The first stereoselective total synthesis of a new antitumour and anti-inflammatory neolignan, surinamensinol A†‡
Parigi Raghavendar Reddy and
Biswanath Das*
Division of Natural Products Chemistry, CSIR-Indian Institute of Chemical Technology, Hyderabad 500 607, India. E-mail: biswanathdas@yahoo.com; Fax: +91-40-27193198
First published on 25th November 2013
Abstract
The stereoselective total synthesis of an antitumour and anti-inflammatory 8-O-4′-neolignan, surinamensinol A has been accomplished starting from two aldehydes, 3,4,5-trimethoxy benzaldehyde and vanillin. The key steps involve an asymmetric reduction using a chiral oxazaborolidine complex, a Sharpless asymmetric dihydroxyllation and a Mitsunobu reaction. This is the first report of the total synthesis of surinamensinol A.
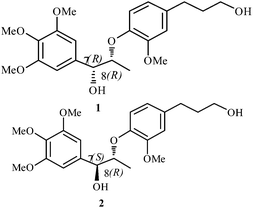
Surinamensinols A (1) and B (2), two new diastereomeric 8-O-4′-neolignans along with several other phenolics have recently been isolated from the rhizomes of the aquatic plant, Acorus gramineus (Araceae).1 The first compound bears (7R, 8R) configuration while the latter (7S, 8R) (Fig. 1). Compounds 1 and 2 were found to possess potent cytotoxicity against the A549 cell line and moderate activity against the SK-OV-3, SK-MEL-2 and HCT-15 cell lines.1 Both compounds 1 and 2 also exhibited impressive anti-inflammatory activity. They significantly inhibited the NO levels in LPS-stimulated BV-2 cells.1 In continuation to our work2 on the construction of natural products herein we disclose the total synthesis of 1. This is the first report of the synthesis of this molecule. The synthesis of some 8-O-4′-oxyneolignan derivatives have been reported,3 but earlier synthetic approaches were different compared to our present work. Our current approach involves two non-chiral easily available aldehydes as the starting materials. The reagents are also commercially available and the synthetic steps are limited.
The retrosynthetic analysis (Scheme 1) indicates that surinamensinol (1) can be synthesized from the chiral diol 3 and the TBS ether 4. Compounds 3 and 4 can, in turn, be obtained from 3,4,5-trimethoxy benzaldehyde (5) and vanillin (6), respectively.
For the synthesis of the chiral diol 3, 3,4,5-trimethoxy benzaldehyde (5) was treated with vinyl magnesium bromide to produce the alcohol 7 (Scheme 2).4 The alcohol 7 was oxidized with IBX and the corresponding ketone was subjected to chiral reduction using (R)-(+) 2-methyl-CBS-oxazaborolodine and BH3SMe2 to furnish the chiral alcohol 8 (ee 91%).5 The free hydroxyl group of 8 was protected as a Bn-ether (9) by treatment with BnBr and NaH. Compound 9 underwent a Sharpless asymmetric dihydroxylation6 with AD-mix β in t-BuOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to furnish the diol 10 along with its minor diastereomer (diastereomeric ratio 82
1) to furnish the diol 10 along with its minor diastereomer (diastereomeric ratio 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18) which was separated out. The primary hydroxyl group of 10 was tosylated with TsCl to form the mono-hydroxy compound 11. The subsequent reduction of 11 with LiAlH4 afforded the desired fragment 3 in a high yield.7
18) which was separated out. The primary hydroxyl group of 10 was tosylated with TsCl to form the mono-hydroxy compound 11. The subsequent reduction of 11 with LiAlH4 afforded the desired fragment 3 in a high yield.7
For the synthesis of another fragment 4, vanillin (6) was subjected to a Wittig reaction with Ph3PCHCOOEt to form the unsaturated ester 12 (ref. 8) (Scheme 3) with an E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z ratio of 92
Z ratio of 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8. The reduction of 12 with NaBH4 in the presence of NiCl2·6H2O followed by the protection of the phenolic hydroxyl group by treatment with BnBr and NaH and subsequent reduction with LiAlH4 afforded the saturated alcohol 13. The hydroxyl group of 13 was protected as a TBS ether (14) by using TBSCl and imidazole and compound 14 was then hydrogenated in the presence of 10% Pd–C to produce fragment 4.9
8. The reduction of 12 with NaBH4 in the presence of NiCl2·6H2O followed by the protection of the phenolic hydroxyl group by treatment with BnBr and NaH and subsequent reduction with LiAlH4 afforded the saturated alcohol 13. The hydroxyl group of 13 was protected as a TBS ether (14) by using TBSCl and imidazole and compound 14 was then hydrogenated in the presence of 10% Pd–C to produce fragment 4.9
Finally, the coupling of the two fragments 3 and 4 was carried out by a Mitsunobu reaction10 using Ph3P and DEAD and the resulting product on treatment with p-TsOH in MeOH followed by hydrogenation in the presence of 10% Pd–C yielded the target molecule, surinamensinol A (1) (Scheme 4) with 90% ee. The physical and spectral properties of the compound were compared to those reported for the natural product.1
 | ||
| Scheme 4 Reagents and conditions: (a) (i) Ph3P, DEAD, dry THF, reflux; (ii) p-TsOH, MeOH, room temperature; (iii) 10% Pd–C, H2, room temperature, 40%. | ||
In conclusion, we have demonstrated the first stereoselective total synthesis of the bioactive neolignan, surinamensinol A starting from two easily available aldehydes, 3,4,5-trimethoxy benzaldehyde and vanillin. Chiral reduction, asymmetric dihydroxylation and the Mitsunobu reaction are the key steps in the present synthesis. The overall yield of 1 starting from 5 is 10% involving sixteen steps.
Acknowledgements
The authors thank CSIR and UGC, New Delhi for financial assistance. They are also thankful to NMR, Mass and IR divisions of CSIR-IICT for spectral recording.References
- K. H. Kim, E. Moon, H. K. Kim, J. Y. Oh, S. Y. Kim, S. U. Choi and K. R. Lee, Bioorg. Med. Chem. Lett., 2012, 22, 6155 CrossRef CAS PubMed.
- (a) G. C. Reddy, P. Balasubhramanyam, N. Salvanna, T. S. Reddy and B. Das, Bioorg. Med. Chem. Lett., 2012, 22, 2415 CrossRef PubMed; (b) Ch. Sudhakar, P. R. Reddy, G. C. Kumar, P. Sujitha and B. Das, Eur. J. Org. Chem., 2012, 1253 CrossRef CAS; (c) D. B. Shinde, B. S. Kanth, M. Srilatha and B. Das, Synthesis, 2012, 469 CAS; (d) Ch. R. Reddy, B. Veeranjaneyulu, S. Nagendra and B. Das, Helv. Chim. Acta, 2013, 96, 505 CrossRef CAS; (e) P. R. Reddy, Ch. Sudhakar, J. N. Kumar and B. Das, Helv. Chim. Acta, 2013, 96, 289 CrossRef CAS.
- (a) C. E. Rye and D. Barker, Eur. J. Med. Chem., 2013, 60, 240 CrossRef CAS PubMed; (b) S. Kumar Das, S. Kumar Das and G. Panda, Eur. J. Org. Chem., 2010, 5100 CrossRef CAS; (c) A.-L. Lee and S. V. Ley, Org. Biomol. Chem., 2003, 1, 3957 RSC; (d) C. Curti, F. Zanardi, L. Battistini, A. Sartori, G. Rassu, L. Pinna and G. Casiraghi, J. Org. Chem., 2006, 71, 8552 CrossRef CAS PubMed; (e) M. Nagaraju, R. Chandra and B. B. Gawali, Synlett, 2012, 23, 1485 CrossRef CAS PubMed.
- C. Sudhakar, P. R. Reddy, C. G. Kumar, P. Sujitha and B. Das, Eur. J. Org. Chem., 2012, 1253 CrossRef CAS.
- S. Tamura, A. Shiomi, T. Kimura and N. Murakami, Bioorg. Med. Chem. Lett., 2010, 20, 2082 CrossRef CAS PubMed.
- K. B. Sharpless, W. Amberg, Y. L. Bennani, G. A. Crispino, J. Hartung, K. S. Jeong, H. L. Kwong, K. Morikawa and Z. M. Wang, J. Org. Chem., 1992, 57, 2768 CrossRef CAS.
- H. J. Yuan, Y. Y. Cheng, S. Qian, X. Xiao and Y. Wu, Chin. Chem. Lett., 2010, 21, 127 CrossRef CAS PubMed.
- B. Das, B. Veeranjaneyulu, P. Balasubramanyam and M. Srilatha, Tetrahedron: Asymmetry, 2010, 21, 2762 CrossRef CAS PubMed.
- S. P. Chavan and Ch. Praveen, Tetrahedron Lett., 2005, 46, 1939 CrossRef CAS PubMed.
- O. Mistunobu and M. Yamada, Bull. Chem. Soc. Jpn., 1967, 40, 2380 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: experimental procedures, copies of 1H and 13C NMR spectra of products. See DOI: 10.1039/c3ra45419c |
| ‡ Part 68 in the series, synthetic studies on natural products. |
| This journal is © The Royal Society of Chemistry 2014 |



![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHMgBr, THF, 0 °C to room temperature, 85%; (b) (i) IBX, DMSO, CH2Cl2, 0 °C to room temperature; (ii) (
CHMgBr, THF, 0 °C to room temperature, 85%; (b) (i) IBX, DMSO, CH2Cl2, 0 °C to room temperature; (ii) (