Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Induced CD of iron(II) clathrochelates: sensing of the structural and conformational alterations of serum albumins†
Vladyslava
Kovalska
 *ab,
Marina
Kuperman
a,
Mykhaylo
Losytskyy
a,
Serhii
Vakarov
*ab,
Marina
Kuperman
a,
Mykhaylo
Losytskyy
a,
Serhii
Vakarov
 bc,
Slawomir
Potocki
bc,
Slawomir
Potocki
 d,
Sergiy
Yarmoluk
a,
Yan
Voloshin
d,
Sergiy
Yarmoluk
a,
Yan
Voloshin
 e,
Oleg
Varzatskii
e,
Oleg
Varzatskii
 bc and
Elzbieta
Gumienna-Kontecka
bc and
Elzbieta
Gumienna-Kontecka
 *d
*d
aInstitute of Molecular Biology and Genetics, NASU, 150 Zabolotnogo St., 03143 Kyiv, Ukraine. E-mail: v.kovalska@gmail.com
bSC Princeton Biomolecular Research Labs, 26A Saperne pole St., 01042, Kyiv, Ukraine
cInstitute of General and Inorganic Chemistry, 32/34 Palladin Av., 03080 Kyiv, Ukraine
dFaculty of Chemistry, University of Wroclaw, 14 F. Joliot-Curie St., 50-383 Wroclaw, Poland. E-mail: elzbieta.gumienna-kontecka@chem.uni.wroc.pl
eNesmeyanov Institute of Organoelement Compounds RAS, 28 Vavilova St., 119991, Moscow, Russia
First published on 5th December 2018
Abstract
An ability of inherently achiral macrobicyclic metal complexes iron(II) clathrochelates to acquire an induced CD (ICD) output in the visible spectral range upon interaction with bovine serum albumin (BSA) was recently discovered. In the present work, the CD-reporting properties of iron(II) clathrochelates to proteins and the thermodynamic parameters of their binding to albumins are evaluated. It is shown that iron(II) clathrochelates functionalized by six ribbed carboxyphenylsulfide groups are able to discriminate between serum albumins of relative structure (here human and bovine albumins) by giving distinct ICD spectra. Besides, by the variation of the shape and intensity of CD bands, these cage metal complexes reflect the pH-triggered alterations of the tertiary structure of albumins. The constitutional isomerism (ortho-, meta- or para-isomers) of terminal carboxyphenylsulfide groups of iron(II) clathrochelates strongly affects both the character of their ICD output upon binding with proteins and the parameters of the formed guest–host associates. Using isothermal titration calorimetry, it was determined that cage metal complexes bearing meta- and ortho-isomers of carboxyphenylsulfide groups possess higher association constants (Ka ∼ 2 × 104 M−1) and clathrochelate-to-BSA binding ratios (n = 2) than the para-isomer (Ka ∼ 5 × 103 M−1, n = 1). The iron(II) clathrochelates are suggested to be potential molecular three-dimensional scaffolds for the design of CD-sensitive reporters able to recognize specific elements of protein surfaces.
Significance to metallomicsBiological macromolecules exhibiting inherent chirality are able to induce asymmetry of achiral organic and coordination compounds and, therefore, may cause an appearance of a signal in their CD spectra. These induced CD (ICD) bands are very sensitive to the arrangement of the binding sites of hosting biomolecules. As a result, the chirality of small molecules gained upon their binding to proteins may reflect both the structural alterations and the conformation transitions of proteins (to be observed via changes in the ICD spectra). In the present work we demonstrate that hexafunctionalized iron(II) clathrochelates (that are inherently achiral) give a CD output in the visible range upon their supramolecular binding to serum albumins (HSA and BSA). Since the reported clathrochelates are able to reflect alterations of albumin structure and conformational transitions by their ICD outputs, we suggest that these cage metal complexes are prospective three-dimensional molecular scaffolds for the design of probes giving chirooptical output for use in studies of proteins. |
1. Introduction
Circular dichroism (CD) spectroscopy is a powerful spectral technique for studies of protein–ligand interactions.1 Biological macromolecules exhibiting inherent chirality are able to induce asymmetry of achiral organic and coordination compounds, and therefore may cause an appearance of a signal in their CD spectra.2–4 These induced CD (ICD) bands are very sensitive to the arrangement of the binding sites of hosting biomolecules.4 As a result, the chirality of small molecules gained upon their binding to proteins may reflect both the structural alterations and the conformation transitions of proteins (to be observed via changes in the ICD spectra). Thus molecules giving an ICD response upon interaction with proteins are studied as potential reporters with the ability to discriminate structural variants of proteins connected with various diseases via reflection of their structural differences.Serum albumin is a main transport protein of blood plasma, and its certain structural variations may act as markers of various pathological processes, such as albumin glycation in diabetes,5–7 oxidation in liver disease,6,8 N-terminal modifications in ischemia/reperfusion,6 and allosteric modifications by tumor metabolites in cancer.9
Various organic compounds and metal complexes have been reported to be ICD probes due to their binding to proteins and sensitivity to their structural alterations.10,11 In particular, an adenosine derivative that senses the hydrophobic cavity of bovine serum albumin (BSA) was reported,12 as well as a bilirubin molecule that is able to signal the conformational alternations of human serum albumin (HSA) caused by a variation of pH.13 Diazepam and dansylglycine were reported to be site-specific ligands for albumin binding site 2 (vide infra).2,14cis-Parinaric and retinoic acids were used as ligands for the hydrophobic interior cavity of β-lactoglobulin.2,15 A derivative of cobalt porphyrin was proposed to serve as a chiral probe for a few globular proteins, such as albumin, lysozyme and insulin.16 A well-known organic dye, Congo Red, was used in ICD studies as an indicator of the alterations in the structure of albumin regarding its binding sites and the formation of amyloid aggregates.17
A wide range of cage complexes with an encapsulated metal ion, known as clathrochelates, have been prepared and characterized to date.18–20 Metal complexes of this type, which are formed by rigid quasiaromatic polyazomethine macropolycyclic ligands, are metal-centered compounds because their caging (encapsulating) ligands do not exist in the metal-free form due to thermodynamic reasons.19 Iron(II) clathrochelates exhibit unique chemical and biological activities, and allow easy chemical modification of their macropolycyclic frameworks as molecular scaffolds. Data on their biological activity as submicromolecular inhibitors in the transcription systems of T7 RNA and Taq DNA polymerases,21–25 and as antifibrillogenic agents,26–28 together with axially coordinated phthalocyanines,29 were recently highlighted. The ability of these cage metal complexes to form supramolecular assemblies with serum albumins has been established using steady state and time-resolved fluorescent spectroscopy methods.30–32
Iron(II) clathrochelates with mono- and dicarboxyphenylsulfide substitution that are inherently CD-silent have been recently reported to give a strong CD output in the visible spectral range upon their binding to BSA.31 The induction of chirality has been explained by selective fixation of the metal centered framework of a clathrochelate in one of the two (Λ or Δ) trigonally antiprismatic distorted conformations upon host–guest assembly with BSA. It was shown that the intensity of the ICD signal is affected by the constitutional isomerism of the macrobicyclic reporter molecule (in particular, by ortho-, meta- or para-positions of its terminal carboxyl groups).31
The development of supramolecular compounds, particularly metal complexes, for protein surface recognition, and application as potential sensors, affinity tags, tools for protein immobilization etc., is a prospective research and practical area.33 The specificity of recognition is supposed to be achieved via non-covalent interactions between large areas of the host protein surface and multiple binding groups of a guest compound.34 In this light, the discovered ICD sensitivity of clathrochelates to albumins generates an interest in further studies of these macrocyclic metal complexes as potential reporters for proteins.
In the present work, we explore the ability of iron(II) clathrochelates bearing six carboxyphenylsulfide ribbed groups to sense and reflect the structural alterations and conformational changes of tertiary structure of proteins using ICD. For this study, “relative structure” proteins, i.e. human and bovine serum albumins, were used; their conformation transitions were triggered by pH variation. All the constitutional isomers of a hexacarboxyphenylsulfide iron(II) clathrochelate (i.e. ortho-, meta-, or para-positions of its terminal carboxyl group, Scheme 1) were tested as the reporters. The characterization of clathrochelate-to-albumin association was monitored by CD and complemented by protein fluorescence quenching studies. Isothermal titration calorimetry (ITC) was used to determine the thermodynamics of host–guest assembly of these cage metal complexes with BSA. Through the obtained data, the perspective of application of iron(II) clathrochelates as chirooptical probes for the sensing of proteins is explored.
2. Materials and methods
2.1. Materials
The clathrochelates were prepared as described elsewhere.35 BSA and HSA were obtained commercially (fraction V from Sigma-Aldrich® and fraction V protease free from Fisher Bioreagents®, respectively). 0.05 M Tris–HCl aqueous buffer (pH 7.9), 0.05 M aqueous phosphate buffers (pH 5 and pH 6), and 0.05 M aqueous acetate buffer (pH 3.7) were used as solvents.2.2. Preparation of the solutions for protein fluorescence quenching studies
Aqueous solutions of BSA and HSA (concentration of 0.2 mg ml−1 – 3 μM) were prepared by dissolution of their weighed amounts in corresponding buffer. 2 mM stock DMSO solutions of the clathrochelates were prepared, and an aliquot was added to Tris–HCl buffer solution of a chosen protein. Since the amount of added DMSO solution was rather small (up to 2.5% of the total volume), the protein's concentration remained practically unchanged (0.2 mg ml−1), while that of the clathrochelate changed from 0 to 15 μM.2.3. Fluorescence assay
To study the quenching of albumin fluorescence upon the binding of iron(II) clathrochelates, the fluorescence spectra of the protein itself and after the addition of a DMSO solution of clathrochelate were recorded. Albumin fluorescence was excited at 280 and 295 nm, and the signal was recorded at λmax of corresponding emission. Fluorescence spectra were measured at 5 nm width of the excitation and emission slits at room temperature on a Cary Eclipse fluorescence spectrophotometer (Varian) in a 1 cm × 1 cm quartz cell. To avoid possible errors caused by differences in the media used for the fluorescent and ITC experiments (Tris–HCl aqueous buffer and sodium acetate aqueous solution, respectively), fluorescence quenching measurements were also performed in sodium acetate aqueous solution. The observed differences were found to be insignificant.An increase in the clathrochelate concentration led to the simultaneous increase in the optical density at maxima of BSA excitation and/or emission, thus causing a decrease of observed fluorescence intensity because of the so-called “inner filter effect” (IFE), as well as due to the reabsorption process.36,37 These effects should be taken into account for the correct interpretation of the experimental data.37–39 The correction factor for these effects can be calculated using eqn (1):39
| Icor = Iobs × 10(Dex+Dem)/2 | (1) |
2.4. UV-vis spectroscopy
The UV-vis absorbance spectra of BSA–clathrochelate assemblies were recorded at room temperature on a Specord M-40 spectrophotometer (Carl Zeiss, Germany). An aliquot of 2 mM stock DMSO solution of the cage complex was added to 0.05 M Tris–HCl aqueous buffer solution, pH 7.9. The concentration of clathrochelate was varied from 0 to 15 μM.2.5. ICD spectroscopy
The CD spectra were recorded on a Jasco J-1500 spectropolarimeter at room temperature in the 300–600 nm range, in 0.1 nm steps with a scan of 3001 points; three scans were averaged for each of the ICD spectra. The data expressed as ellipticity (mdeg) were obtained in mdeg units directly from the instrument. Tris–HCl aqueous buffer (pH 7.9), phosphate aqueous buffers (pH 5 and pH 6) and acetate aqueous buffer (pH 3.7) were used for the preparation of stock solutions of the proteins as well as for working solutions with an albumin-to-clathrochelate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio (calbumin = 8 × 10−5 mol l−1, cclt = 4 × 10−5 mol l−1). It should be mentioned that the observed fluorescence quenching is rather low at such a molar ratio. But due to the peculiarities of the experiment (higher sensitivity of the fluorescence method compared to the CD method), absolute concentrations used in CD studies were much higher as compared to the ones used in fluorescence studies (albumin concentration equals 8 × 10−5 mol l−1 and 3 × 10−6 mol l−1 respectively). Thus, a significantly higher percentage of clathrochelate molecules is bound to albumins at a 2
1 molar ratio (calbumin = 8 × 10−5 mol l−1, cclt = 4 × 10−5 mol l−1). It should be mentioned that the observed fluorescence quenching is rather low at such a molar ratio. But due to the peculiarities of the experiment (higher sensitivity of the fluorescence method compared to the CD method), absolute concentrations used in CD studies were much higher as compared to the ones used in fluorescence studies (albumin concentration equals 8 × 10−5 mol l−1 and 3 × 10−6 mol l−1 respectively). Thus, a significantly higher percentage of clathrochelate molecules is bound to albumins at a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio for the conditions of the CD experiment, and induced CD was clearly observed.
1 molar ratio for the conditions of the CD experiment, and induced CD was clearly observed.
The clathrochelate binding site was evaluated by displacement of clathrochelate upon addition of site markers: (i) warfarin,3,41 and (ii) ibuprofen.13 A 50-fold excess (2 × 10−3 M) of warfarin or ibuprofen was added to a mixture of protein (calbumin = 8 × 10−5 mol l−1) and clathrochelate (cclt = 4 × 10−5 mol l−1).
2.6. ITC experiments
A 5 mM solution of clathrochelate in 0.15 M aqueous sodium acetate was added to a 0.5 mM solution of BSA in 0.15 M aqueous sodium acetate thus obtaining a working solution with clathrochelate-to-protein molar ratios from 0 to 3. ITC experiments were carried out on a Nano ITC calorimeter (TA Instruments), equipped with a standard 1.0 ml cell (24K Gold). The stock solution of clathrochelate was added to the cell using a 250 μl syringe. The calorimetric experiments were operated by Nano ITC Run v. 2.2.3 software. All the experimental data were evaluated using the NanoAnalyze v. 2.4.1 program package and an independent model was used for their interpretation. In each case, control experiments were performed and the enthalpies of dilution of the components were subtracted from the data for their supramolecular assembly.3. Results and discussion
3.1. Supramolecular binding of the hexacarboxyl-terminated iron(II) clathrochelates to BSA and HSA
BSA and HSA are heart-shaped proteins, the crystal structures of which are very similar.41 The molecule of HSA consists of three helical domains (I, II and III), each comprising two subdomains (A and B; Fig. 1). The drug binding site 1 (Sudlow's I) is a binding pocket within a core in the subdomain IIA. Drug binding site 2 (Sudlow's II) is located in subdomain IIIA.42–44 Despite the similarity of BSA and HSA molecular structures, the proteins possess certain regions with substantial differences in amino acid sequences;45 the BSA molecule was reported to be less conformationally flexible than HSA.46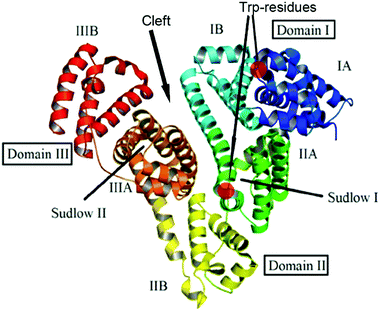 | ||
| Fig. 1 Structure of the albumin molecule (adopted from ref. 48). | ||
The type of serum albumin, BSA versus HSA, was shown to affect the association of various drugs/toxins, such as warfarin, ochratoxin A and phenylbutazone,46,47 with site 1, i.e. the stability constants and competing capacity. The structural distinctions between the binding sites of BSA and HSA are suggested to cause variations of the ICD output of clathrochelates upon their association.
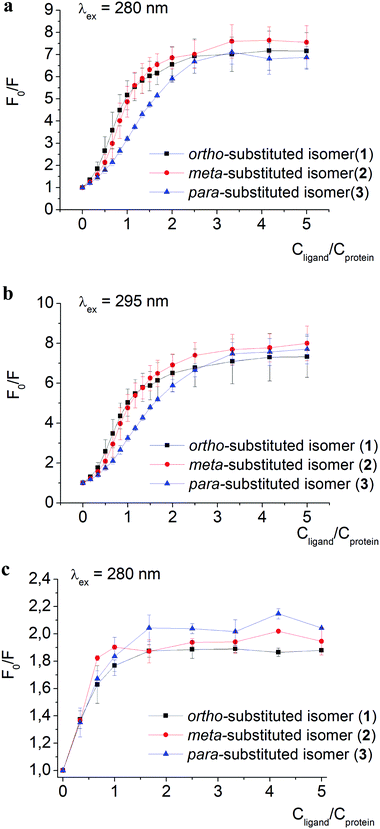
The quenching of proteins’ intrinsic fluorescence observed in the presence of clathrochelate compounds (Fig. 2 and Fig. S1a–i, ESI†) evidenced the formation of their host–guest supramolecular associates. The fluorescence intensity was much more affected by clathrochelate addition in the case of BSA (6.9–7.5-fold quenching, Fig. 2a) than in that of HSA (2–2.4-fold quenching, Fig. 2c). Shifts of the corresponding emission maxima (up to 20 nm to the short-wavelength range for BSA, and up to 9 nm to the long-wavelength range for HSA) were observed (Fig. S1 and S2, ESI†).
Tryptophan (Trp) residues are the brightest fluorophores in albumin, and characteristics of Trp emission (the emission intensity and maximum) are sensitive to the surrounding medium.49,50 Apart from Trp, also tyrosine (Tyr) residues contribute to the intrinsic fluorescence of proteins;51 BSA and HSA contain twenty and eighteen Tyr residues per protein macromolecule, respectively.2,41 However, in comparison to Trp, the fluorescence of Tyr residues is less intense and independent of the local environment.
A weak effect of fluorescence quenching and a small shift of the emission maximum observed upon iron(II) clathrochelate binding to HSA might be explained by the low accessibility of the single Trp-214 residue that is hidden within a protein globule (subdomain IIA). On the other hand, the BSA macromolecule contains two Trp residues: surface-located Trp-134 (subdomain IB), and pocket-buried Trp-213 (subdomain IIA, Fig. 1).50 The efficiency of BSA fluorescence quenching by hexacarboxyphenylsulfide iron(II) clathrochelates is similar to that of the ortho- and para-isomers of di-substituted macrobicyclic analogs, but is smaller than that caused by binding of the di-meta-substituted cage complex.31,50
The Stern–Volmer plots for BSA–clathrochelate assemblies (Fig. 2a and b) suggest that ortho- and meta-isomers (complexes 1 and 2, respectively) possess similar fluorescence quenching patterns (with a bend of the corresponding curves at a clathrochelate-to-BSA 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio), while for para-substituted analog 3 the increase is more gradual (with a bend at a molar ratio of approximately 3
2 molar ratio), while for para-substituted analog 3 the increase is more gradual (with a bend at a molar ratio of approximately 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). For HSA, the quenching patterns for iron(II) clathrochelate isomers are of similar shape (Fig. 2c and Fig. S3, ESI†) with the bend values close to a 1
1). For HSA, the quenching patterns for iron(II) clathrochelate isomers are of similar shape (Fig. 2c and Fig. S3, ESI†) with the bend values close to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. The observed behavior could be explained by “multiple quenching” of two Trp residues in BSA, and “single” quenching of one Trp residue in HSA.
1 molar ratio. The observed behavior could be explained by “multiple quenching” of two Trp residues in BSA, and “single” quenching of one Trp residue in HSA.
To estimate the effect of the clathrochelate binding on the fluorescence of both Trp and Tyr amino acids, we performed quenching experiments (Fig. 2a–c and Fig. S3, ESI†) upon excitation at 280 nm, where both Trp and Tyr are excited, and at 295 nm where only Trp residues are excited.49 The data clearly show similar quenching patterns upon both used excitation wavelengths for clathrochelate assemblies with BSA or HSA. This indicates that the binding of clathrochelates to albumins quenches mainly Trp emission, and only slightly that of Tyr residues.
Thus we suggest that the quenching of albumin fluorescence is caused by the binding of clathrochelates near Trp-213 and Trp-134 of BSA, or Trp-214 of HSA; the binding most probably occurs at site 1 located in protein subdomain IIA.
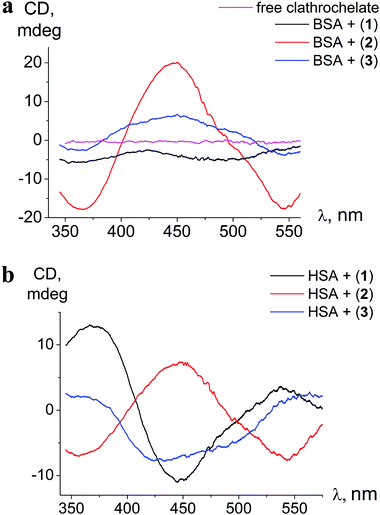 | ||
| Fig. 3 ICD spectra of clathrochelates 1–3 in the presence of BSA (a) and HSA (b); calbumin = 8 × 10−5 mol l−1, cclt= 4 × 10−5 mol l−1. Spectra are recorded in 0.05 M Tris HCl pH 7.9. | ||
The binding to albumins induces CD bands that are distinct for HSA and BSA, in terms of intensity in the case of clathrochelate meta-isomer 2, and also in the shape for ortho- and para-substituted analogs 1 and 3, respectively (Fig. 3a and b). meta-Isomer 2 shows a positive-sign ICD band with a maximum at 446 nm and higher intensity of the CD signal. The ICD band of para-substituted clathrochelate 3 bound to BSA is less intense, but has a similar shape to that of meta-isomer 2 (dominant positive-sign band at 450 nm). In the presence of HSA, the spectrum of para-isomer 3 changes its shape exhibiting a “double peak” negative-sign band with the main maximum near 430 nm. The ICD spectrum of ortho-substituted clathrochelate 1 in the presence of BSA is of low intensity and located in the negative range; with HSA it is much more intense and contains a negative band with a maximum at 447 nm.
The intensities of ICD bands of hexa-carboxyl-terminated iron(II) clathrochelates in the presence of BSA are substantially lower than those of their dicarboxyl-terminated analogs reported earlier.31 Moreover, for all the di- and mono-substituted carboxyphenylsulfide cage metal complexes the shape of ICD bands in the presence of BSA was similar, with an intense negative maximum at 450 nm.31
Hexa-carboxyphenylsulfide iron(II) clathrochelates reveal the ICD sensitivity to related serum albumins, and are capable of discriminating between BSA and HSA hosts, giving different ICD spectra. This spectral discrimination is affected by the constitutional isomerism of the macrobicyclic complexes: it is more pronounced for ortho- and para-substituted cage complexes, 1 and 3, respectively, and substantially less manifested in the case of meta-isomer 2.
The differences in the sequences and thus structures of albumins lead to the alterations of the arrangement of the drug binding sites of BSA and HSA. Hence, variations of ICD outputs of BSA- and HSA-bound hexacarboxy-terminated iron(II) clathrochelates are suggested to be caused by distinct arrangements of BSA– and HSA–clathrochelate assemblies that come from the structural distinctions of BSA and HSA binding sites. The fluorescent data obtained here clearly indicate that the clathrochelate–protein binding is associated with albumin's drug site 1.
3.2. Sensing of albumin conformational transitions by ICD of clathrochelates
To estimate the effect of albumin tertiary structure alterations on the ICD response given by iron(II) clathrochelates, the protein conformation changes were triggered by the variation of pH (Table 1).13,52–57 The acidic F-form mainly concerns unfolding of domain III, while in the basic B-form an alteration of the conformation of domain I occurs.53 In the neutral N-form, an interaction between site 1 and site 2 is suggested, while in the B-form, the distance between the corresponding subdomains increases and this interaction disappears.58| Conformer | F (fast) | ↔ | N (normal) | ↔ | B (basic) |
|---|---|---|---|---|---|
| pH range | 2.5–3.5 | 5–7 | 9–10 | ||
| Transition pH | 3.5–5 | 7–9 |
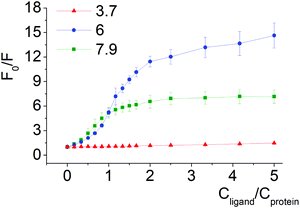
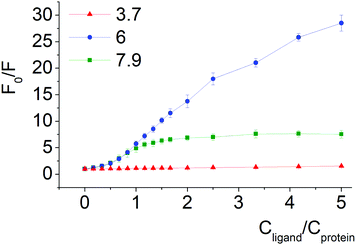
Changes in ICD spectra of the protein–clathrochelate assemblies with pH variations were studied for both albumins. The corresponding fluorescence quenching experiments were performed only for BSA since its intrinsic emission is more affected by assembling with clathrochelate (see Section 3.1.2).
Quenching of BSA intrinsic fluorescence depends on the constitutional isomerism of the iron(II) clathrochelate terminal carboxy groups (Fig. 4, 5 and Fig. S6, ESI†). Maximal variation of quenching versus pH was observed for para-isomer 3 (from 2- to 37-fold, upon the F–N transition and N-forms of BSA, respectively, (Fig. S5, ESI†)). Also, constitutional isomerism affected the quenching patterns of these cage metal complexes (Fig. S5, ESI†).
It has to be mentioned that the pH-dependent experiments were performed in a range of pH that includes the albumin isoelectric point and pK of iron(II) clathrochelates. Both proteins have the same isoelectric point with pI about 4.7;49,52 pK values for the hexacarboxyphenylsulfide cage metal complexes were estimated by potentiometric titrations, and are in the range 5.5–6.3, depending on constitutional isomerism (data not presented).
Processes of dissociation of terminal carboxyl groups of clathrochelates are considered to affect the albumin–clathrochelate non-covalent interactions, leading to strong quenching of Trp fluorescence at pH 6, as compared to pH 3.7. This also indicates the proximity of the guest clathrochelate to a Trp residue in the formed assembly.
Moreover, the small effect of iron(II) clathrochelates on BSA fluorescence in acidic media could be due to the conformation transition (spatial separation) of the domains III and I (Fig. 2). In the N-form, the binding of the clathrochelate molecule to site I (domain I) could be additionally stabilized by spatial interactions with proximal domain III, thus separation of this domain upon the decrease of pH promotes re-arrangement or dissociation of the clathrochelate–protein associate.
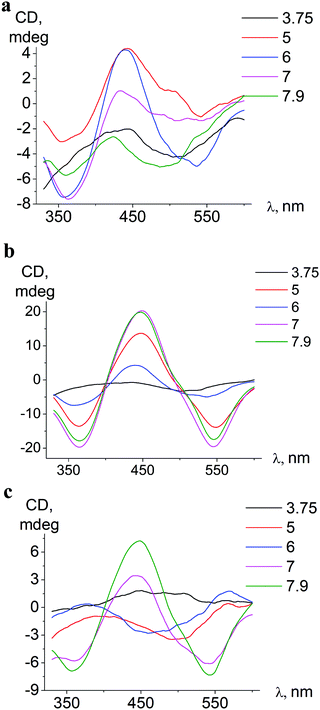 | ||
| Fig. 6 pH-Dependent ICD spectra of ortho-isomer 1 bound to BSA (a), meta-isomer 2 bound to BSA (b), and meta-isomer 2 bound to HSA (c). | ||
The conformation alterations of a host protein molecule triggered by pH variation cause changes of the ICD spectra of the guest clathrochelate; the changes depend on the origin of serum albumin (BSA or HSA).
In associates with HSA, iron(II) clathrochelates change the shape and intensity of their ICD-bands upon pH variation, indicating rearrangements59 of protein–clathrochelate assemblies. The changes were the most pronounced for meta-isomer 2 (Fig. 6).
Upon pH-triggered conformational modifications of BSA, the intensity and maxima of ICD-bands of meta- and para-clathrochelates were changed, while in the case of ortho-clathrochelate also the shape of the bands was altered (Fig. 6a, b and Fig. S5, ESI†).
In acidic medium (pH 3.7) all iron(II) clathrochelate isomers showed low intensity ICD spectra (up to ΔCD = 2.2 mdeg for ortho-isomer 1 with BSA); together with a very low albumin fluorescence quenching degree (Fig. 4 and 5) this may evidence clathrochelate–albumin assembly dissociation.
In the pH range 5–9 the bands of ICD spectra of clathrochelate–albumin assemblies are the most intense (Fig. 6a–c and Fig. S5, ESI†) and the ability to discriminate between BSA and HSA hosts via the difference of ICD signals is the most pronounced. The variations of the ICD spectra probably reflect rearrangement of the albumin–clathrochelate assembly that may occur upon passing through clathrochelate pK points (at pH 5.5–6.3), and also at more basic pH (7–8) may be caused by change of protein conformation from the N- towards the B-form.
Thus iron(II) clathochelates in assemblies with albumin change the shape and intensity of the CD spectra with a variation of the pH of the medium, and consequently with protein tertiary structure transitions. This points to the high sensitivity of clathrochelate CD output to the arrangement of the structure of their associates with host proteins. Thus, it allows suggesting these cage metal complexes as potential tools for monitoring of protein conformation changes.
3.3. Competitive binding studies using ibuprofen and warfarin as site specific binders
To evaluate the preferable iron(II) clathrochelate binding site in serum albumins, we carried out experiments of clathrochelate displacement by specific binders of albumin sites 1 and 2, warfarin and ibuprofen, respectively.3,13,40 These site-specific binders show high affinity to albumin, with measured binding constants of about 2.5 × 105 M−1 for warfarin,60 and 2 × 106 M−1 for ibuprofen.61 Upon binding to serum albumin, neither warfarin nor ibuprofen exhibit any ICD spectra in the visible spectral range.3 Therefore, evaluation of the iron(II) clathrochelate preference to certain binding sites is performed by monitoring the changes in the ICD spectra of clathrochelate upon the addition of each of these site-specific binders.For the competitive binding studies on serum albumin with site-specific ligands, the para-substituted constitutional isomer 3 was used since it shows the binding ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to albumin (see Section 3.4), and certain specificity to only one of the binding sites could be expected. Generally, an addition of low concentrations of site-specific ligands to the clathrochelate–protein assembly did not influence the clathrochelate ICD output. Only the presence of a high excess of the site-specific binders (from 30-fold excess) resulted in changes of the ICD-band intensity (Fig. 7).
1 to albumin (see Section 3.4), and certain specificity to only one of the binding sites could be expected. Generally, an addition of low concentrations of site-specific ligands to the clathrochelate–protein assembly did not influence the clathrochelate ICD output. Only the presence of a high excess of the site-specific binders (from 30-fold excess) resulted in changes of the ICD-band intensity (Fig. 7).
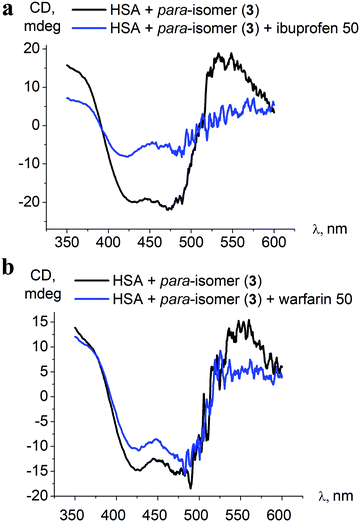 | ||
| Fig. 7 Initial ICD spectra of the para-isomer 3–HSA assembly and those after addition of a 50-fold excess of albumin site-specific drugs ibuprofen (a) and warfarin (b). | ||
Despite the affinity of the site-specific binders being a few orders of magnitude higher than that determined for clathrochelate 3 (2.5 × 105 M−1 for warfarin, 2 × 106 M−1 for ibuprofen, over 5.3 × 103 M−1 for 3, Table 2), only in the case of a 50-fold excess of ibuprofen over the clathrochelate 3–HSA assembly was a noticeable decrease of the ICD band intensity, up to 2.5-fold, observed (Fig. 7). This indicates that warfarin and ibuprofen displace the iron(II) clathrochelate from its associate with albumin only insignificantly, and thus the competitive binding studies do not provide specific information about the binding site preference.
| Compound | ΔHa (kJ mol−1) | ΔSa (J mol−1 K−1) | ΔGa (kJ mol−1) | K a (103 mol l−1) | n |
|---|---|---|---|---|---|
a ΔH, ΔS and ΔG are the changes of enthalpy, entropy and Gibbs free energy, respectively, upon the binding of clathrochelates to BSA.
b
K
a is a clathrochelate-to-BSA association constant.
c
n is the stoichiometry of the interaction, thus also the calculated number of the clathrochelates molecules per BSA macromolecule as a host. The area under each peak was integrated and fit to an independent binding model to determine the binding affinity (Ka), enthalpy (H) and stoichiometry (n). Since the temperature (T) is held constant throughout, the free energy (ΔG) of the binding reaction can be determined by: ΔG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka. ITC directly measures ΔH, so the change in entropy (ΔS) can be determined by: ΔS = (ΔH − ΔG)/T. The independent model assumes n-independent binding sites and close thermodynamic parameters of each site. The binding ratio 2 Ka. ITC directly measures ΔH, so the change in entropy (ΔS) can be determined by: ΔS = (ΔH − ΔG)/T. The independent model assumes n-independent binding sites and close thermodynamic parameters of each site. The binding ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was obtained for ortho- and meta-isomers, thus both isomers bind to BSA with similar Ka. 1 was obtained for ortho- and meta-isomers, thus both isomers bind to BSA with similar Ka.
|
|||||
| 1 | −9.1 | 53.2 | −24.9 | 24 | 1.95 |
| 2 | −17.2 | 27.2 | −25.3 | 28 | 1.9 |
| 3 | −23.2 | −6.5 | −19.3 | 5.3 | 0.9 |
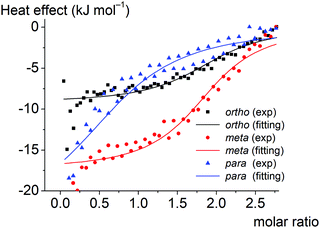
3.4. Calorimetric study of the supramolecular clathrochelate-to-BSA association
Thermodynamic parameters of supramolecular BSA-to-clathrochelate assemblies were determined using the ITC technique (Fig. 8); the obtained values are summarized in Table 2. The difference between the binding modes of ortho- and meta-isomers and the para-isomer is clearly seen from the shapes of the plots of the binding heat effect versus the clathrochelate-to-BSA molar ratio (Fig. 8), as well as from the values of the corresponding thermodynamic parameters (Table 2). Indeed, ΔG values for meta- and ortho-isomers of clathrochelate complexes are similar, while that for the para-substituted cage complex is smaller. According to this, the binding constant values Ka are almost the same for the ortho- and meta-isomers 1 and 2 (2.4 × 104 and 2.8 × 104 M−1, respectively), while for para-functionalized compound 3 this value is 5 times lower (5.3 × 103 M−1). These data are in accordance with the results of the fluorescent quenching studies (Fig. 2a and b), where ortho- and meta-isomers 1 and 2 quench the BSA emission with the same intensity, while para-isomer 3 demonstrates less intense quenching. Thus, the supramolecular binding of the ortho- and meta-substituted iron(II) clathrochelates to BSA is suggested to be more favorable as compared to their para-isomer 3.Enthalpy (ΔH) and entropy (ΔS) changes for these assemblies are also affected by the constitutional isomerism of the iron(II) clathrochelates (Table 2). Both hydrophobic interactions (ΔS > 0) and intermolecular attractions (ΔH < 0) contribute to the binding of the ortho- and meta-substituted iron(II) cage complexes 1 and 2 to BSA. In the case of their para-isomer 3, we suggest that its assembly with BSA is driven mainly by intermolecular attractions (ΔH < 0), while a contribution of hydrophobic interactions does not exceed the entropy losses caused by the decrease in conformational degrees of freedom upon the corresponding protein-to-clathrochelate supramolecular binding (thus, total ΔS < 0). The influence of hydrophobic effects on binding decreases in the order ortho- > meta- > para-, while enthalpy effects increase (a predominant role of the energy from non-covalent interactions).
The obtained results can be also explained by the peculiarities of the spatial arrangements of the terminal functionalizing carboxyl groups of the clathrochelate guests. In the case of para-isomer 3 of the hexacarboxyphenylsulfide clathrochelate, an external “shell” of the molecule is formed by the “sticking out” terminal polar carboxyl groups, which hampers the ability of this molecule to enter the hydrophobic interactions. In the case of the ortho- and meta-substituted clathrochelate molecules 1 and 2, their terminal carboxyl groups are positioned closer to the cage framework, thus making their inclusion into the hydrophobic cavities of protein macromolecules easier and their supramolecular binding is energetically more favorable. As for the enthalpy effect, we suppose that the para-isomer 3 has a higher local dipole moment of ribbed substituents as compared to those for meta- and ortho-substituted analogs 1 and 2, thus causing an increase in the energy of dipole–dipole interactions.
The number of the iron(II) clathrochelate molecules per BSA macromolecule (n) also depends on their constitutional isomerism. This ratio is estimated as n = 2 for the ortho- and meta-isomers 1 and 2 and n = 1 for the para-substituted analog 3. This suggests that one additional site on the BSA globule is available for the “stable binding” of the ortho- and meta-substituted clathrochelates. This could be explained by the higher number of geometric configurations of the clathrochelate molecule (i.e. the variations of the shape) available due to the rotation of “inherently asymmetric” ortho- and meta-functionalized ribbed substituents compared to the “inherently symmetric” para-functionalized substituents. Higher structural variability allows more flexible tuning of the iron(II) clathrochelate geometry to the shape of the binding site and promotes more precise adsorption of carboxyphenylsulfude groups to the complimentary binding groups of the protein. The independent binding model suggests that two molecules of each clathrochelate isomer bind to albumin with similar Ka. Hence we could consider two “thermodynamically similar” sites on the BSA molecule where ortho- and meta-isomers are able to bind.
Among studied clathrochelates, the para-isomer is the most sensitive to albumin binding site structure distinctions (discriminating HSA and BSA) at neutral and slightly basic pH. This isomer strongly reflects changes that the albumin molecule undergoes when passing from acidic to slightly basic pH, and the quenching of protein fluorescence (i.e. effect on the Trp residue) at slightly basic media is most pronounced for this isomer. The clathrochelate-to-albumin binding ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (established by ITC) and strong effect on protein fluorescence allow suggesting that the exact binding site for this isomer is Sudlow site 1 of the albumin molecule. Thus, despite the lower binding constant values compared with ortho- and meta-clathrochelate isomers, the para-isomer is considered most suitable as a structure sensitive CD-reporting molecule for albumins.
1 (established by ITC) and strong effect on protein fluorescence allow suggesting that the exact binding site for this isomer is Sudlow site 1 of the albumin molecule. Thus, despite the lower binding constant values compared with ortho- and meta-clathrochelate isomers, the para-isomer is considered most suitable as a structure sensitive CD-reporting molecule for albumins.
4. Conclusions
In summary, cage metal complexes iron(II) clathrochelates have shown potency as molecular three-dimensional scaffolds for the design of CD-sensitive reporters able to recognize specific elements of protein surfaces. These important unconventional properties may lead to their possible future use in biochemistry- and medicine-related fields.Here we show that hexa-carboxyphenylsulfide iron(II) clathrochelates discriminate between proteins of similar structure, in this case HSA and BSA, giving distinct ICD spectra. These cage metal complexes sense the variation of the arrangement of binding sites of these particular proteins. Binding of clathrochelates to site 1 of albumins is suggested based on protein fluorescence quenching studies.
These iron(II) clathrochelates bound to albumin could reflect the transitions of the protein conformation by the changes of the band profile and intensity of their CD spectra. In this work, such changes of ICD bands were observed together with the variation of medium pH that in turn triggered the alteration of the tertiary structure of albumin.
The above described reporting properties depend on the constitutional isomerism of the ribbed carboxyphenyl substituents and are the most pronounced for the ortho- and para-isomers of the iron(II) clathrochelate. The para-isomer clathrochelate is suggested as the most appropriate structure-sensitive CD-reporter for albumin studies and discrimination.
The thermodynamic parameters of the clathrochelate-to-BSA assemblies were estimated by ITC, which showed that constitutional isomerism determines the characteristics of such assemblies. Thus meta- and ortho-isomers have higher binding constants (Ka ∼ 2 × 104 M−1) and clathrochelate-to-albumin binding ratios (n = 2) than the para-isomer (Ka ∼ 5 × 103 M−1, n = 1).
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The project leading to these results has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 778245. The synthesis of cage complexes was supported by the Russian Science Foundation (grant 16-13-10475).References
- C. Wolf and K. Bentley, Chirality sensing using stereodynamic probes with distinct electronic circular dichroism output, Chem. Soc. Rev., 2013, 42, 5408–5424 RSC.
- N. Berova, L. Di Bari and G. Pescitelli, Application of electronic circular dichroism in configurational and conformational analysis of organic compounds, Chem. Soc. Rev., 2007, 36, 914–931 RSC.
- D. Venturini, A. de Souza, I. Caracelli, N. Morgon, L. da Silva-Filho and V. Ximenes, Induction of axial chirality in divanillin by interaction with bovine serum albumin, PLoS One, 2017, 12, e0178597 CrossRef PubMed.
- F. Zsila, Z. Bikádi, I. Fitos and M. Simonyi, Probing protein binding sites by circular dichroism spectroscopy, Curr. Drug Discovery Technol., 2004, 1, 133–153 CrossRef CAS.
- Y. Lopukhin, G. Dobretsov and Y. Gryzunov, Conformational changes in albumin molecule: a new response to pathological process, Bull. Exp. Biol. Med., 2000, 130, 615–619 CrossRef CAS.
- K. Oettl and R. Stauber, Physiological and pathological changes in the redox state of human serum albumin critically influence its binding properties, Br. J. Pharmacol., 2007, 151, 580–590 CrossRef CAS.
- A. Arasteh, S. Farahi, M. Habibi-Rezaei and A. Moosavi-Movahedi, Glycated albumin: an overview of the in vitro models of an in vivo potential disease marker, J. Diabetes Metab. Disord., 2014, 13, 49 CrossRef.
- A. Ivanov, E. Korolenko, E. Korolik, S. Firsov, R. Zhbankov, M. Marchewka and H. Ratajczak, Chronic liver and renal diseases differently affect structure of human serum albumin, Arch. Biochem. Biophys., 2002, 408, 69–77 CrossRef CAS.
- M. Moergel, P. Kämmerer, K. Schnurr, M. Klein and B. Al-Nawas, Spin electron paramagnetic resonance of albumin for diagnosis of oral squamous cell carcinoma (OSCC), Clin. Oral Investig., 2012, 16, 1529–1533 CrossRef.
- P. Mineo, N. Micali, V. Villari, M. G. Donato and E. Scamporrino, Reading of protein surfaces in the native state at micromolar concentrations by a chirogenetic porphyrin probe, Chemistry, 2012, 18, 12452–12457 CrossRef CAS.
- D. Tedesco and C. Bertucci, Induced circular dichroism as a tool to investigate the binding of drugs to carrier proteins: classic approaches and new trends, J. Pharm. Biomed. Anal., 2015, 113, 34–42 CrossRef CAS.
- M. Nag, K. Bera, S. Chakraborty and S. Basak, Sensing of hydrophobic cavity of serum albumin by an adenosine analogue: fluorescence correlation and ensemble spectroscopic studies, J. Photochem. Photobiol., B, 2013, 127, 202–211 CrossRef CAS.
- N. Berova, Comprehensive chiroptical spectroscopy, Hoboken, NJ, Wiley, 2012, 665–705 Search PubMed.
- F. S. Graciani and V. F. Ximenes, Investigation of human albumin-induced circular dichroism in dansylglycine, PLoS One, 2013, 8, e76849 CrossRef CAS.
- F. Zsila, T. Imre, P. T. Szabó, Z. Bikádi and M. Simonyi, Induced chirality upon binding of cis-parinaric acid to bovine beta-lactoglobulin: spectroscopic characterization of the complex, FEBS Lett., 2002, 520, 81–87 CrossRef CAS.
- P. Mineo, N. Micali, V. Villari, M. G. Donato and E. Scamporrino, Reading of protein surfaces in the native state at micromolar concentrations by a chirogenetic porphyrin probe, Chemistry, 2012, 18, 12452–12457 CrossRef CAS.
- D. N. de Vasconcelos and V. F. Ximenes, Albumin-induced circular dichroism in Congo red: applications for studies of amyloid-like fibril aggregates and binding sites, Spectrochim. Acta, Part A, 2015, 150, 321–330 CrossRef.
- Y. Z. Voloshin, N. A. Kostromina and R. Kramer, Clathrochelates: synthesis, structure and properties, Elsevier Science, Amsterdam, Oxford, 2002 Search PubMed.
- Y. Z. Voloshin, I. Belaya and R. Krämer, Cage Metal Complexes clathrochelates revisited, Springer, 2017 Search PubMed.
- S. Tomyn, S. I. Shylin, D. Bykov, V. Ksenofontov, E. Gumienna-Kontecka, V. Bon and I. O. Fritsky, Indefinitely stable iron(IV) cage complexes formed in water by air oxidation, Nat. Commun., 2017, 8, 14099 CrossRef CAS.
- V. V. Novikov, O. A. Varzatskii, V. V. Negrutska, Y. N. Bubnov, L. G. Palchykovska, I. Y. Dubey and Y. Z. Voloshin, Size matters, so does shape: inhibition of transcription of T7 RNA polymerase by iron(II) clathrochelates, J. Inorg. Biochem., 2013, 124, 42–45 CrossRef CAS PubMed.
- O. A. Varzatskii, V. V. Novikov, S. V. Shulga, A. S. Belov, A. V. Vologzhanina, V. V. Negrutska, I. Y. Dubey, Y. N. Bubnov and Y. Z. Voloshin, Copper-promoted reductive homocoupling of quasi-aromatic iron(II) clathrochelates: boosting the inhibitory activity in a transcription assay, Chem. Commun., 2014, 50, 3166–3168 RSC.
- O. A. Varzatskii, S. V. Shul'ga, A. S. Belov, V. V. Novikov, A. V. Dolganov, A. V. Vologzhanina and Y. Z. Voloshin, Copper(I)- and copper(0)-promoted homocoupling and homocoupling-hydrodehalogenation reactions of dihalogenoclathrochelate precursors for C–C conjugated iron(II) bis-cage complexes, Dalton Trans., 2014, 43, 17934–17948 RSC.
- A. Belov, A. Vologzhanina, V. Novikov, V. Negrutska, I. Dubey, Z. A. Mikhailova, E. G. Lebed and Y. Voloshin, Synthesis of the first morpholine-containing iron(II) clathrochelates: a new class of efficient functionalized transcription inhibitors, Inorg. Chim. Acta, 2014, 421, 300–306 CrossRef CAS.
- O. A. Varzatskii, A. V. Vologzhanina, V. V. Novikov, S. V. Vakarov, R. V. Oblap and Y. Z. Voloshin, Inhibition of DNA synthesis in the transcription system of Taq DNA polymerase by various iron and cobalt(II) tris-dioximate clathrochelates: in vitro study and X-ray structure of leader inhibitors, the carboxyl-terminated macrobicyclic complexes, Inorg. Chim. Acta, 2018, 482, 90–98 CrossRef CAS.
- V. Kovalska, S. Chernii, V. Cherepanov, M. Losytskyy, V. Chernii, O. Varzatskii, A. Naumovets and S. Yarmoluk, The impact of binding of macrocyclic metal complexes on amyloid fibrillization of insulin and lysozyme, J. Mol. Recognit., 2017, 30, e2622 CrossRef.
- V. Kovalska, V. Cherepanov, M. Losytskyy, S. Chernii, A. Senenko, V. Chernii, I. Tretyakova, S. Yarmoluk and S. Volkov, Anti-fibrillogenic properties of phthalocyanines: effect of the out-of-plane ligands, Bioorg. Med. Chem., 2014, 22, 6918–6923 CrossRef CAS.
- Y. Voloshin, V. Novikov and Y. Nelyubina, Recent advances in biological applications of cage metal complexes, RSC Adv., 2015, 5, 72621–72637 RSC.
- V. B. Kovalska, M. Y. Losytskyy, S. V. Chernii, V. Y. Chernii, I. M. Tretyakova, S. M. Yarmoluk and S. V. Volkov, Towards the anti-fibrillogenic activity of phthalocyanines with out-of-plane ligands: correlation with self-association proneness, Biopolym. Cell, 2013, 29, 473–479 CAS.
- M. Y. Losytskyy, V. B. Kovalska, O. A. Varzatskii, A. M. Sergeev, S. M. Yarmoluk and Y. Z. Voloshin, Interaction of the iron(II) cage complexes with proteins: protein fluorescence quenching study, J. Fluoresc., 2013, 23, 889–895 CrossRef CAS.
- V. B. Kovalska, S. V. Vakarov, M. V. Kuperman, M. Y. Losytskyy, E. Gumienna-Kontecka, Y. Z. Voloshin and O. A. Varzatskii, Induced chirality of cage metal complexes switched by their supramolecular and covalent binding, Dalton Trans., 2018, 47, 1036–1052 RSC.
- S. V. Vakarov, M. V. Kuperman, V. B. Kovalska and O. A. Varzatskii, Functionalization of the Fe(II) clathrochelates and the effect of the modification on their binding to albumins, Ukr. Chem. J., 2015, 81, 116–120 CAS.
- S. van Dun, C. Ottmann, L.-G. Milroy and L. Brunsveld, Supramolecular chemistry targeting proteins, J. Am. Chem. Soc., 2017, 139, 13960–13968 CrossRef CAS PubMed.
- S. H. Hewitt and A. J. Wilson, Metal complexes as “protein surface mimetics”, Chem. Commun., 2016, 52(63), 9745–9756 RSC.
- I. Belaya, G. Zelinskii, A. Belov, O. Varzatskii, V. Novikov, Y. Z. Voloshin, A. Dolganov, H. Kozlowski, Ł. Szyrwiel, Y. Bubnov and Y. Voloshin, Synthesis, spectra and properties of the first protono- and ionogenic tris-dioximate iron(II) clathrochelates, Polyhedron, 2012, 40, 32–39 CrossRef CAS.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, Kluwer Academic/Plenum, New York, London, 2nd edn, 1999 Search PubMed.
- Q. Gu and J. E. Kenny, Improvement of inner filter effect correction based on determination of effective geometric parameters using a conventional fluorimeter, Anal. Chem., 2009, 81, 420–426 CrossRef CAS.
- B. Birdsall, R. W. King, M. R. Wheeler, C. A. Lewis, S. R. Goode, R. B. Dunlap and G. Roberts, Correction for light absorption in fluorescence studies of protein–ligand interactions, Anal. Biochem., 1983, 132, 353–361 CrossRef CAS.
- M. Kubista, R. Sjoback, S. Eriksson and B. Albinsson, Experimental correction for the inner-filter effect in fluorescence spectra, Analyst, 1994, 119, 417–419 RSC.
- S. S. Sur, L. D. Rabbani, L. Libman and E. Breslo, Fluorescence studies of native and modified neurophysins. Effects of peptides and pH, Biochemistry, 1979, 18, 1026–1036 CrossRef CAS.
- S. Patel, K. K. Sharma and A. Datta, Competitive binding of Chlorin p6 and Dansyl-L-Proline to Sudlow's site II of human serum albumin, Spectrochim. Acta, Part A, 2015, 138, 925–931 CrossRef CAS.
- K. A. Majorek, P. J. Porebski, A. Dayal, M. D. Zimmerman, K. Jablonska, A. J. Stewart, M. Chruszcz and W. Minor, Structural and immunologic characterization of bovine, horse, and rabbit serum albumins, Mol. Immunol., 2012, 52, 174–182 CrossRef CAS.
- J. Ghuman, P. A. Zunszain, I. Petitpas, A. A. Bhattacharya, M. Otagiri and S. Curry, Structural basis of the drug-binding specificity of human serum albumin, J. Mol. Biol., 2005, 353, 38–52 CrossRef CAS.
- K. M. Sand, M. Bern, J. Nilsen, H. T. Noordzij, I. Sandlie and J. T. Andersen, Unraveling the nteraction between FcRn and albumin: opportunities for design of albumin-based therapeutics, Front. Immunol., 2014, 5, 682 Search PubMed.
- S. Curry, H. Mandelkow, P. Brick and N. Franks, Crystal structure of human serum albumin complexed with fatty acid reveals an asymmetric distribution of binding sites, Nat. Struct. Biol., 1998, 5, 827–835 CrossRef CAS.
- Y. Akdogan, J. Reichenwallner and D. Hinderberger, Evidence for water-tuned structural differences in proteins: an approach emphasizing variations in local hydrophilicity, PLoS One, 2012, 7, e45681 CrossRef CAS.
- M. Poór, Y. Li, G. Matisz, L. Kiss, S. Kunsági-Máté and T. Koszegi, Quantitation of species differences in albumin–ligand interactions for bovine, human and rat serum albumins using fluorescence spectroscopy: a test case with some Sudlow's site I ligands, J. Lumin., 2014, 145, 767–773 CrossRef.
- N. Morade, M. R. Ashrafi-Kooshk, S. Ghobadi, M. Shahlaei and R. Khodarahmi, Spectroscopic study of drug-binding characteristics of unmodified and pNPA-based acetylated human serum albumin: Does esterase activity affect microenvironment of drug binding sites on the protein?, J. Lumin., 2015, 160, 351–361 CrossRef.
- L. Stella, A. L. Capodilupo and M. Bietti, A reassessment of the association between azulene and [60]fullerene. Possible pitfalls in the determination of binding constants through fluorescence spectroscopy, Chem. Commun., 2008, 4744–4746 RSC.
- D. C. Carter and J. X. Ho, Structure of serum albumin, Adv. Protein Chem., 1994, 45, 153–203 CrossRef CAS.
- A. Sułkowskaa, M. Maciążek-Jurczyka, B. Bojko, J. Równicka, I. Zubik-Skupień, E. Temba, D. Pentak and W. W. Sułkowski, Competitive binding of phenylbutazone and colchicine to serum albumin in multidrug therapy: a spectroscopic study, J. Mol. Struct., 2008, 881, 97–106 CrossRef.
- P. J. Sadler and A. Tucker, pH-induced structural transitions of bovine serum albumin. Histidine pKa values and unfolding of the N-terminus during the N to F transition, Eur. J. Biochem., 1993, 212, 811–817 CrossRef CAS.
- N. El Kadi, N. Taulier, J. Y. Le Huérou, M. Gindre, W. Urbach, I. Nwigwe, P. C. Kahn and M. Waks, Unfolding and refolding of bovine serum albumin at acid pH: ultrasound and structural studies, Biophys. J., 2006, 91, 3397–3404 CrossRef CAS.
- B. Ahmad, S. Parveen and R. H. Khan, Effect of albumin conformation on the binding of ciprofloxacin to human serum albumin: a novel approach directly assigning binding site, Biomacromolecules, 2006, 7, 1350–1356 CrossRef CAS.
- M. Dockal, D. C. Carter and F. Rüker, Conformational transitions of the three recombinant domains of human serum albumin depending on pH, J. Biol. Chem., 2000, 275, 3042–3050 CrossRef CAS.
- M. Amiri, K. Jankeje and J. R. Albani, Characterization of human serum albumin forms with pH. Fluorescence lifetime studies, J. Pharm. Biomed. Anal., 2010, 51, 1097–1102 CrossRef CAS.
- A. Del Giudice, C. Dicko, L. Galantini and N. V. Pavel, Time-Dependent pH Scanning of the Acid-Induced Unfolding of Human Serum Albumin Reveals Stabilization of the Native Form by Palmitic Acid Binding, J. Phys. Chem. B, 2017, 121, 4388–4399 CrossRef CAS.
- K. Yamasaki, T. Maruyama, K. Yoshimoto, Y. Tsutsumi, R. Narazaki, A. Fukuhara, U. Kragh-Hansen and M. Otagiri, Interactive binding to the two principal ligand binding sites of human serum albumin: effect of the neutral-to-base transition, Biochim. Biophys. Acta, 1999, 1432, 313–323 CrossRef CAS.
- Z. Ferenc, Z. Bikádi and M. Simonyi, Circular dichroism spectroscopic studies reveal pH dependent binding of curcumin in the minor groove of natural and synthetic nucleic acids, Org. Biomol. Chem., 2004, 2, 2902–2910 RSC.
- J. Wilting, W. F. van der Giesen, L. H. Janssen, M. M. Weideman, M. Otagiri and J. H. Perrin, The effect of albumin conformation on the binding of warfarin to human serum albumin. The dependence of the binding of warfarin to human serum albumin on the hydrogen, calcium, and chloride ion concentrations as studied by circular dichroism, fluorescence, and equilibrium dialysis, J. Biol. Chem., 1980, 255, 3032–3037 CAS.
- S. Evoli, D. L. Mobley, R. Guzzi and B. Rizzuti, Multiple binding modes of ibuprofen in human serum albumin identified by absolute binding free energy calculations, Phys. Chem. Chem. Phys., 2016, 18, 32358–32368 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8mt00278a |
| This journal is © The Royal Society of Chemistry 2019 |