Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solid phase synthesis in the development of magnetic resonance imaging probes
Liam
Connah
a and
Goran
Angelovski
 *ab
*ab
aMR Neuroimaging Agents, Max Planck Institute for Biological Cybernetics, Tuebingen, Germany. E-mail: goran.angelovski@tuebingen.mpg.de
bLaboratory of Molecular and Cellular Neuroimaging, International Center for Primate Brain Research (ICPBR), Center for Excellence in Brain Science and Intelligence Technology (CEBSIT), Chinese Academy of Sciences (CAS), Shanghai, PR China
First published on 11th November 2020
Abstract
MRI has emerged as a very important tool in biomedical research and is an essential diagnostic method in clinical radiology today. Although it can be utilised as a standalone technique, the inherent low sensitivity of the method has led to the development of contrast agents (CAs) in order to improve the specificity of the measurement. Nevertheless, the preparation of such probes is often challenging using standard solution phase chemistry, resulting in limitations in CA diversity and ultimately their broader applications. Solid phase synthesis (SPS) has emerged as an alternative synthetic methodology that can assist in circumventing these issues to enable more complex and specific derivatives to be developed. This article aims to provide a concise overview of the strategies employed for MRI CAs developed using SPS synthetic methodologies and evaluate the outlook for the approach in future CA synthesis. Specifically, the development of ligands for T1-weighted imaging, chemical exchange saturation transfer and bioresponsive MRI CAs synthesised directly via SPS are discussed.
Introduction
The invention and development of imaging techniques such as magnetic resonance imaging, positron emission tomography, single-photon emission computed tomography, computed tomography and optical imaging have been fundamental in the fields of diagnostic and molecular imaging.1,2 Imaging probes for each technique are either necessary to conduct the experiment or aim to improve the specificity of the technique. Molecules based on paramagnetic ions (for the use in MRI or OI), radio metals (for PET or SPECT) or organic dyes (for OI) have been deeply investigated for a wide range of applications/targets. MRI has emerged as a leading imaging modality due to its ability to obtain highly spatially resolved, three dimensional anatomical images, without the use of ionising radiation that is fundamentally required in both PET and SPECT. Additionally, the unlimited penetration depth of MRI offers a unique advantage over OI, which suffers in this regard and is thus unsuitable for the study of deep tissue. Finally, the versatility of available MRI methodologies such as T1- or T2-weighted, CEST, heteronuclear or hyperpolarized MRI offers a great deal of possibilities to provide very specific information that is not obtainable using any other imaging modality.Although MRI can be utilised as a standalone technique, the inherent low sensitivity of the method has led to the development of CAs in order to improve the specificity of the measurement.3,4 CAs can be categorised in a variety of ways based on their chemical structure, magnetic properties, biodistribution and contrast mechanisms.5 Each of these mechanisms and the principles of MRI have been concisely reviewed elsewhere, and a spectrum of CAs developed for each application.6–12
The most common method of MRI CA synthesis is standard solution phase chemistry. Indeed, these protocols have been used to develop a range of agents including clinically available MRI CAs. The design of these probes is relatively simple, so their preparation can be achieved easily following these protocols. However, as the field of molecular imaging continuously expands, there is a need for CAs which can be utilised to study specific biological events and/or processes. Therefore, bifunctional probes with increased functionality and specificity are required. This in turn requires more complex CA design to include additional moieties, which improve CA potency or biocompatibility. Consequently, the increased complexity of the probes can often lead to more challenging synthesis and arduous purification procedures.13–15 Alternative synthetic methods such as solid phase synthesis (SPS) offer specific advantages over standard solution phase techniques, which can be employed to circumvent these issues and enable more ‘complex’ and specific derivatives to be developed. Indeed various radiopharmaceuticals,16–27 lanthanide luminescent polyaminopolycarboxylate complexes28–36 and other peptide–metal complex conjugates have been developed using SPS protocols.37 Furthermore, examples of the use of peptide-based building blocks for supramolecular MRI CAs have been covered in a recent review article and will be also discussed here.38 More recently, an article describing the SPS of molecular heterooligonuclear lanthanoid complexes as a platform for luminescence or MRI applications has also been published.39 SPS is instrumental in the development of these specific probes, showing how it can be utilised as a special tool in the synthesis of molecular imaging agents and of similar compounds for applications unrelated to metal chelation.40 To expand on the elegance of the SPS methodology and its application potential, this review aims to provide a concise overview of the strategies employed in the development of MRI CAs using it and evaluate the outlook of the approach in future CA synthesis. Focus will be placed in areas related to polyaminopolycarboxylate ligands for T1-weighted imaging, CEST and bioresponsive MRI CAs. Furthermore, as the mainstay of peptide production occurs via SPS methodologies, there have been many reports of CAs developed via the solution phase coupling of MRI reporter ligands to peptides prepared by SPS. These are noted; however, the essence of this work is to discuss the methodologies and applications of MRI CAs synthesised directly via an SPS platform.
SPS
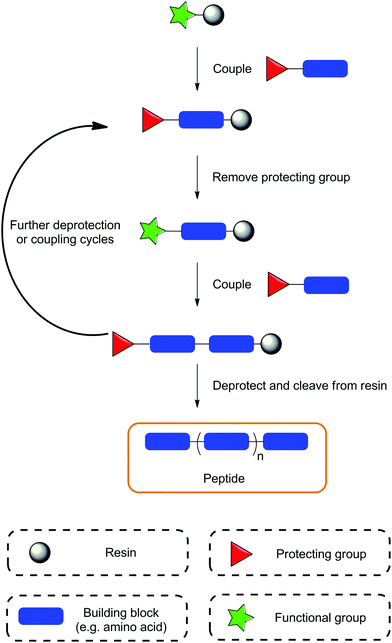
SPS, originally developed as SPPS – solid phase peptide synthesis, was established in the 1960s by Bruce Merrifield, revolutionising the field of peptide synthesis and earning him the Nobel Prize in 1984.41,42 The concept of this methodology relies on the use of a resin in which the compound of interest can be anchored, and subsequent synthetic steps performed. The SPS technique has been reviewed extensively in a range of publications so it will not be discussed in great depth here;43–46 instead, a brief summary of the technique is provided in the following paragraphs (Fig. 1).The term ‘solid-phase’ refers to the use of a resin, typically polystyrene with 1–2% divinylbenzene utilised as a cross-linking agent; the synthesis occurs on this resin. These materials usually have sizes of 35–150 microns and are insoluble in common solvents. One of the key steps in SPS is the swelling of the resin, which allows for all the linker units and surface functionalities to be exposed for further reactions. The linker itself is a specialised functional group, which serves linking the resin bead to the substrate. It plays an essential role in the SPS process and careful consideration is required when choosing the resin, as the linker deprotection conditions must be orthogonal to conditions required to complete the peptide synthesis. The correct and most efficient swelling solvent is dependent on the type of resin used; for polystyrene based resins these are usually tetrahydrofuran, dichlormethane or N,N-dimethylformamide. For the synthesis of more polar compounds, PEGylated resins are recommended allowing for the use of more polar solvents.
Compared to standard solution phase protocols, SPS offers various advantages such as: (i) rapid synthesis, (ii) a reduction in the number of purification steps, (iii) minimal requirement for reaction condition optimisation, (iv) minimal physical losses, and (v) potential automation of the process. Limitations of SPS include difficulties in the final purification steps, which most often originate from incomplete reactions resulting in byproducts and deletion sequences, or alternatively, from the use of impure reagents that cause product contamination. Furthermore, reaction monitoring is usually conducted using either qualitative colourmetric resin tests or by performing a micro cleavage procedure and analysing the resulting compound using standard analytical techniques.47 This can be inconvenient as the qualitative tests are not always conclusive and the micro cleavage procedure requires additional time. Finally, the cost of reagents used in SPS can prove to be a limitation. Coupling agents can be quite expensive and the requirement for large excesses of reagents used in coupling reactions may not be economical when using more expensive building blocks. Despite this, for small to medium scale synthesis of highly diverse compounds, SPS is a highly favourable synthetic method.
After selection of the desired resin and the target sequence, the sequential attachment of protected amino acids/building blocks is usually carried out using coupling protocols utilising various coupling agents, such as HATU/HBTU, PyBOP, DIC/EDC etc. Once coupled, a series of deprotection, coupling and washing procedures are performed to obtain the desired compound, which is then removed from the resin under specific cleavage conditions (Fig. 1). The synthesis of peptides conventionally proceeds in the C-to-N direction, producing compounds with an amino N-terminal; however there have also been reports of synthesis in the opposite direction (N-to-C, inverse SPS) enabling additional flexibility in compound development.48,49 Initially, SPS was carried out using a Boc/tert-butyl protecting group approach, known as the ‘Boc strategy’, which required harsh deprotection conditions, e.g. hydrofluoric acid (to cleave the peptide from the resin) or trifluoroacetic acid (to cleave the Boc protecting group). This approach was successful for the synthesis of large peptides/proteins, but gradually became less popular due to the necessity to use highly toxic hydrofluoric acid. Consequently, Boc was replaced with the base labile Fmoc protecting group to provide a second alternative approach, which is now the main synthetic strategy utilised in SPS. Additional protecting groups are usually used for any orthogonal functional moieties, as is the case in solution phase synthesis; however, the deprotection conditions of the resin must be considered as to not prematurely remove the whole sequence from the resin by mistake.
SPS in the chemistry of MRI probes
MRI CAs based on polyaminopolycarboxylate ligand scaffolds can often be challenging to handle due to their polarity, size and other factors relating to difficulty in synthesis and purification. Furthermore, development of agents for diverse applications requires the production of a variety of structures, which is often challenging. SPS has proved to be an important tool in circumventing these issues by facilitating such complicated synthesis and increasing probe functionality through the simple introduction of targeting vectors (e.g. specific peptide), fluorescent dyes and other functional molecules in simple procedures. Additionally, the nature of SPS allows for combinatorial processes, which enable the rapid production of compound libraries to screen for ideal characteristics in a much more straightforward, timely and efficient manner compared to the solution phase alternative.44,45 These inherent advantages of SPS have thus been exploited in MRI CA development, examples of which are discussed throughout the following sections.T 1-Weighted CAs
Proton T1-weighted MRI CAs operate by shortening the longitudinal and transverse relaxation times of water protons in their immediate vicinity. Typically, T1-weighted CAs are composed of paramagnetic ions (gadolinium or manganese) complexed by organic scaffolds, whose purpose is to tightly bind and prevent the release of free metal ions which can potentially bring toxic effects.4,5,7,50–52 The vast majority of T1-weighted MRI CAs reported are based on Gd3+ complexes with polyaminopolycarboxylate organic scaffolds, either as acyclic or cyclic chelators. Gd3+ offers the greatest advantages for 1H T1-weighted MRI due to its high number of unpaired electrons and slow electronic relaxation rate. The ‘gold standard’ Gd3+-based CA utilises a DOTA scaffold, which is cyclic and offers high kinetic and thermodynamic stability when compared to acyclic chelators. In terms of efficiency, the relaxivity of T1-weighted CAs can be modulated through various parameters, such as the number of inner sphere water molecules, their exchange with the bulk water and the overall rotational correlation time of the complex. Significant research has been undertaken in optimising systems to produce higher relaxivities while attempting to maintain excellent thermodynamic stability and kinetic inertness, resulting in the production of a myriad of Gd3+-based macrocyclic compounds at the research level. Various strategies have been explored in order to further improve the specificity or functionality of gadolinium based CAs, including the use of macromolecular systems, incorporation of targeting vectors and the introduction of functional molecules (e.g. dyes).3,53–56While macromolecular systems offer various advantages, such as increased local Gd3+ concentration, their size can ultimately limit their applications. Alternatively, peptides possess excellent properties for molecular imaging applications such as high bio-compatibly, specific target uptake and retention, high in vivo stability and simple preparation.57 This allows for probes with specific peptide sequences to be exploited for developing cell permeable and target-specific probes. These types of probes are necessary to ensure accumulation in the desired region, thus enabling visualisation of targets with low concentrations, compensating somewhat for the lack of sensitivity of MRI compared to other imaging methods. They also offer a high degree of synthetic flexibility by utilising orthogonal protections of amino acids to conjugate various moieties in a precise stepwise manner. Hence, the desired use of peptides conjugated to DOTA scaffold gave rise to development of various SPS methodologies that have been widely explored for the preparation of potential MRI CAs and radiopharmaceuticals.58,59 The discussion below highlights the examples of T1-weighted MRI CAs developed for the use as intracellular, target-specific or multimeric probes.
Intracellular CAs
For molecular imaging, the intracellular environment offers a range of potential targets for study. However, the vast majority of MRI CAs developed to date are only applicable to the extracellular environment. One of the main reasons for this is the more complex CA chemical design required to facilitate cellular uptake through various mechanisms in order to investigate intracellular biological phenomena.60–62 Another issue relates to a lowering of relaxivity values and hence reduction of the MRI signal they produce due to the compartmentalisation of the probes.63 SPS provides appropriate tools to tackle synthetic issues and has been a common choice for the preparation of intracellular CAs.Cell-penetrating peptides (CPPs) are unique peptide sequences that can import attached cargo molecules across cell membranes into the intracellular space.64,65 Combinations of CPPs and MRI contrast agents have found applications in the tracking of intracellular species and pre-labelled cells (Fig. 2).66 Initially, reports focused on conjugating tris-tBu-DOTA to a HIV-tat peptide constructed exclusively of either L- or D-amino acids using SPS protocols.67,68 The resulting peptides showed efficient uptake into mammalian cells in concentrations relevant for MRI studies. Specifically, the Gd-DOTA-D-Tat peptide was demonstrated to internalise in human Jurkat leukemia cells, showing a T1 relaxation enhancement (r1 = 7.94 ± 0.11 mM−1 s−1, 4.7 T) in preliminary MRI experiments.68 The simple strategy of combining gadolinium chelates with CPPs using SPS for intracellular imaging applications has been adopted by other research groups and are highlighted below.
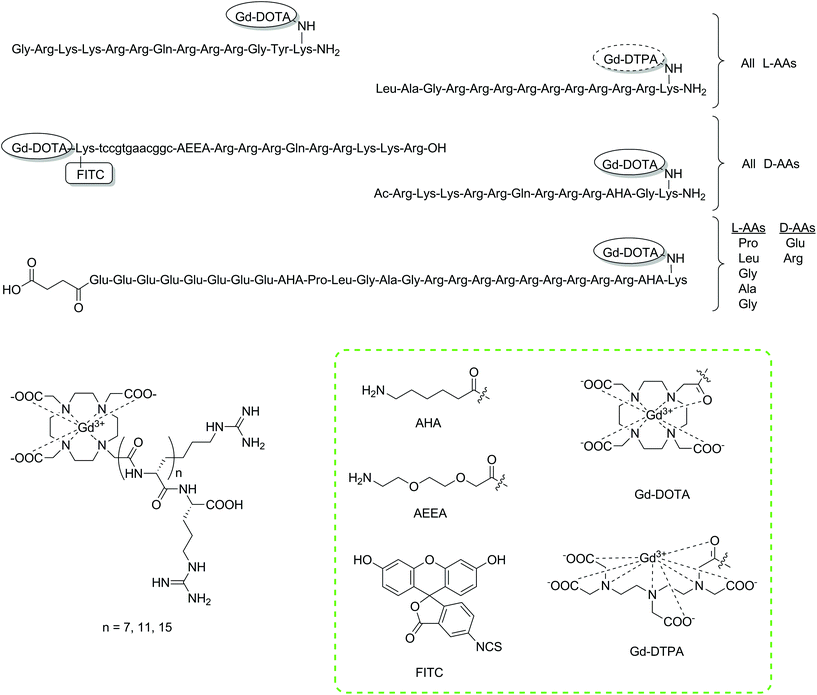 | ||
| Fig. 2 Chemical structures of the discussed intracellular CAs constructed using SPS protocols.62,67–69,73,75 Lower case letters a, t, g and c represent PNA building blocks adenine, thymine, guanine and cytosine, respectively. The tags with Gd-DOTA/Gd-DTPA have been used to display the type of chelator/CA included at this point. Coupling of these moieties will give the monoamide derivatives, namely DOTA/DTPA-monoamide (shown in the green box). | ||
The direct monitoring of mRNA is another attempted application of intracellular CAs. The monitoring of such a target could provide information associated with gene expression patterns, potentially detect changes in disease states and open up the possibility to pre-emptive therapy. Two reported studies have focused on the SPS development of molecular imaging agents to monitor mRNA.69,70 Initially, two molecular imaging probes were designed consisting of Gd-DOTA, FITC, a CPP and a PNA sequence.69 Differences in the PNA sequence were designed to allow one of the two probes to bind to the mRNA of a red fluorescent protein (dsRed), while the other was intended as a control. A Wang resin of low substitution (0.30 mmol g−1) was used as the solid support to couple the CPP (D-Tat57-49). From here, a spacer unit was introduced and the PNA sequence assembled. Afterwards, Fmoc-Lys(Dde)-OH was coupled to provide two amines (α and ε), which were attachment points for the tris-tBu-DOTA ester unit and FITC after deprotection of the amino protecting groups, Fmoc and Dde, respectively. The authors note that the use of SPS as the synthetic strategy provided several advantages, such as allowing milder reaction conditions to be used and preventing side product formation. Furthermore, the process could be repeated on an automated synthesiser if desired, due to the continuous nature of the preparation. Both probes were incubated with 3T3 cells showing intracellular accumulation and a statistically significant relaxation enhancement after applying only 0.5 μM of the probe.
In the follow-up study, the same SPS synthetic platform was used to introduce three derivatives of the HIV-1 Tat CPP to evaluate their effectiveness.70 Utilising a SPS building block approach, the introduction of the peptides was simple and further allowed for changes in the multimodal probe, without significant modification to the SPS synthetic scheme. Assessment of the selected PNA-conjugated MRI probe was performed with a series of in vitro and in vivo experiments, which showed that significant MR contrast enhancement could be achieved in cells with concentrations of 2.5 μM. The binding of the antisense PNA-conjugate could be proven with in vitro cell-free assays, but lacked specificity in transgenic cells due to nonspecific entrapment of the contrast agents. The in vivo biodistribution studies showed encouraging signs, as they exhibited no toxicity for the CA. On the other hand, significant mobility resulted in accumulation in the liver and bladder possibly due to high lipophilicity. Nevertheless, these multimodal probes showed encouraging signs for future development.
Polyarginine residues have also displayed the capability to facilitate cellular uptake. Through simple coupling procedures, two studies explored combinations of polyarginine (8, 12 and 16 monomeric units) and Gd-DOTA as potential intracellular CAs to study biological phenomena.62,71 All CAs were prepared using SPS commencing from a Wang resin, coupling the desired number of arginine monomers and finally the DO3A macrocycle as the tris-tBu ester. Subsequent cleavage from the resin and treatment with Gd(OH)3 afforded the final contrast agents, which demonstrated cellular uptake.
Two studies were reported, in which a Gd3+ complex based on a linear DTPA chelator was functionalised with a polyarginine CPP targeting mesenchymal stem cells. Successful internalisation of the conjugate resulted in an increase in intracellular r1, without affecting cell viability.72,73 The same group applied a similar system to HepG2 cells showing comparable results,74 while a hairpin CPP conjugated to DOTA via SPS was utilised to target MMP-2 in human ovary adenocarcinoma cell lines.75
The use of SPS in the examples provided above has demonstrated the high degree of flexibility in customisation that SPS affords when using a building block approach. Consequently, this allows for the rapid synthesis of such conjugates compared to a ‘classical’ solution-phase approach. Particularly, SPS showed to be the major method of choice for the preparation of CPP-conjugates with Gd-chelates. Although the number of studies for this type of T1-weighted CA is limited, CPP-conjugates remain an interesting platform for molecular imaging and therapeutics with significant work being performed for applications with other imaging modalities.66,76
Target-specific CAs
Currently, MRI CAs available for clinical use lack specificity for monitoring pathologies. To improve the scope of their application, steps towards developing target-specific probes have been explored using targeting vectors, as these can enable accumulation of the CA at a site of interest.77 Furthermore, the targeting process can also improve retention of the probe through anchoring or internalisation, thereby reducing the rate of washout and enabling extensive studies.Peptide sequences are often used as targeting vectors for receptors on cells. The simplicity in which these sequences can be synthesised using Fmoc SPS has increased the flexibility and customisation in MRI probes for targeting purposes. General strategies can be developed to allow modification of conjugates either through targeting choice or number/type of MRI reporter to accommodate a vast number of applications.
For instance, SPS has been utilised to assemble a series of PDAP dendrimers functionalised with a PNA sequence, Gd-DO3A-type complexes and a cyclised insulin-like growth factor 1 (IGF1) peptide D(Cys-Ser-Lys-Cys).78 This tetrapeptide was previously shown to internalise PNA into malignant cells, which overexpress IGF1 receptors via a process known as endocytosis. The rationale behind using the dendrimeric approach was to increase the number of Gd-DO3A conjugates per molecule, thereby increasing the local concentration of the probe and attempting to combat the inherent low sensitivity of MRI. Indeed, this approach has been utilised regularly in MRI CA development with several examples of probes conjugated to nanoparticles, such as liposomes and dendrimers.53 Furthermore, the effect of increasing the molecular weight and size has profound effects on the observed relaxivities at clinically relevant magnetic fields.
PDAP dendron synthesis was simply achieved via Fmoc SPS and then functionalised with the targeting peptide, a spacer moiety, PNA and either two, four, eight or sixteen Gd-DO3A-type complexes (G1 to G4 dendrons, respectively, Fig. 3). In vitro MRI experiments on tube phantoms were performed for the G1 and G3 PDAP hybridisation probes and compared to PAMAM dendrimers (32 units) functionalised with Gd-DOTA- or Gd-DTPA-derived chelates at 3 T. The results revealed an expected trend of increasing calculated r1 relaxivities per mM of Gd3+ with increasing size. This led the authors to postulate that attachment of these probes to mRNA would additionally increase their size and further enhance r1 allowing discrimination between bound and unbound variants.
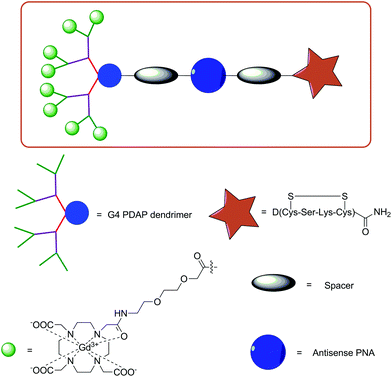 | ||
| Fig. 3 Illustration of the design of a targeted G4 PDAP dendrimeric MRI CA synthesised using Fmoc SPPS protocols.78 | ||
Preliminary data from AsPC1 pancreas cancer xenographs also found that a minimum of eight Gd-DO3A-type complexes were required to provide detectable contrast with up to sixteen conjugates permitted to not perturb uptake of the probes.78,79
Fmoc SPS protocols have been utilised to develop a binary targeted MRI CA for human lung adenocarcinoma cells A549 (Fig. 4a).80 The peptide sequence included both Arg-Gly-Asp (RGD) and Asn-Gly-Arg (NGR) motifs, which display high binding affinities for αvβ3 integrins and aminopeptidase N (CD13) receptors, respectively, both of which are prevalent in tumour cells. Strategies for targeting with either RGD or NGR have been widely exploited in diagnostics and therapeutics.81–87 A lysine residue was also incorporated into the peptide sequence to enable simple coupling of pre-prepared tris-tBu-DOTA using standard coupling procedures. The inclusion of two binding motifs demonstrates the simple customisation available using such synthetic techniques. The longitudinal relaxivity of the dual-targeting CA was greater (7.55 mM−1 s−1vs. 6.36 mM−1 s−1 and 4.25 mM−1 s−1, respectively, 11.7 T) than that of a similar mono-targeted derivative and a non-specific commercially available CA, Magnevist®.
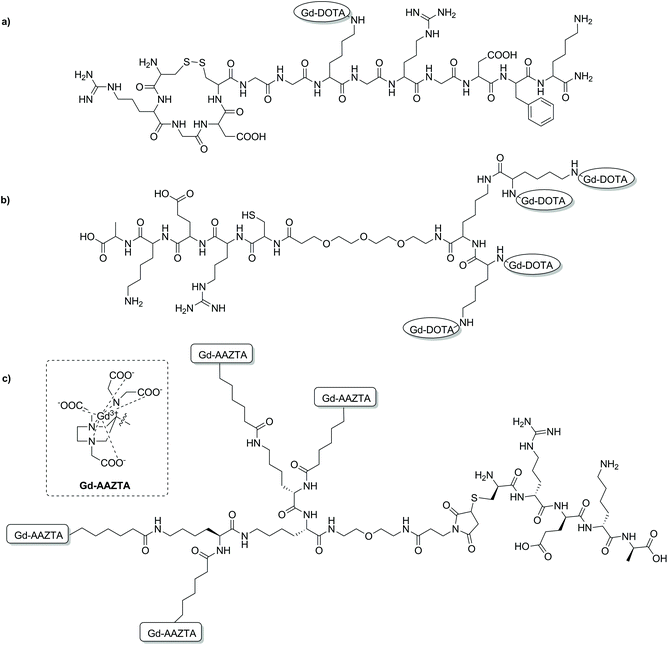 | ||
| Fig. 4 Chemical structures of some targeted MRI CAs discussed in this section. (a) A binary targeting CA consisting of both RGD and NGR binding sequences;80 (b) a multimeric DOTA-based CA with a CREKA peptide for prostate cancer imaging;93 (c) a prostate cancer targeted multimeric AAZTA-based CA.99 | ||
An alternative strategy for developing NGR-functionalised Gd-DTPA-type conjugates has also been reported.88 The strategy involved the assembly of a small peptide sequence, including target specific NGR on a MBHA resin using SPS. Using both Boc and Fmoc chemistry, selective acylation of the oligopeptide terminal was performed, followed by deprotection of a lysine side chain. The primary amine of the lysine residue was utilised to couple an isocyanate-DTPA-type macrocycle derivative to give the final structure. Subsequent removal from the resin and complexation with Gd3+ provided the final targeted CA. Longitudinal relaxivity studies were performed at 1.5 T and 20 °C, revealing an expected increase in r1 for the larger targeted CA (increase in molecular tumbling time) compared to unspecific Gd-DTPA (9.8 vs. 4.2 mM−1 s−1).
Various MRI CAs for the molecular imaging of prostate cancer have been developed via SPS. In order to target fibronectin-fibrin complexes present in malignant prostate cancer tumours, various peptides coupled to Gd-DOTA- or Gd-DTPA-type chelates were investigated (Fig. 4). Initial studies looked at the use of a cyclic decapeptide (CLT1) conjugated to a single Gd-DTPA complex via DTPA bisanhydride, or to four Gd-DOTA monoamide complexes.89,90 Both of these targeted CAs demonstrated sufficient contrast enhancement in atherosclerosis and tumour models; however, limitations in application arose due to limited solubility of the peptide in water, therefore causing high tissue retention and potential toxic effects.89–92 Simple exchange of CLT1 for a water soluble fibronectin-fibrin targeting peptide, CREKA, resulted in a molecular probe with improved pharmacological properties for prostate cancer imaging (Fig. 4b).93 In a more recent study, the efficacy of four Gd-DOTA-type peptides for high-risk prostate cancer has been investigated. The design of each molecular agent was identical, consisting of an extradomain B fibronectin (EDB) binding peptide, a PEG spacer unit and a single Gd-DOTA complex.94 Synthesis of each probe was carried out using standard Fmoc-SPS techniques, followed by conjugation of a DOTA-NCS derivative as the final step before peptide cleavage and complexation with Gd3+. The SPS synthetic scheme enabled the efficient and timely synthesis of peptide–CA conjugates with high yield. Each of these conjugates displayed enhanced r1 relaxivities compared to Gd-DOTA and Gd-HP-DO3A (4.1–4.7 vs. 2.9 mM−1 s−1, 1.5 T, 37 °C), with robust and prolonged signal enhancement observed in PC3 tumour bearing mice. The results of this study are yet another demonstration of the advantages of peptide-based targeted CAs for molecular imaging and of the SPS technique in the development of such probes.
Fibrin is also a useful molecular target for in vivo monitoring of tumours and thrombus, which led to development of different types of peptide-based fibrin-targeting probes.95 An example of such a probe combined four Gd-DTPA-like chelates and a specific fibrin-targeting peptide via simple Fmoc SPS. The resulting MRI CA provided high relaxivity per molecule (42 mM−1 s−1, 20 MHz, 25 °C) giving strong signal enhancement in a Neuroblastoma tumor model. Ultimately, this probe was showed to be efficient for tumour delineation and monitoring fibrin deposits during the progression of the tumour and subsequent anti-thrombotic therapy.96
SPS has been utilised as an ideal platform to build a bifunctional Gd-AAZTA tetrameric system, which was coupled to various targeting vectors for bioconjugation.97 Commencing from a bis-(2-aminoethyl)-ether trityl resin, two lysine coupling steps gave a tri-lysine branched core with four amino groups for coupling with an AAZTA derivative (Fig. 4c). Subsequent cleavage from the resin gave a tetrameric unit with a primary amine, which was then converted to a maleimide, benzaldehyde or cyclooctyne for biomolecule conjugation. As a proof of concept, a known fibrin-binding peptide from the CA EP-2104R was utilised as the targeting vector.98 Relaxivity measurements showed an increase in r1 relaxivity per Gd3+ for the fibrin peptide conjugate compared to the ‘unlabelled’ tetramer and the original monomeric Gd-AAZTA unit (18.5 ± 0.3 mM−1 s−1vs. 16.4 ± 0.2 and 7.1 mM−1 s−1 respectively, 0.5 T, 25 °C).97 In a follow-up study, the maleimide derivative was synthesised and combined with the CREKA peptide for prostate cancer detection in a similar method to that described previously.93,99 The authors hypothesised that an improvement in contrast could be achieved by using their AAZTA-derived tetramer due to the favourable relaxometric properties of AAZTA (compared to DOTA monoamide). High molecular relaxivity was observed for the targeted probe (18.2 mM−1 s−1 per Gd3+ or 72.8 mM−1 s−1 per molecule, 0.5 T, 25 °C), allowing for higher contrast to be achieved in vivo with a lower dose of the compound relative to current clinically approved CAs. Experiments on an orthotopic PC3 tumour mouse model at 7 T showed significant contrast enhancement 10 min post injection compared to a similar AAZTA-derived tetramer with a ‘scrambled’ peptide (Fig. 5).
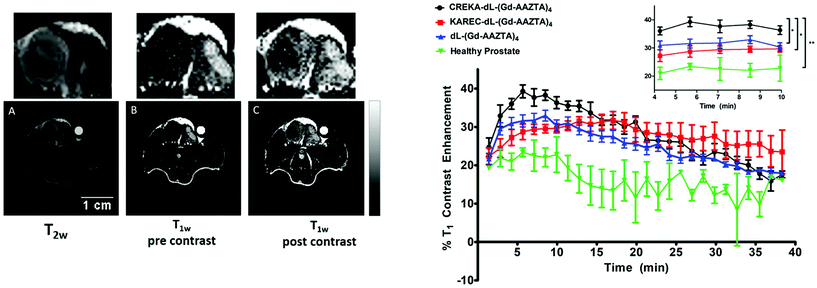 | ||
| Fig. 5 In vivo MRI results from a multimeric AAZTA targeted CA (Fig. 4c) in PC3 orthotopic tumour bearing mice. Left: 7 T MRI images of prostate cancer in the mouse. (A) T2-Weighted image for tumour localisation; (B) T1-weighted image pre-injection of the CA; (C) T1-weighted image of the tumour region at 12 min post injection. Right: T1 contrast enhancement (%) over time of the targeted CA (black), non-targeting scrambled peptide derivate (red) and non-functionalised variant (blue) in PC3 prostate cancer bearing mice. The green line represents the T1 contrast enhancement of the targeted CA in healthy mice. Adapted with permission from ref. 99. Copyright © 2018 International Society for Magnetic Resonance in Medicine. | ||
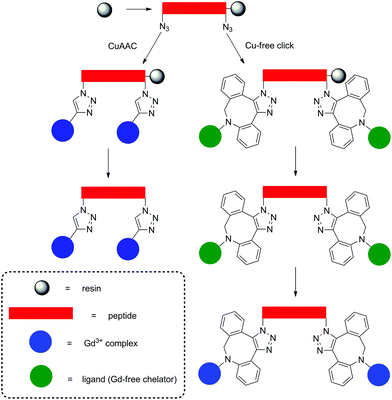
Strategies for MRI CAs developed via SPS techniques described so far generally follow similar protocols, namely, synthesis of the peptide–CA conjugate on-resin, before cleavage to give the deprotected ligand and finally complexation with Gd3+ in solution. This methodology is robust and applicable with the final complexation procedures being carried out in conditions ranging from mild (room temperature, neutral pH) to more extreme, e.g. elevated temperature or acidic/basic pH. More robust conditions are sometimes required to ensure complexation, however, this procedure can also present problems when dealing with sensitive moieties. Therefore alternate approaches have been demonstrated in the development of a polyadenylic acid targeted MRI CA, which could potentially be used in reporting on global mRNA anabolism.100 Specifically, a series of Gd-DOTA-type complexes were attached to a PNA sequence via CuAAC on resin (Fig. 6). This is a high yielding, selective reaction performed under mild conditions, thus ideal for use with sensitive biomolecules or as an orthogonal method of attachment for two molecules. As a result, CuAAC has found numerous applications in SPS in the synthesis of peptides, nucleotides, cyclic peptides etc.101 In order to perform the click reaction, azide monomers were introduced into the peptide sequence with SPS and reacted with an alkyne functionalised Gd-DO3A monoamide complex (Fig. 7). Relaxivity measurements showed an increase for the multimeric probe and its supramolecular complex with RNA versus the monomeric chelate (5.6 and 6.6 mM−1 s−1 per Gd3+, respectively, 20 MHz, 298 K). The use of SPS circumvented the tedious and laborious workup procedures usually required for solution-phase CuAAC reactions; instead, simple washing steps efficiently removed the waste materials. The authors note that for application, the inclusion of a moiety capable of cellular uptake would be required. This can be simply included through minor modifications of the SPS scheme as shown in the previously discussed examples. In addition, between 160–200 Gd3+ ions would be localised to the microenvironment with an average poly(rA) tail of mRNA, which is comparable to G6 PAMAM dendrimers, however with increased specificity. Another approach to using click reactions for DOTA-type macrocycles was also demonstrated.102 Here, SPS was utilised to develop a PEGylated MRI CA with three Gd-DO3A-monoamide units and a terminal thiol functional group (Fig. 7). The DO3A-monoamide units were attached to the PEGylated molecule as the deprotected ligand using a Cu-free click reaction. The azide on solid phase was reacted with a dibenzocyclooctyne DOTA derivative and complexed with Gd3+ before deprotection from the resin. The final CA was incorporated into a larger Au nanoparticle system with a surface enhanced resonance Raman scattering CA to form a multi CA for tumour targeting.
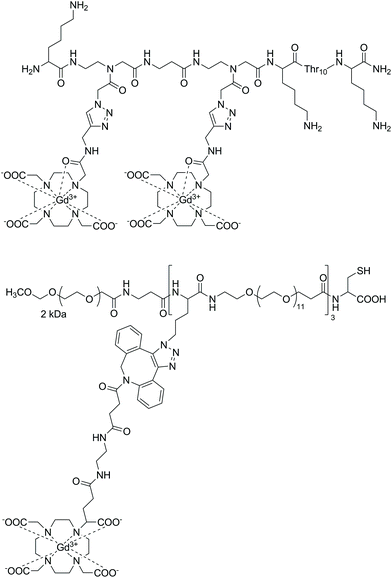 | ||
| Fig. 7 Examples of probes assembled using on-resin click chemistry methods.100,102 Upper: A polyadenylic acid targeted MRI CA synthesised with CuAAC on resin. Lower: A PEGylated MRI CA assembled using Cu-free click chemistry methods. | ||
Finally, SPS has also found applications in the development of multimodal imaging probes. The aim of such probes is to enhance the strengths and circumvent the disadvantages of individual imaging modalities through their synergistic combination.103 A mixed solid/solution phase procedure to develop a multimodal molecular probe for thrombus imaging has been reported.104 Specifically, this report described the synthesis of four bimodal probes, all of which possessed an identical fibrin binding peptide. After coupling of either fluorescein isothiocyanate for optical imaging or DOTA monoamide for PET or MRI, the probe was cleaved from the resin, a DMSO cyclisation step performed and then a solution coupling to introduce the moiety for the second imaging modality. Complexation of DOTA monoamide was then carried out either with 64Cu (PET) or Gd3+ (MRI). Each probe was assessed for binding to human fibrin and the relaxivities of the Gd-containing variants were measured showing an increased r1 when bound to the target protein due to an increased molecular weight (18.8–21.2 mM−1 s−1, 1.4 T, 37 °C, 30 μM fibrin gel in Tris-buffered saline). Although, the specific example discussed here follows a mixed solution/solid phase approach, principally, systems could also be designed exclusively following SPS protocols.
Another example of a mixed solution/SPS approach to a multimodal MRI/optical targeted imaging probe was reported for a gastrin releasing peptide, which is overexpressed in a large number of tumours.105 In this report, a simple building block approach was applied to afford a probe comprised of a BN peptide and a TTDA-NP acyclic ligand on-resin. Subsequently, the construct was cleaved from the resin, complexed with Gd3+ and conjugated with a NIR organic dye, Cy5.5. Studies performed both in vitro and in vivo showed the capability of the dual-modal agent to efficiently target PC-3 tumour cells producing significant MR and fluorescence signal enhancement.
Self-aggregating peptide CAs
Supramolecular structures, such as micelles, liposomes and dendrimers have gained significant attention for nanomedicinal purposes.106–108 Such aggregates can be fine-tuned to exhibit specific properties with beneficial pharmacokinetic properties. Peptides are another ideal building block for such applications due to high customisability and unique control in the synthetic process. These properties have led to a range of peptide-based self-aggregating/assembling systems being developed as potential diagnostic and theranostic agents.38,109,110 More recently, the development of a peptide-based hydrogel as an MRI CA has been described (Fig. 8).111 The design of such self-aggregating MRI CA systems is usually composed of a Gd3+ complex, a peptide backbone and possibly additional moieties to assist in the aggregation process. In this latest study, the CAs were made up of two functional components, namely, a peptide-polymer and either a DOTA- or DTPA-type scaffold for Gd3+ chelation. As with many peptide-based molecules, the synthesis of such compounds was carried out with SPS protocols following the Fmoc/tBu strategy, before complexation post cleavage from the resin. Placing the MR reporter at the N-terminus did not impede the self-aggregation process and formation of secondary structures. In the aggregated state, the relaxivity of the hydrogels was found to be 12.0 and 12.1 mM−1 s−1 (DTPA- and DOTA-type CAs respectively, neutral pH and 21.5 MHz), which is 2–3 times greater than that of the low molecular weight analogues. Additional cytotoxicity studies on a metastasizing mouse mammary adenocarcinoma cell line showed encouraging results and the potential for application as a diagnostic agent.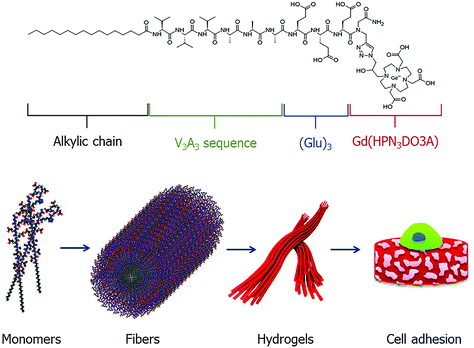 | ||
| Fig. 8 A schematic representation of a self-aggregating system composed of an alkyl chain, peptide sequence and Gd-complex. The monomeric unit self assembles to form ordered fibers, which undergo Ca2+ ion mediated cross-linking to form hydrogels which can be utilized in applications such as tissue engineering. Reproduced with permission from ref. 38. Copyright © 2019 European Peptide Society and John Wiley & Sons, Ltd. | ||
Other applications
Outside of the major applications already discussed, SPS has been reported as a useful tool in the development MRI CAs with high relaxivity, probes for the detection of peptide–protein binding events and as precursors towards biodegradable macromolecular agents for blood pool imaging (Fig. 9).112–115 By incorporating a rigid amino acid Gd3+ chelate such as DOTAla into a peptide chain, significant control and optimisation of the rotational dynamics could be attained enabling the design of CAs with high relaxivities at low and high field.112 Indeed, the CAs reported in this study showed superiority over commercially available CAs such as MS-325/HSA at high field (4.5–5.5 vs. 3.7 mM−1 s−1, 500 MHz, 37 °C). Attaching similar amino acid DOTA conjugates (DOTAlys, DOTAPhe) to a Gal-80 binding peptide was also shown to affect the binding specificity of a peptide–protein system, which in turn could significantly assist in identifying new metal-ion based systems for MRI diagnostic applications.114 With safety concerns surrounding the excretion of macromolecules, biodegradable derivatives have been designed to show accelerated clearance, minimising Gd3+ retention in tissues.116 One biodegradable macromolecular CA was prepared using a (N6-lysyl)lysine DOTA monoamide monomer assembled using the Fmoc SPS methodology.115 Post cleavage from the resin, the monomeric unit was used in a condensation polymerisation reaction with dithiobis(succinimidylpropionate), before complexation with Gd3+ to form the final biodegradable macromolecular structure. Subsequent in vitro and in vivo studies revealed significant r1 relaxivity (8.25 mM−1 s−1, 1.5 T, 37 °C), high kinetic stability and efficient excretion following degradation by endogenous thiols. Such properties are advantageous for anticipated applications in MR cancer and cardiovascular imaging.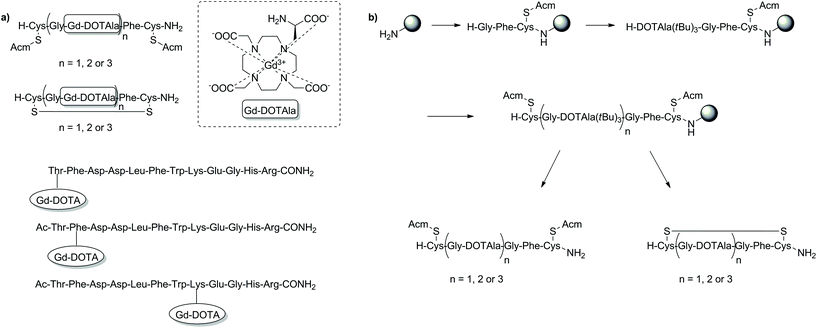 | ||
| Fig. 9 Examples of T1-weighted MRI CAs developed for other applications than those highlighted thus far using SPS techniques. (a) SPS synthesised T1 MRI CAs: with high relaxivity (top two examples) and as probes for the detection of peptide–protein binding events (bottom three examples); (b) the SPS scheme for preparation of the DOTAla-containing peptide conjugates. Gd3+ addition is performed in solution after cleavage from the resin to give the MRI CA that is then appended with Gd-DOTAla.112–114 | ||
Chemical exchange saturation transfer CAs
CEST is a modern, alternative MRI methodology that exploits the magnetization transfer between two exchanging pools of protons from a molecule (CEST agent) and bulk water.117–119 These pools of protons operate at different frequencies, thus allowing selective irradiation of one pool (e.g. amide protons of the CEST probe) to consequently observe a reduction in magnetization of the second pool (bulk water) upon the exchanging of protons. This can also be extended to CEST agents with multiple exchanging pools of protons, enabling multicolour and ratiometric CEST. The necessity to irradiate a pool of protons in order to generate the CEST image is one of the greatest inherent advantages of CEST MRI, as it provides complete control and ability to selectively generate contrast. CEST utilises both endogenous (metabolites, amino acids and small molecules) and exogenous (129Xe) diamagnetic species as well as paramagnetic compounds for a variety of applications. While much success has been found when using diamagnetic species, the small shift difference of the exchangeable protons from water can create limitations leading to the need for diamagnetic CEST agents with incredibly slow exchange rates. The use of paramagnetic CEST (paraCEST) probes aims to limit these disadvantages by ensuring a larger shift difference between the pools of protons. This is often achieved using lanthanide ions with anisotropic f-electron configurations (e.g. Eu3+, Tm3+ and Dy3+) complexed with macrocyclic ligands such as DOTA-tetraamide. The advantages of SPS have also been exploited in the development of individual paraCEST complexes and applied to the large scale screening of a vast number of compounds in combinatorial approaches.Often the conjugation of DOTA-type chelators to peptides is performed through a carboxylate of the macrocycle to the N-terminal of a specific peptide (Fig. 10). The reverse methodology has also been applied to conjugate an amino functionalised DOTA-type moiety to the C-terminus of a peptide, thus diversifying potential applications.120,121 The general approach for this mixed solution/SPS synthetic approach included a DO3A/DOTA-type macrocycle prepared in solution with the carboxylate arms free to couple to a Wang resin. After attachment to the resin via one of the carboxylates, the remaining free acids were reprotected as tBu esters and further modifications were performed on the solid support to obtain an amine-derivatised DOTA-type product, which was subsequently coupled to the C-terminus of a peptide sequence. In the final phase, the compounds were cleaved from the resin and complexed with Tm3+ to produce the desired paraCEST agents. The product was able to detect urokinase plasminogen activator. The selection of an appropriate α-amino group with a labile protecting group was essential to this strategy and highlighted within these studies. In the initial work, Cbz was utilised as the protecting group, however the typical method of deprotection is incompatible with SPS (hydrogenation with Pd/C catalyst). Therefore, an unconventional approach was adopted to cleave Cbz on resin through treatment with diethylaluminium chloride/thioanisole at −78 °C. Although the deprotection procedure was successful, the harsh conditions also removed the whole DOTA-type construct from the resin, consequently reducing the final yield. In the follow-up study, the Cbz protection was replaced with the SPS-friendly Fmoc group before loading on the polymeric support. This ensured simpler deprotection and therefore higher yields of the final product. The new synthetic methods reported in these two studies demonstrated an alternative approach to utilising DOTA-type chelators in conventional Fmoc SPS for the functionalisation of peptides at the C-terminus.
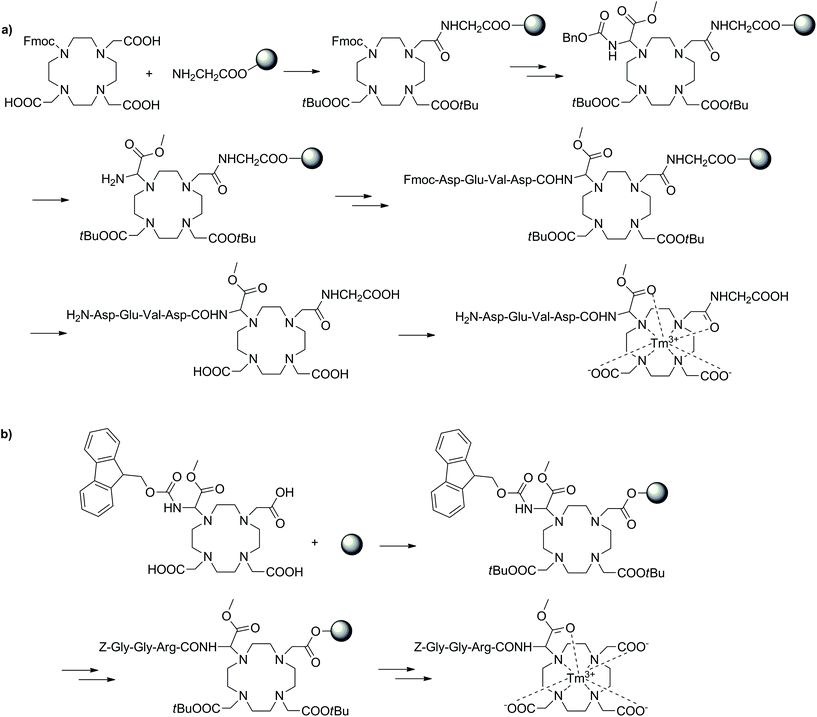 | ||
| Fig. 10 Two SPS schemes showing different approaches towards similar paraCEST CAs. (a) Utilising the Cbz protecting group, (b) using a Fmoc protected DOTA building block.120,121 | ||
Improving the sensitivity of paraCEST agents is of paramount importance to their application. In order to enhance the performance of CEST probes, the influence of different molecular properties on the key parameters governing the sensitivity (e.g. water exchange for CEST) must be well understood.122 Traditionally, this has been approached by studying individual CAs. However, not only is this process time-consuming, it also is incredibly difficult to develop a complete understanding of the influence of multiple parameters on the signal. SPS provides a platform which can be used as a combinatorial tool to allow for the fast screening of molecules. This approach has been adopted to identify key factors that influence water exchange in paraCEST agents.123,124 Here, two libraries of 80 and 76 on-bead Eu3+ DOTA-tetraamide complexes were produced as potential paraCEST agents following similar synthetic protocols. Specifically, commencing from a TentaGel resin, a small linker was introduced to increase the space between the beads and the macrocycle. Next, the tris-tBu-DO3A ester was coupled and the esters removed with TFA. The three acetate arms were then treated with ethylene diamine to afford the typical DOTA-tetraamide structure commonly used in paraCEST agents. Subsequently, two peptoid residues, of varying hydrophobicity, size and charge, were introduced onto each arm before complexation with EuCl3 to give the final on-bead paraCEST agents (Fig. 11). CEST imaging was then performed to identify compounds which enhanced the CEST effect. From this first study, it was identified that negatively charged groups in the immediate vicinity of the DOTA scaffold improved the CEST signal.123 Therefore, the follow-up study focused on modifying the outer residues with a variety of amino acids, amines, anhydrides and carboxylic acids to assess a range of physicochemical properties (Fig. 12).124 Overall, the results of this combinatorial study indicated, in a fast and straightforward manner, that negatively charged compounds with less sterically crowded groups contribute favourably towards CEST properties and should be included in the design of future DOTA-tetraamide paraCEST CAs. Moreover, this also demonstrated the significant advantages to adopting a SPS approach, in which a library of compounds can be synthesised with high yields, in order to simultaneously assess the performance of several probes in a timely manner and further aid CA design.
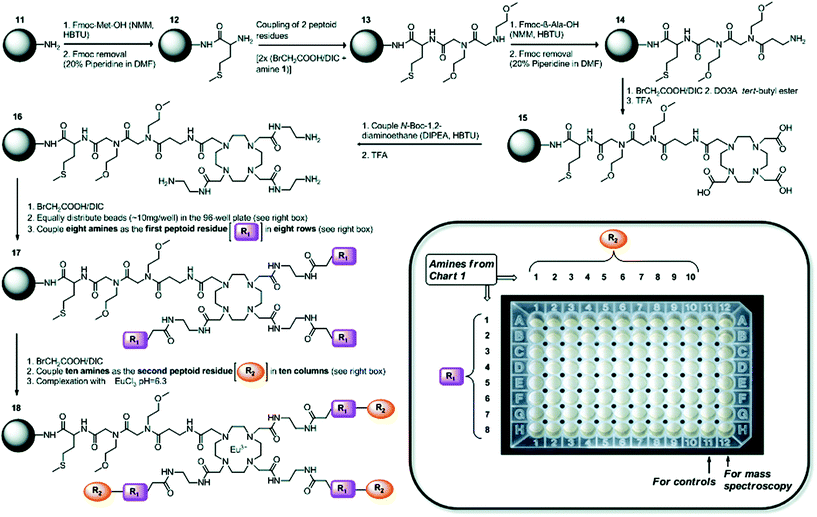 | ||
| Fig. 11 Synthetic scheme towards a library of on-bead Eu3+ DOTA-tetraamide complexes as potential paraCEST agents. R1 and R2 are different amine peptoid residues of varying hydrophobicity, size and charge, which were used to prepare a library of paramagnetic Eu3+ DOTA-tetraamide complexes and study their paraCEST properties in a high-throughput manner. Reproduced with permission from ref. 123. Copyright © 2011, American Chemical Society. | ||
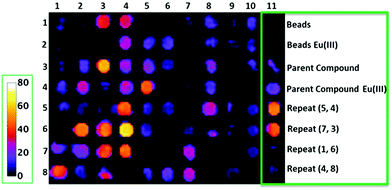 | ||
| Fig. 12 On-bead CEST images of a library of Eu3+ DOTA-tetraamide complexes showing the percent change in bulk water signal intensity. Numbers in the rows and columns represent different structural subunits that were used for creation of the library in order to add the physicochemical diversity to the prepared and investigated Eu3+ DOTA-tetraamide complexes. Reproduced with permission from ref. 124. Copyright © 2017, John Wiley & Sons, Ltd. | ||
Furthermore, a similar combinatorial approach was adopted to synthesise and screen Eu-DOTA-tetraamide complexes against cancer cell lines, identifying one compound for future investigation.125 This strategy can identify potential theranostic agents, further indicating how SPS combinatorial chemistry can be utilised for the screening of compounds from a variety of applications.
The development of 129Xe biosensors for hyperCEST has also been reported following a building block SPS approach.126 HyperCEST is a combination of hyperpolarization, which provides significant signal amplification, and CEST to detect between two states, which in this case are encapsulated and free 129Xe. To this end, the authors reported a system combining a crytophane macrocycle (for Xe encapsulation), a fluorophore tag and biotin as a targeting moiety on a TentaGel resin. The use of such a synthetic strategy enables the development of highly sensitive probes capable of labelling and visualising a variety of biological targets, which would otherwise be MR-inaccessible in vivo. Furthermore, the reported compound can be considered a model synthetic system for future 129Xe hyperCEST CAs, with modifications easily accessible through simple exchanging of individual components in the SPS scheme. It is also important to note that the macrocycles employed for 129Xe encapsulation are entirely different from the polyaminopolycarboxylate scaffolds discussed throughout this work, showing that SPS can serve as a versatile synthetic platform for a variety of applications.
Bioresponsive CAs
Bioresponsive CAs are of great interest in developing fundamental understandings of biological and pathological processes rather than their final effects. These agents, also known as smart CAs (SCAs) are unique as they can alter their MR properties in response to a physical–chemical stimulus in their microenvironment, often through the modulation of hydration number or rotational correlation time.127–132 This in turn enables the reporting of functional changes in living systems and gives further insight into specific biological events. In recent years, this research area has expanded significantly, and much focus has been placed on developing a wide variety of SCAs responsive to a range of conditions/targets including enzymatic activity, pH changes and metal ions (Ca2+, Zn2+etc.). While many SCAs have been reported, the development of such systems can include arduous synthetic and purification procedures. This in turn can impose restrictions in the diversity of developed SCAs by limiting the further addition of functionalities. Ultimately, these shortcomings can limit the broader utilisation of SCAs. SPS offers an alternative to laborious solution-phase techniques and while there are limited examples of SCAs synthesised via this route, the benefits of such a method will allow for increased diversity in future SCAs.Enzyme-responsive CAs have been widely reported since initial studies by Meade and co-workers.133 In order to develop efficient SCAs that can be modulated by enzymes, both the effective delivery of the agent to the site of interest and relaxation enhancement are vital. While the first enzyme-responsive MRI CA achieved relaxation enhancement through hydration modulation of the Gd3+ centre, attachment of small molecule agents to macromolecules also increases relaxivity due to a phenomenon known as RIME. Furthermore, the targeted macromolecules can be associated with specific disease states, allowing for localisation of the probe. A strategy to develop an enzyme-responsive MRI SCA modulated by RIME has been reported using Fmoc SPS to assemble the construct on a PAC-PEG-PS resin (Fig. 13a).134 Two SCAs were designed composed of a masking group (three lysine residues), a HSA binding moiety, a glycine linker and a Gd-DTPA-type MRI reporter. The pro-RIME agents were designed to have poor affinity to HSA in absence of TAFI (an enzyme associated with thrombotic disease) due to the positively charged lysine residues. When exposed to TAFI, the lysine residues are subsequently cleaved and the resulting bioactivated construct is capable of binding HSA to a greater extent, thus leading to an increase in relaxivity (11.1 to 24.5 and 9.8 to 26.5 mM−1 s−1, 37 °C, 20 MHz). This strategy allows the detection of targets in the submicromolar concentration range and can be applied to target other enzymes through modulation of the masking and targeting groups.
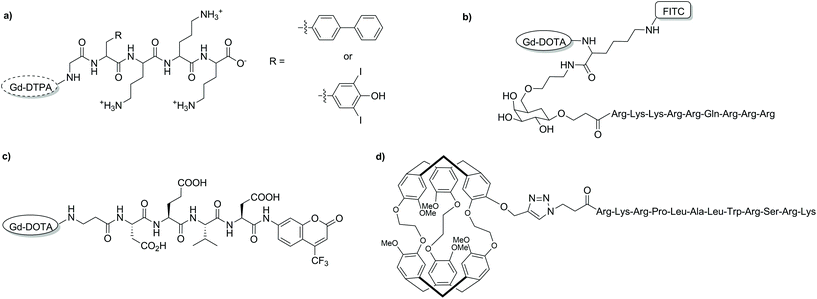 | ||
| Fig. 13 Chemical structures of some of the discussed bioresponsive MRI CAs developed with SPS. (a) a RIME modulated CA, (b) an intracellular T1 CA, (c) a cryptophane-based 129Xe biosensor, and (d) a dual modal 19F MRI/optical imaging probe.134–137 | ||
Intracellular delivery is also an important aspect to monitoring certain enzymes. This issue has been tackled by adopting a SPS building block approach to develop an MRI CA sensitive to β-galactosidase.135 The CA was comprised of a cell penetrating peptide (D-Tat49-57) capable of transporting the agent into the cell, a galactose derivative as the enzyme cleavable site, Gd-DOTA derivative as the MR reporter and FITC as a second functionality for optical imaging (Fig. 13b). The efficient combination of these functional units explicitly demonstrates the advantages of SPS. In addition, utilising a building block approach enables the development of a procedure in which each of the individual functionalities can be easily interchanged for another building block, allowing for high levels of customisation. The Gd-DOTA-k(FR)-Gal-CPP contrast agent developed in this study showed an increased longitudinal relaxivity (16.8 ± 0.6 mM−1 s−1, 21 °C, 123 MHz) compared to Gd-DOTA, likely due to a longer rotational correlation time. The CA demonstrated efficient cellular internalisation in C6/LacZ cells where β-galactosidase cleaved the galactose moiety, which in turn also separates the CPP from the Gd-DOTA-FITC block, leading to entrapment of the CA inside the cell. This study represents a successful blueprint for the development of an enzyme-responsive CA, which can be used for cell imaging with MRI and optical imaging.
Heteronuclear MRI with 19F is attractive as it has a high NMR sensitivity while also being absent in living animals, thus does not suffer from intrinsic background signals. SPS has been used to develop an off/on dual modal 19F MRI and optical probe to detect protease activity (Fig. 13c).136 In order to combine the various components, a similar building block approach to what has been described before was adopted. In this case DOTA-type chelator, a caspase-3 sensitive peptide sequence (DEVD) and a fluorinated fluorescent motif (AFC) were combined through Fmoc-SPS methodologies. Like most CAs developed through SPS, complexation with Gd3+ was performed post-deprotection from the resin. Without the target enzyme, the probe was in the so-called ‘off’ state. In this form, the 19F MRI signal was low due to a significantly short T2 caused by the paramagnetic relaxation enhancement effect of Gd3+; moreover, the fluorescence emission of AFC was low. Upon addition of caspase-3, the central peptide linker was cleaved, splitting the Gd-DOTA-type fragment from the AFC dye leading to an increase in both 19F MRI signal and fluorescence intensity.
The first enzyme-responsive 129Xe NMR biosensor was assembled using mixed solution/SPS protocols.137 Specifically, an MMP-7 peptide substrate was assembled on-resin and modified to contain an azide terminal functionality (Fig. 13d). A monopropargyl-cryptophane macrocycle was synthesised using solution-phase techniques before being combined with the peptide via on-resin CuAAC with high yield. The resulting probe was capable of producing changes in the chemical shift of 129Xe upon enzymatic cleavage with picomolar amounts of MMP-7. This synthetic strategy was replicated in a later study to develop a Cy3 labelled, folate-cryptophane 129Xe biosensor.138
In our group, we employed SPS as an alternate synthetic methodology to synthesise bismacrocyclic calcium-responsive MRI probes, which previously showed a strong response in to Ca2+ in cellular model systems.139–141 For this, we also utilised a building block approach, which included two different DO3A-type macrocycles and an EGTA-derived chelator, all synthesised in solution prior to assembly on resin. Initiating from a Rink amide resin, a short peptide linker (Lys(Mtt)-Gly-Gly) was assembled following standard SPS techniques, followed by conversion of the NH2 terminal to a carboxylic acid through treatment with succinic anhydride. The DO3A- and EGTA-type building blocks were then introduced sequentially in the following order: DO3A-EGTA-DO3A, before introducing biotin. Concurrently, we also revealed useful SPS chemistry for the removal of phthalimide as an amino protecting group with ethylene diamine at room temperature, as well as the use of alkylation to introduce the final DO3A-type building block. The whole construct was then deprotected and removed from then resin in one step before complexation with Gd3+ to give the final SCA (Fig. 14). The probe displayed a significant response to Ca2+ (118 and 150% enhancement in r1 and r2 respectively upon Ca2+ addition), which was shown to be greater than that of the non-functionalised precursor and its activity with avidin was also assessed. This proof-of-concept study combined previous concepts shown in the development of MRI CAs using SPS and demonstrated its applicability in the diversification of SCAs. Consequently, this approach opens a new realm of possibilities in the development of these types of CAs for use in biological systems.
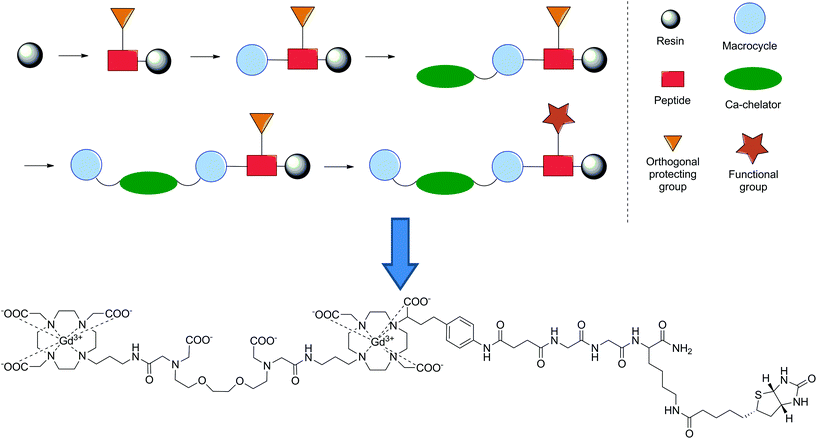 | ||
| Fig. 14 Schematic representation of the synthetic methodology utilised to develop a Ca2+-responsive MRI CA and its final chemical structure.141 | ||
Conclusions
The invention of the SPS technique has added significantly to the toolbox of methodologies available to the synthetic chemists. The inherent advantages of this methodology have seen its widespread use in many chemical fields, including that of molecular imaging. In this review, the key strategies and applications of SPS in the development of T1, CEST and bioresponsive MRI CAs have been highlighted, ranging from increasing the diversity of products, to being used as a platform to perform large scale synthesis and screening of products. As the majority of MRI CAs are aimed towards biological application, SPS offers an ideal platform to intricately combine multiple building blocks (targeting moieties, MRI agent, other imaging modality) in a simple, efficient manner and increase the scope of their use. Furthermore, the combinatorial aspect offers numerous advantages in identifying key CAs and properties of specific CAs in a timely manner, which is impossible to match with standard solution phase synthesis. Overall, this makes SPS a viable alternative to standard synthetic procedures in the chemical design and production of MRI CAs, offering to provide valuable solutions for the development of future molecular imaging probes.Abbreviations
| AA | Amino acid |
| AAZTA | 6-Amino-6-methylperhydro-1,4-diazepine tetraacetate |
| AFC | 7-Amido-4-trifluoromethylcoumaryl |
| BN | Bombesin analogue |
| Boc | t-Butyloxycarbonyl |
| CAs | Contrast agents |
| CD13 | Aminopeptidase N receptors |
| CEST | Chemical exchange saturation transfer |
| CPP | Cell-penetrating peptide |
| CT | Computed tomography |
| CuAAC | Copper-catalysed azide alkyne cycloaddition |
| Cy3/5.5 | Cyanine 3 or 5.5 fluorescent dye, respectively |
| DIC | 1,3-Diisopropylcarbodiimide |
| DO3A | 1,4,7,10-Tetraazacyclododecane-1,4,7-triacetate |
| DOTA | 1,4,7,10-Tetraazacyclododecane-1,4,7-tetraacetic acid |
| DTPA | Diethylenetriaminepentaacetic acid |
| EDC | N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide |
| EGTA | Ethylenediaminetetraacetic acid |
| FITC | Fluorescein isothiocyanate |
| Fmoc | Fluorenylmethyl |
| HSA | Human serum albumin |
| HATU | O-(7-Azabenzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate |
| HBTU | O-(Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium |
| HyperCEST | Hyperpolarised chemical exchange saturation transfer |
| IGF1 | Insulin-like growth factor 1 |
| MMP-2 | Matrix metalloproteinase-2 |
| MMP-7 | Matrix metalloproteinase-7 |
| MRI | Magnetic resonance imaging |
| mRNA | Messenger ribonucleic acid |
| Mtt | 4-Methyltrityl |
| NGR | Asn-Gly-Arg |
| OI | Optical imaging |
| PAMAM | Polyamidoamine |
| paraCEST | Paramagnetic chemical exchange saturation transfer |
| PDAP | Polydiamidopropanoyl |
| PEG | Polyethylene glycol |
| PET | Positron emission tomography |
| PNA | Peptide nucleic acid |
| PyBOP | Benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate |
| RGD | Arg-Gly-Asp |
| RIME | Receptor induced magnetisation enhancement |
| SCAs | Smart contrast agents |
| SPECT | Single-photon emission computed tomography |
| SPPS | Solid phase peptide synthesis |
| SPS | Solid phase synthesis |
| TTDA-NP | (N-(1-Methylene(p-isothiocyanatophenol)di(carboxymethyl) triazadodecanedioic acid |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The financial support from the German Research Foundation (DFG, grant AN 716/7-1) and the German Federal Ministry of Education and Research (BMBF, e:Med program: FKZ: 01ZX1503) is gratefully acknowledged. Open Access funding provided by the Max Planck Society.Notes and references
- M. L. James and S. S. Gambhir, A Molecular Imaging Primer: Modalities, Imaging Agents, and Applications, Physiol. Rev., 2012, 92, 897–965 CrossRef CAS.
- M. A. Pysz, S. S. Gambhir and J. K. Willmann, Molecular imaging: current status and emerging strategies, Clin. Radiol., 2010, 65, 500–516 CrossRef CAS.
- P. Caravan, Strategies for increasing the sensitivity of gadolinium based MRI contrast agents, Chem. Soc. Rev., 2006, 35, 512–523 RSC.
- R. B. Lauffer, Paramagnetic metal complexes as water proton relaxation agents for NMR imaging: theory and design, Chem. Rev., 1987, 87, 901–927 CrossRef CAS.
- C. F. G. C. Geraldes and S. Laurent, Classification and basic properties of contrast agents for magnetic resonance imaging, Contrast Media Mol. Imaging, 2009, 4, 1–23 CrossRef CAS.
- J. Wahsner, E. M. Gale, A. Rodríguez-Rodríguez and P. Caravan, Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers, Chem. Rev., 2019, 119, 957–1057 CrossRef CAS.
- A. E. Merbach, L. Helm and É. Tóth, The chemistry of contrast agents in medical magnetic resonance imaging, Wiley, Chichester, 2nd edn, 2013 Search PubMed.
- M. T. McMahon, J. W. M. Bulte, A. A. Gilad and P. C. M. van Zijl, Chemical Exchange Saturation Transfer Imaging: Advances and Applications, Pan Stanford Publishing, 2017 Search PubMed.
- V. C. Pierre and M. J. Allen, Contrast Agents for MRI: Experimental Methods, Royal Society of Chemistry, 2018 Search PubMed.
- E. B. Adamson, K. D. Ludwig, D. G. Mummy and S. B. Fain, Magnetic resonance imaging with hyperpolarized agents: methods and applications, Phys. Med. Biol., 2017, 62, R81–R123 CrossRef CAS.
- S. Sinharay and M. D. Pagel, Advances in Magnetic Resonance Imaging Contrast Agents for Biomarker Detection, Annu. Rev. Anal. Chem., 2016, 9, 95–115 CrossRef.
- E. Terreno and S. Aime, MRI Contrast Agents for Pharmacological Research, Front. Pharmacol., 2015, 6, 290 Search PubMed.
- L. Lattuada, A. Barge, G. Cravotto, G. B. Giovenzana and L. Tei, The synthesis and application of polyamino polycarboxylic bifunctional chelating agents, Chem. Soc. Rev., 2011, 40, 3019–3049 RSC.
- F. Luca and C. Peter, Strategies for the Preparation of Bifunctional Gadolinium(III) Chelators, Curr. Org. Synth., 2011, 8, 535–565 CrossRef.
- G. B. Giovenzana, L. Lattuada and R. Negri, Recent Advances in Bifunctional Paramagnetic Chelates for MRI, Isr. J. Chem., 2017, 57, 825–832 CrossRef CAS.
- P. Kumar, S. K. Tripathi, C.-P. Chen, N. Mehta, B. Paudyal, E. Wickstrom and M. L. Thakur, Evaluation of a PACAP Peptide Analogue Labeled with 68Ga Using Two Different Chelating Agents, Cancer Biother. Radiopharm., 2016, 31, 29–36 CrossRef CAS.
- P. Kumar, S. K. Tripathi, C. P. Chen, E. Wickstrom and M. L. Thakur, Evaluating Ga-68 Peptide Conjugates for Targeting VPAC Receptors: Stability and Pharmacokinetics, Mol. Imaging Biol., 2019, 21, 130–139 CrossRef CAS.
- A. Pirisedigh, V. Blais, S. Ait-Mohand, K. Abdallah, B. J. Holleran, R. Leduc, Y. L. Dory, L. Gendron and B. Guérin, Synthesis and Evaluation of a 64Cu-Conjugate, a Selective δ-Opioid Receptor Positron Emission Tomography Imaging Agent, Org. Lett., 2017, 19, 2018–2021 CrossRef CAS.
- B. Guérin, S. Ait-Mohand, M.-C. Tremblay, V. Dumulon-Perreault, P. Fournier and F. Bénard, Total Solid-Phase Synthesis of NOTA-Functionalized Peptides for PET Imaging, Org. Lett., 2010, 12, 280–283 CrossRef.
- G. Fischer, B. Wängler and C. Wängler, Optimized solid phase-assisted synthesis of dendrons applicable as scaffolds for radiolabeled bioactive multivalent compounds intended for molecular imaging, Molecules, 2014, 19, 6952–6974 CrossRef.
- J. J. Peterson, R. H. Pak and C. F. Meares, Total Solid-Phase Synthesis of 1,4,7,10-Tetraazacyclododecane-N,N′,N′′,N′′′-tetraacetic Acid-Functionalized Peptides for Radioimmunotherapy, Bioconjugate Chem., 1999, 10, 316–320 CrossRef CAS.
- S. M. Okarvi and I. AlJammaz, A convenient and efficient total solid-phase synthesis of DOTA-functionalized tumor-targeting peptides for PET imaging of cancer, EJNMMI Res., 2019, 9, 88 CrossRef.
- M. Briand, M. L. Aulsebrook, T. L. Mindt and G. Gasser, A solid phase-assisted approach for the facile synthesis of a highly water-soluble zirconium-89 chelator for radiopharmaceutical development, Dalton Trans., 2017, 46, 16387–16389 RSC.
- K. Ogawa, A. Ishizaki, K. Takai, Y. Kitamura, A. Makino, T. Kozaka, Y. Kiyono, K. Shiba and A. Odani, Evaluation of Ga-DOTA-(D-Asp)n as bone imaging agents: D-aspartic acid peptides as carriers to bone, Sci. Rep., 2017, 7, 13971 CrossRef.
- J. Lau, D. Kwon, E. Rousseau, Z. Zhang, J. Zeisler, C. F. Uribe, H.-T. Kuo, C. Zhang, K.-S. Lin and F. Bénard, [68Ga]Ga/[177Lu]Lu-BL01, a Novel Theranostic Pair for Targeting C-X-C Chemokine Receptor 4, Mol. Pharmaceutics, 2019, 16, 4688–4695 CrossRef CAS.
- A. Lucia Tornesello, M. Lina Tornesello and F. M. Buonaguro, An Overview of Bioactive Peptides for in vivo Imaging and Therapy in Human Diseases, Mini-Rev. Med. Chem., 2017, 17, 758–770 CrossRef.
- B. Mitran, Z. Varasteh, A. Abouzayed, S. S. Rinne, E. Puuvuori, M. De Rosa, M. Larhed, V. Tolmachev, A. Orlova and U. Rosenström, Bispecific GRPR-Antagonistic Anti-PSMA/GRPR Heterodimer for PET and SPECT Diagnostic Imaging of Prostate Cancer, Cancers, 2019, 11, 1371 CrossRef CAS.
- C. F. W. Becker, D. Clayton, G. Shapovalov, H. A. Lester and G. G. Kochendoerfer, On-Resin Assembly of a Linkerless Lanthanide(III)-Based Luminescence Label and Its Application to the Total Synthesis of Site-Specifically Labeled Mechanosensitive Channels, Bioconjugate Chem., 2004, 15, 1118–1124 CrossRef CAS.
- H. L. Handl, J. Vagner, H. I. Yamamura, V. J. Hruby and R. J. Gillies, Lanthanide-based time-resolved fluorescence of in cyto ligand–receptor interactions, Anal. Biochem., 2004, 330, 242–250 CrossRef CAS.
- C. R. De Silva, J. Vagner, R. Lynch, R. J. Gillies and V. J. Hruby, Optimization of time-resolved fluorescence assay for detection of europium–tetraazacyclododecyltetraacetic acid-labeled ligand–receptor interactions, Anal. Biochem., 2010, 398, 15–23 CrossRef CAS.
- A. Niedźwiecka, F. Cisnetti, C. Lebrun and P. Delangle, Femtomolar Ln(III) Affinity in Peptide-Based Ligands Containing Unnatural Chelating Amino Acids, Inorg. Chem., 2012, 51, 5458–5464 CrossRef.
- J. Hovinen and P. M. Guy, Bioconjugation with Stable Luminescent Lanthanide(III) Chelates Comprising Pyridine Subunits, Bioconjugate Chem., 2009, 20, 404–421 CrossRef CAS.
- C. S. Bonnet, M. Devocelle and T. Gunnlaugsson, Luminescent lanthanide-binding peptides: sensitising the excited states of Eu(III) and Tb(III) with a 1,8-naphthalimide-based antenna, Org. Biomol. Chem., 2012, 10, 126–133 RSC.
- T. Nakamura, S. Mizukami, M. Tanaka and K. Kikuchi, Efficient Formation of Luminescent Lanthanide(III) Complexes by Solid-Phase Synthesis and On-Resin Screening, Chem. – Asian J., 2013, 8, 2685–2690 CrossRef CAS.
- J. Peuralahti, H. Hakala, V.-M. Mukkala, K. Loman, P. Hurskainen, O. Mulari and J. Hovinen, Introduction of Lanthanide(III) Chelates to Oligopeptides on Solid Phase, Bioconjugate Chem., 2002, 13, 870–875 CrossRef CAS.
- K. Brückner, R. Zitterbart, O. Seitz, S. Beck and M. W. Linscheid, Solid Phase Synthesis of Short Peptide-Based Multimetal Tags for Biomolecule Labeling, Bioconjugate Chem., 2014, 25, 1069–1077 CrossRef.
- G. Dirscherl and B. König, The Use of Solid-Phase Synthesis Techniques for the Preparation of Peptide–Metal Complex Conjugates, Eur. J. Org. Chem., 2008, 597–634 CrossRef CAS.
- C. Diaferia, E. Gianolio and A. Accardo, Peptide-based building blocks as structural elements for supramolecular Gd-containing MRI contrast agents, J. Pept. Sci., 2019, 25, e3157 CrossRef.
- E. Kreidt, W. Leis and M. Seitz, Direct solid-phase synthesis of molecular heterooligonuclear lanthanoid-complexes, Nat. Commun., 2020, 11, 1346 CrossRef CAS.
- P. Lejault, K. Duskova, C. Bernhard, I. E. Valverde, A. Romieu and D. Monchaud, The Scope of Application of Macrocyclic Polyamines Beyond Metal Chelation, Eur. J. Org. Chem., 2019, 6146–6157 CrossRef CAS.
- R. B. Merrifield, Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide, J. Am. Chem. Soc., 1963, 85, 2149–2154 CrossRef CAS.
- R. B. Merrifield, Solid Phase Synthesis (Nobel Lecture), Angew. Chem., Int. Ed. Engl., 1985, 24, 799–810 CrossRef.
- M. Stawikowski and G. B. Fields, Introduction to Peptide Synthesis, Curr. Protoc. Protein Sci., 2002, 18, 18 Search PubMed.
- D. M. M. Jaradat, Thirteen decades of peptide synthesis: key developments in solid phase peptide synthesis and amide bond formation utilized in peptide ligation, Amino Acids, 2018, 50, 39–68 CrossRef CAS.
- W. Chan and P. D. White, Fmoc Solid Phase Peptide Synthesis, Oxford University Press, 2000 Search PubMed.
- B. Merrifield, Solid phase synthesis, Science, 1986, 232, 341–347 CrossRef CAS.
- F. Gaggini, A. Porcheddu, G. Reginato, M. Rodriquez and M. Taddei, Colorimetric Tools for Solid-Phase Organic Synthesis, J. Comb. Chem., 2004, 6, 805–810 CrossRef CAS.
- N. Thieriet, F. Guibé and F. Albericio, Solid-Phase Peptide Synthesis in the Reverse (N→C) Direction, Org. Lett., 2000, 2, 1815–1817 CrossRef CAS.
- R. Sasubilli and W. G. Gutheil, General Inverse Solid-Phase Synthesis Method for C-Terminally Modified Peptide Mimetics, J. Comb. Chem., 2004, 6, 911–915 CrossRef CAS.
- P. Hermann, J. Kotek, V. Kubíček and I. Lukeš, Gadolinium(III) complexes as MRI contrast agents: ligand design and properties of the complexes, Dalton Trans., 2008, 3027–3047 RSC.
- L. M. De León-Rodríguez, A. F. Martins, M. Pinho, N. Rofsky and A. D. Sherry, Basic MR Relaxation Mechanisms & Contrast Agent Design, J. Magn. Reson. Imaging, 2015, 42, 545–565 CrossRef.
- P. Caravan, J. J. Ellison, T. J. McMurry and R. B. Lauffer, Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications, Chem. Rev., 1999, 99, 2293–2352 CrossRef CAS.
- S. Aime, D. D. Castelli, S. G. Crich, E. Gianolio and E. Terreno, Pushing the Sensitivity Envelope of Lanthanide-Based Magnetic Resonance Imaging (MRI) Contrast Agents for Molecular Imaging Applications, Acc. Chem. Res., 2009, 42, 822–831 CrossRef CAS.
- D. Delli Castelli, E. Gianolio, S. Geninatti Crich, E. Terreno and S. Aime, Metal containing nanosized systems for MR-Molecular Imaging applications, Coord. Chem. Rev., 2008, 252, 2424–2443 CrossRef CAS.
- M. Botta and L. Tei, Relaxivity Enhancement in Macromolecular and Nanosized GdIII-Based MRI Contrast Agents, Eur. J. Inorg. Chem., 2012, 2012, 1945–1960 CrossRef CAS.
- P. Caravan, Protein-Targeted Gadolinium-Based Magnetic Resonance Imaging (MRI) Contrast Agents: Design and Mechanism of Action, Acc. Chem. Res., 2009, 42, 851–862 CrossRef CAS.
- S. Lee, J. Xie and X. Chen, Peptide-Based Probes for Targeted Molecular Imaging, Biochemistry, 2010, 49, 1364–1376 CrossRef CAS.
- L. M. De León-Rodríguez and Z. Kovacs, The Synthesis and Chelation Chemistry of DOTA–Peptide Conjugates, Bioconjugate Chem., 2008, 19, 391–402 CrossRef.
- Z. Kovacs and L. M. D. Leon-Rodriguez, Conjugation of 1,4,7,10-Tetraazacyclododecane-1,4,7,10-Tetracetic Acid (DOTA) and its Derivatives to Peptides: Synthesis, Applications and Future Prospects, Mini-Rev. Org. Chem., 2007, 4, 281–291 CrossRef CAS.
- D. L. Morse and R. J. Gillies, Molecular imaging and targeted therapies, Biochem. Pharmacol., 2010, 80, 731–738 CrossRef CAS.
- T. Skotland, Molecular imaging: challenges of bringing imaging of intracellular targets into common clinical use, Contrast Media Mol. Imaging, 2012, 7, 1–6 CrossRef CAS.
- M. J. Allen, K. W. MacRenaris, P. N. Venkatasubramanian and T. J. Meade, Cellular Delivery of MRI Contrast Agents, Chem. Biol., 2004, 11, 301–307 CrossRef CAS.
- S. Aime, C. Cabella, S. Colombatto, S. Geninatti Crich, E. Gianolio and F. Maggioni, Insights into the use of paramagnetic Gd(III) complexes in MR-molecular imaging investigations, J. Magn. Reson. Imaging, 2002, 16, 394–406 CrossRef.
- H. Derakhshankhah and S. Jafari, Cell penetrating peptides: A concise review with emphasis on biomedical applications, Biomed. Pharmacother., 2018, 108, 1090–1096 CrossRef CAS.
- G. Guidotti, L. Brambilla and D. Rossi, Cell-Penetrating Peptides: From Basic Research to Clinics, Trends Pharmacol. Sci., 2017, 38, 406–424 CrossRef CAS.
- V. Kersemans and B. Cornelissen, Targeting the Tumour: Cell Penetrating Peptides for Molecular Imaging and Radiotherapy, Pharmaceuticals, 2010, 3, 600–620 CrossRef CAS.
- R. Bhorade, R. Weissleder, T. Nakakoshi, A. Moore and C.-H. Tung, Macrocyclic Chelators with Paramagnetic Cations Are Internalized into Mammalian Cells via a HIV-Tat Derived Membrane Translocation Peptide, Bioconjugate Chem., 2000, 11, 301–305 CrossRef CAS.
- A. M. Prantner, V. Sharma, J. R. Garbow and D. Piwnica-Worms, Synthesis and Characterization of a Gd-DOTA-D-Permeation Peptide for Magnetic Resonance Relaxation Enhancement of Intracellular Targets, Mol. Imaging, 2003, 2, 333–341 CrossRef CAS.
- W. Su, R. Mishra, J. Pfeuffer, K.-H. Wiesmüller, K. Ugurbil and J. Engelmann, Synthesis and cellular uptake of a MR contrast agent coupled to an antisense peptide nucleic acid – cell–penetrating peptide conjugate, Contrast Media Mol. Imaging, 2007, 2, 42–49 CrossRef CAS.
- R. Mishra, W. Su, R. Pohmann, J. Pfeuffer, M. G. Sauer, K. Ugurbil and J. Engelmann, Cell-Penetrating Peptides and Peptide Nucleic Acid-Coupled MRI Contrast Agents: Evaluation of Cellular Delivery and Target Binding, Bioconjugate Chem., 2009, 20, 1860–1868 CrossRef CAS.
- M. J. Allen and T. J. Meade, Synthesis and visualization of a membrane-permeable MRI contrast agent, J. Biol. Inorg. Chem., 2003, 8, 746–750 CrossRef CAS.
- M. Liu, Y.-M. Guo, Q.-F. Wu, J.-L. Yang, P. Wang, S.-C. Wang, X.-J. Guo, Y.-Q. Qiang and X.-Y. Duan, Paramagnetic particles carried by cell-penetrating peptide tracking of bone marrow mesenchymal stem cells, a research in vitro, Biochem. Biophys. Res. Commun., 2006, 347, 133–140 CrossRef CAS.
- M. Liu, Y.-M. Guo, J.-L. Yang, P. Wang, L.-Y. Zhao, N. Shen, S.-C. Wang, X.-J. Guo and Q.-F. Wu, Application of Cell Penetrating Peptide in Magnetic Resonance Imaging of Bone Marrow Mesenchymal Stem Cells, Acta Biochim. Biophys. Sin., 2006, 38, 865–873 CrossRef CAS.
- Y.-M. Guo, M. Liu, J.-L. Yang, X.-J. Guo, S. Wang, X.-Y. Duan and P. Wang, Intercellular imaging by a polyarginine derived cell penetrating peptide labeled magnetic resonance contrast agent, diethylenetriamine pentaacetic acid gadolinium, Chin. Med. J., 2007, 120, 50–55 CrossRef CAS.
- X.-H. Zhai, M. Liu, X.-J. Guo, S. Wang, H.-X. Zhang and Y.-M. Guo, SKOV-3 cell imaging by paramagnetic particles labeled with hairpin cell-penetrating peptides, Chin. Med. J., 2011, 124, 111–117 CAS.
- S. Silva, J. A. Almeida and N. Vale, Combination of Cell-Penetrating Peptides with Nanoparticles for Therapeutic Application: A Review, Biomolecules, 2019, 9, 22 CrossRef.
- S. Lacerda, Targeted Contrast Agents for Molecular MRI, Inorganics, 2018, 6, 129 CrossRef CAS.
- N. V. Amirkhanov, I. Dimitrov, A. W. Opitz, K. Zhang, J. P. Lackey, C. A. Cardi, S. Lai, N. J. Wagner, M. L. Thakur and E. Wickstrom, Design of (Gd-DO3A)n-polydiamidopropanoyl-peptide nucleic acid-D(Cys-Ser-Lys-Cys) magnetic resonance contrast agents, Biopolymers, 2008, 89, 1061–1076 CrossRef CAS.
- N. V. Amirkhanov, K. Zhang, M. R. Aruva, M. L. Thakur and E. Wickstrom, Imaging Human Pancreatic Cancer Xenografts by Targeting Mutant KRAS2 mRNA with [111In]DOTAn-Poly(diamidopropanoyl)m-KRAS2 PNA-d(Cys-Ser-Lys-Cys) Nanoparticles, Bioconjugate Chem., 2010, 21, 731–740 CrossRef CAS.
- Y. Yang, J. Zhou and K. Yu, Design, synthesis, and in vitro evaluation of a binary targeting MRI contrast agent for imaging tumor cells, Amino Acids, 2014, 46, 449–457 CrossRef CAS.
- K. Temming, R. M. Schiffelers, G. Molema and R. J. Kok, RGD-based strategies for selective delivery of therapeutics and imaging agents to the tumour vasculature, Drug Resist. Updates, 2005, 8, 381–402 CrossRef CAS.
- P.-H. Wu, A. E. Opadele, Y. Onodera and J.-M. Nam, Targeting Integrins in Cancer Nanomedicine: Applications in Cancer Diagnosis and Therapy, Cancers, 2019, 11, 1783 CrossRef CAS.
- F. Araste, K. Abnous, M. Hashemi, S. M. Taghdisi, M. Ramezani and M. Alibolandi, Peptide-based targeted therapeutics: Focus on cancer treatment, J. Controlled Release, 2018, 292, 141–162 CrossRef CAS.
- C. Schreiber and B. Smith, Molecular Imaging of Aminopeptidase N in Cancer and Angiogenesis, Contrast Media Mol. Imaging, 2018, 2018, 1–15 CrossRef.
- F. Wang and Z. Liu, in Advanced Topics in Science and Technology in China, Springer, Berlin, Heidelberg, 2013, pp. 513–538 Search PubMed.
- M. E. G. Moral and T. J. Siahaan, Conjugates of Cell Adhesion Peptides for Therapeutics and Diagnostics Against Cancer and Autoimmune Diseases, Curr. Top. Med. Chem., 2017, 17, 3425–3443 CrossRef CAS.
- M. Tan and Z.-R. Lu, Integrin Targeted MR Imaging, Theranostics, 2011, 1, 83–101 CrossRef CAS.
- S. Langereis, A. Dirksen, B. F. M. De Waal, M. H. P. Van Genderen, Q. G. De Lussanet, T. M. Hackeng and E. W. Meijer, Solid-Phase Synthesis of a Cyclic NGR-Functionalized GdIIIDTPA Complex, Eur. J. Org. Chem., 2005, 2534–2538 CrossRef CAS.
- X. Wu, S. M. Burden-Gulley, G.-P. Yu, M. Tan, D. Lindner, S. M. Brady-Kalnay and Z.-R. Lu, Synthesis and Evaluation of a Peptide Targeted Small Molecular Gd-DOTA Monoamide Conjugate for MR Molecular Imaging of Prostate Cancer, Bioconjugate Chem., 2012, 23, 1548–1556 CrossRef CAS.
- F. Ye, E.-K. Jeong, Z. Jia, T. Yang, D. Parker and Z.-R. Lu, A Peptide Targeted Contrast Agent Specific to Fibrin-Fibronectin Complexes for Cancer Molecular Imaging with MRI, Bioconjugate Chem., 2008, 19, 2300–2303 CrossRef CAS.
- X. Wu, N. Balu, W. Li, Y. Chen, X. Shi, C. M. Kummitha, X. Yu, C. Yuan and Z.-R. Lu, Molecular MRI of atherosclerotic plaque progression in an ApoE-/- mouse model with a CLT1 peptide targeted macrocyclic Gd(III) chelate, Am. J. Nucl. Med. Mol. Imaging, 2013, 3, 446–455 Search PubMed.
- F. Ye, E.-K. Jeong, D. Parker and Z.-R. Lu, Evaluation of CLT1-(Gd-DTPA) for Cancer MR Molecular Imaging in a Mouse Breast Cancer Model, Bopuxue Zazhi, 2011, 2, 325–330 CAS.
- X. Wu, G. Yu, D. Lindner, S. M. Brady-Kalnay, Q. Zhang and Z.-R. Lu, Peptide targeted high-resolution molecular imaging of prostate cancer with MRI, Am. J. Nucl. Med. Mol. Imaging, 2014, 4, 525–536 CAS.
- Y. Li, Z. Han, S. Roelle, A. DeSanto, R. Sabatelle, R. Schur and Z.-R. Lu, Synthesis and Assessment of Peptide Gd–DOTA Conjugates Targeting Extradomain B Fibronectin for Magnetic Resonance Molecular Imaging of Prostate Cancer, Mol. Pharmaceutics, 2017, 14, 3906–3915 CrossRef CAS.
- B. L. Oliveira and P. Caravan, Peptide-based fibrin-targeting probes for thrombus imaging, Dalton Trans., 2017, 46, 14488–14508 RSC.
- L. Chaabane, L. Tei, L. Miragoli, L. Lattuada, M. von Wronski, F. Uggeri, V. Lorusso and S. Aime, In Vivo MR Imaging of Fibrin in a Neuroblastoma Tumor Model by Means of a Targeting Gd-Containing Peptide, Mol. Imaging Biol., 2015, 17, 819–828 CrossRef CAS.
- M. Tripepi, F. Capuana, E. Gianolio, F. Kock, A. Pagoto, R. Stefania, G. Digilio and S. Aime, Synthesis of High Relaxivity Gadolinium AAZTA Tetramers as Building Blocks for Bioconjugation, Bioconjugate Chem., 2018, 29, 1428–1437 CrossRef CAS.
- K. Overoye-Chan, S. Koerner, R. J. Looby, A. F. Kolodziej, S. G. Zech, Q. Deng, J. M. Chasse, T. J. McMurry and P. Caravan, EP-2104R: A Fibrin-Specific Gadolinium-Based MRI Contrast Agent for Detection of Thrombus, J. Am. Chem. Soc., 2008, 130, 6025–6039 CrossRef CAS.
- A. Pagoto, M. Tripepi, R. Stefania, S. Lanzardo, D. Livio Longo, F. Garello, F. Porpiglia, M. Manfredi, S. Aime and E. Terreno, An efficient MRI agent targeting extracellular markers in prostate adenocarcinoma, Magn. Reson. Med., 2019, 81, 1935–1946 CrossRef CAS.
- X. Wang, M. Milne, F. Martinez, T. J. Scholl and R. H. E. Hudson, Synthesis of a poly(Gd(III)-DOTA)-PNA conjugate as a potential MRI contrast agent via post-synthetic click chemistry functionalization, RSC Adv., 2017, 7, 45222–45226 RSC.
- V. Castro, H. Rodríguez and F. Albericio, CuAAC: An Efficient Click Chemistry Reaction on Solid Phase, ACS Comb. Sci., 2016, 18, 1–14 CrossRef CAS.
- L. Litti, N. Rivato, G. Fracasso, P. Bontempi, E. Nicolato, P. Marzola, A. Venzo, M. Colombatti, M. Gobbo and M. Meneghetti, A SERRS/MRI multimodal contrast agent based on naked Au nanoparticles functionalized with a Gd(III) loaded PEG polymer for tumor imaging and localized hyperthermia, Nanoscale, 2018, 10, 1272–1278 RSC.
- A. Y. Louie, Multimodality Imaging Probes: Design and Challenges, Chem. Rev., 2010, 110, 3146–3195 CrossRef CAS.
- R. Uppal, K. L. Ciesienski, D. B. Chonde, G. S. Loving and P. Caravan, Discrete Bimodal Probes for Thrombus Imaging, J. Am. Chem. Soc., 2012, 134, 10799–10802 CrossRef CAS.
- Y.-H. Lin, K. Dayananda, C.-Y. Chen, G.-C. Liu, T.-Y. Luo, H.-S. Hsu and Y.-M. Wang, In vivo MR/optical imaging for gastrin releasing peptide receptor of prostate cancer tumor using Gd-TTDA-NP-BN-Cy5.5, Bioorg. Med. Chem., 2011, 19, 1085–1096 CrossRef CAS.
- Y. Min, J. M. Caster, M. J. Eblan and A. Z. Wang, Clinical Translation of Nanomedicine, Chem. Rev., 2015, 115, 11147–11190 CrossRef CAS.
- A. Kakkar, G. Traverso, O. C. Farokhzad, R. Weissleder and R. Langer, Evolution of macromolecular complexity in drug delivery systems, Nat. Rev. Chem., 2017, 1, 0063 CrossRef CAS.
- Y. H. Choi and H.-K. Han, Nanomedicines: current status and future perspectives in aspect of drug delivery and pharmacokinetics, Int. J. Pharm. Invest., 2018, 48, 43–60 CrossRef CAS.
- M. Vaccaro, G. Mangiapia, L. Paduano, E. Gianolio, A. Accardo, D. Tesauro and G. Morelli, Structural and Relaxometric Characterization of Peptide Aggregates Containing Gadolinium Complexes as Potential Selective Contrast Agents in MRI, ChemPhysChem, 2007, 8, 2526–2538 CrossRef CAS.
- S. R. Bull, M. O. Guler, R. E. Bras, T. J. Meade and S. I. Stupp, Self-Assembled Peptide Amphiphile Nanofibers Conjugated to MRI Contrast Agents, Nano Lett., 2005, 5, 1–4 CrossRef CAS.
- E. Gallo, C. Diaferia, E. Di Gregorio, G. Morelli, E. Gianolio and A. Accardo, Peptide-Based Soft Hydrogels Modified with Gadolinium Complexes as MRI Contrast Agents, Pharmaceuticals, 2020, 13, 19 CrossRef CAS.
- E. Boros, M. Polasek, Z. Zhang and P. Caravan, Gd(DOTAla): A Single Amino Acid Gd-complex as a Modular Tool for High Relaxivity MR Contrast Agent Development, J. Am. Chem. Soc., 2012, 134, 19858–19868 CrossRef CAS.
- L. M. De León-Rodríguez, A. Ortiz, A. L. Weiner, S. Zhang, Z. Kovacs, T. Kodadek and A. D. Sherry, Magnetic Resonance Imaging Detects a Specific Peptide–Protein Binding Event, J. Am. Chem. Soc., 2002, 124, 3514–3515 CrossRef.
- L. M. De León-Rodriguez, Z. Kovacs, G. R. Dieckmann and A. D. Sherry, Solid-Phase Synthesis of DOTA–Peptides, Chem. – Eur. J., 2004, 10, 1149–1155 CrossRef.
- Z. Ye, X. Wu, M. Tan, J. Jesberger, M. Grisworld and Z.-R. Lu, Synthesis and evaluation of a polydisulfide with Gd–DOTA monoamide side chains as a biodegradable macromolecular contrast agent for MR blood pool imaging, Contrast Media Mol. Imaging, 2013, 8, 220–228 CrossRef CAS.
- Z.-R. Lu and X. Wu, Polydisulfide Based Biodegradable Macromolecular Magnetic Resonance Imaging Contrast Agents, Isr. J. Chem., 2010, 50, 220–232 CrossRef CAS.
- A. D. Sherry and M. Woods, Chemical exchange saturation transfer contrast agents for magnetic resonance imaging, Annu. Rev. Biomed. Eng., 2008, 10, 391–411 CrossRef CAS.
- G. Liu, X. Song, K. W. Y. Chan and M. T. McMahon, Nuts and bolts of chemical exchange saturation transfer MRI, NMR Biomed., 2013, 26, 810–828 CrossRef CAS.
- B. Wu, G. Warnock, M. Zaiss, C. Lin, M. Chen, Z. Zhou, L. Mu, D. Nanz, R. Tuura and G. Delso, An overview of CEST MRI for non-MR physicists, EJNMMI Phys., 2016, 3, 19 CrossRef CAS.
- B. Yoo and M. D. Pagel, Peptidyl Molecular Imaging Contrast Agents Using a New Solid Phase Peptide Synthesis Approach, Bioconjugate Chem., 2007, 18, 903–911 CrossRef CAS.
- B. Yoo, V. R. Sheth and M. D. Pagel, An amine-derivatized, DOTA-loaded polymeric support for Fmoc solid phase peptide synthesis, Tetrahedron Lett., 2009, 50, 4459–4462 CrossRef CAS.
- A. Rodríguez-Rodríguez, M. Zaiss, D. Esteban-Gómez, G. Angelovski and C. Platas-Iglesias, Paramagnetic chemical exchange saturation transfer agents and their perspectives for application in magnetic resonance imaging, Int. Rev. Phys. Chem., 2021, 40, 51–79 CrossRef.
- R. Napolitano, T. C. Soesbe, L. M. De León-Rodríguez, A. D. Sherry and D. G. Udugamasooriya, On-Bead Combinatorial Synthesis and Imaging of Chemical Exchange Saturation Transfer Magnetic Resonance Imaging Agents To Identify Factors That Influence Water Exchange, J. Am. Chem. Soc., 2011, 133, 13023–13030 CrossRef CAS.
- J. Singh, V. Rustagi, S. Zhang, A. D. Sherry and D. G. Udugamasooriya, On-bead combinatorial synthesis and imaging of europium(III)-based paraCEST agents aids in identification of chemical features that enhance CEST sensitivity, Magn. Reson. Chem., 2017, 55, 747–753 CrossRef CAS.
- V. Rustagi and D. G. Udugamasooriya, Identification of side arm-modified DOTA scaffolds as multi-site binding ligands for cancer cells over normal cells, Bioorg. Med. Chem. Lett., 2019, 29, 126619 CrossRef CAS.
- J. Jayapaul and L. Schröder, Complete Generation of a 129Xe Biosensor on the Solid Support by Systematic Backbone Assembly, Bioconjugate Chem., 2018, 29, 4004–4011 CrossRef CAS.
- G. Angelovski, Heading toward Macromolecular and Nanosized Bioresponsive MRI Probes for Successful Functional Imaging, Acc. Chem. Res., 2017, 50, 2215–2224 CrossRef CAS.
- G.-L. Davies, I. Kramberger and J. J. Davis, Environmentally responsive MRI contrast agents, Chem. Commun., 2013, 49, 9704–9721 RSC.
- D. V. Hingorani, A. S. Bernstein and M. D. Pagel, A review of responsive MRI contrast agents: 2005–2014, Contrast Media Mol. Imaging, 2015, 10, 245–265 CrossRef CAS.
- M. Carril, Activatable probes for diagnosis and biomarker detection by MRI, J. Mater. Chem. B, 2017, 5, 4332–4347 RSC.
- M. C. Heffern, L. M. Matosziuk and T. J. Meade, Lanthanide Probes for Bioresponsive Imaging, Chem. Rev., 2014, 114, 4496–4539 CrossRef CAS.
- D. Parrott, W. S. Fernando and A. F. Martins, Smart MRI Agents for Detecting Extracellular Events In Vivo: Progress and Challenges, Inorganics, 2019, 7, 18 CrossRef CAS.
- R. A. Moats, S. E. Fraser and T. J. Meade, A Smart Magnetic Resonance Imaging Agent That Reports on Specific Enzymatic Activity, Angew. Chem., Int. Ed. Engl., 1997, 36, 726–728 CrossRef CAS.
- A. L. Nivorozhkin, A. F. Kolodziej, P. Caravan, M. T. Greenfield, R. B. Lauffer and T. J. McMurry, Enzyme-Activated Gd3+ Magnetic Resonance Imaging Contrast Agents with a Prominent Receptor-Induced Magnetization Enhancement, Angew. Chem., Int. Ed., 2001, 40, 2903–2906 CrossRef CAS.
- A. Keliris, T. Ziegler, R. Mishra, R. Pohmann, M. G. Sauer, K. Ugurbil and J. Engelmann, Synthesis and characterization of a cell-permeable bimodal contrast agent targeting β-galactosidase, Bioorg. Med. Chem., 2011, 19, 2529–2540 CrossRef CAS.
- S. Mizukami, R. Takikawa, F. Sugihara, M. Shirakawa and K. Kikuchi, Dual-Function Probe to Detect Protease Activity for Fluorescence Measurement and 19F MRI, Angew. Chem., Int. Ed., 2009, 48, 3641–3643 CrossRef CAS.
- Q. Wei, G. K. Seward, P. A. Hill, B. Patton, I. E. Dimitrov, N. N. Kuzma and I. J. Dmochowski, Designing 129Xe NMR Biosensors for Matrix Metalloproteinase Detection, J. Am. Chem. Soc., 2006, 128, 13274–13283 CrossRef CAS.
- N. S. Khan, B. A. Riggle, G. K. Seward, Y. Bai and I. J. Dmochowski, Cryptophane-Folate Biosensor for 129Xe NMR, Bioconjugate Chem., 2015, 26, 101–109 CrossRef CAS.
- G. Angelovski, P. Fouskova, I. Mamedov, S. Canals, E. Toth and N. K. Logothetis, Smart Magnetic Resonance Imaging Agents that Sense Extracellular Calcium Fluctuations, ChemBioChem, 2008, 9, 1729–1734 CrossRef CAS.
- G. Angelovski, S. Gottschalk, M. Milošević, J. Engelmann, G. E. Hagberg, P. Kadjane, P. Andjus and N. K. Logothetis, Investigation of a Calcium-Responsive Contrast Agent in Cellular Model Systems: Feasibility for Use as a Smart Molecular Probe in Functional MRI, ACS Chem. Neurosci., 2014, 5, 360–369 CrossRef CAS.
- L. Connah, R. Joshi, S. Vibhute, G. Gambino, J. D. G. Correia and G. Angelovski, Solid-Phase-Supported Approach for the Preparation of Bioresponsive and Multifunctional MRI Probes, Org. Lett., 2019, 21, 5378–5382 CrossRef CAS.
| This journal is © the Partner Organisations 2020 |