterials, such as SiO2 and BaSO4.23–25 For instance, it was reported that the size of BaSO4 is reduced from 85 nm to 54 nm with the increase of ethanol percentage in ethanol–water mixed solvent from 30% to 70%.24 The effect of water and ethanol amount on the morphology of NaMnY3 could be attributed to the solvent interactions with the precursors, manganese chloride and sodium fluoride.26 It is well known that water has higher degree of porosity than ethanol. Increasing the ET/DW ratio will decrease the solvent polarity and the interfacial energy with the particles, which prevents the aggregation of the particles due to water swelling effect and makes the system more homogeneous.27,28 On the other hand, the reason for the formation of cubic particles may lie in an unusual inherent characteristic of NaMnF3.29 The ethanol solvent with relatively longer chain than water may change the order of the free energies of different facets through their interactions with the specific facets of NaMnF3 crystals.30 This alternation may significantly affect the relative growth rates of different facets and lead to the crystals with cubic morphology.
3.2. Upconversion luminescence properties
Fig. 5a and b show the room-temperature UC emission spectra of NaMnF3 nanocubes doped with various concentrations of Er3+ and Yb3+ ions. In comparison with Er3+/Yb3+ codoped routine rare-earth based fluoride nanocrystals which typically exhibit multiple-band emissions in the visible spectral region, a single-band emission in the spectral range of 640–690 nm is detected for all the NaMnF3 nanocubes doped with different amount of Er3+/Yb3+ (1–3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5–20 mol%) upon excitation at 980 nm, which is assigned to the 4F9/2 → 4I15/2 transition of Er3+ ions. It should be noted that though single-band red emission has been realized in several host materials, it is still challenging to obtain red emission with the high chromatic purity in NaMnF3 host.31,32 In NaMnF3 nanocubes, the red-to-green intensity ratios in all samples are larger than 40, which indicate that the present materials are favorable for applications in deep-tissue bioimaging (Fig. S1†). In addition, the full width at half maximum (FWHM) of the red-emitting band is measured to be 27 nm, which is comparable to that for KMnF3:Yb3+/Er3+ nanocrystals (20 nm), but is narrower than the red emission bands of ZrO2:Yb3+/Er3+ nanocrystals (42 nm) or Y2O3:Yb3+/Er3+ nanocrystals (75 nm).10,33,34
5–20 mol%) upon excitation at 980 nm, which is assigned to the 4F9/2 → 4I15/2 transition of Er3+ ions. It should be noted that though single-band red emission has been realized in several host materials, it is still challenging to obtain red emission with the high chromatic purity in NaMnF3 host.31,32 In NaMnF3 nanocubes, the red-to-green intensity ratios in all samples are larger than 40, which indicate that the present materials are favorable for applications in deep-tissue bioimaging (Fig. S1†). In addition, the full width at half maximum (FWHM) of the red-emitting band is measured to be 27 nm, which is comparable to that for KMnF3:Yb3+/Er3+ nanocrystals (20 nm), but is narrower than the red emission bands of ZrO2:Yb3+/Er3+ nanocrystals (42 nm) or Y2O3:Yb3+/Er3+ nanocrystals (75 nm).10,33,34
To gain more information on the UC mechanism, the pumping power dependence of UC luminescence intensity is studied. For an unsaturated UC process, the UC emission intensity (I) increases in proportion to the excitation power (P) according to the power law I ∝ Px, and generally, the measured slope of x is indicative of an upconversion process, which involves at least n photons, where n is the smallest integer greater than x or equal to x if x is an integer.35,36 Fig. 5c shows the log–log plots of the red luminescence intensity in 2 mol% Er3+/20 mol% Yb3+ doped NaMnF3 nanocubes as a function of excitation intensity at 980 nm. The result indicates that, a slope n value of 1.83 is obtained for the red emission band, indicating that two-photon processes are involved for generating the UC emissions in the present sample. It is also noteworthy that, the single-band feature of Er3+/Yb3+ codoped NaMnF3 can be remained well in the broad excitation power density range of 7.5–20 mW cm−2 (Fig. S2†).
According to the energy matching and quadratic dependence on excitation power, the possible UC mechanisms for the single-band red emission are discussed based on the simplified energy levels of Er3+, Yb3+ and Mn2+ ions. As illustrated in Fig. 5d, the Er3+ ion can be firstly excited to the 4I11/2 state through an energy transfer process from a Yb3+ ion, and then further jumped to the 4F7/2 state by absorbing the energy from another Yb3+ ion. Then Er3+ ion can be nonradiatively relaxed to two lower levels, 2H11/2 and 4S3/2, resulting in the green (2H11/2 → 4I15/2 and 4S3/2 → 4I15/2) UC emissions, and even further relaxed to 4F9/2 level to generate red (4F9/2 → 4I15/2) emission. However, with the presence of large amount of Mn2+ ions in NaMnF3 host, the interaction between Er3+ and Mn2+ plays an important role on modifying the UC emissions. Due to the close proximity and excellent overlap of energy levels of the Er3+ and Mn2+ ions in the host lattices, a nonradiative energy transfer from the 2H11/2 and 4S3/2 levels of Er3+ to the 4T1 level of Mn2+, which is followed by back-energy transfer to the 4F9/2 level of Er3+.10,31 The large red-to-green intensity ratios in all samples suggests that an extremely efficient exchange-energy transfer process occurs between the Er3+ and Mn2+ ions.
As is known, the luminescence intensity from rare-earth ions strongly depends on the doping level, and the proper doping is indispensable to achieve maximum intensity.9,37 The UC luminescence intensities are compared between the NaMnF3 nanocubes doped with various concentrations of Er3+ and Yb3+ ions (Fig. S3†). In the condition of 20 mol% Yb3+ in NaMnF3 nanocubes, the sample with Er3+: 2 mol% irradiates brightest red luminescence, which indicates that the further increase of Er3+ concentration does not benefit luminescence intensity. In the previous publication, Du et al. reported that, the optimum Er3+ concentration in Ca0.65La0.35F2.35 host should be 2 mol%, which is consistent with our results.38 On the other hand, with 2 mol% Er3+ concentration, the red emission increases with the Yb3+ concentration increases from 5 mol% to 15 mol%, and further increase of Yb3+ concentration results in the decrease of red emission due to concentration quenching effect.20 The above comparative studies suggest that our NaMnF3:Er3+/Yb3+ nanocrystals fabricated by present synthetic procedure own the strongest single-band emission feature at the dopant concentrations of Er3+ (2 mol%) and Yb3+ (15 mol%).
It should be noted that, in the previously published paper, Zhang et al. synthesized Er3+/Yb3+ doped NaMnF3 nanocrystals in the mixed solvents of 1-octadecene and oleic acid at 300 °C, and the strongest emission appears at Er3+ (25 mol%) and Yb3+ (25 mol%).11 In contrast, in our samples fabricated in ethanol at 80 °C, the dopant ions (Er3+ + Yb3+) cannot reach very high level (≤22%), since the excessive Yb3+ ions in ethanol prefer to react with NaF to form Na5Yb9F32 crystal, rather than doped into NaMnF3 host. The dopant ions can occupy the Mn2+ sites in the host crystals. It is reasonable to assume that, some Mn2+ sites are favourable for dopant ions when fabricated by our method. With the increase of reaction temperature, the dopants may occupy more Mn2+ sites. Therefore, the distance and interaction between dopants are different in samples fabricated by different methods, which results in distinct luminescence properties.
Among the investigated fluorides, hexagonal-phase NaYF4 is known as one of the most efficient host lattices for both downconversion and UC processes.39,40 To evaluate the luminescence efficiency of NaMnF3 host, a reference sample, hexagonal-phase NaYF4 doped with 2 mol% Er3+ and 15 mol% Yb3+ has been prepared by a modified version of the procedure described previously (Fig. S4†).41,42 Fig. 6 shows the comparison of UC luminescence spectra of NaYF4 and NaMnF3 doped with 2 mol% Er3+ and 15 mol% Yb3+ ions, respectively. The same amounts of samples are measured at the same experimental condition. Under the 980 nm excitation, the NaYF4 sample shows multi-peak emissions in the green and red regions, though pure red emission is detected from NaMnF3 sample. Despite the emission difference, the red emission intensity of NaMnF3:Er3+/Yb3+ is 1.4 times stronger and overall (green-plus-red) emissions are 1.2 times greater than those of NaYF4:Er3+/Yb3+ sample, indicating that NaMnF3 is a promising host material for deep tissue bioimaging. Such a red-emission enhancement should mostly arise from the efficient cross-relaxation of energy between Mn2+ and Er3+ ions.
 | ||
| Fig. 6 Comparison of upconversion luminescence spectra of hexagonal-phase NaYF4 and NaMnF3 host nanocrystals doped with 2 mol% Er3+ and 15 mol% Yb3+ ions, respectively. | ||
4. Conclusions
In summary, we have demonstrated the fabrication of uniform and monodispersed NaMnF3 nanocubes by a facile low-temperature solution-based method at ambient conditions. It is revealed that the proper controlling of the reaction temperature and solvent is critical for the formation of NaMnF3 nanocubes. Though the NaMnF3 nanocubes can be formed in the temperature range of 25–80 °C, higher temperature is favorable to obtain uniform and smooth products. The ethanol solvent is essential for the formation of NaMnY3 nanocubic assemblies. Doping Yb3+ ion lower than 20 mol% is required to preserve the single-phase and morphology of obtained materials. As a result of efficient energy transfer between the dopant Er3+ ion and host Mn2+ ion, remarkably pure red UC emissions were generated in the dopant concentration ranges of Er3+/Yb3+ (1–3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5–20 mol%). The strongest red emission in these Er3+/Yb3+ doped nanocrystals has been realized at the dopant concentrations of Er3+ (2 mol%) and Yb3+ (15 mol%). The achieved red emission is 1.4 times stronger and overall (green-plus-red) emissions are 1.2 times greater than those of NaYF4:Er3+/Yb3+ phosphor.
5–20 mol%). The strongest red emission in these Er3+/Yb3+ doped nanocrystals has been realized at the dopant concentrations of Er3+ (2 mol%) and Yb3+ (15 mol%). The achieved red emission is 1.4 times stronger and overall (green-plus-red) emissions are 1.2 times greater than those of NaYF4:Er3+/Yb3+ phosphor.
Acknowledgements
We gratefully acknowledge the William March Scholar program and the Iowa State University Presidential Initiative for Interdisciplinary Research and Health Research Initiative (ISU-HRI) for support of this work.References
- M. Ito, C. Goutaudier, Y. Guyot, K. Lebbou, T. Fukuda and G. Boulon, J. Phys.: Condens. Matter, 2004, 16, 1501–1521 CrossRef CAS.
- H. T. Sun, C. L. Yu, Z. C. Duan, L. Wen, J. J. Zhang, L. L. Hu and S. X. Dai, Opt. Mater., 2006, 28, 448–452 CrossRef CAS PubMed.
- L. Q. Xiong, Z. G. Chen, Q. W. Tian, T. Y. Cao, C. J. Xu and F. Y. Li, Anal. Chem., 2009, 81, 8687–8694 CrossRef CAS PubMed.
- S. A. Hilderbrand, F. W. Shao, C. Salthouse, U. Mahmood and R. Weissleder, Chem. Commun., 2009, 28, 4188–4190 RSC.
- H. S. Mader, P. Kele, S. M. Saleh and O. S. Wolfbeis, Curr. Opin. Chem. Biol., 2010, 14, 582–596 CrossRef CAS PubMed.
- F. Vetrone, J. C. Boyer, J. A. Capobianco, A. Speghini and M. Bettinelli, J. Appl. Phys., 2004, 96, 661–667 CrossRef CAS PubMed.
- K. Konig, J. Microsc., 2000, 200, 83–104 CrossRef CAS.
- G. Tian, Z. J. Gu, X. X. Liu, L. J. Zhou, W. Y. Yin, L. Yan, S. Jin, W. L. Ren, G. M. Xing, S. J. Li and Y. L. Zhao, J. Phys. Chem. C, 2011, 115, 23790–23796 CAS.
- Z. H. Bai, H. T. Sun, T. Hasegawa, M. Fujii, F. Shimaoka, Y. Miwa, M. Mizuhata and S. Hayashi, Opt. Lett., 2010, 35, 1926–1928 CrossRef CAS PubMed.
- J. Wang, F. Wang, C. Wang, Z. Liu and X. G. Liu, Angew. Chem., Int. Ed., 2011, 50, 10369–10372 CrossRef CAS PubMed.
- Y. Zhang, J. D. Lin, V. Vijayaragavan, K. K. Bhakoo and T. T. Y. Tan, Chem. Commun., 2012, 48, 10322–10324 RSC.
- J. H. Zeng, T. Xie, Z. H. Li and Y. D. Li, Cryst. Growth Des., 2007, 7, 2774–2777 CAS.
- M. Li, X. F. Yu, W. Y. Yu, J. Zhou, X. N. Peng and Q. Q. Wang, J. Phys. Chem. C, 2009, 113, 20271–20274 CAS.
- M. Y. Xie, X. N. Peng, X. F. Fu, J. J. Zhang, G. L. Lia and X. F. Yu, Scr. Mater., 2009, 60, 190–193 CrossRef CAS PubMed.
- J. H. Zeng, J. Su, Z. H. Li, R. X. Yan and Y. D. Li, Adv. Mater., 2005, 17, 2119–2123 CrossRef CAS.
- C. X. Li, Z. W. Quan, J. Yang, P. P. Yang and J. Lin, Inorg. Chem., 2007, 46, 6329–6337 CrossRef CAS PubMed.
- R. Montazami, S. Liu, Y. Liu, D. Wang, Q. Zhang and J. R. Heflin, J. Appl. Phys., 2011, 109, 104301 CrossRef PubMed.
- R. Montazami, C. M. Spillmann, J. Naciri and B. R. Ratna, Sens. Actuators, A, 2012, 178, 175 CrossRef CAS PubMed.
- R. Montazami, D. Wang and J. R. Heflin, Int. J. Smart Nano Mater., 2012, 3, 204 CrossRef CAS.
- Z. Bai, H. Lin, J. Johnson, S. C. Rong Gui, K. Imakita, R. Montazami, M. Fujii and N. Hashemi, J. Mater. Chem. C, 2014, 2, 1736–1741 RSC.
- K. W. Kramer, D. Biner, G. Frei, H. U. Gudel, M. P. Hehlen and S. R. Luthi, Chem. Mater., 2004, 16, 1244–1251 CrossRef.
- H. X. Mai, Y. W. Zhang, R. Si, Z. G. Yan, L. D. Sun, L. P. You and C. H. Yan, J. Am. Chem. Soc., 2006, 128, 6426–6436 CrossRef CAS PubMed.
- K. S. Rao, K. E. Hami, T. Koday, K. Matsushigue and K. Makino, J. Colloid Interface Sci., 2005, 289, 125–131 CrossRef CAS PubMed.
- V. Ramaswamy, R. M. Vimalathithan and V. Ponnusamy, J. Ceram. Process. Res., 2011, 12, 173–175 Search PubMed.
- X. Zhang, Y. Li and C. Cao, J. Mater. Chem., 2012, 22, 13918–13921 RSC.
- P. B. Khoza, M. J. Moloto and L. M. Siklrwivhilu, J. Nanotechnol., 2012, 2012 Search PubMed.
- C. J. Kim and M. S. Kwon, Electron. Mater. Lett., 2009, 5, 113–117 CAS.
- M. Thirumavalavan, K. L. Huang and J. F. Lee, Materials, 2013, 6, 4198–4212 CrossRef CAS PubMed.
- L. Qi, J. Ma, H. Cheng and Z. Zhao, Colloids Surf., A, 1996, 108, 117–126 CrossRef CAS.
- S. Tanvir and L. Qiao, Nanoscale Res. Lett., 2012, 7, 226 CrossRef PubMed.
- G. Tian, Z. J. Gu, L. J. Zhou, W. Y. Yin, X. X. Liu, L. Yan, S. Jin, W. L. Ren, G. M. Xing, S. J. Li and Y. L. Zhao, Adv. Mater., 2012, 24, 1226–1231 CrossRef CAS PubMed.
- Z. H. Bai, M. Fujii, K. Imakita and S. Hayashi, Microporous Mesoporous Mater., 2013, 173, 43–46 CrossRef CAS PubMed.
- D. Matsuura, Appl. Phys. Lett., 2002, 81, 4526–4528 CrossRef CAS PubMed.
- G. Y. Chen, Y. G. Zhang, G. Somesfalean, Z. G. Zhang, Q. Sun and F. P. Wang, Appl. Phys. Lett., 2006, 89, 163105 CrossRef PubMed.
- M. Pollnau, D. R. Gamelin, S. R. Luthi, H. U. Gudel and M. P. Hehlen, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 3337–3346 CrossRef CAS.
- H. T. Sun, L. L. Hu, C. L. Yu, G. Zhou, Z. C. Duan, J. J. Zhang and Z. H. Jiang, Chem. Phys. Lett., 2005, 408, 179–185 CrossRef CAS PubMed.
- Z. H. Bai, M. Fujii, Y. Mori, Y. Miwa, M. Mizuhata, H. T. Sun and S. Hayashi, Opt. Lett., 2011, 36, 1017–1019 CrossRef CAS PubMed.
- P. Du, Z. Xia and L. Liao, Mater. Res. Bull., 2011, 46, 543–546 CrossRef CAS PubMed.
- F. Wang and X. G. Liu, J. Am. Chem. Soc., 2008, 130, 5642–5643 CrossRef CAS PubMed.
- G. Y. Chen, T. Y. Ohulchanskyy, R. Kumar, H. Agren and P. N. Prasad, ACS Nano, 2010, 4, 3163–3168 CrossRef CAS PubMed.
- G. S. Yi, H. C. Lu, S. Y. Zhao, G. Yue, W. J. Yang, D. P. Chen and L. H. Guo, Nano Lett., 2004, 4, 2191–2196 CrossRef CAS.
- G. Y. Chen, H. C. Liu, G. Somesfalean, H. J. Liang and Z. G. Zhang, Nanotechnology, 2009, 20, 385704 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra10723c |
| This journal is © The Royal Society of Chemistry 2014 |


