Selective oxidation of benzylic alcohols via synergistic bisphosphonium and cobalt catalysis†
Jia
Ding‡
ab,
Shuaishuai
Luo‡
ab,
Yuanli
Xu
c,
Qing
An
a,
Yi
Yang
 *c and
Zhiwei
Zuo
*c and
Zhiwei
Zuo
 *b
*b
aSchool of Physical Science and Technology, ShanghaiTech University, Shanghai 201210, China
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China. E-mail: zuozhw@sioc.ac.cn
cInnovation Center for Chenguang High Performance Fluorine Material, Key Laboratory of Green Chemistry of Sichuan Institutes of Higher Education, Sichuan University of Science and Engineering, Zigong, CN 643000, China
First published on 8th March 2023
Abstract
A synergistic photocatalytic system using a bisphosphonium catalyst and a cobalt catalyst has been developed, enabling the selective oxidation of benzylic alcohols under oxidant-free and environmentally benign conditions. High efficiencies have been obtained for a variety of alcohol substrates, and exclusive selectivity for aldehyde products has been achieved across the board. Furthermore, this photocatalytic system proved to be efficient when performed under continuous-flow conditions, even using a simple and easily assembled continuous-flow setup.
Central to the recent emergence of photoredox catalysis, the development of photocatalysts has played a critical role in achieving novel and sustainable transformations.1 Organophosphorus molecules have long been recognized for their unique electronic properties and optical behaviour.2,3 Despite extensive research in organic electronics, fluorescence imaging and optical sensing, the utilization of organophosphorus compounds in photoredox catalysis remains largely underdeveloped compared with other types of organic chromophore such as acridinium dyes.4–9 Photoexcited bisphosphonium 3, which can be easily prepared from 2,2′-bis(diphenylphosphino)-1,1′-binaphthyl (BINAP),10 showcases an outstanding oxidizing capacity in comparison with the most commonly used Mes-Acr+ photocatalyst; nevertheless, only limited examples have been reported that include the oxidative cyclization of alkenes and the polymerization of vinyl ethers.11–13 The synergistic incorporation of photocatalysts with transition metal catalysts has emerged as a powerful platform to expand the synthetic realm of radical-mediated transformations.14 Herein, we report the first synergistic catalytic system using a bisphosphonium photocatalyst and a cobalt catalyst for the selective photocatalytic oxidation of benzylic alcohols.
The selective oxidation of alcohols to aldehydes and ketones is an essential process in organic synthesis, which constantly drives the development of new catalytic strategies to achieve higher levels of efficiency and economic value under environmentally benign and practical conditions.15–17 Eminently, the photochemical hydrogen evolution strategy has enabled the development of efficient dehydrogenation processes under oxidant-free and ambient conditions, enabling the oxidation of alcohols in an atom-economical fashion with molecular hydrogen as the sole side product.18–30 Recently, Wu and co-workers have developed dual catalytic systems with nickel ions acting as the proton reduction catalyst and CdSe quantum dots or eosin Y being used as the photocatalyst, achieving the selective oxidation of benzylic alcohols in aqueous medium.31,32 Kanai, Mitsunuma and co-workers recently demonstrated a triple catalytic system employing a Mes-Acr+ photocatalyst, a nickel catalyst and a thiophosphate organocatalyst to oxidize aliphatic secondary alcohols into ketones.33 Considering the synthetic utility of alcohol oxidation reactions and the commonly encountered scalability problem with photocatalytic transformations,34 efficient photocatalytic systems that are adaptable in continuous flow conditions remain in high demand. Here we disclose a practical bisphosphonium/Co catalytic system that can enable the selective oxidation of benzylic alcohols in batch as well as continuous-flow conditions (Fig. 1).
 | ||
| Fig. 1 Photocatalytic selective oxidation of benzylic alcohols via synergistic bisphosphonium and cobalt catalysis. | ||
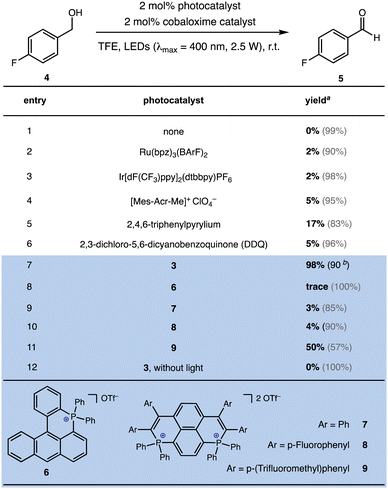
p-Fluorobenzylic alcohol was initially chosen as the template substrate to initiate reaction optimization. Under the irradiation of LED light (light intensity: 2.5 W cm−2, λmax = 400 nm), a variety of photocatalysts were examined with the commonly utilized H2 evolution catalyst CoIII(dmgH)2pyCl.35 As demonstrated in Fig. 2, the use of oxidizing polypyridyl metal catalysts such as Ru(bpz)32+ or Ir[dF(CF3)ppy]2(dtbbpy)+ led to only a trace amount of the aldehyde product, and a large amount of the alcohol remained in solution. The use of more oxidizing organophotocatalysts, including [Mes-Acr-Me]+, 2,4,6-triphenylpyrylium or DDQ resulted in a slight improvement of the catalytic efficiency. In striking contrast, the utilization of bisphosphonium catalyst 3 enabled the full conversion of benzylic alcohol 4 to benzaldehyde 5 with 98% yield. Importantly, the possible generation of benzoic acid was examined under scrutiny and ruled out via GCMS and 1H NMR, validating the high selectivity of aldehyde production. Several other types of cationic P-containing conjugated arene were evaluated and compared side by side with catalyst 3. Phosphonium salt 6 (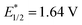 vs. SCE), which was previously reported to exhibit intense fluorescence in aqueous medium,36,37 failed to deliver any desired product in this case. Bisphosphapyrenium salts (7–9), which possess the dicationic P-containing motif with close resemblance to bisphosphonium salt 3, were also tested,38,39 and only the salt with electron-deficient arene substitution (9,
vs. SCE), which was previously reported to exhibit intense fluorescence in aqueous medium,36,37 failed to deliver any desired product in this case. Bisphosphapyrenium salts (7–9), which possess the dicationic P-containing motif with close resemblance to bisphosphonium salt 3, were also tested,38,39 and only the salt with electron-deficient arene substitution (9, 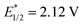 vs. SCE) exhibited a moderate catalytic efficiency. These results further indicated the importance of the high oxidative capacity of bisphosphonium 3 (
vs. SCE) exhibited a moderate catalytic efficiency. These results further indicated the importance of the high oxidative capacity of bisphosphonium 3 (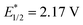 vs. SCE). Moreover, control experiments revealed the critical role of light and photocatalysts.
vs. SCE). Moreover, control experiments revealed the critical role of light and photocatalysts.
With the environmentally benign catalysts and operationally simple conditions in hand, the reaction scope was further investigated. As exhibited in Fig. 3, a variety of primary and secondary benzylic alcohols can be oxidized to the corresponding carbonyl products smoothly using bisphosphonium catalyst 3 and the cobaloxime catalyst. Importantly, the seemingly trivial influence of the electronic properties of the benzylic motif on the catalytic efficiency was observed, as both electronically donating groups such as p-alkyl (12) and p-methoxyl (14) as well as electronically withdrawing groups such as p-chloro (19 and 31), p-fluoro (25) and 3,4-dichloro (20) can be well-tolerated. Furan and benzofuran, which are particularly prone to degradation under oxidative conditions, can also be tolerated in this oxidant-free photocatalytic transformation (products 22 and 28). Critically, in the cases of 23 and 30 where two different hydroxyl groups were presented in the same molecule, only the benzylic hydroxyl group was selectively oxidized and the primary hydroxyl group distal to the phenyl ring was left intact. Furthermore, the allylic alcohol with a conjugated phenyl substitution can be converted to the corresponding carbonyl product 32.
 | ||
| Fig. 3 Reaction scope. Batch reactions were performed at the 0.2 mmol scale, and isolated yields are shown. aYields were determined via GC-FID due to volatile products. b4 mol% bisphosphonium 3 and 4 mol% cobaloxime were used. cMeCN was used instead of TFE. See ESI† for experimental details. | ||
To further evaluate the synthetic potential of this synergistic bisphosphonium/Co catalytic system, scaled up reactions in an easily-assembled continuous-flow setup were conducted (Fig. 3). Due to the photon absorption and utilization efficiency in scaled-up vessels, photocatalytic transformations oftentimes can suffer from low conversions at the larger scale, posing significant limitations on synthetic applications. The fast conversion rate (most substrates can reach full conversion in 2 hours) and the homogeneous dispersion of all components in the TFE solvent enabled a smooth transfer from batch conditions to continuous-flow conditions, without the need for any further optimization of the catalysts. With a commonly occurring PTFE tubing coil used as the photoreactor, a series of benzaldehydes and aromatic ketones can be easily prepared at production rates of ∼5–13 mmol day−1 with satisfactory yields.
Primary mechanistic investigations shed some light on the mechanism of this synergistic catalytic system (Fig. 4). Stern–Volmer quenching experiments between p-methoxybenzylic alcohol and photocatalyst 3 indicated a feasible photoinduced single electron transfer (SET) event which will oxidize the arene to the corresponding radical cation intermediate. Furthermore, kinetic isotope experiments with PhCH2OH and α-deuterated PhCD2OH conducted in different vessels revealed a large primary KIE of 2.1, whereas the same set of KIE experiments with PhCH2OH and PhCH2OD rendered a KIE of 1.1. Intermolecular competition KIE experiments with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of PhCH2OH and PhCD2OH in the same vessel, as well as intramolecular competition experiments with PhCHDOH, indicated a primary KIE of 2.0. These results unambiguously validate the cleavage of the α-C–H bond of benzylic alcohol as the turnover limiting step. Furthermore, kinetic analysis revealed first order kinetics in catalyst 3 as well as in CoIII(dmgH)2pyCl at low concentration. Based on these observations, we propose that the oxidation process starts via SET between the benzylic alcohol and photoexcited 3, which will generate a radical cation intermediate with a weakened benzylic C–H bond. Subsequent Co-mediated hydrogen atom transfer will deliver the desired carbonyl product after deprotonation. The resultant [CoIII–H] complex then reacts with H+ to release H2. Finally, SET between the reduced radical anion 3 (E1/2 = −0.55 V vs. SCE) and [CoIII] (E1/2 = −0.16 V vs. SCE) completes both catalytic cycles. This synergistic bisphosphonium/Co catalytic system enables the selective oxidation of benzylic alcohols under batch as well as continuous-flow conditions.
1 ratio of PhCH2OH and PhCD2OH in the same vessel, as well as intramolecular competition experiments with PhCHDOH, indicated a primary KIE of 2.0. These results unambiguously validate the cleavage of the α-C–H bond of benzylic alcohol as the turnover limiting step. Furthermore, kinetic analysis revealed first order kinetics in catalyst 3 as well as in CoIII(dmgH)2pyCl at low concentration. Based on these observations, we propose that the oxidation process starts via SET between the benzylic alcohol and photoexcited 3, which will generate a radical cation intermediate with a weakened benzylic C–H bond. Subsequent Co-mediated hydrogen atom transfer will deliver the desired carbonyl product after deprotonation. The resultant [CoIII–H] complex then reacts with H+ to release H2. Finally, SET between the reduced radical anion 3 (E1/2 = −0.55 V vs. SCE) and [CoIII] (E1/2 = −0.16 V vs. SCE) completes both catalytic cycles. This synergistic bisphosphonium/Co catalytic system enables the selective oxidation of benzylic alcohols under batch as well as continuous-flow conditions.
Conflicts of interest
There are no conflicts to declare.Notes and references
- N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075–10166 CrossRef CAS PubMed.
- T. Baumgartner and R. Réau, Chem. Rev., 2006, 106, 4681–4727 CrossRef CAS PubMed.
- T. Baumgartner, Acc. Chem. Res., 2014, 47, 1613–1622 CrossRef CAS PubMed.
- M. P. Duffy, W. Delaunay, P. A. Bouit and M. Hissler, Chem. Soc. Rev., 2016, 45, 5296–5310 RSC.
- S. O. Jeon and J. Y. Lee, J. Mater. Chem., 2012, 22, 4233–4243 RSC.
- P. Federmann, H. K. Wagner, P. W. Antoni, J. M. Morsdorf, J. L. Perez Lustres, H. Wadepohl, M. Motzkus and J. Ballmann, Org. Lett., 2019, 21, 2033–2038 CrossRef CAS PubMed.
- A. Belyaev, P. T. Chou and I. O. Koshevoy, Chem. – Eur. J., 2020, 27, 537–552 CrossRef PubMed.
- T. Delouche, A. Vacher, T. Roisnel, M. Cordier, J.-F. Audibert, B. Le Guennic, F. Miomandre, D. Jacquemin, M. Hissler and P.-A. Bouit, Mater. Adv., 2020, 1, 3369–3377 RSC.
- E. Regulska and C. Romero-Nieto, Mater. Today Chem., 2021, 22, 100604 CrossRef CAS.
- S. Nieto, P. Metola, V. M. Lynch and E. V. Anslyn, Organometallics, 2008, 27, 3608 CrossRef CAS.
- H. Cheng, X. Wang, L. Chang, Y. Chen, L. Chu and Z. Zuo, Sci. Bull., 2019, 64, 1896–1901 CrossRef CAS PubMed.
- Z. Yang, J. Chen and S. Liao, ACS Macro Lett., 2022, 11, 1073–1078 CrossRef CAS PubMed.
- X. Zhang, Y. Jiang, Q. Ma, S. Hu and S. Liao, J. Am. Chem. Soc., 2021, 143, 6357–6362 CrossRef CAS PubMed.
- J. Twilton, C. Le, P. Zhang, M. H. Shaw, R. W. Evans and D. W. C. MacMillan, Nat. Rev. Chem., 2017, 1, 0052 CrossRef CAS.
- I. Borthakur, A. Sau and S. Kundu, Coord. Chem. Rev., 2022, 451, 214257 CrossRef CAS.
- C. Gunanathan and D. Milstein, Science, 2013, 341, 1229712 CrossRef PubMed.
- R. H. Crabtree, Chem. Rev., 2017, 117, 9228–9246 CrossRef CAS PubMed.
- Q.-Y. Meng, J.-J. Zhong, Q. Liu, X.-W. Gao, H.-H. Zhang, T. Lei, Z.-J. Li, K. Feng, B. Chen, C.-H. Tung and L.-Z. Wu, J. Am. Chem. Soc., 2013, 135, 19052–19055 CrossRef CAS PubMed.
- Y.-W. Zheng, B. Chen, P. Ye, K. Feng, W. Wang, Q.-Y. Meng, L.-Z. Wu and C.-H. Tung, J. Am. Chem. Soc., 2016, 138, 10080–10083 CrossRef CAS PubMed.
- G. Zhang, X. Hu, C.-W. Chiang, H. Yi, P. Pei, A. K. Singh and A. Lei, J. Am. Chem. Soc., 2016, 138, 12037–12040 CrossRef CAS PubMed.
- L. Niu, H. Yi, S. Wang, T. Liu, J. Liu and A. Lei, Nat. Commun., 2017, 8, 14226 CrossRef PubMed.
- B. Chen, L.-Z. Wu and C.-H. Tung, Acc. Chem. Res., 2018, 51, 2512–2523 CrossRef CAS PubMed.
- X. Hu, G. Zhang, F. Bu, X. Luo, K. Yi, H. Zhang and A. Lei, Chem. Sci., 2018, 9, 1521–1526 RSC.
- H. Cao, H. Jiang, H. Feng, J. M. C. Kwan, X. Liu and J. Wu, J. Am. Chem. Soc., 2018, 140, 16360–16367 CrossRef CAS PubMed.
- A. Wimmer and B. König, Adv. Synth. Catal., 2018, 360, 3277–3285 CrossRef CAS PubMed.
- J. B. McManus, J. D. Griffin, A. R. White and D. A. Nicewicz, J. Am. Chem. Soc., 2020, 142, 10325–10330 CrossRef CAS PubMed.
- M. Kojima and S. Matsunaga, Trends Chem., 2020, 2, 410–426 CrossRef CAS.
- L. Huang, T. Ji, C. Zhu, H. Yue, N. Zhumabay and M. Rueping, Nat. Commun., 2022, 13, 809 CrossRef CAS PubMed.
- X. Wang, Y. Li and X. Wu, ACS Catal., 2022, 12, 3710–3718 CrossRef CAS.
- K. Ohmatsu and T. Ooi, Nat. Synth, 2023, 2, 209–216 Search PubMed.
- L.-M. Zhao, Q.-Y. Meng, X.-B. Fan, C. Ye, X.-B. Li, B. Chen, V. Ramamurthy, C.-H. Tung and L.-Z. Wu, Angew. Chem., Int. Ed., 2017, 56, 3020–3024 CrossRef CAS PubMed.
- X.-J. Yang, Y.-W. Zheng, L.-Q. Zheng, L.-Z. Wu, C.-H. Tung and B. Chen, Green Chem., 2019, 21, 1401–1405 RSC.
- H. Fuse, H. Mitsunuma and M. Kanai, J. Am. Chem. Soc., 2020, 142, 4493–4499 CrossRef CAS PubMed.
- D. Cambié, C. Bottecchia, N. J. W. Straathof, V. Hessel and T. Noël, Chem. Rev., 2016, 116, 10276–10341 CrossRef PubMed.
- J. L. Dempsey, B. S. Brunschwig, J. R. Winkler and H. B. Gray, Acc. Chem. Res., 2009, 42, 1995–2004 CrossRef CAS PubMed.
- A. Belyaev, Y.-T. Chen, Z.-Y. Liu, P. Hindenberg, C.-H. Wu, P.-T. Chou, C. Romero-Nieto and I. O. Koshevoy, Chem. – Eur. J., 2019, 25, 6332–6341 CrossRef CAS PubMed.
- Z. Yang, J. Chen and S. Liao, ACS Macro Lett., 2022, 11, 1073–1078 CrossRef CAS PubMed.
- T. Delouche, A. Vacher, E. Caytan, T. Roisnel, B. Le Guennic, D. Jacquemin, M. Hissler and P.-A. Bouit, Chem. – Eur. J., 2020, 26, 8226–8229 CrossRef CAS PubMed.
- T. Delouche, A. Vacher, T. Roisnel, M. Cordier, J.-F. Audibert, B. Le Guennic, F. Miomandre, D. Jacquemin, M. Hissler and P.-A. Bouit, Mater. Adv., 2020, 1, 3369–3377 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc00532a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |