Electrical conductivity and DFT investigations of a 2D CuI-TCNQII− framework†
Ashley L.
Sutton
 ,
Brendan F.
Abrahams
,
Brendan F.
Abrahams
 *,
Christopher J.
Commons
*,
Christopher J.
Commons
 ,
A. David
Dharma
,
Lars
Goerigk
,
A. David
Dharma
,
Lars
Goerigk
 ,
Simon G.
Hardin
,
Simon G.
Hardin
 ,
Timothy A.
Hudson
and
Richard
Robson
,
Timothy A.
Hudson
and
Richard
Robson
School of Chemistry, University of Melbourne, Victoria, 3010, Australia. E-mail: bfa@unimelb.edu.au; Tel: +61 3 8344 0341
First published on 24th October 2023
Abstract
An investigation of the coordination polymer, [Cu2(Me2pz)(TCNQ)] (Me2pz = 2,5-dimethylpyrazine) is described. The structure of [Cu2(Me2pz)(TCNQ)] is comprised of 2D sheets with mean plane sheet-to-sheet separations closer than that of graphene sheets in graphite. Electrical conducticity measurements on the framework indicate semiconductor behaviour with a σ300K = 9.8 × 10−8 S cm−1 and an Ea = 0.43(3) eV. Density functional theory studies reveal a band structure comprised predominatly by donor TCNQ and acceptor Me2pz ligand-p oribtals. This investigation also resulted in the formation of Cu(TCNQ) (phase I) from the reaction mixture that yielded [Cu2(TCNQ)(Me2pz)]. A redetermination of the crystal structure of Cu(TCNQ) is reported.
Introduction
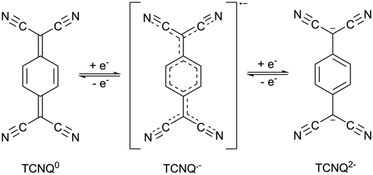
In recent years, there has been considerable effort to improve the charge-transport properties of porous coordination polymers.1,2 Some of the most prominent and highly conductive systems have been planar or near-planar 2D frameworks. These include [Ni3(BHT)2] (H6BHT = benzenehexathiol), and (Me2NH2)2[Fe2(can)3] (H2can = chloranilic acid), which have room temperature conductivities of 0.15 S cm−1 and 1.4 × 10−2 S cm−1 respectively.3,4 The high electrical conductivity of each material is attributed to ligand mixed valency.7,7,8,8-Tetracyanoquinodimethane, TCNQ, has attracted significant attention as a mixed-valent partner in organic charge-transfer complexes.5 Perhaps one of the most prominent examples of charge transfer complexes is tetrathiafulvalene-7,7,8,8-tetracyanoquinodimethane, TTF-TCNQ, which displays metallic conductivity (>105 S cm−1 at 58 K).6 Three accessible oxidation states of TCNQ exist, which leads to a considerable potential for mixed valency. The neutral form of TCNQ is a strong π acceptor, while the radical monoanion, which may be accessed through a one electron reduction, can be considered a weak π-donor. A second one electron reduction leads to the formation of the dianion which is a strong π donor (Fig. 1). Although the dianion is susceptible to air oxidation it can be stabilised by coordination to metal centres and through its participation in charge transfer interactions with π-acceptors.7–18
Not surprisingly, given that each of the nitrile groups has the potential to act as a donor group, there has been long-term interest in metal-TCNQ materials. Cu(TCNQ) has been the focus of particular attention, as its semi-conducting properties depend upon the method of preparation. Variability in the electrical conductivity of Cu(TCNQ) was explained by Dunbar and co-workers who discovered that Cu(TCNQ) existed in two structural forms, a kinetic product (phase I) and a thermodynamic product (phase II).19 Both phases act as semiconductors with phase I offering superior electrical conductivity.
As part of the general interest in metal complexes of TCNQ, we have directed our efforts towards the generation of TCNQ2− coordination polymers.7–16 As part of an investigation into CuI2(TCNQ−II) and CuI2(F4TCNQ−II) coordination polymers we reported the synthesis and structure of [Cu2(Me2pz)(TCNQ)] (Me2pz = 2,5-dimethylpyrazine).14 This 2D framework displayed structural features that warranted further attention. In particular, it is noted that the mean planes of the 2D sheets are separated by a distance of 3.20 Å which is shorter than the separation of graphene sheets in graphite (3.41 Å).20 Given the ability of TCNQ to act as a mixed-valent species, we have further explored physical properties of [Cu2(Me2pz)(TCNQ)] which are reported herein. This investigation is complemented by density functional theory (DFT) calculations which provide insight into electrical conductivity pathways.
Results and discussion
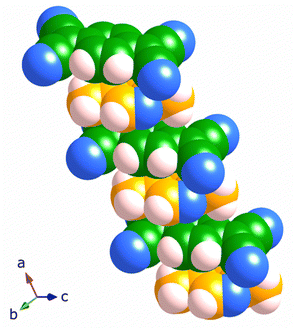
The structure of [Cu2(Me2pz)(TCNQ)], is comprised of crystallographically equivalent trigonal planar copper(I) centres, which are coordinated by two TCNQ2− anions and one Me2pz ligand.14 Each TCNQ2− anion coordinates four copper centres at the corners of a rectangle to form a [Cu2(TCNQ)] sheet. The remaining copper(I) sites are occupied by bridging Me2pz ligands (Fig. 2). Whilst the Me2pz ligand is co-planar with the mean plane of the sheet, the aromatic core of the TCNQ2− is rotated from the mean plane by approximately 13°. Sheets stack parallel to the [4 0 1] direction. Face-to-face interactions between Me2pz and TCNQ2− ligands of neighbouring sheets, results in the generation of infinite π–π stacks (Fig. 3). This stacking of the 2D [Cu2(Me2pz)(TCNQ)] networks results in an intersheet Cu⋯Cu separation of 3.20 Å.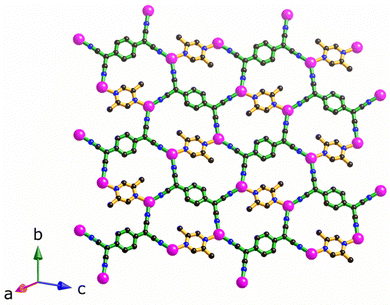 | ||
| Fig. 2 The 2D sheet structure of [Cu2(Me2pz)(TCNQ)]; carbon, nitrogen and copper atoms in black, blue and pink respectively. | ||
Replacement of Me2pz with the chelating ligand N,N′-tetramethyl-ethylenediamine (Me4en) yields a very similar 2D framework with composition [Cu2(TCNQ)(Me4en)2], whilst 2-aminopyrazine yields an open 3D framework.14 Clearly the type of co-ligand used can have a considerable impact on the dimensionality of the polymeric material.
Charge-transfer interactions commonly occur in X4TCNQ2− (X = H or F) based compounds and in certain instances result in unusual and interesting properties.15–18 The degree of charge transfer can be estimated by various methods5,21–24 that are commonly linked to an estimation of the of the charge (q) of the TCNQ species. The Kistenmacher equation is an empirical relationship represented by the formula:
| q = A[c/(b + d)] + B |
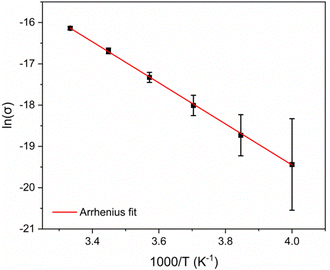
The short sheet-to-sheet separation and the infinite mixed-stacks of Me2pz and TCNQ2− prompted an examination of the electrical conductivity of [Cu2(Me2pz)(TCNQ)]. Although many X4TCNQ2− coordination polymers have been reported,15 there have been relatively few conductivity studies.16,25 In this current work two-point pressed-pellet conductivity measurements reveal a value of σ = 9.8 × 10−8 S cm−1 at 300 K. Variable temperature measurements between 250 and 300 K, show an increase in conductivity with temperature, indicating semiconductor behaviour. Current density-electric field strength plots for [Cu2(Me2pz)(TCNQ)] are presented in Fig. S2.1 (ESI†). Through application of the Arrhenius model, an estimation of the activation energy (Ea) of 0.43(3) eV was determined (Fig. 4).
With such significant π–π interactions it is somewhat surprising the charge-transport is not greater, especially given that non-TCNQ frameworks, albeit those utilising TTF-type ligands, have displayed considerable through-space charge-transport.26,27
The electronic band structure of [Cu2(Me2pz)(TCNQ)] has been examined using solid-state DFT. The calculated band structure and density of states plots at the HSE0628//PBE29-D3(BJ)30,31 level of theory are presented in Fig. 5. The conduction and valence bands of the [Cu2(Me2pz)(TCNQ)] framework are comprised predominantly of ligand p orbitals from the Me2pz and TCNQ2− respectively (see ESI,† Fig. S3.1). This is unsurprising, given the mixed stack packing arrangement of TCNQ2- and Me2pz. As expected, Me2pz ligands act as the acceptor whilst the TCNQ2− ligands serve as donors. The calculated electronic bandgap of 1.7 eV, effectively represents the difference between the ionisation energy of the donor (TCNQ2−) and the electron affinity of the acceptor (Me2pz). There is a very minor contribution from the Cu-d orbitals to the valence band which alters the valence energy, although such a small contribution is unlikely to indicate metal–ligand mediated charge-transport.
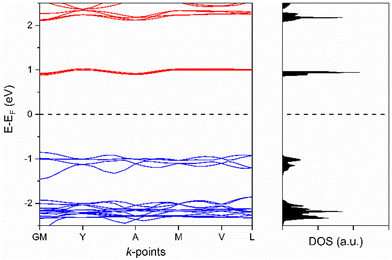 | ||
| Fig. 5 Band structure and density of states (DOS) for [Cu2(Me2pz)(TCNQ)]. The valence and conduction bands are shown in blue and red respectively. | ||
The conduction and valence band dispersions are moderate for a coordination polymer at 190 and 120 meV respectively. The largest dispersion in the conduction band occurs along the G–Y vector in reciprocal space, which corresponds to a vector along stacks of the Me2pz ligands. We note it may be possible to generate a framework with enhanced semiconductor behaviour through the use of a ligand with a comparable geometric profile to Me2pz but with a higher electron affinity and high degree of charge delocalisation.
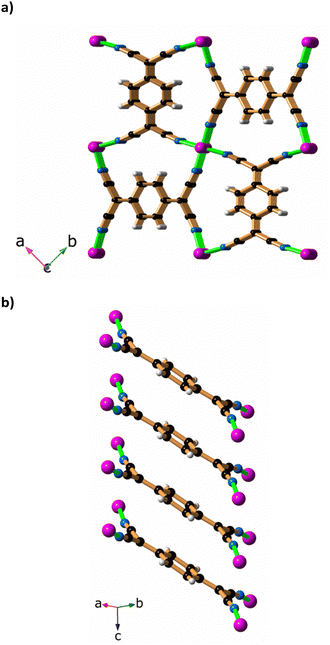
A final interesting aspect of this work relates to the air oxidation of the sample that occurs if the crystals of [Cu2(Me2pz)(TCNQ)] remain in the mother liquor. Over a period of days the orange [Cu2(Me2pz)(TCNQ)] crystals are accompanied by black, phase I Cu(TCNQ) crystals which were suitable for X-ray crystallography. It would appear that precautions to exclude atmospheric oxygen failed to prevent the slow oxidation of the TCNQ dianion to the radical monoanionic form. It was uncertain as to whether the Cu(TCNQ) crystals formed from the orange [Cu2(Me2pz)(TCNQ)] crystals or grew independently from the mother liquor. When less stringent conditions relating to exclusion of air are employed, the orange [Cu2(Me2pz)(TCNQ)] crystals are accompanied by crystals of Cu(TCNQ) which form at the same time, suggesting that Cu(TCNQ) is formed directly from the mother liquor. The original structure determination of the phase I crystals was hampered by the quality of the crystals, which were produced by mixing either TCNQ or TCNQ− with Cu(I) in acetonitrile.19 The crystallographic problems included severe disorder and significant pseudomerohedral twinning. The original crystal structure was reported with cell dimensions a = b = 11.266(2) Å, c = 3.8878(8) Å, α = β = γ = 90°. Although the cell dimensions were consistent with a tetragonal cell, a satisfactory refinement in tetragonal symmetry could not be achieved because of the poor quality of the crystalline product. The structure was ultimately solved and refined in the monoclinic space group, Pn, using isotropic displacement parameters. The R1 and wR2 values of 23.2 and 48.1% reflect the poor quality of the crystals. The Cu(TCNQ) crystals obtained from oxidation of TCNQ2−, as reported in this current work, were examined using X-ray crystallography and a tetragonal cell of similar dimensions to those previously found by Dunbar and co-workers was identified.32 The subsequent structure determination was achieved in the tetragonal space group P42/mnm. The data set collected on this crystal was not ideal, but it did yield an Rint value of 4.28%. Refinement of the structure resulted in a wR2 (all data) value of 19.96% and R1 (I > 2σ(I)) value of 6.33%. Within the structure all the TCNQ anions form stacks that extend in the c-direction. The TCNQ anions are inclined to the c-axis at an angle of 30.3(4)°. Whilst all TCNQ anions within a stack must be parallel, each TCNQ anion is disordered over two symmetry related sites. Similarly, Cu(I) centres are disordered over sites lying along the direction of the c axis. The disorder with both the TCNQ anion and the Cu(I) centres does not allow unambiguous identification of the network topology. A view along the direction of the TCNQ stacks is presented in Fig. 6a whilst Fig. 6b shows a single stack from side-on.
The generation of Cu(TCNQ) from the dianionic form of TCNQ, shares similarities with an approach employed in the formation of metal anilate coordination polymers.33,34 The slow air oxidation of the tetrahydroxy aromatic form to the dianionic benzoquinone represents a favourable synthetic pathway in the formation of crystallographically usable crystals of the metal anilate polymer. It appears in such circumstances that crystal growth is promoted by the slow generation of the oxidised form of the ligand which is incorporated in the final product.
Conclusions
Measurement of the electrical conductivity of the 2D coordination polymer, [Cu2(Me2pz)(TCNQ)] revealed modest semiconducting behaviour with an electrical conductivity of 9.8 × 10−8 S cm−1 and an activation energy of 0.43 eV. Solid-state DFT analysis reveals that the conduction bands are predominantly p-orbitals from TCNQ2− whilst the valence band consists largely of Me2pz p-orbitals and thus the electrical conductivity would appear to result from a charge-transfer interaction between neighbouring TCNQ2− and Me2pz ligands within infinite stacks that extend in the a direction. The results described in this report provide a clear example of the ability of the TCNQ in its coordinated dianionic form to participate in charge transfer interactions which result in semiconductor behaviour.The reaction conditions that yielded [Cu2(TCNQ)(Me2pz)] have also permitted slow air oxidation of the TCNQ dianion and in the process generated crystallographically useful crystals of Cu(TCNQ) (phase I). This result provides encouragement that other elusive metal-TCNQ materials may be generated by the slow air oxidation of the TCNQ dianion.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The support of the Australian Research Council is gratefully acknowledged. ALS thank the Australian Government for an Australian Postgraduate Award. This research was undertaken with the assistance of resources and services from the National Computational Infrastructure (NCI), within the National Computational Merit Allocation Scheme (project fk5).References
- L. Sun, M. G. Campbell and M. Dinca, Angew. Chem., Int. Ed., 2016, 55, 3566–3579 CrossRef CAS PubMed.
- R. Murase, C. F. Leong and D. M. D'Alessandro, Inorg. Chem., 2017, 56, 14373–14382 CrossRef CAS PubMed.
- T. Kambe, R. Sakamoto, K. Hoshiko, K. Takada, M. Miyachi, J. H. Ryu, S. Sasaki, J. Kim, K. Nakazato, M. Takata and H. Nishihara, J. Am. Chem. Soc., 2013, 135, 2462–2465 CrossRef CAS.
- J. A. DeGayner, I. R. Jeon, L. Sun, M. Dinca and T. D. Harris, J. Am. Chem. Soc., 2017, 139, 4175–4184 CrossRef CAS PubMed.
- K. P. Goetz, D. Vermeulen, M. E. Payne, C. Kloc, L. E. McNeil and O. D. Jurchescu, J. Mater. Chem. C, 2014, 2, 3065–3076 RSC.
- M. J. Cohen, L. B. Coleman, A. F. Garito and A. J. Heeger, Phys. Rev. B: Solid State, 1974, 10, 1298–1307 CrossRef CAS.
- B. F. Abrahams, T. A. Hudson and R. Robson, Cryst. Growth Des., 2008, 8, 1123–1125 CrossRef CAS.
- B. F. Abrahams, R. W. Elliott, T. A. Hudson and R. Robson, Cryst. Growth Des., 2010, 10, 2860–2862 CrossRef CAS.
- B. F. Abrahams, R. W. Elliott, T. A. Hudson and R. Robson, CrystEngComm, 2012, 14, 351–354 RSC.
- B. F. Abrahams, R. W. Elliott, T. A. Hudson and R. Robson, Cryst. Growth Des., 2013, 13, 3018–3027 CrossRef CAS.
- B. F. Abrahams, R. W. Elliott and R. Robson, Aust. J. Chem., 2014, 67, 1871–1877 CrossRef CAS.
- T. H. Le, A. Nafady, A. N. T. Vo, R. W. Elliott, T. A. Hudson, R. Robson, B. F. Abrahams, L. L. Martin and A. M. Bond, Inorg. Chem., 2014, 53, 3230–3242 CrossRef CAS.
- M. R. Saber, A. V. Prosvirin, B. F. Abrahams, R. W. Elliott, R. Robson and K. R. Dunbar, Chem. – Eur. J., 2014, 20, 7593–7597 CrossRef CAS PubMed.
- B. F. Abrahams, R. W. Elliott, T. A. Hudson, R. Robson and A. L. Sutton, Cryst. Growth Des., 2015, 15, 2437–2444 CrossRef CAS.
- B. F. Abrahams, R. W. Elliott, T. A. Hudson, R. Robson and A. L. Sutton, CrystEngComm, 2018, 20, 3131–3152 RSC.
- R. W. Elliott, A. L. Sutton, B. F. Abrahams, D. M. D’Alessandro, L. Goerigk, C. Hua, T. A. Hudson, R. Robson and K. F. White, Inorg. Chem., 2021, 60, 13658–13668 CrossRef CAS PubMed.
- T. A. Hudson and R. Robson, Cryst. Growth Des., 2009, 9, 1658–1662 CrossRef CAS.
- A. L. Sutton, B. F. Abrahams, D. M. D'Alessandro, R. W. Elliott, T. A. Hudson, R. Robson and P. M. Usov, CrystEngComm, 2014, 16, 5234–5243 RSC.
- R. A. Heintz, H. Zhao, X. Ouyang, G. Grandinetti, J. Cowen and K. R. Dunbar, Inorg. Chem., 1999, 38, 144–156 CrossRef CAS.
- J. D. Bernal, Proc. R. Soc. London, Ser. A, 1924, A106, 749 Search PubMed.
- T. J. Kistenmacher, T. J. Emge, A. N. Bloch and D. O. Cowan, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1982, 38, 1193–1199 CrossRef.
- T. C. Umland, S. Allie, T. Kuhlmann and P. Coppens, J. Phys. Chem., 1988, 92, 6456–6460 CrossRef CAS.
- S. Matsuzaki, R. Kawata and K. Toyoda, Solid State Commun., 1980, 33, 403–405 CrossRef CAS.
- J. S. Chappell, A. N. Bloch, W. A. Bryden, M. Maxfield, T. O. Poehler and D. O. Cowan, J. Am. Chem. Soc., 1981, 103, 2442–2443 CrossRef CAS.
- S. Shimomura, N. Yanai, R. Matsuda and S. Kitagawa, Inorg. Chem., 2011, 50, 172–177 CrossRef CAS PubMed.
- T. C. Narayan, T. Miyakai, S. Seki and M. Dinca, J. Am. Chem. Soc., 2012, 134, 12932–12935 CrossRef CAS PubMed.
- S. S. Park, E. R. Hontz, L. Sun, C. H. Hendon, A. Walsh, T. Van Voorhis and M. Dinca, J. Am. Chem. Soc., 2015, 137, 1774–1777 CrossRef CAS PubMed.
- A. V. Krukau, O. A. Vydrov, A. F. Izmaylov and G. E. Scuseria, J. Chem. Phys., 2006, 125, 224106 CrossRef PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- Crystallographic details for Cu(TCNQ), C12H4N4Cu M = 267.73, tetragonal, a = b = 11.2444(6), c = 3.8703(15) Å, V = 489.3(2) Å3, T = 100.0(1) K, Z = 2, P42/mnm, 1459 reflections measured, 295 independent reflections, Rint = 0.0428, wR2 (all data) = 0.1996, R1 (I >2σ(I)) = 0.0633. CIF deposited with Cambridge Crystallographic Data Centre (CCDC), deposition number 2291474†.
- B. F. Abrahams, T. A. Hudson, L. J. McCormick and R. Robson, Cryst. Growth Des., 2011, 11, 2717–2720 CrossRef CAS.
- C. J. Kingsbury, B. F. Abrahams, D. M. D'Alessandro, T. A. Hudson, R. Murase, R. Robson and K. F. White, Cryst. Growth Des., 2017, 17, 1465–1470 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of Kistenmacher relationship calculations, electrical conductivity and computational details. CCDC 2291474. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3tc03237j |
| This journal is © The Royal Society of Chemistry 2023 |