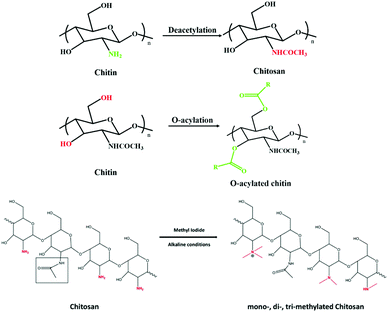
Applications of deep eutectic solvents in the extraction, dissolution, and functional materials of chitin: research progress and prospects
Jiake
Wang
,
Changchang
Teng
and
Lifeng
Yan
 *
*
Hefei National Laboratory for Physical Sciences at the Microscale, and Department of Chemical Physics, iCHEM, University of Science and Technology of China, Jinzai Road 96, Hefei, 230026, Anhui, China. E-mail: lfyan@ustc.edu.cn
First published on 13th December 2021
Abstract
As a recyclable and inexhaustible renewable resource, chitin is one of the most abundant polysaccharides in nature. It has excellent biocompatibility, biodegradability, and non-toxicity and has been widely used in food packaging, tissue engineering, and other fields. Deep eutectic solvents (DESs) are regarded as a new generation of green solvents due to their excellent biocompatibility and stability. Recently, the use of DESs to process chitin has also received widespread attention. In this review, we first introduced DESs and chitin, then discussed the relationship between the properties of DESs and the applications in the extraction, dissolution, and functional materials of chitin. In addition, the related applications are classified and introduced. The opportunities and challenges for DESs when dealing with chitin systems are also summarized.
Introduction
With the development of science and technology, people's quality of life has been greatly improved. However, while enjoying the convenience brought about by modern technology, energy and environmental issues have become increasingly prominent and have attracted widespread attention from all countries. Due to people's inadequate anticipation of the negative effects of highly developed industries and unfavorable prevention, it has led to three major global crises: resource shortage, environmental pollution, and ecological destruction. Sustainable development has become the theme of the 21st century.The development of environmentally friendly materials using widely existing substances in nature can effectively solve environmental problems, and researchers have made a lot of effort for research.1–3 Among these natural materials, chitin has been favored by researchers because of its wide sources and excellent properties in nature. Chitin is composed of β-(1,4) linked N-acetyl-glucosamine units, which is the second-largest natural polymer after cellulose with an annual biosynthesis amount of about 10 billion tons, and it is a recyclable and inexhaustible renewable resource.1,4,5 As the most abundant nitrogen-containing biopolymer in nature, chitin is mainly distributed in the shells of marine arthropods, such as shrimps and crabs, the shells of insects, the shells and bones of mollusks, and the cell walls of higher plants.6–8 Chitin and its derivatives (such as chitosan and etherified chitin) have excellent biocompatibility, non-toxicity, biodegradability, and physiological activity.9,10 Due to these superior properties, chitin and its derivatives have a wide range of applications in the fields of textiles, medicine, food, cosmetics, and bioengineering.11–20
Despite the abundance and excellent properties of chitin, two critical factors hinder the production and utilization of chitin:
The first factor is the extraction and separation of chitin. So far, the main source of chitin is the shells of shrimp and crabs, which are composed of 20%–40% protein, 20%–50% calcium carbonate, and 15%–40% chitin.21–23 Therefore, the traditional process of extracting chitin inevitably goes through two steps: deproteinization and demineralization. At present, the industry uses chemical methods and biological methods to carry out these steps.24,25 In the traditional chemical process, demineralization is achieved by treating with dilute hydrochloric acid to remove calcium carbonate, and deproteinizing is performed by treating the product with sodium hydroxide at high temperatures. The process of chemical treatment involves the use of a large number of acid and alkali, which causes great corrosion to the equipment. In addition, the washing process produced large plenty of wastewater, which caused great pollution to the environment. Biological strategies, such as enzyme treatment and microbial treatment, can avoid the use of acid and alkali and obtain high-quality chitin. The chitin obtained by biological strategies has higher molecular weight and crystallinity than that obtained by chemical treatment25,26 However, biological processes require a lot of time, high energy, and cost, and the removal of minerals and proteins is incomplete, which limits the use of this strategy.
The second factor is the dissolution process of chitin. Chitin is a very stubborn polymer due to its high degree of crystallinity and the existence of a large number of hydrogen bonds between its molecules, which cannot be dissolved in conventional solvents (such as water, dilute acids, and alkalis, and organic solvents).27,28 Poor solubility brings great challenges to chitin-related applications. To date, there are two types of traditional solvents for chitin: aqueous and non-aqueous solvents (LiCl/DMAC and saturated CaCl2·2H2O/methanol).29–35 For aqueous solvents, there are mainly three types of solvents: strong acid solution, inorganic salt solution, and alkaline solution. (1) Chitin can be dissolved in high concentrations of hydrochloric, sulfuric, and phosphoric acid solutions. However, in the process of dissolution, the molecular weight of chitin will be greatly reduced and the degree of deacetylation will be enhanced, resulting in poor performance of the prepared material. In addition, the use of large amounts of acid also causes inevitable pollution to the environment. (2) It has been found that chitin can also be dissolved in inorganic salt solutions, such as LiSCN, CaCl2, and NaI solutions. However, there are few studies on this type of solvent, and the related dissolution mechanism is not clear. (3) The alkaline solvent system has attracted much attention due to its mild dissolution process and high solubility. It is found that chitin has good solubility in the alkali metal/urea system (KOH, NaOH, LiOH/urea). By the freezing and thawing method, chitin and alkali metals interact with each other by electrostatic interaction and hydrogen bond, which weakens the force between chitin molecules. Meanwhile, urea molecules block the aggregation of chitin molecules. The synergistic effect between urea and alkali metals makes chitin have good solubility in the aqueous solution of alkali metals. However, the dissolution process of this system takes a long time, and the solubility of chitin is limited, so it is difficult to expand to industrial production. For non-aqueous solvent systems, there are disadvantages of solvent instability and high toxicity, and they are difficult to produce on a large scale. For example, although the LiCl/DMAC system has a good dissolution effect on chitin, the toxicity of Li+ and DMAC will cause environmental pollution. In addition, this solvent system is very sensitive to water, and the existence of water will greatly reduce the dissolution effect. These shortcomings seriously hinder the use of non-aqueous solvents.
In recent years, ionic liquids (ILs), ionic compounds consisting of charged anion and cation pairs whose melting points are below a certain temperature (e.g. 100 °C), have attracted attention for their excellent solubility and separation properties.36,37 Researchers have also made great progress in separating and dissolving chitin with ionic liquids.38–42 However, the high preparation cost, high toxicity, and difficult recovery of ionic liquids hinder the application of ionic liquids in chitin.43 Finding a solvent with good performance and environmental protection is a huge challenge.
Deep eutectic solvents (DESs) are multi-component eutectic mixtures composed of hydrogen bond acceptors (such as quaternary ammonium salts) and hydrogen bond donors (polyols and polycarboxylic acids, etc.) in a certain molar ratio.44,45 They not only have many advantages similar to ionic liquids (such as low vapor pressure, stability, and conductivity) but more importantly, DESs are considered as a new generation of green solvents because of their biocompatibility, low cost, biodegradability, wide source and other advantages that ionic liquids cannot achieve. Based on these advantages, people have conducted in-depth research on DESs.45–48 Great progress has also been made in the study of DESs related to chitin.
The separation and application of chitin have been summarized wonderfully in other reviews.49–52 In this mini-overview, we begin with a brief introduction to chitin and DESs. The progress of DESs related to chitin (including extraction, dissolution, and related materials) is also summarized. Finally, the development of DESs and chitin is summarized and prospected.
Chitin and DESs
Chitin
As one of the oldest and most abundant polysaccharides, chitin is widely found in nature. As the origin of life, there are more than 180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kinds of animals and 20
000 kinds of animals and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kinds of plants in the ocean. The main source of chitin is shrimp and crab shells in the sea, which are inexhaustible. Chitin is a linear polymer composed of 1000 to 3000 acetylglucosamine residues connected to each other through 1,4 glycosidic bonds. As mentioned above, natural chitin has a high degree of crystallinity, including α, β, and γ crystal forms.53,54 The α-chitin is composed of two antiparallel chains and has strong intermolecular hydrogen bonds, which is the most important and most stable form in existence. The high crystallization and a large number of hydrogen bonds in the molecular chain of chitin make it difficult to dissolve. In addition, the low reactivity of chitin limits its application (Fig. 1).
000 kinds of plants in the ocean. The main source of chitin is shrimp and crab shells in the sea, which are inexhaustible. Chitin is a linear polymer composed of 1000 to 3000 acetylglucosamine residues connected to each other through 1,4 glycosidic bonds. As mentioned above, natural chitin has a high degree of crystallinity, including α, β, and γ crystal forms.53,54 The α-chitin is composed of two antiparallel chains and has strong intermolecular hydrogen bonds, which is the most important and most stable form in existence. The high crystallization and a large number of hydrogen bonds in the molecular chain of chitin make it difficult to dissolve. In addition, the low reactivity of chitin limits its application (Fig. 1).
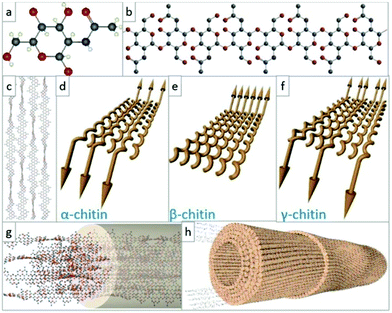 | ||
| Fig. 1 The structure and configuration of chitin. (a and b) Ball-and-stick model of the dGlcNAc monomer, where red is oxygen, blue is nitrogen, and gray is carbon atom; (c) schematic diagram of chitin molecular chain arrangement; (d–f) schematic diagrams of the molecular chain orientations in the polymorphic configurations of different types of chitin; (g and h) molecular chain packaging diagram and nanofiber packaging diagram in chitin nanofibers.54 Copyright 2021 Elsevier Ltd. | ||
To solve these problems, researchers have adopted two strategies to use chitin: find a suitable solvent to use natural chitin directly and modify chitin to obtain higher-value chitin derivatives (such as chitosan). Chitosan is the product of partial or complete N-deacetylation of natural chitin. Generally speaking, it can be called chitosan if more than 55% of the n-acetyl group is removed, which is the most important derivative of chitin because of its better solubility and higher reactivity. Chitin and chitosan are widely used in food, textile, agriculture, medicine, and other fields for their excellent properties such as biodegradability, biocompatibility, non-toxicity, and bacteriostasis.
As described in the introduction, the existing solvent system has disadvantages such as poor solubility, high pollution, and difficulty in mass production. Researchers are eager to find a new generation of green solvents to replace the existing solvents and realize the direct use of chitin. DESs, as a new generation of environmentally friendly solvents, provide the possibility for the large-scale utilization of chitin (the dissolving of chitin by DESs will be described in detail in the following sections).
DESs
DESs consist of hydrogen bond donors (HBDs) and hydrogen bond acceptors (HBAs) with strong hydrogen bond interaction and electrostatic interaction and can be prepared by directly heating and stirring raw materials. Normally, DESs are two-component or three-component systems. HBAs are composed of quaternary ammonium salts and metal salts (such as choline chloride, betaine, LiCl, ZnCl2, etc.) while HBDs are composed of polyols, polyacids, and polyamines (such as ethylene glycol, lactic acid, oxalic acid, urea, etc.). They have good biocompatibility, biodegradability, stability, conductivity, and 100% atomic utilization. Due to the above advantages, DESs have been widely used in the extraction and separation of target components of biomass, smelting of precious metals, preparation of gels, synthesis of organic reactions, and other aspects.DESs related to chitin are shown in Table 1, which can not only be used to extract and separate chitin but also can interact with chitin to prepare chitin nanofibers, deacetylated chitin (chitosan), magnetic chitin, etc. In addition, with its excellent solubility, DESs can directly dissolve chitin to prepare chitin-related materials. Moreover, DESs can also be used as modifiers to modify chitin and its derivatives. Due to the limited space of this article, we will only focus on DESs related to the application of chitin in the following paragraphs. For the application and progress of DESs in other areas, readers can refer to other wonderful reviews.55,56
| Applications | HBAs | HBDs | Molar ratio | Raw materials | Ref. |
|---|---|---|---|---|---|
| Dissolution of chitin | Choline chloride | Urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
α-Chitin | 62 |
| Choline bromide | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||||
| Betaine hydrochloride | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
||||
| Choline chloride | Thiourea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
|||
| 1-Allyl-3-methylimidazolium chloride | Thiourea | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
α-Chitin | 63 | |
| 1-Ethyl-3-methylimidazolium chlorides | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
||||
| 1-Butyl- and 1-ethyl-3-methylimidazolium bromides | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
||||
| Extraction and conversion of chitin | Choline chloride | Thiourea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Lobster shells | 64 |
| Urea | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
||||
| Glycerol | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
||||
| Malonic acid | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
||||
| Choline chloride | Malonic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Lobster shell | 65 | |
| Malic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
||||
| Lactic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
||||
| Levulinic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||||
| Choline chloride | Malic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Shrimp shells | 66 | |
| Choline chloride | DL-Lactic acid | Not given | Skimmed black soldier fly | 67 | |
| Glycerol | |||||
| Urea | |||||
| Oxalic acid | |||||
| N-Butyric acid | |||||
| Betaine | DL-Lactic acid | ||||
| Glycerol | |||||
| Urea | |||||
| Oxalic acid | |||||
| N-Butyric acid | |||||
| Betaine HCl | Urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Shrimp shells | 68 | |
| Choline chloride | Urea | ||||
| Ethylene glycol | |||||
| Glycerol | |||||
| Choline chloride | Lactic acid (85%) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Shrimp shells | 69 | |
| Malonic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
||||
| Urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||||
| Citric acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
||||
| Preparation of chitin nanofibers and nanocrystals | Choline chloride | Thiourea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Chitin (the degree of acetylation was 94.1%) | 74 |
| Choline chloride | ZnCl2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
α-Chitin | 75 | |
| Choline chloride | Ferric chloride hexahydrate | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
α-Chitin | 76 | |
| Choline chloride | Oxalic acid dihydrate | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Chitin from crab shell | 77 | |
| Citric acid monohydrate | |||||
| Malonic acid | |||||
| Lactic acid | |||||
| DL-Malic | |||||
| Chitin composites | Choline chloride | Malonic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Chitosan (Mw = 900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
78 |
| Choline chloride | Lactic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Chitosan (deacetylation degree of 76%) | 79 | |
| Choline chloride | Lactic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Chitosan (deacetylation degree of 81.7% and 76%) | 80 | |
| Malic acid | |||||
| Citric acid 1-hydrate | |||||
| Glycerol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||||
| Choline chloride | Lactic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Chitosan (deacetylation degree of 72.2% and 83.4%) | 81 | |
| Choline chloride | Citric acid-1-hydrate | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Chitosan (deacetylation degree of 90%) | 82 | |
| Choline chloride | Lactic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Shrimp shells | 83 | |
| 1,4-Butanediol | |||||
| Ethylene glycol | |||||
| Urea | |||||
| 1,6-Hexanediol | |||||
| Choline chloride | Urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Chitosan and carboxymethyl cellulose | 84 | |
| Zn(OAc)2·2H2O | Urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
Shrimp shells | 85 | |
| Choline chloride | Lactic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
White button mushrooms | 86 | |
| Urea | |||||
| Thiourea | |||||
| Betaine | Urea | ||||
| Lactic acid | |||||
| Choline chloride | Methacrylic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Chitosan | 87 | |
| Betaine | Methacrylic acid and H2O | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|||
| Choline chloride | Lactic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Chitosan | 88 | |
| Chitin modification | Choline chloride | Urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Chitosan | 89 |
| Choline chloride | Glycerol | ||||
| L-Lactic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Shells of red shrimp | 90 | ||
| D/L/DL-Malic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1 2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
||||
| Citric acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 2 2 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
||||
| Choline chloride | Acetic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Chitin from shrimp shells | 91 | |
| Malic acid |
Structure–property relationships between DESs and chitin
As a new generation of green solvents, DESs have unique properties. For example, compared with ionic liquids, DESs have the advantages of a wide range of sources, simple preparation, low toxicity, and low cost, while the thermal stability, non-flammability, biodegradability, and excellent solubility of DESs make it possible to replace organic solvents. By regulating the molar ratio and types of HBAs and HBDs, the properties of DESs such as density, viscosity, pH, and polarity can be tuned. Moreover, these properties influence each other and are temperature dependent so that we can choose the optimal DESs based on the needs of the application. In view of the limited space here, we focus on the relationship between the properties of DESs and the applications of chitin, hoping to provide a reference for selecting suitable DESs for the valorization of chitin in the future. As for the structure–property relationships of DESs, readers can refer to the previous wonderful reviews.56–59As shown in Table 2, we have listed several typical examples of the applications of DESs to chitin, and discussed the structure–property relationships between DESs and chitin.| Applications | HBAs | HBDs | Molar ratio | T f (K) | Viscosity at 298 K (cP) | Density at 298 K (g ml−1) | pH | Ref. |
|---|---|---|---|---|---|---|---|---|
| Dissolution of chitin | Choline chloride | Urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
285 | 750 | 1.21 | 10.07 | 61 |
| Extraction and conversion of chitin | Glycerol | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
290 | 281 | 1.18 | 4.47 | 63 | |
| Malonic acid | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
283 | 1638 | 1.25 | 1.28 | |||
| Oxalic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
307 | 597 | 1.15 | 1.22 | 64 | ||
| Ethylene glycol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
237 | 37 | 1.12 | 4.38 | 65 | ||
| Preparation of chitin nanofibers and nanocrystals | Malonic acid | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
283 | 1638 | 1.25 | 1.28 | 76 |
As mentioned earlier, dissolving chitin begins by breaking numerous hydrogen bonds between the molecules. For this reason, choosing DESs with greater polarity can better dissolve chitin (such as ChCl/urea, ChCl/thiourea). The solvent parameters of the Kamlet–Taft (KT) model (hydrogen-bond acidity (α), hydrogen-bond basicity (β), and polarity (π*)) can also provide a reference for dissolving chitin. DESs with higher α and β values have better hydrogen bond donating/receiving ability, which makes it easier to dissolve chitin.57,60,61
The presence of H+ can effectively separate chitin from organisms, choosing DESs with lower pH (such as ChCl/oxalic acid, ChCl/lactic acid, ChCl/citric acid) can result in chitin with higher purity. Moreover, due to the advantages of the low vapor pressure of DESs, using a microwave instead of the traditional heating method can extract chitin efficiently and quickly.
Viscosity and density are important parameters of DESs. Due to a large number of hydrogen bonds, van der Waals interactions, and ionic interactions between the various components, the density and viscosity of DESs are relatively high and decrease with increasing temperature. DESs with lower viscosity and density can better penetrate the structure of biomass, thereby efficiently extracting chitin. The proton acid-catalyzed cleavage is an important mechanism for the preparation of chitin nanofibers. Therefore, DESs with lower pH are usually used to prepare chitin nanofibers.
In the following chapters, we will carry out an in-depth analysis based on specific applications.
Related applications of DES-chitin system
Dissolution of chitin
Because of the obstinate nature of chitin, the dissolving of chitin must break the high strength hydrogen bond between its molecules, which requires the solvent to have high hydrogen bond breaking ability and ionic strength. There are a lot of hydrogen and ionic bonds between the components of DESs, and they are highly soluble to many substances such as lignin, hemicellulose and even cellulose. Therefore, using DESs to dissolve chitin has become the focus of research.The dissolution of chitin by DESs can be traced back to 2013. Sharma et al. studied the dissolution of α-chitin using different DESs by heating, microwave, and ultrasound. Typically, chitin was added to DESs and the mixture was heated to 100 °C, using microwave and ultrasonic assistance can reduce the time required for dissolution (Fig. 2).62 Using choline chloride as the HBA and thiourea as the HBD, DESs with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 had the highest solubility (9 wt%). Replacing the HBD with urea also has a good solubility to chitin (up to 7 wt%). However, when the hydrogen bond donor is replaced with glycol and glycerol, chitin cannot dissolve in DESs, possibly due to their poor ability to break hydrogen bonds. These phenomena indicated that HBDs played an important role in chitin dissolution. It is noteworthy that chitosan was insoluble in DESs, indicating the effect of the chitin acetamido group on dissolution. This study opens the way for DESs to dissolve chitin.
2 had the highest solubility (9 wt%). Replacing the HBD with urea also has a good solubility to chitin (up to 7 wt%). However, when the hydrogen bond donor is replaced with glycol and glycerol, chitin cannot dissolve in DESs, possibly due to their poor ability to break hydrogen bonds. These phenomena indicated that HBDs played an important role in chitin dissolution. It is noteworthy that chitosan was insoluble in DESs, indicating the effect of the chitin acetamido group on dissolution. This study opens the way for DESs to dissolve chitin.
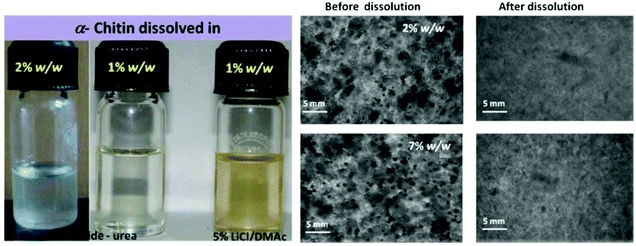 | ||
Fig. 2 (a) Optical photographs of α-chitin (2% w/w) solubilized in choline chloride/urea DES and 5% LiCl–N,N-dimethylacetamide; (b) phase contrast microscopic photograph (1006) of α-chitin (2% and 7% w/w) in choline chloride–thiourea 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 before and after dissolution (ultrasonication assisted heating).62 Copyright 2013 Royal Society of Chemistry. 2 before and after dissolution (ultrasonication assisted heating).62 Copyright 2013 Royal Society of Chemistry. | ||
Idenoue et al. mixed thiourea with imidazolium ILs to prepare an ionic liquid DES.63 The introduction of thiourea increased the solubility of chitin (2 wt%–5 wt%) (Fig. 3). The substituents and counterions on the imidazole ring also have a great influence on the solubility of DES, but the specific mechanism was not clear. It is worth mentioning that after the chitin was dissolved, a transparent solution with no obvious color was formed. This DES had great application potential in the conversion and functionalization of chitin in the future.
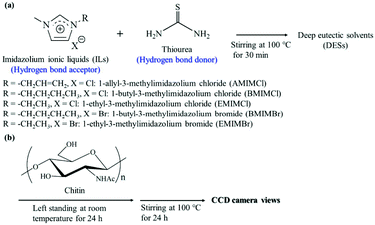 | ||
| Fig. 3 (a) Preparation of DESs and (b) dissolution experiment procedure of chitin in DESs.63 Copyright 2019 Multidisciplinary Digital Publishing Institute. | ||
Above all, DESs have great potential for dissolving chitin. However, the large number of hydrogen bonds and Lewis acid–base interactions in DES components complicate the mechanism of dissolving chitin by DESs, and relatively few DESs were reported for chitin dissolution. The viscosity of DESs and the solvent parameters of the Kamlet–Taft (K–T) model can provide a reference for dissolving chitin. The DES system dissolving chitin is a huge treasure trove, which needs researchers to explore many new efficient DESs systems and realize the large-scale application.
Extraction and conversion of chitin
As mentioned before, in the process of extracting chitin, minerals and proteins need to be removed, which involves the use of a large amount of acid and alkali and will cause environmental pollution. Therefore, finding a green way to extract chitin has become a great concern. Recently, it has been discovered that in addition to the dissolution of chitin, DESs can also replace traditional strong acids and bases to extract chitin.As shown in Fig. 4, as early as 2017, Zhu et al. designed four DESs with choline chloride as the hydrogen bond acceptor, while thioureas, urea, glycerol, and malonic acid as hydrogen bond donors to extract chitin.64 High purity chitin was extracted from lobster shells and compared with chitin extracted by chemical methods. Among them, the DES composed of choline chloride and malonic acid had the best extraction effect, the purity of chitin was the highest, and chitin with two crystallinities (67.2 ± 0.9% for sample MA-S and 80.6 ± 0.8%% for sample MA-P) was obtained. Not only that, the DES extraction method obtained a higher chitin yield than the chemical extraction method (21% and 16% for comparison), and the extracted chitin also has the potential for application in adsorption materials and tissue engineering materials. This work proves the potential of DESs in extracting chitin in a green, environmentally friendly, and efficient way, and is expected to realize the commercialization of chitin extraction.
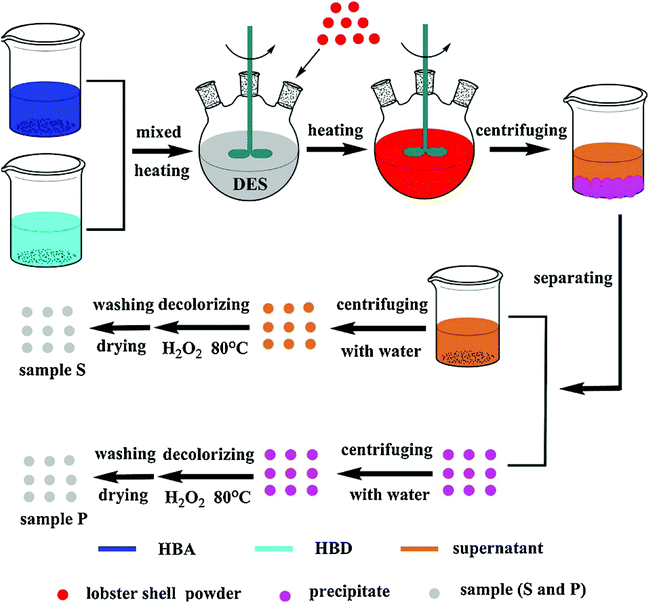 | ||
| Fig. 4 Schematic diagram of the DES extraction process.64 Copyright 2017 Elsevier Ltd. | ||
Later, Hong et al. in the same research group studied the effect of pH on the extraction of chitin from lobster shells by regulating hydrogen bond donors.65 The results showed that the acidity and temperature of DES have significant effects on the extraction effect and molecular weight of chitin, and the highest purity of chitin can be extracted from DES composed of choline chloride and malonic acid. Moreover, they successfully converted CaCO3 into a more valuable salt, calcium levulinate. This work opens up the possibility of extracting chitin of different molecular weights in a green way (Fig. 5).
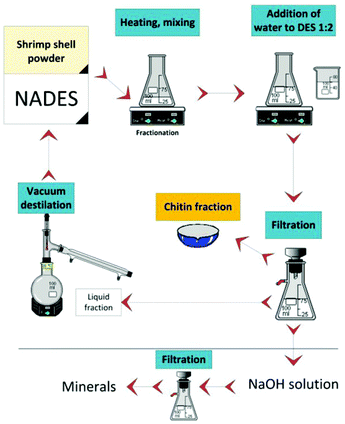 | ||
| Fig. 5 Sustainable process for chitin and minerals fractionation from shrimp shell by NADES.65 Copyright 2020 De Gruyter. | ||
Similarly, Huang et al. prepared natural DESs (NADESs) with natural substances, choline chloride and malic acid in 2018, and achieved the successful extraction of chitin from DESs.66 Meanwhile, high-quality chitin with a crystallinity of up to 71% was prepared by a microwave-assisted method. The whole process did not involve the use of toxic chemicals, and was an efficient and facile method. It provides a green and simple strategy for extracting chitin from DESs, which has great potential for application in the future.
In another work, Zhou et al. prepared a series of DESs based on betaine and choline chloride and studied the factors affecting the extraction effect of Hermetia illucens chitin.67 After being reused three times, DESs still had a good extraction effect. The results showed that the H+ released in DESs contributed to demineralization, and a large number of hydrogen bonds in DESs was the key factor of deproteinization. This report provides a reference for extracting chitin from natural DESs.
Recently, Zhao et al. extracted chitin from lobster shells using a two-step process.68 Firstly, the chitin was demineralized by citric acid. Then, the residual part was deproteinized by using DES with the assistance of the microwave method, and high-purity chitin was extracted. Besides, DESs can be recycled several times without loss of extraction efficiency. Bradić et al. extracted chitin with a purity of up to 98% using a system of choline chlorine–lactic acid in a zero-pollution process with a yield of approximately 90% and achieved the recycling and continuous use of DESs.69 These works provided the possibility of large-scale production in the future.
The strategy of using DESs to extract chitin is still in its infancy. However, due to its environmental protection and high efficiency, DESs have great application potential in the extraction of chitin, and they provide the possibility for the industrial production of chitin. So far, the extraction effect of acidic DES (such as ChCl/lactic acid, ChCl/oxalic acid) is better, the release of H+ in acidic DESs and the existence of a large number of hydrogen bonds can effectively extract high-purity chitin, and the use of the microwave method can also greatly improve the extraction efficiency. We hope that in the future, more and more people will participate in the research of extracting chitin from DESs, conduct in-depth research on the extraction mechanism, and expand this system from the laboratory scale to commercial scale.
Preparation of chitin nanofibers and nanocrystals. Chitin nanofibers and nanocrystals (ChNFs and ChNCs) are widely used in composite materials, medical health, and tissue engineering due to their low density, high surface area, good biocompatibility, and mechanical properties.51,70,71 As early as 2014, Mukesh et al. successfully obtained chitin nanofibers using DESs composed of choline chloride and thiourea. However, after replacing thiourea with urea, nanofibers cannot be prepared, indicating the important role of hydrogen bond donors. And they successfully applied nanofibers to calcium alginate beads as an enforcement filler, which proved the possibility of preparing chitin nanofibers with DESs.72 Later, as shown in Fig. 6, Hong et al. proposed a method using a DES composed of choline chloride and zinc chloride as a solvent and catalyst. After adding acetic anhydride or acetic acid, acetylation or esterification and the hydrolysis of chitin occur simultaneously in one step. DESs acted as a reaction solvent and also played an effective catalytic role. This work provides a new method for the preparation of functionalized chitin nanocrystals.73
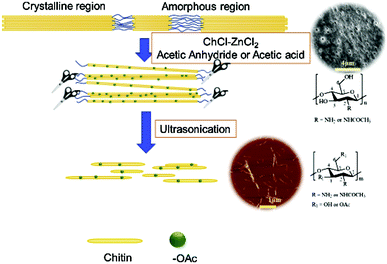 | ||
| Fig. 6 Schematic diagram of the one-step manufacturing of nanocrystals (CNC).73 Copyright 2019 Elsevier Ltd. | ||
Recently, Hong et al. prepared a DES with ferric chloride hexahydrate and choline chloride at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.74 The synergistic effect of Lewis acid and Brønsted acid promotes the effective hydrolysis of chitin. Meanwhile, with the aid of ultrasound, ChNCs were prepared from chitin in a DES with a yield of 88.5% and crystallinity of 89.2%. Moreover, the prepared nanocrystal has proved its application value in stabilizing emulsion. Yuan et al. studied the effect of various acidic DESs on the production of chitin nanocrystals.75 The experiments showed that the acid promoted the hydrolysis and acetylation of chitin. During the DES treatment process, the removal of amorphous areas increased the crystallinity of the chitin nanofibers.
1.74 The synergistic effect of Lewis acid and Brønsted acid promotes the effective hydrolysis of chitin. Meanwhile, with the aid of ultrasound, ChNCs were prepared from chitin in a DES with a yield of 88.5% and crystallinity of 89.2%. Moreover, the prepared nanocrystal has proved its application value in stabilizing emulsion. Yuan et al. studied the effect of various acidic DESs on the production of chitin nanocrystals.75 The experiments showed that the acid promoted the hydrolysis and acetylation of chitin. During the DES treatment process, the removal of amorphous areas increased the crystallinity of the chitin nanofibers.
Similar to the extraction process of chitin, the synergistic effect of Lewis acid and Brown acid facilitates the hydrolysis of chitin, thereby preparing chitin nanofibers. By using microwave-assisted and ultrasound methods, the efficiency of preparing chitin nanofibers can be improved. During the preparation process, the reaction reagents can also be directly added to the DESs to obtain functionalized nanofibers. These methods raise green strategies for the preparation of functional ChNFs and ChNCs, which have great application prospects in the future.
Plasticizing of chitosan films
Due to the non-biodegradable and low recycling rate, a large number of discarded plastics enter the ocean from the land and permeate into the food chain. “White pollution” – plastic pollution is a serious threat to the ecological environment. Replacing petroleum-based plastics with biodegradable and green natural poly (polysaccharides) has aroused people's interest.76,77 Among them, chitosan, the green derivative of chitin, is regarded as a good alternative to petroleum-based plastics for its excellent biodegradability, antibacterial properties, wide source, and film-forming ability. However, the poor mechanical properties and stability of chitosan membranes restrict their application. Recently, the use of environmentally friendly small molecule DESs to plasticize chitosan membrane to improve the performance has aroused people's interest.Sokolova et al. prepared DES-plasticized chitosan films based on choline chloride and malonic acid by the solution casting method, and the influence of the DES content on film morphology, structure, thermal stability, and mechanical properties was studied.78 The strong interaction between DESs and chitosan made it have a good plasticizing effect on chitosan film. Similarly, Almeida et al. took a DES (prepared with choline chloride and lactic acid in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) as the plasticizer and prepared the chitosan film through a knife-coating technique to explore the effect of the DES content on the chitosan film. Experiments showed that the addition of a DES leads to the enhancement of mechanical properties, better water vapor permeability, and water absorption capacity. The plasticized chitosan film has good application potential in food packaging.
1 molar ratio) as the plasticizer and prepared the chitosan film through a knife-coating technique to explore the effect of the DES content on the chitosan film. Experiments showed that the addition of a DES leads to the enhancement of mechanical properties, better water vapor permeability, and water absorption capacity. The plasticized chitosan film has good application potential in food packaging.
Galvis-Sánchez et al. studied the effect of NADESs based on choline chloride on chitosan films prepared by thermo-compression molding.80 The results showed that the morphology, mechanical properties, and water resistance of chitosan films can be effectively regulated by selecting the type of DES and the degree of deacetylation of chitosan. Therefore, this work opens a new perspective for the production of chitosan films for specific purposes. Jakubowska et al. studied the effect of DES-based on choline chloride and lactic acid on the properties of chitosan films.81 The flexibility and oxidation resistance of the DES plasticized chitosan film was significantly increased, meanwhile, the water vapor transmission rate was also improved. The modified chitosan film shows great application potential in packaging materials.
In addition to the indirect plasticization described above, DESs can also be used to prepare chitin/chitosan films and achieve direct plasticization. After mixing choline chloride, citric acid, and chitosan, Galvis-Sánchez et al. prepared Chit–ChCl–CA films by thermo-compression molding, which exhibited high flexibility, transparency, and water vapor permeability.82 Saravana et al. prepared chitin films using chitin extracted from shrimp shells by DESs as raw materials.83 They obtained high-purity chitin using a DES composed of choline chloride–malonic acid and prepared a chitin film. Moreover, the mechanical properties, swelling properties, and biodegradability of the prepared films are comparable to those of standard films.
Due to the antibacterial, non-toxic, and biodegradable properties of chitin, chitin film is a good alternative to replace petroleum-based plastics. Through the modification of DESs, a functionalized chitin film can be obtained, which has promising application prospect in the future.
Chitin composites
In addition to the above studies, the applications of DESs in chitin composites have also been studied.Wong et al. first mixed chitin with carboxymethyl cellulose (CMC) at different mass ratios and then studied the effect of the DES composed of choline chloride and urea on the chitin–CMC film.84 The DES modified membrane had a great improvement in electrical conductivity and had good thermal stability, and is an ideal material for polymer electrolyte membranes.
Feng et al. adopted urea–Zn(OAc)2·2H2O as the DES, which removed calcium carbonate and loaded Zn2+ through ion exchange, and removed proteins by the aggregation of urea clusters.85 Meanwhile, Zn2+ provided excellent antibacterial properties for the composite membrane. Therefore, in a simple and green method, a chitin/zinc antibacterial complex was prepared from shrimp shells in one step. This work provides a reference for the direct conversion of waste shrimp shells into chitin/metal complexes.
Recently, Kim et al. extracted ten types of chitosan–glucan complexes (CGCs) with different properties from mushroom using five DESs.86 By selecting different DESs, CGCs with different properties and structures can be obtained, which provides the possibility of subsequent functionalization and application in diverse areas. Ma et al. prepared magnetic chitin using DES as the functional monomer, showing excellent recognition and selectivity, and then realized the selective separation of catechins in black tea.87 Vorobiov et al. embedded the DES-based on choline chloride and lactic acid into the chitosan film, which had a conductivity of 2.5 mS cm−1 and a voltage window of 1.6 V.88 The chitosan film after compounding showed potential application in energy storage materials.
The DES strategy can prepare chitin composite materials under mild and environmentally friendly conditions. These preparation processes were simple and environmentally friendly, and have good application prospect in the subsequent preparation of various functional biomaterials.
Chitin modification
As mentioned above, due to the poor solubility and low reactivity of chitin, the modification of chitin has become one of the most important research directions of chitin, where the use of green and safe DESs to modify chitin/chitosan has attracted research interest. The reactions related to chitin modification are shown in Fig. 7.Bangde et al. achieved the methylation of chitosan by using the DES of choline chloride–urea and choline chloride–glycerol in 2016.89 Not only that, they replaced methyl iodide with lipase and dimethyl carbonate due to the good biocompatibility of the DES. This investigation has realized the green methylation conversion of chitosan in the DES for the first time.
Feng et al. obtained O-acylated chitin with a variety of choline chloride-based natural deep eutectic solvents (NADESs) directly from shrimp shells.90 They pointed out that H+ can simultaneously take part in demineralization, deproteinization, and acetylation. Under the best conditions, O-malate chitin can achieve a purity of 98.6% and a degree of substitution of 0.46, which has good antibacterial and anti-tumor effects. Recently, Vicente et al. studied the mechanism of deacetylation of chitin by a DES and obtained chitosan under mild conditions.91 After deacetylation at 120 °C for 24 h using the DES of choline chloride–malic acid, the degree of deacetylation of chitin reached 40%, and the low reactivity and solubility of chitin were greatly improved.
These studies have enriched the applications of chitin and provided a simple, effective and environmentally friendly approach to obtain chitin derivatives, showing great application prospects.
Recycling of DESs
Considering the large-scale industrial application of the DES–chitin system, the recovery of DESs is very important. However, most attention has been paid to the application of DESs in chitin treatment, and only a few studies have reported the recovery of DESs.At the earliest in 2014, Mukesh et al. separated chitin and DESs by centrifugation when preparing chitin nanofibers, and then removed the water in the DES solution by rotary evaporation with a DES yield of 84%.72 Moreover, the recycled DES can be reused, and the properties of the chitin nanofibers obtained are equivalent to those prepared using original DESs.
In the process of extracting chitin with a natural DES (NADES) composed of choline chloride and malic acid, Huang et al. separated chitin and NADES by centrifugation, and the recovered NADES was directly reused without any treatment.66 The results showed that NADES can be reused three times without affecting the extraction of chitin. However, after more than three times, too much protein would accumulate in NADES, resulting in too much viscosity to be used to extract chitin. Similarly, Feng et al. studied the recovery effect of the DES composed of choline chloride–malic acid, and they showed that the DES had a good extraction effect after at least five cycles of recycling.
Zhao et al. studied the repeatability of DESs in detail in the process of extracting chitin from shrimp shells using a microwave-assisted method.68 The results showed that DES can be reused at least five times. However, with the increase in the number of repetitions, the hydrogen bond of the DES was destroyed, resulting in decreased instability. Meanwhile, due to the increase of proteins and other impurities, the viscosity of DESs would gradually increase, resulting in a poor extraction effect.
So far, in the DES recycling process, the chitin and DESs have been separated by centrifugation or filtration. However, some soluble minerals and proteins would continue to remain in DESs, which would affect the recycling effect with the increase of recycling times. In addition, the presence of water will greatly affect the performance of the DES. Therefore, it is necessary to remove water from the recovered DES solution. However, the low vapor pressure of DES determines that water can only be removed by evaporating. This is undoubtedly an inefficient and energy-intensive way.
It is worth studying to find a suitable solvent to remove proteins and minerals in DESs and to replace water with low boiling point solvents.
Summary and outlook
Chitin and its derivatives have huge application potential due to their wide range of sources, biodegradability, biocompatibility, and non-toxicity. The use of environmentally friendly DESs for the dissolution, modification, and preparation of chitin-related materials has also made great progress.However, the application of DESs to chitin is still in its infancy, and a lot of research is needed to make the system mature and even commercialized. For example, the mechanism of DESs dissolving chitin is still not clear, and its solubility is relatively poor, which limits its application. Chitin treatment with DESs is a complex system, which is affected by many factors such as viscosity, pH, polarity, and reaction conditions of DESs. However, people often ignore the mechanism of DES processing, which can provide theoretical guidance for people. Further studies are needed to determine the appropriate DESs and reaction conditions through structural design and synthesis to achieve the specific application value of chitin.
In addition, considering the large-scale application, the recycling of DESs is also worthy of research. At present, the process of recycling DESs is relatively complicated, requiring steps such as filtration, centrifugation, and rotary steaming, which are undoubtedly energy-consuming and not environmentally friendly, and the processing efficiency of DESs often drops significantly after processing chitin after 3 cycles. Finding an effective recovery method, such as the use of low-boiling anti-solvents to reduce the energy consumption of rotary evaporation, is conducive to the rapid industrialization of DESs.
Moreover, there are too few kinds of DESs to deal with chitin, and most of them focus on DESs with ChCl as the HBA. In fact, the properties of DESs vary significantly with the types and properties of HBAs and HBDs. We can achieve the efficient utilization of chitin by selecting the best DESs. This requires us to conduct in-depth and extensive research, explore and summarize the properties of different DESs, and use to achieve the efficient utilization of chitin. In the process of chitin processing, we tend to ignore the use of minerals and proteins and discard them as waste. In the following research, we should strive to achieve the full utilization of biomass.
The work of extracting chitin with DESs has only been studied since 2017, and there are still many areas that need improvement, and the whole application of chitin based marine bioresources is also a key point. Developing more green and efficient DESs and realizing the recycling of DESs require joint efforts, but we are confident that one day, DES processing chitin systems will be successfully industrialized.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Key R&D Program of China (No. 2020YFA0710700), the National Natural Science Foundation of China (No. 51873201 and 51673180), and the Fundamental Research Funds for the Central Universities (No. YD2060002015).Notes and references
- P. Kandra, M. M. Challa and H. Kalangi Padma Jyothi, Appl. Microbiol. Biotechnol., 2012, 93, 17–29 CrossRef PubMed.
- Y. H. Choi and R. Verpoorte, Curr. Opin. Food Sci., 2019, 26, 87–93 CrossRef.
- B. Kudłak, K. Owczarek and J. Namieśnik, Environ. Sci. Pollut. Res. Int., 2015, 22, 11975–11992 CrossRef PubMed.
- J.-I. Kadokawa, Int. J. Biol. Macromol., 2019, 123, 732–737 CrossRef CAS PubMed.
- M. Rinaudo, Prog. Polym. Sci., 2006, 31, 603–632 CrossRef CAS.
- D. H. Bartlett and F. Azam, Science, 2005, 310, 1775–1777 CrossRef CAS PubMed.
- W. M. F. B. W. Nawawi, M. Jones, R. J. Murphy, K.-Y. Lee, E. Kontturi and A. Bismarck, Biomacromolecules, 2020, 21, 30–55 CrossRef CAS PubMed.
- H. Wang, K. U. Rehman, X. Liu, Q. Yang, L. Zheng, W. Li, M. Cai, Q. Li, J. Zhang and Z. Yu, Biotechnol. Biofuels, 2017, 10, 304 CrossRef PubMed.
- B. Duan, X. Zheng, Z. Xia, X. Fan, L. Guo, J. Liu, Y. Wang, Q. Ye and L. Zhang, Angew. Chem., Int. Ed., 2015, 54, 5152–5156 CrossRef CAS PubMed.
- M.-S. Hong, G.-M. Choi, J. Kim, J. Jang, B. Choi, J.-K. Kim, S. Jeong, S. Leem, H.-Y. Kwon, H.-B. Hwang, H.-G. Im, J.-U. Park, B.-S. Bae and J. Jin, Adv. Funct. Mater., 2018, 28, 1705480 CrossRef.
- X. Shen, J. L. Shamshina, P. Berton, G. Gurau and R. D. Rogers, Green Chem., 2016, 18, 53–75 RSC.
- B. Duan, C. Chang, B. Ding, J. Cai, M. Xu, S. Feng, J. Ren, X. Shi, Y. Du and L. Zhang, J. Mater. Chem. A, 2013, 1, 1867–1874 RSC.
- C. King, J. L. Shamshina, G. Gurau, P. Berton, N. F. A. F. Khan and R. D. Rogers, Green Chem., 2017, 19, 117–126 RSC.
- J. Huang, Y. Zhong, L. Zhang and J. Cai, Adv. Funct. Mater., 2017, 27, 1701100 CrossRef.
- K. Zhu, S. Shi, Y. Cao, A. Lu, J. Hu and L. Zhang, Carbohydr. Polym., 2019, 212, 361–367 CrossRef CAS PubMed.
- Y. Huang, Z. Zhong, B. Duan, L. Zhang, Z. Yang, Y. Wang and Q. Ye, J. Mater. Chem. B, 2014, 2, 3427–3432 RSC.
- D. Xu, J. Huang, D. Zhao, B. Ding, L. Zhang and J. Cai, Adv. Mater., 2016, 28, 5844–5849 CrossRef CAS PubMed.
- B. Duan, X. Gao, X. Yao, Y. Fang, L. Huang, J. Zhou and L. Zhang, Nano Energy, 2016, 27, 482–491 CrossRef CAS.
- B. Duan, K. Shou, X. Su, Y. Niu, G. Zheng, Y. Huang, A. Yu, Y. Zhang, H. Xia and L. Zhang, Biomacromolecules, 2017, 18, 2080–2089 CrossRef CAS PubMed.
- B. Duan, H. Gao, M. He and L. Zhang, ACS Appl. Mater. Interfaces, 2014, 6, 19933–19942 CrossRef CAS PubMed.
- M. Gortari and R. Hours, Electron. J. Biotechnol., 2013, 16, 14–14 Search PubMed.
- V. L. Pachapur, K. Guemiza, T. Rouissi, S. J. Sarma and S. K. Brar, J. Chem. Technol. Biotechnol., 2016, 91, 2331–2339 CrossRef CAS.
- S. Hong, Q. Yang, Y. Yuan, L. Chen, Y. Song and H. Lian, Carbohydr. Polym., 2019, 205, 236–243 CrossRef CAS PubMed.
- P. Beaney, J. Lizardi-Mendoza and M. Healy, J. Chem. Technol. Biotechnol., 2005, 80, 145–150 CrossRef CAS.
- I. Younes, O. Ghorbel-Bellaaj, R. Nasri, M. Chaabouni, M. Rinaudo and M. Nasri, Process Biochem., 2012, 47, 2032–2039 CrossRef CAS.
- I. Younes, S. Hajji, V. Frachet, M. Rinaudo, K. Jellouli and M. Nasri, Int. J. Biol. Macromol., 2014, 69, 489–498 CrossRef CAS PubMed.
- K. M. Rudall, in Adv. Insect Physiol, ed. J. W. L. Beament, J. E. Treherne and V. B. Wigglesworth, Academic Press, 1963, vol. 1, pp. 257–313 Search PubMed.
- C. Liu, G. Wang, W. Sui, L. An and C. Si, ACS Sustainable Chem. Eng., 2017, 5, 4690–4698 CrossRef CAS.
- A. Einbu, S. N. Naess, A. Elgsaeter and K. M. Vårum, Biomacromolecules, 2004, 5, 2048–2054 CrossRef CAS PubMed.
- F. Feng, Y. Liu and K. Hu, Carbohydr. Res., 2004, 339, 2321–2324 CrossRef CAS PubMed.
- X. Hu, Y. Du, Y. Tang, Q. Wang, T. Feng, J. Yang and J. F. Kennedy, Carbohydr. Polym., 2007, 70, 451–458 CrossRef CAS.
- H. Xu, Z. Fang, W. Tian, Y. Wang, Q. Ye, L. Zhang and J. Cai, Adv. Mater., 2018, 30, 1801100 CrossRef PubMed.
- Y. Fang, R. Zhang, B. Duan, M. Liu, A. Lu and L. Zhang, ACS Sustainable Chem. Eng., 2017, 5, 2725–2733 CrossRef CAS.
- M. Poirier and G. Charlet, Carbohydr. Polym., 2002, 50, 363–370 CrossRef CAS.
- C. L. de Vasconcelos, P. M. Bezerril, M. R. Pereira, M. F. Ginani and J. L. C. Fonseca, Carbohydr. Res., 2011, 346, 614–618 CrossRef CAS PubMed.
- A. Pinkert, K. N. Marsh, S. Pang and M. P. Staiger, Chem. Rev., 2009, 109, 6712–6728 CrossRef CAS PubMed.
- M. E. Zakrzewska, E. Bogel-Łukasik and R. Bogel-Łukasik, Energy Fuels, 2010, 24, 737–745 CrossRef CAS.
- Y. Qin, X. Lu, N. Sun and R. D. Rogers, Green Chem., 2010, 12, 968–971 RSC.
- S. S. Silva, J. F. Mano and R. L. Reis, Green Chem., 2017, 19, 1208–1220 RSC.
- P. Walther, A. Ota, A. Müller, F. Hermanutz, F. Gähr and M. R. Buchmeiser, Macromol. Mater. Eng., 2016, 301, 1337–1344 CrossRef CAS.
- J.-I. Kadokawa, A. Takegawa, S. Mine and K. Prasad, Carbohydr. Polym., 2011, 84, 1408–1412 CrossRef CAS.
- M. Shimo, M. Abe and H. Ohno, ACS Sustainable Chem. Eng., 2016, 4, 3722–3727 CrossRef CAS.
- A. Pinkert, K. N. Marsh, S. Pang and M. P. Staiger, Chem. Rev., 2009, 109, 6712–6728 CrossRef CAS PubMed.
- C. Alvarez-Vasco, R. Ma, M. Quintero, M. Guo, S. Geleynse, K. K. Ramasamy, M. Wolcott and X. Zhang, Green Chem., 2016, 18, 5133–5141 RSC.
- Q. Liu, X. Zhao, D. Yu, H. Yu, Y. Zhang, Z. Xue and T. Mu, Green Chem., 2019, 21, 5291–5297 RSC.
- Á. Miguel, R. P. Fornari, N. García, A. Bhowmik, D. Carrasco-Busturia, J. M. García-Lastra and P. Tiemblo, ChemSusChem, 2020, 13, 5523–5530 CrossRef PubMed.
- J. A. Sirviö and J. P. Heiskanen, ChemSusChem, 2017, 10, 455–460 CrossRef PubMed.
- X. Tan, W. Zhao and T. Mu, Green Chem., 2018, 20, 3625–3633 RSC.
- B. Krajewska, Enzyme Microb. Technol., 2004, 35, 126–139 CrossRef CAS.
- X. Mao, N. Guo, J. Sun and C. Xue, J. Cleaner Prod., 2017, 143, 814–823 CrossRef CAS.
- B. Duan, Y. Huang, A. Lu and L. Zhang, Prog. Polym. Sci., 2018, 82, 1–33 CrossRef CAS.
- N. Özel and M. Elibol, Carbohydr. Polym., 2021, 262, 117942 CrossRef PubMed.
- Y. Zhong, J. Cai and L.-N. Zhang, Chin. J. Polym. Sci., 2020, 38, 1047–1060 CrossRef CAS.
- M. V. Tsurkan, A. Voronkina, Y. Khrunyk, M. Wysokowski, I. Petrenko and H. Ehrlich, Carbohydr. Polym., 2021, 252, 117204 CrossRef CAS PubMed.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- Q. Zhang, K. De Oliveira Vigier, S. Royer and F. Jérôme, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
- H. Xu, J. Peng, Y. Kong, Y. Liu, Z. Su, B. Li, X. Song, S. Liu and W. Tian, Bioresour. Technol., 2020, 310, 123416 CrossRef CAS PubMed.
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Chem. Rev., 2021, 121, 1232–1285 CrossRef CAS PubMed.
- T. El Achkar, H. Greige-Gerges and S. Fourmentin, Environ. Chem. Lett., 2021, 19, 3397–3408 CrossRef CAS.
- Y. Ma, Q. Xia, Y. Liu, W. Chen, S. Liu, Q. Wang, Y. Liu, J. Li and H. Yu, ACS Omega, 2019, 4, 8539–8547 CrossRef CAS PubMed.
- Q. Xia, Y. Liu, J. Meng, W. Cheng, W. Chen, S. Liu, Y. Liu, J. Li and H. Yu, Green Chem., 2018, 20, 2711–2721 RSC.
- M. Sharma, C. Mukesh, D. Mondal and K. Prasad, RSC Adv., 2013, 3, 18149–18155 RSC.
- S. Idenoue, K. Yamamoto and J.-I. Kadokawa, ChemEngineering, 2019, 3, 90 CrossRef CAS.
- P. Zhu, Z. Gu, S. Hong and H. Lian, Carbohydr. Polym., 2017, 177, 217–223 CrossRef CAS PubMed.
- S. Hong, Y. Yuan, Q. Yang, P. Zhu and H. Lian, Carbohydr. Polym., 2018, 201, 211–217 CrossRef CAS PubMed.
- W.-C. Huang, D. Zhao, N. Guo, C. Xue and X. Mao, J. Agric. Food Chem., 2018, 66, 11897–11901 CrossRef CAS PubMed.
- P. Zhou, J. Li, T. Yan, X. Wang, J. Huang, Z. Kuang, M. Ye and M. Pan, Carbohydr. Polym., 2019, 225, 115255 CrossRef PubMed.
- D. Zhao, W.-C. Huang, N. Guo, S. Zhang, C. Xue and X. Mao, Polymers, 2019, 11, 409 CrossRef PubMed.
- B. Bradić, U. Novak and B. Likozar, Green Process. Synth., 2020, 9, 13–25 Search PubMed.
- T. Lertwattanaseri, N. Ichikawa, T. Mizoguchi, Y. Tanaka and S. Chirachanchai, Carbohydr. Res., 2009, 344, 331–335 CrossRef CAS PubMed.
- M. V. Tzoumaki, T. Moschakis, V. Kiosseoglou and C. G. Biliaderis, Food Hydrocolloids, 2011, 25, 1521–1529 CrossRef CAS.
- C. Mukesh, D. Mondal, M. Sharma and K. Prasad, Carbohydr. Polym., 2014, 103, 466–471 CrossRef CAS PubMed.
- S. Hong, Y. Yuan, Q. Yang, L. Chen, J. Deng, W. Chen, H. Lian, J. D. Mota-Morales and H. Liimatainen, Carbohydr. Polym., 2019, 220, 211–218 CrossRef CAS PubMed.
- S. Hong, Y. Yuan, K. Zhang, H. Lian and H. Liimatainen, Nanomaterials, 2020, 10, 869 CrossRef CAS PubMed.
- Y. Yuan, S. Hong, H. Lian, K. Zhang and H. Liimatainen, Carbohydr. Polym., 2020, 236, 116095 CrossRef CAS PubMed.
- A. Rafique, K. Mahmood Zia, M. Zuber, S. Tabasum and S. Rehman, Int. J. Biol. Macromol., 2016, 87, 141–154 CrossRef CAS PubMed.
- J. M. Lagaron and A. Lopez-Rubio, Trends Food Sci. Technol., 2011, 22, 611–617 CrossRef CAS.
- M. P. Sokolova, M. A. Smirnov, A. A. Samarov, N. V. Bobrova, V. K. Vorobiov, E. N. Popova, E. Filippova, P. Geydt, E. Lahderanta and A. M. Toikka, Carbohydr. Polym., 2018, 197, 548–557 CrossRef CAS PubMed.
- C. M. R. Almeida, J. M. C. S. Magalhães, H. K. S. Souza and M. P. Gonçalves, Food Hydrocolloids, 2018, 81, 456–466 CrossRef CAS.
- A. C. Galvis-Sánchez, M. C. R. Castro, K. Biernacki, M. P. Gonçalves and H. K. S. Souza, Food Hydrocolloids, 2018, 82, 478–489 CrossRef.
- E. Jakubowska, M. Gierszewska, J. Nowaczyk and E. Olewnik-Kruszkowska, Carbohydr. Polym., 2021, 255, 117527 CrossRef CAS PubMed.
- A. C. Galvis-Sánchez, A. M. M. Sousa, L. Hilliou, M. P. Gonçalves and H. K. S. Souza, Green Chem., 2016, 18, 1571–1580 RSC.
- P. S. Saravana, T. C. Ho, S.-J. Chae, Y.-J. Cho, J.-S. Park, H.-J. Lee and B.-S. Chun, Carbohydr. Polym., 2018, 195, 622–630 CrossRef CAS PubMed.
- C. Y. Wong, W. Y. Wong, R. Walvekar, K. S. Loh, M. Khalid and K. L. Lim, J. Mol. Liq., 2018, 269, 675–683 CrossRef CAS.
- M. Feng, X. Lu, K. Jiang, J. Zhang, J. Xin, C. Shi, K. Wang and S. Zhang, Green Chem., 2018, 20, 2212–2217 RSC.
- H. Kim, S. Kang, K. Li, D. Jung, K. Park and J. Lee, Int. J. Biol. Macromol., 2021, 169, 122–129 CrossRef CAS PubMed.
- W. Ma, Y. Dai and K. H. Row, Electrophoresis, 2018, 39, 2039–2046 CrossRef CAS PubMed.
- V. K. Vorobiov, M. A. Smirnov, N. V. Bobrova and M. P. Sokolova, Mater. Lett., 2021, 283, 128889 CrossRef CAS.
- P. S. Bangde, R. Jain and P. Dandekar, ACS Sustainable Chem. Eng., 2016, 4, 3552–3557 CrossRef CAS.
- M. Feng, X. Lu, J. Zhang, Y. Li, C. Shi, L. Lu and S. Zhang, Green Chem., 2019, 21, 87–98 RSC.
- F. A. Vicente, M. Huš, B. Likozar and U. Novak, ACS Sustainable Chem. Eng., 2021, 9, 3874–3886 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |